Abstract
The interthalamic adhesion (ITA) is an understudied neuroanatomical structure that forms a bridge of tissue connecting the thalamus of each hemisphere across the midline whose functional significance remains largely unknown. The likelihood of ITA absence has been reported in some studies to be increased in males, but findings have been inconsistent. We used magnetic resonance imaging to investigate the size and absence of the ITA and their relationship to thalamic volume, putative indices of white matter integrity (fractional anisotropy and mean diffusivity) within the anterior thalamic radiation and neuropsychological functions in 233 (129M/104F) healthy volunteers (age range = 8 to 68). To ensure high reliability in this study 2 operators independently rated the absence of the ITA and measured its size for all individuals. The ITA was absent in 4% of all individuals with no sex differences in its absence. Females had greater ITA size compared to males overall with both groups demonstrating nonlinear age-associated changes across the age range examined. ITA size among females correlated significantly with thalamus volume and lower mean diffusivity in the anterior thalamic radiation. Path modeling indicated that ITA size statistically mediated the relationship between age and attention among females. Our findings provide evidence for sex differences in ITA size across the lifespan, which are associated with the surrounding thalamic anatomy and neuropsychological functions.
Keywords: interthalamic adhesion, magnetic resonance imaging, diffusion tensor imaging, thalamus, sex effects
Introduction
The interthalamic adhesion (ITA) is a small bridge of tissue between the thalami, which is comprised of neurons and/or neuropil, and has received little attention in the literature. Laslo et al (2005) reported a specific circular organization of the neurons contained within the ITA that could represent in-vivo correlates of neurospheres and the Golgi morphology comprising these neurons tends to be highly variable across individuals, but with fusiform neurons being the most characteristic (Malobabic et al 1990). Several features of the ventricular system, including the ITA do not become apparent until the fetal period, around the second trimester of gestation (O’Rahilly and Müller 1990; Rosales et al, 1968). The ITA can critically influence the pattern of pressure distribution within the cerebral ventricles (Cheng et al 2010) and when the third ventricle enlarges with age there may be concomitant atrophy of the ITA (Rosales et al 1968).
Several post-mortem studies suggest that the ITA may be absent in otherwise healthy individuals and that this effect may be more pronounced in males compared to females. In a post-mortem study of 100 age- and sex-matched healthy volunteers Allen and Gorski (2001) reported that the ITA was present in 78% of females, but only 68% of males when viewed in the midsagittal plane. These authors further reported that among individuals who had this structure it was, on average, 53.3% larger in females compared to males despite the fact that male brains were larger than females. In contrast, another post-mortem study by Park et al (1993) reported that the ITA was absent in only 11.6% of the 146 individuals examined, but sex differences were not investigated.
The use of in-vivo techniques such as magnetic resonance (MR) imaging may be particularly suited for investigation of the ITA. Protocols for its identification, however, have been largely inconsistent across studies. In a meta-analysis Trzesniak et al (2011) reported that its absence ranged from 2.3% to 22.3% in healthy volunteers with 4 of 11 studies reporting rates below 10%. Few MR imaging studies have developed quantitative measures of the ITA and investigated its relationship to age and sex. It is conceivable that sex differences in the prevalence of the ITA may be related to aberrant neurodevelopmental processes that are more often observed among males (Raz et al 1994) and that size/volume differences between the sexes (e.g., Luders et al 2009) may permit females to “compensate” for overall brain size differences. Sen et al (2005) reported that the average transverse length of the ITA was longer in males compared to females whereas the vertical and anteroposterior lengths were longer in females. Although these authors reported no association between age and transverse, vertical and anteroposterior length of the thalamus, the ITA became thin and lengthened with age. Shimizu et al (2008) reported ITA ratings (defined as the number of consecutive coronal images showing this structure) were higher in healthy women compared to healthy men. In contrast, using morphometry, Ceyhan et al (2008) reported no differences between 89 healthy males and females in ITA size. Consistent with this latter study there are additional data suggesting that ITA size does not depend directly on overall brain size (Malobabić et al 1987).
Little is known regarding the relationship between the ITA and its surrounding anatomy. Thalamic nuclei may be divided into 3 groups: lateral, medial and anterior with the medial aspect forming a bridge of tissue (i.e., interthalamic adhesion) across the two thalami. Moreover, fiber tractography studies have demonstrated that the anterior thalamic radiation consists of fibers between the mediodorsal thalamic nuclei (where the interthalamic adhesion forms a bridge of tissue) and the frontal cortex (Zhou et al., 2003). Animal studies indicate that the ITA may be functionally important for providing the transfer of information across hemispheres (Hirayasu and Wada, 1992). In one study investigating the relationship between the ITA and thalamus Shimizu et al (2008) reported no significant association between mediodorsal nucleus volumes and ITA ratings among healthy volunteers. The anterior thalamic radiation is the major white matter bundle projection system within the brain that traverses the internal capsule and is a component of circuitry crucial to the formation of new memories. More specifically, these fibers extend from the anterior thalamic nuclei to the frontal lobes and cingulate regions by projecting from the hypothalamus and limbic structures (Zhou et al., 2003). Diffusion tensor imaging tractography studies (Kamali et al 2010) demonstrate the feasibility of reconstructing this fiber tract and its connectivity with the prefrontal cortex thereby permitting in-vivo investigation of the anterior thalamic radiation. To our knowledge, however, no studies have investigated the relationship between the ITA and the anterior thalamic radiation using diffusion tensor imaging tractography.
