Abstract
Background
The independent association of recent infection with venous thromboembolism is uncertain. The purpose of the study was to test both overall infection (site unspecified) and specific infection sites as potential risk factors for deep vein thrombosis and pulmonary embolism adjusting for other known venous thromboembolism factors.
Methods
Using Rochester Epidemiology Project (REP) resources, we identified all Olmsted County, MN residents with objectively-diagnosed incident deep vein thrombosis or pulmonary embolism over the 13-year period, 1988–2000 (cases; n=1303), and 1–2 residents without venous thromboembolism matched to each case on age, sex and incident venous thromboembolism date (controls; n=1494). These case-control sets were analyzed using conditional logistic regression. Data were collected on recent infection and infection site(s), body mass index, smoking, current or recent hospitalization with and without surgery, nursing home confinement, active cancer, trauma or fracture, leg paresis, prior superficial vein thrombosis, transvenous catheter/pacemaker, ischemic heart disease, congestive heart failure, chronic lung or renal disease, serious liver disease, asthma, diabetes mellitus, hormone therapy, and among women, hormonal contraception and pregnancy/post-partum.
Results
Five hundred thirteen (39.4%) cases and 189 (12.7%) controls had an infection in the previous 92 days (OR=4.5; 95%CI: 3.6, 5.5; p<0.0001). In a multivariable analysis adjusting for common venous thromboembolism risk factors, pneumonia as well as symptomatic urinary tract, oral, intra-abdominal and systemic blood stream infections were associated with significantly increased odds of venous thromboembolism.
Conclusion
Infection as a whole, as well as specific infection sites in particular are independent risk factors for venous thromboembolism and should be considered as potential indications for venous thromboembolism prophylaxis.
Keywords: deep vein thrombosis, pulmonary embolism, thrombophlebitis, infection, epidemiology
INTRODUCTION
Venous thromboembolism is a major public health problem; over 500,000 incident or recurrent venous thromboembolism events occur in the US annually.1–3 Survival after venous thromboembolism is reduced, especially after pulmonary embolism;4 ~25% of incident pulmonary embolism patients suffer sudden death. To improve survival, the occurrence of venous thromboembolism must be reduced. Of all venous thromboembolism occurring in the community, about 50% are related to, but about 50% are unrelated to, current or recent hospitalization for surgery or medical illness.3,5 Currently, venous thromboembolism prophylaxis is only recommended for hospitalized patients.6 If all hospitalized patients received universally-effective prophylaxis, only about half of the venous thromboembolism burden in the community would be prevented. To further reduce the venous thromboembolism burden, better methods are needed to identify the non-hospitalized individual at risk for venous thromboembolism. However, among Olmsted County residents with incident or recurrent venous thromboembolism unrelated to current or recent hospitalization over the six-year period, 2005–2010, 40.5% and 63.5% were idiopathic, respectively (with idiopathic defined as not having active cancer, recent nursing home confinement, trauma, fracture, immobilization, leg paresis, hormone therapy, or among women, recent hormonal contraception, or pregnancy/post-partum).3
Infection is common and has been associated with venous thromboembolism7–9, and could account for a substantial burden of incident or recurrent venous thromboembolism currently labeled as “idiopathic”. Identification of infection and particularly, infection sites, as independent risk factor(s) for venous thromboembolism would allow providers to stratify venous thromboembolism risk among non-hospitalized individuals, target prophylaxis and reduce the occurrence of venous thromboembolism currently labeled as “idiopathic”. Previous studies reporting infection as a venous thromboembolism risk factor used administrative (ICD-8 or -9 CM code) data (UK Health Improvement Network7; Danish National Registry of Patients8; Health and Retirement Study [Medicare beneficiaries from CMS]9) to identify venous thromboembolism cases. We have shown that these codes have very poor predictive value for identifying objectively-diagnosed venous thromboembolism when compared to direct medical record review.10 Thus, the validity of these previous studies is uncertain.
To address these limitations, we performed a population-based case-control study nested within the population of Olmsted County, MN to estimate the magnitude of risk of venous thromboembolism attributable to active infection that included the entire spectrum of infection-associated venous thromboembolism occurring in the community. We took advantage of Rochester Epidemiology Project (REP) resources to identify all Olmsted County residents with incident venous thromboembolism as well as matched controls drawn from the same population. Combining information on incident venous thromboembolism cases with active infection for each individual afforded us the opportunity to evaluate whether overall infection and infection sites are potential risks factor for deep vein thrombosis and pulmonary embolism across the full spectrum of venous thromboembolism severity ranging from symptomatic to fatal events, alone or after adjusting for other known venous thromboembolism factors.