More conspicuously there are no data investigating the functional significance of the ITA, especially given prior work documenting sex differences in ITA size. Some findings indicate that the mediodorsal nuclei of the thalamus (Van der Werf et al., 2003) and anterior thalamic radiation (Booth et al 2013) have both been linked to neurocognitive functioning, thus providing an opportunity to examine whether the interthalamic adhesion is driving this effect. We used mediation analysis to examine the relationship between age and neuropsychological functioning through ITA size to specifically address the functional correlates of size differences between the sexes across the agespan. In this regard a mediational model provides more information compared to simple correlational analysis, which are indirect; thus, the “mediator” is believed to cause the outcome variable. Lastly, we believe the investigation of these correlations would be useful in the context of expanding our knowledge regarding the relationship of the interthalamic adhesion in relationship to its surrounding neuroanatomy that could potentially inform neuroradiological, neurosurgical and neuroanatomical studies.
In this study we used high resolution MR images acquired at 3T in a large cohort of 233 healthy individuals across a wide age range (8–68) to: (1) identify the overall rate at which the ITA was absent in our cohort; (2) examine sex differences in the absence/size of the ITA; (3) investigate age-associated changes in ITA size across the lifespan; and (4) examine the relationship between the size of the ITA with thalamic volume, the anterior thalamic radiation, and its functional significance. To ensure high reliability in this study 2 operators independently rated the absence of the ITA and measured its size for all individuals.
Materials and Methods
Subjects
Two-hundred thirty-three (129M/104F) healthy volunteers (age range = 8 to 68 years) were recruited through local advertisements and by word of mouth in the community. Mean (SD) age of the sample was 30.4 years (SD = 14.8). All individuals were classified as either right or left-handed based on a modified version of the Edinburgh Inventory (Oldfield, 1971). The total number of right and left handed items was scored and the laterality quotient was computed according to the following formula: [(Total R − Total L)/(Total R + Total L)] yielding a range from +1.00 (totally dextral) to −1.00 (totally sinistral). Mean laterality quotient score for the cohort was .72 (SD = .50). Exclusion criteria for all subjects included: (1) denial of any lifetime history of a major mood or psychotic disorder as determined by clinical interview using the SCID-NP (First et al 2002) or K-SADS-PL (Kaufman et al, 1997); (2) intellectual disability; (3) documented learning disability; (4) MR imaging contraindications; (5) pregnancy; and (6) significant medical illness. Written informed consent was obtained from participants or if the participant was a minor, from a parent or guardian. All minors provided written assent. This study was approved by the Institutional Review Board of the North Shore – Long Island Jewish Health System.
Image Acquisition
MR imaging exams were conducted at the North Shore University Medical Center on a General Electric 3 Tesla HDx whole body superconducting imaging system. All scans were reviewed by a radiologist for gross anatomic pathology that would preclude participation in this study. Scans were also reviewed by a member of the research team and any scan with significant artifacts was repeated. We minimized movement by stabilizing the head with cushions prior to scanning. We acquired 216 coronal SPGR images through the whole head using a 3D 1mm thick slice acquisition with the following image parameters: TR = 7.5 ms, TE = 3 ms, matrix = 256 × 256, FOV = 240 mm). In addition, we acquired a diffusion tensor imaging scan that included a total of 36 DTI volumes from each subject, including 31 volumes with diffusion gradients applied along non-parallel directions with b = 1000 s/mm2 and, and 5 volumes without diffusion weighting (b = 0). Each volume consisted of 51 contiguous 2.5 mm axial slices acquired parallel to the anterior-posterior (AC-PC) commissural line using a ramp sampled, spin-echo, single shot echo-planar imaging (EPI) method (TR = 1400ms, TE = min, matrix = 128×128, FOV = 240mm).
Interthalamic Adhesion
Following alignment along the anterior and posterior commissures in ITK-SNAP (www.itksnap.org) (Yushkevich et al 2006) (Version 2.0.0) 2 operators independently rated the absence of the ITA using information available from all 3 planes. When present, both operators independently traced the ITA on the single midsagittal image where it appeared smallest in size in the midline. We then computed the average size of the ITA between the two operators for all individuals and used this value as the dependent measure in analyses. We were unable to trace the ITA across multiple slices in the parasagittal plane due to our inability to discriminate it from the surrounding thalamic anatomy.
Thalamic Volume
Right and left thalamic volumes were measured using the FreeSurfer package analysis suite (version 5.0.0), which is documented extensively and freely available for online download (http://surfer.nmr.mgh.harvard.edu/). Details regarding this automated approach have been described in prior work (e.g., Fischl et al., 2004). MR images underwent motion correction, inhomogeneity corrections, and gray/white matter segmentation along with identification of the thalamus (Fischl et al., 2002; Fischl et al., 2004) for measurements, which were then visually inspected for accuracy.
Probabilistic Tractography of the Anterior Thalamic Radiation
DTI data were processed and analyzed using the FMRIB Software Library (FSL) (www.fmrib.ox.ac.uk/fsl/). Head motion and eddy current induced distortions were corrected through affine registration of the diffusion weighted images to the first B0 image. The gradient directions were corrected according to the rotation parameters. Non-brain tissue was removed using the Brain Extraction Tool. The DTIFIT tool was then used to fit a diffusion tensor model to the raw diffusion data at each voxel using weighted least squares.
We utilized probabilistic tractography within the FSL package to identify the anterior thalamic radiation tracts connecting the thalamus as described in our previous study (Peters et al 2014). Using the Bedpostx tool, the local (i.e., within-voxel) probability density functions of the principal diffusion direction were estimated using Markov Chain Monte Carlo sampling; a spatial probability density function across voxels was then estimated based on these local probability density functions using the probtrackx tool. The resulting spatial probability density function permitted the visualization of a “connectivity distribution” between any given voxel in the brain and every other voxel. Seed regions were masked in MNI space and linearly registered to each subject’s native DTI space. The parameters for Probtrackx included: number of samples = 5000, curvature threshold = 0.2, step length = 0.5mm, and number of steps per sample = 2000.
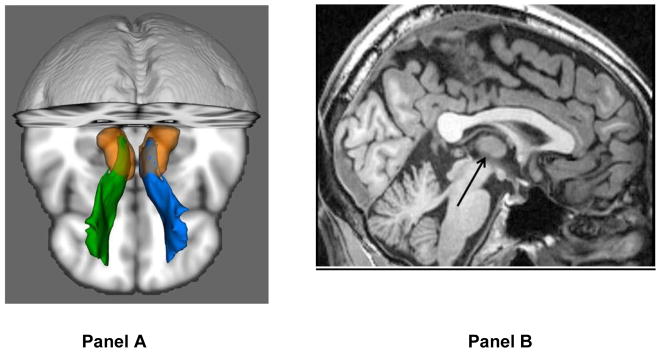
Seed regions, way-points and exclusion mask were defined on the MNI152_T1 template in 1×1×1mm in MNI space, and normalized from MNI space to each subject’s DTI space. A seedmask of the thalamus was derived from the HarvardOxford subcortical atlas (Desikan et al. 2006), as provided in FSL, and then manually edited according to the MNI152 T1 template to exclude the medial and lateral geniculate nuclei. Prefrontal white matter was used as a way-point, derived by segmenting the white matter from the prefrontal cortical regions in the HarvardOxford atlas (Desikan et al. 2006). Fibers were terminated when they reached the prefrontal white matter. In addition, only fibers traversing the anterior limb of the internal capsule were included. The second way-point was defined on 3 coronal slices in the anterior section of the anterior limb of the internal capsule. Fibers traveling through occipital, temporal, parietal, sensory-motor (including the supplementary motor area) gray matter, as defined using the HarvardOxford cortical atlas (Desikan et al. 2006), were excluded. This mask was manually expanded to exclude fibers traveling into the brainstem or the contralateral hemisphere. The bilateral anterior thalamic radiation of each subject was thresholded at a normalized probability value of 0.005 (Figure 1).
Figure 1. Illustration of the Anterior Thalamic Radiation using Probabilistic Tractography (Panel A) and Interthalamic Adhesion on a Midsagittal Slice (Panel B).
Note: Images are in radiologic convention (green = right and blue = left). Arrow points to interthalamic adhesion.
Neuropsychological Assessment
Subjects were administered a battery of neuropsychological tests designed to assess a wide range of functions. Tests were grouped into 6 domains by using z-score transformations such that higher scores were indicative of better performance. A global measure was also computed as the average of these scores. Domains (along with Chronbach’s coefficient alpha and sample sizes in parentheses) included: (1) Speed of Processing (.69): Brief Assessment of Cognition in Schizophrenia - Symbol Coding (n = 168), Trail Making Test - Part A (n = 196); (2) Attention (.84): Continuous Performance Test - identical pairs, average of d’ 2 (n = 164), 3 (n = 162) and 4 (n = 162); (3) Spatial Working Memory: Wechsler Memory Scale 3rd edition - spatial span (n = 168); (4) Verbal Functioning (.64): Controlled Oral Word Association Test – total words (n = 195), Animal Naming Test (n = 195), UMd Letter-Number Span Task (n = 168), Hopkins Verbal Learning Test revised – immediate recall, total correct words (n = 168); (5) Visual Learning: Brief Visuospatial Memory Test revised - total recall (n = 166); and (6) Executive Functioning (.69): Neuropsychological Assessment Battery - mazes subtest (n = 167), Wisconsin Card Sorting Test - categories completed & percent errors (n = 164 and n = 162), Trail Making Test - Part B (n = 196). A global measure of neuropsychological functioning was computed as the average of these 6 domains.
Statistical Analysis
In all analyses we examined the distribution of measures by visual inspection of scatterplots and from skewness and kurtosis to ensure that they did not deviate from normality. One case was considered an outlier for thalamus volume asymmetry (> 3SD from the mean) and was excluded from analyses investigating thalamus volume. We used mixed models analyses to investigate ITA size, thalamus volume, and fractional anisotropy and mean diffusivity within the anterior thalamic region using separate statistical models. In each model sex was the between subjects factor and hemisphere was a within subjects factor; the exception being ITA size. In each model we tested the main effects of sex, age and age2 and the interactions of age × sex, age2 × sex, age × sex × hemisphere and age2 × sex × hemisphere. Age2 was included in the model to identify possible quadratic effects across the agespan. In analyses of ITA size we used total (sum of the right and left hemispheres) thalamus volume as a statistical covariate and for analyses investigating the thalamus we used total intracranial volume. Relationships between age and dependent measures were fit using both linear and quadratic (c + a * age + b * age2) models within the NLS2 package in R. Partial correlation analysis was used to examine the relationship between ITA size with thalamic volume and fractional anisotropy and mean diffusivity within the anterior thalamic radiation separately in males and females while controlling for age2. All analyses were two-tailed with alpha set to .05.
Path models investigating structure-function relations involving the ITA included all six neuropsychological domains, ITA size, age and age2, and were conducted separately for males and females. We included age2 to model the nonlinear relationship between age and ITA size. The relationship between age and neuropsychological functioning was determined to be linear (using regression plots); hence no paths were included between age2 and the neuropsychological measures in the models. An indirect effect was considered significant based on two criteria described by Kline (2010, p. 165). First, as described by Cohen and Cohen (1983) “If all its component unstandardized path coefficients are statistically significant at the same level α, then the whole indirect effect can be taken as statistically significant at the same level α, too.” Second, where paths between age, parameter and a neuropsychological domain were all significant, a test recommended by Baron and Kenny (1986), based on the approximate standard error estimates for the indirect effects by Sobel (1986) was used to determine whether significant mediation was present (Kline, 2010; pp.165). If and are unstandardized coefficients for paths X → M and M → Y then the product estimates the unstandardized indirect effect of X on Y through M. If SEa and SEb are the corresponding standard errors, then Sobel’s estimated standard error of ab is . In large samples, the ratio ab/SEab follows approximately a normal distribution and hence a test (Sobel’s test) can be utilized to reject or accept the null hypothesis that the unstandardized indirect effect is zero.
Results
Of the 233 healthy volunteers in this study both operators agreed that the ITA was present in 222 individuals and absent in 9 individuals. Operator I rated the ITA present in two individuals for which operator II rated it absent. There were no individuals for whom operator II rated the ITA present and operator I rated it as absent. Kappa between the operators was .90 (SE = .07); 95% CI = .75 to 1.04 with the strength of agreement considered “very good.”
Mixed models analyses revealed a significant main effect of sex (F = 14.09, df = 217, p < .001) such that females had larger ITA size overall compared to males. In addition, the main effect of age2 was statistically significant (F = 10.51, df = 217, p = .001). Figure 2 illustrates ITA size across the age-span modeled using a quadratic curve; the age at which the ITA achieved its maximum size was 15 in males and 23 in females. Neither the interactions of sex with age nor sex with age2 were statistically significant suggesting that age-associated changes were comparable in males and females across the age-span.
Figure 2. Interthalamic Adhesion Size (mm2) across the Agespan Modeled Using a Quadratic Curve in Males and Females.
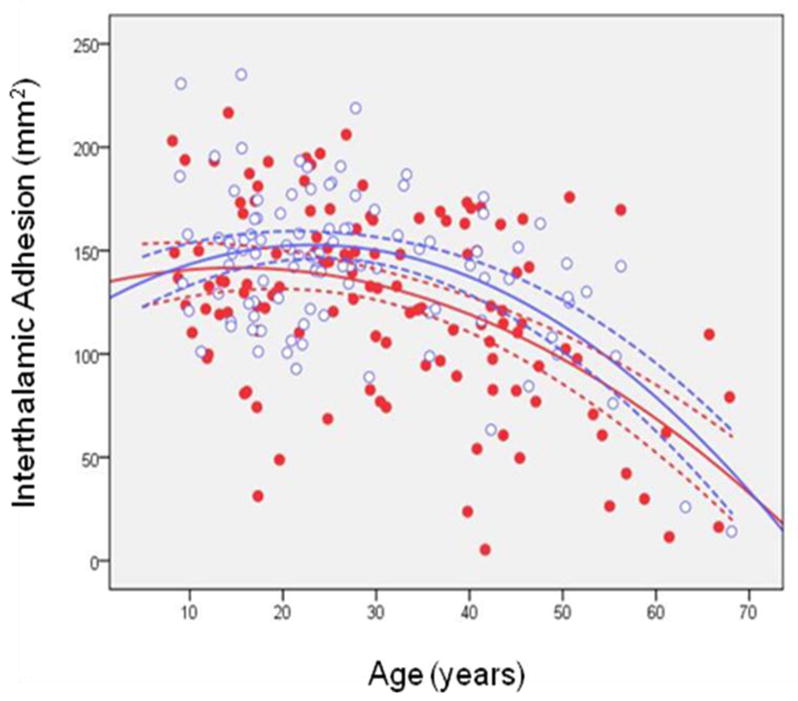
Note: Dotted lines represent the 95% confidence intervals. Red dots are males and open blue circles are females.
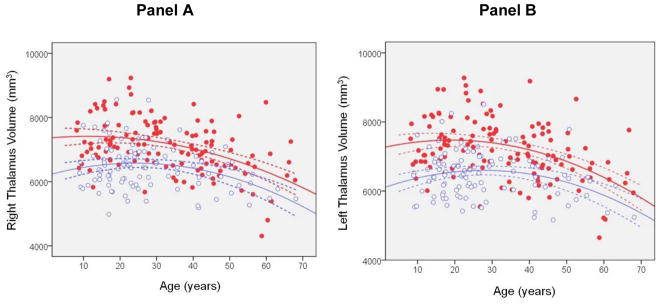
Mixed models analysis of thalamus volume revealed a significant main effect of sex (F = 6.13, df = 227, p = .014) such that males had larger volume of the thalamus compared to females overall. A significant main effect of hemisphere indicated that thalamus volume was significantly larger in the left compared to the right hemisphere (F = 11.28, df = 218, p < .001). The main effect of age2 was statistically significant (F = 11.21, df = 228, p = .001) indicating that thalamus volume changes across the age-span were nonlinear. There was a significant sex-by-hemisphere interaction (F = 8.93, df = 231, p = .003) such that males had larger left compared to right thalamus volume (t = 4.50, df = 130, p < .001) in contrast to females who did not demonstrate this asymmetry. Males also had larger left hemisphere thalamus volume compared to females (t = 3.14, df = 256, p = .002). The sex × age2 × hemisphere interaction was statistically significant (F = 6.30, df = 200, p < .001). Posthoc analyses revealed that for right thalamus volume females had a greater quadratic trajectory across the age-span compared to males (t = 2.19, df = 243, p = .029; figure 3). In addition, males had a greater quadratic trajectory across the age-span in the left compared to the right thalamus (t = 3.96, df = 190, p < .001). The age at which the right thalamus achieved its maximum size was 13 in males and 23 in females determined from the quadratic model. In contrast, the peak age at which the left thalamus achieved its maximum size was 19 in males and 28 in females also using the quadratic model.
Figure 3. Trajectory of Right (panel A) and Left (Panel B) Thalamus Volume (mm3) Modeled using a Quadratic Curve Across the Agespan in Males and Females.
Note: Dashed lines represent the 95% confidence intervals. Red dots are males and open blue circles are females.
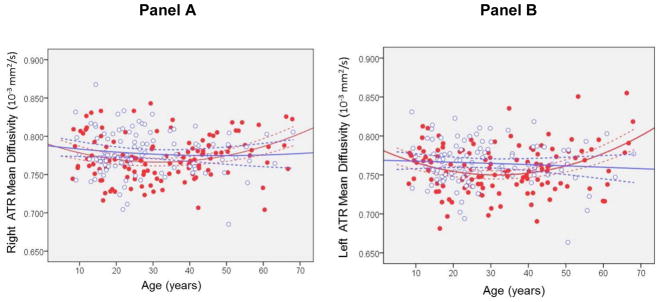
Investigation of the anterior thalamic radiation using mixed models revealed significant main effects of hemisphere overall for fractional anisotropy (F = 175.87, df = 224, p < .001) and mean diffusivity (F = 149.81, df = 220, p < .001) such that fractional anisotropy was higher and mean diffusivity was lower in the left compared to the right hemisphere. There were significant main effects of age (F = 5.60, df = 221, p = .019) and age2 for mean diffusivity (F = 6.53, df = 221, p = .011). There was a significant main effect of sex for mean diffusivity (F = 5.78, df = 220, p = .017) such that males had lower mean diffusivity overall compared to females. Figure 4 illustrates mean diffusivity across the age-span modeled using a quadratic curve separately in males and females. There were no significant interactions of sex with hemisphere, age or age2. The 3 way interactions of sex × age × hemisphere and sex × age2 × hemisphere were not statistically significant for either fractional anisotropy or mean diffusivity (ps > .05). The age at which mean diffusivity achieved its minima was 32 in males and 36 in females.
Figure 4. Mean diffusivity in the Right (Panel A) and Left (Panel B) Anterior Thalamic Radiation Modeled Using a Quadratic Curve Across the Agespan Separately in Males and Females.
Note: ATR=Anterior thalamic radiation. Dashed lines represent the 95% confidence intervals. Red dots are males and open blue circles are females. Units are 10−3 mm2/s.
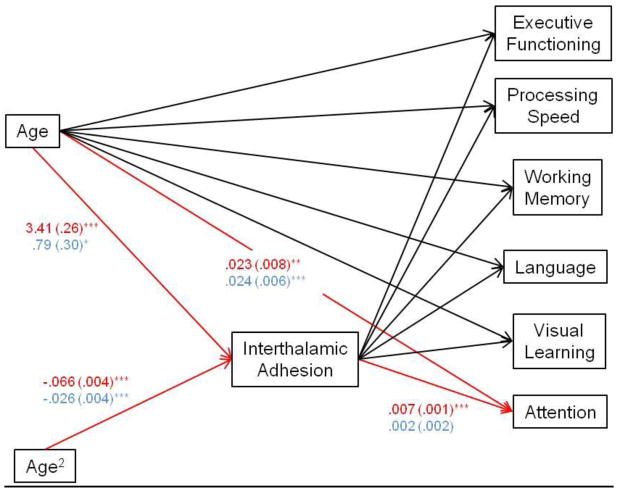
ITA size correlated significantly with total thalamus volume (r = .30, df = 98, p = .003) and mean diffusivity in the anterior thalamic radiation (r = −.26, df = 94, p = .01) among females, but not males (ps > .05). ITA size did not correlate significantly with either asymmetry in thalamus volume, or asymmetry in either fractional anisotropy or mean diffusivity in the anterior thalamic radiation. We assessed whether ITA size mediated the relationship between age (including both the age and age2 terms) with the neuropsychological domains. ITA size significantly mediated the relationship between age and performance on the attention domain across the observed age range among females with no significant effects evident for males. There were no significant differences between males and females for any of the neuropsychological domains. (ps > .05). The path diagrams for males and females, including significant maximum likelihood estimates, are illustrated in Figure 5.
Figure 5. Path Diagram Reflecting Significant Maximum Likelihood Estimate Values (Standard Errors in parentheses) for Female (Red) and Male (Blue) Healthy Volunteers.
Note: Significance level of maximum likelihood estimates: *** p < .001, ** p < .005 and * p < .01. Red lines reflect significant maximum likelihood estimates for significant mediation model in females.
Discussion
To our knowledge this is the largest study to investigate the ITA in healthy individuals. Prior studies have yielded inconsistent findings regarding the absence of this structure in otherwise healthy volunteers and, more specifically, how sex may potentially mediate this effect. Thus, an important finding from our study is that the absence of the ITA was approximately 4%, which is consistent with, but at the lower range observed in a meta-analysis that included healthy volunteers (Trzesniak et al 2011). The lower rate of absence in our study compared to prior work may relate to methodologic issues in defining the ITA and our use of high resolution MR images acquired at 3T using thin slices to visualize this structure. No sex differences were observed in the absence of the ITA in our study. Investigation of age-related effects revealed nonlinear (quadratic) changes in ITA size that were comparable between sexes from childhood through older age, but with females demonstrating larger size consistently across the agespan (Figure 2) consistent with prior post-mortem (Allen and Gorski 2001) and MR imaging (Sen et al 2005) work. It should be noted that this finding was observed despite males having both larger thalamus and total intracranial volume compared to females. A significant strength of the current study compared to prior work is the use of two operators to independently rate the absence of the ITA and measure its size for all individuals.
In the current study we observed sex differences in thalamic volume and putative white matter integrity (i.e., mean diffusivity) within one thalamocortical network (i.e., the anterior thalamic radiation) that reciprocally interconnects thalamus and frontal cortex by projecting through the anterior limb of the internal capsule and across diencephalic structures. Moreover, ITA size correlated significantly with total thalamus volume and mean diffusivity in the anterior thalamic radiation among females, but not males. To our knowledge no MR imaging studies have investigated the relationship among thalamus volume, putative white matter integrity of the anterior thalamic radiation and ITA size and it is thus difficult to compare our work to prior studies. In one study, however, Spalleta et al (2013) reported sex differences in the relationship between the thalamus and anterior thalamic radiation among individuals experiencing subclinical apathy. It may also be noteworthy that sexual dimorphism has also been observed in other (relatively) small brain structures including the pituitary gland (Gruner et al 2012) and anterior commissure (Choi et al 2011), but the functional correlates of these sex differences remain largely unknown.
In contrast to interthalamic size, we identified larger thalamus volume in males compared to females, but there was no significant age2 × sex interaction indicating that age-associated changes were comparable between sexes across the age-span. Although prior studies have investigated the relationship between age and thalamic volume, limitations with prior work may include a restricted age range and/or the sole use of linear modeling. In general, however, our results converge with several prior studies (Koolschijn et al 2013; Bramen et al 2011) and, in particular, Xie et al (2012) who reported that age was a significant predictor of thalamus volume in male and female children and adolescents. There are also cross-sectional data implicating age-associated changes in thalamus volume (Van Der Werf et al 2001) that are comparable in males and females (Sullivan et al 2004; Hughes et al 2012). In another study Mohammadi et al (2008) found that thalamic dimensions increased with age until 31–40 years, but then decreased among older individuals suggesting that quadratic modeling may be superior to linear modeling across the agespan examined.
Few studies have investigated sex differences in thalamic asymmetry across the age-span and thus, our study provides new information in that regard. Specifically, we observed greater left compared to right thalamus volume across males and females. Although several prior studies reported larger right compared to left thalamus volume (Xie et al 2012; Lee et al 2006; Coscia et al 2009) a leftward asymmetry was observed in an adult study where larger left thalamus volume was identified following spatial normalization to stereotaxic space (Ahsan et al 2007). Differences among studies may relate to the age range examined and use of manual (including operator bias if cases were not flipped in the right-left hemisphere) versus automated mensuration. Notably, the finding of a significant sex × age2 × hemisphere interaction for thalamus volume indicates that males demonstrated greater age-associated changes in the right thalamus (possibly as a function of having already achieved its maximum size by age 13) compared to their left thalamus and compared to the right thalamus among healthy females. It is conceivable that volume changes associated with healthy aging may be related to neuronal loss, shrinkage of neurons and concomitant reductions in the numbers of synaptic spines (Fjell and Walhovd 2010), but the neural mechanisms underlying this hemispheric bias are unclear.
This study also provides data supporting significant statistical mediation between age (i.e., the independent variable) and neuropsychological functioning (i.e., the dependent variable) through ITA size. In other words, ITA size plays a role in governing the relationship between age and attention functioning, at least among healthy females. Although the current study could not address the mechanisms underlying how ITA size mediates this relationship, the size of the ITA could play a role in more effective connectivity between the two thalamic nuclei as well as cerebrospinal fluid circulation (Nayak and Kv 2010) that have implications for neurocognitive functioning. Although animal data are sparse, one study noted that ITA parameters may be useful for evaluating brain atrophy in animals with “cognitive dysfunction” (Hasegawa et al 2005). We did not observe significant mediation effects between age and neuropsychological functions in males. Sex-specific mediation effects observed in the current study could relate to larger ITA size among females. Such findings, coupled with prior work demonstrating sex differences in attention functioning (Gur et al 2012), could plausibly be mediated, at least in part, by neuroanatomical structures such as the ITA.
There were several limitations of the current study that should be acknowledged. Despite capturing a large age range we did not include children prior to the age of 8 and individuals older than 68, which could have conceivably biased the trajectories. In this regard studies investigating these additional age ranges could provide complementary information. In addition, although we used standard neurocognitive tests in our study it is conceivable that instruments tapping other domains may have further explicated the relationship between ITA size and neuropsychological functions. We also acknowledge that in the current study we used an automated approach for segmentation of the thalamus and differences between this approach and manual mensuration could yield volumetric differences. Also, the thalamus is known to be comprised of multiple nuclei and it is acknowledged that other projection fibers were not investigated in the current study. Moreover, the use of additional techniques such as principal components analysis and/or hierarchical clustering could be useful in further explicating the relationship among variables in this study.
In sum, we report that the ITA was absent in approximately 4% of the healthy cohort we examined without associated sex differences in its absence, and that females demonstrated larger ITA size compared to males overall. Furthermore, ITA size was associated with thalamic volume and measures of anterior thalamic radiation white matter integrity among females. Mediation analysis revealed that ITA size differences were associated with neuropsychological measures of attention functioning in females without any mediation effects observed in males.
Table 1.
Mensuration Results for Regions-of-Interest
| Interthalamic Adhesion (mm2) | (N=129) 125 (46) | (N=104) 142 (36) |
|---|---|---|
| Thalamus (mm3) | ||
| Right Hemisphere | 7092 (832) | 6450 (701) |
| Left Hemisphere | 7204 (858) | 6467 (671) |
| Anterior Thalamic Radiation1 | ||
| Fractional Anisotropy | ||
| Right Hemisphere | .458 (.028) | .448 (.029) |
| Left Hemisphere | .472 (.029) | .463 (.027) |
| Radial Diffusivity | ||
| Right Hemisphere | .564 (.033) | .574 (.033) |
| Left Hemisphere | .543 (.034) | .554 (.033) |
| Mean Diffusivity | ||
| Right Hemisphere | .772 (.029) | .778 (.029) |
| Left Hemisphere | .758 (.030) | .765 (.029) |
| Axial Diffusivity | ||
| Right Hemisphere | 1.189 (.039) | 1.118 (.040) |
| Left Hemisphere | 1.184 (.039) | 1.186 (.035) |
Notes: Data are presented for descriptive purposes only (see text for a description of the statistical analysis plan). Standard deviations are in parentheses. FA values are dimensionless and multiplied by 1000. Axial, radial and mean diffusivity units are 10−3 mm2/s.
Data could not be traced for 8 individuals.
Acknowledgments
This work was supported in part by grants from the National Institute of Mental Health to Dr. Szeszko (R01 MH076995), the NSLIJ Research Institute General Clinical Research Center (M01 RR018535), an Advanced Center for Intervention and Services Research (P30 MH090590) and a Center for Intervention Development and Applied Research (P50 MH080173).
References
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Lemieux L, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. Neuroimage. 2007;38:261–270. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Allen LS, Gorski RA. Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol. 1991 Oct 1;312(1):97–104. doi: 10.1002/cne.903120108. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Booth T, Bastin ME, Penke L, Maniega SM, Murray C, Royle NA, Gow AJ, Corley J, Henderson RD, del Hernández MC, Starr JM, Wardlaw JM, Deary IJ. Brain white matter tract integrity and cognitive abilities in community-dwelling older people: the Lothian Birth Cohort, 1936. Neuropsychology. 2013 Sep;27(5):595–607. doi: 10.1037/a0033354. Epub 2013 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, et al. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cerebral Cortex. 2011;21:636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan M, Adapinar B, Aksaray G, Ozdemir F, Colak E. Absence and size of massa intermedia in patients with schizophrenia and bipolar disorder. Acta Neuropsychiatry. 2008;20:193–8. doi: 10.1111/j.1601-5215.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- Cheng S, Tan K, Bilston LE. The effects of the interthalamic adhesion position on cerebrospinal fluid dynamics in the cerebral ventricles. J Biomech. 2010 Feb 10;43(3):579–82. doi: 10.1016/j.jbiomech.2009.10.002. Epub 2009 Nov 5. [DOI] [PubMed] [Google Scholar]
- Choi MH, Kim JH, Yeon HW, Choi JS, Park JY, Jun JH, Lee BY, Kim HJ, Tack GR, Chung SC. Effects of gender and age on anterior commissure volume. Neurosci Lett. 2011 Aug 15;500(2):92–4. doi: 10.1016/j.neulet.2011.06.010. Epub 2011 Jun 15. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Analysis for the Behavioral Sciences. Taylor & Francis; 1983. Applied Multiple Regression/correlation. [Google Scholar]
- Coscia DM, Narr KL, Robinson DG, Hamilton LS, Sevy S, Burdick KE, et al. Volumetric and shape analysis of the thalamus in first-episode schizophrenia. Hum Brain Mapp. 2009;30:1236–1245. doi: 10.1002/hbm.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Non-patient Edition (SCID-I/NP) [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Gruner P, Christian C, Robinson DG, Sevy S, Gunduz-Bruce H, Napolitano B, Bilder RM, Szeszko PR. Pituitary volume in first-episode schizophrenia. Psychiatry Res. 2012 Jul 30;203(1):100–2. doi: 10.1016/j.pscychresns.2011.09.017. Epub 2012 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012 Mar;26(2):251–65. doi: 10.1037/a0026712. Epub 2012 Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa D, Yayoshi N, Fujita Y, Fujita M, Orima H. Measurement of interthalamic adhesion thickness as a criteria for brain atrophy in dogs with and without cognitive dysfunction (dementia) Vet Radiol Ultrasound. 2005 Nov-Dec;46(6):452–7. doi: 10.1111/j.1740-8261.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Wada JA. Convulsive seizures in rats induced by N-methyl-D–aspartate injection into the massa intermedia. Brain Res. 1992;577:36–40. doi: 10.1016/0006-8993(92)90534-g. [DOI] [PubMed] [Google Scholar]
- Hughes EJ, Bond J, Svrckova P, Makropoulos A, Ball G, Sharp DJ, et al. Regional changes in thalamic shape and volume with increasing age. Neuroimage. 2012;63:1134–1142. doi: 10.1016/j.neuroimage.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Kramer LA, Hasan KM. Feasibility of prefronto-caudate pathway tractography using high resolution diffusion tensor tractography data at 3T. J Neurosci Methods. 2010 Aug 30;191(2):249–54. doi: 10.1016/j.jneumeth.2010.06.026. Epub 2010 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci. 2013;5:106–118. doi: 10.1016/j.dcn.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. 3. Guilford Press; 2010. [Google Scholar]
- Laslo P, Slobodan M, Nela P, Milos M, Rade P, Tatjana I. Specific circular organization of the neurons of human interthalamic adhesion and of periventricular thalamic region. Int J Neurosci. 2005;115:669–679. doi: 10.1080/00207450590524340. [DOI] [PubMed] [Google Scholar]
- Lee JS, Yoo SS, Cho SY, Ock SM, Lim MK, Panych LP. Abnormal thalamic volume in treatment naive boys with Tourette syndrome. Acta Psychiatrica Scandinavica. 2006;113:64–67. doi: 10.1111/j.1600-0447.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009 Nov 11;29(45):14265–70. doi: 10.1523/JofNeuroscience.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malobabić S, Puskas L, Blagotić M. Size and position of the human adhaesio interthalamica. Gegenbaurs Morphol Jahrb. 1987;133:175–180. [PubMed] [Google Scholar]
- Malobabić S, Puskas L, Vujasković G. Golgi morphology of the neurons in frontal sections of human interthalamic adhesion. Acta Anat (Basel) 1990;139(3):234–8. [PubMed] [Google Scholar]
- Mohammadi MR, Hosseini SH, Golalipour MJ. Morphometric measurements of the thalamus and interthalamic adhesion by MRI in the South-East of the Caspian Sea border. Neurosciences. 2008;13:272–275. [PubMed] [Google Scholar]
- Nayak Kv. Unusually large interthalamic adhesion and its clinical importance. International Journal of Anatomical Variations (2010) 2010;3:174–175. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Ventricular system and choroid plexuses of the human brain during the embryonic period proper. Am J Anat. 1990;189:285–302. doi: 10.1002/aja.1001890402. [DOI] [PubMed] [Google Scholar]
- Park IA, Lee HY, Chung IH, Han YP, Shin TS. A Morphologic Study of Interthalamic Adhesions in Korean. Brains Clinical Anatomy. 1993;6:33–36. [Google Scholar]
- Peters BD, Ikuta T, DeRosse P, John M, Burdick KE, Gruner P, Prendergast DM, Szeszko PR, Malhotra AK. Age-related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biol Psychiatry. 2014 Feb 1;75(3):248–56. doi: 10.1016/j.biopsych.2013.05.020. Epub 2013 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz S, Goldstein R, Hopkins TL, Lauterbach MD, Shah F, Porter CL, Riggs WW, Magill LH, Sander CJ. Sex differences in early vulnerability to cerebral injury and their neurodevelopmental implications. 1994 Sep;22(3):244–253. [Google Scholar]
- Rosales RK, Lemay MJ, Yakovlev PI. The development and involution of the massa intermedia with regard to age and sex. J Neuropathol Exp Neurol. 1968;27:166. [PubMed] [Google Scholar]
- Sen F, Ulubay H, Ozeksi P, Sargon MF, Tascioglu AB. Morphometric measurements of the thalamus and interthalamic adhesion by MR imaging. Neuroanatomy. 2005;4:10–12. [Google Scholar]
- Shimizu M, Fujiwara H, Hirao K, Namiki C, Fukuyama H, Hayashi T, et al. Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia. Schizophr Res. 2008;101:331–338. doi: 10.1016/j.schres.2007.12.486. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Some new results on indirect effects and their standard errors in covariance structure models. Sociological Methodology. 1986;16:159–186. [Google Scholar]
- Spalletta G, Fagioli S, Caltagirone C, Piras F. Brain microstructure of subclinical apathy phenomenology in healthy individuals. Hum Brain Mapp. 2013 Dec;34(12):3193–203. doi: 10.1002/hbm.22137. Epub 2012 Ju 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging. 2004;25:185–192. doi: 10.1016/S0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Trzesniak C, Kempton MJ, Busatto GF, de Oliveira IR, Galvão-de Almeida A, Kambeitz J, et al. Adhesio interthalamica alterations in schizophrenia spectrum disorders: A systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:877–886. doi: 10.1016/j.pnpbp.2010.12.024. Epub 2011 Feb 16. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PA, Vuurman E, Uylings HB, et al. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Brain Res Cogn Brain Res. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Xie Y, Chen YA, De Bellis MD. The relationship of age, gender, and IQ with the brainstem and thalamus in healthy children and adolescents: a magnetic resonance imaging volumetric study. J Child Neurol. 2012;27:325–331. doi: 10.1177/0883073811419260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry. 2003;54:427–436. doi: 10.1016/s0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]