METHODS
Study Setting, Population and Design
Olmsted County, MN (2010 census population=144,248), provides a unique opportunity for investigating the natural history of venous thromboembolism.1–9 Rochester, the county seat, is approximately 80 miles from the nearest major metropolitan area. The population-based resources of the REP (see Supplemental Material) link and provide access to the medical records of all Olmsted County residents from all Olmsted County medical care providers, assuring complete ascertainment of most medical conditions. Using these REP resources, we identified all Olmsted County, MN residents with incident deep vein thrombosis and/or pulmonary embolism over the 35-year period, 1966–2000, as previously described.5 We then performed a matched case-control study nested within the Olmsted County population. All Olmsted County residents with a first lifetime objectively-diagnosed deep vein thrombosis or pulmonary embolism during the 13-year period, 1988–2000, were included in the present study as venous thromboembolism cases. Patients with isolated subsegmental pulmonary embolism or gastrocnemius, soleal, cerebral or abdominal vein thrombosis were excluded. The REP also provides an enumeration of the entire Olmsted County population from which controls can be sampled. Using this system, one to two age- (± 1 years) and sex-matched Olmsted County residents without venous thromboembolism who had an episode of medical care within ± 1 year of the case event date and whose medical record number was closest to the case’s medical record number were selected as controls as previously described.11, 12 Since a patient’s medical record number is assigned sequentially and in perpetuity, matching on medical record number assures a similar length of prior medical history among cases and matched controls. The case’s index date was defined as the date of diagnosis of venous thromboembolism, while the date of the episode of medical care closest to the case’s venous thromboembolism diagnosis date was defined as the control’s index date. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Definitions and Measurements
Using explicit criteria, trained and experienced nurse abstractors reviewed all medical records (inpatient and outpatient) in the community for cases and controls who provided consent to review of their medical records for research purposes. All records were reviewed from date first seen by a REP healthcare provider until the earliest of death, date of last medical record follow-up, or 200013 as previously performed.14–17 For cases, data were recorded on the method of diagnosis and type of incident venous thromboembolism event (deep vein thrombosis, pulmonary embolism, or both; chronic thromboembolic pulmonary hypertension). A deep vein thrombosis was categorized as objectively-diagnosed when symptoms or signs of acute deep vein thrombosis were present and the diagnosis was confirmed by venography, compression venous duplex ultrasonography, impedance plethysmography, computed tomographic venography, magnetic resonance imaging or pathology examination of thrombus removed at surgery or autopsy. A pulmonary embolism was categorized as objectively-diagnosed when symptoms and/or signs of acute pulmonary embolism were present and the diagnosed was confirmed by pulmonary angiography, a ventilation/perfusion lung scan interpreted as high probability for pulmonary embolism, computed tomographic pulmonary angiography, magnetic resonance imaging or pathology examination of thrombus removed at surgery or autopsy. Mayo Clinic pathologists performed all autopsy examinations and completed the death certificates of persons dying within Olmsted County during the study period. Infection (see Supplemental Material) was defined as a physician diagnosis of infection recorded in the medical record within the 92 days (365 days ÷ 4, or ~ 3 months) prior to the index venous thromboembolism event for cases, or the index date for controls, and according to CDC/NHSN criteria.18 Infection sites with ≤5% prevalence were combined in a logical manner into larger categories for purposes of analysis.
Statistical Analyses
We tested the association of infection with venous thromboembolism using conditional logistic regression. Any infection (versus no infection) and individual site-specific infections (versus none, adjusted for a summary indicator of the remaining infections) were modeled using conditional logistic regression unadjusted for other risk factors and after adjusting for all important covariates jointly in a multivariable model. We adjusted all models for age at event. Specifically, for each infection site, we adjusted for each previously-identified independent venous thromboembolism risk factor, including patient age at index date (matching variable), BMI, and the following risk factors if they occurred within 92 days prior to the index date: current or recent hospitalization with or without surgery, nursing home confinement, trauma or fracture, active cancer (or diagnosed within 92 days after index), neurologic disease with leg paresis, transvenous catheter (92 days)/pacemaker (ever), and estrogen, progestin or hormonal contraceptives. Prior superficial vein thrombosis was a risk factor if it ever occurred prior to the index date. We considered interactions between venous thromboembolism location (pulmonary embolism ± deep vein thrombosis and deep vein thrombosis alone) and additional variables not consistently shown to be venous thromboembolism risk factors in our previous studies because of their potential as confounders. These additional variables included varicose veins, smoking (ever vs. never), physician diagnosis of chronic renal disease with creatinine > 2 mg/dL for ≥3 months duration, and physician diagnosis of ischemic heart disease (angina pectoris or myocardial infarction), congestive heart failure, serious liver disease, diabetes mellitus, asthma, or chronic lung disease, all if they ever occurred prior to index. We fit a stepwise model using all previously known risk factors to get a list of adjusting variables, and a second stepwise analysis addressing potential confounders and their interaction with type of venous thromboembolism. The p-value to enter and leave was 0.05. When examining any infection versus no infection and for each infection site versus none, adjusted for a summary indicator of the remaining infections, we adjusted for all of the venous thromboembolism risk factors that were significant above. Additionally, we fit a forward stepwise logistic regression allowing each infection to enter the model (p=0.05 to enter and leave). Once we had a final model, we pooled all remaining infections into a single variable, and tested that variable, and all infections that came into the model against no infection, adjusting for the above venous thromboembolism risk factors. Finally, we fit a forward stepwise model with the variable, any infection, and allowed individual infection sites to compete for entry to capture the infection sites that did not fit well in a more general model.
RESULTS
Over the 13-year period, 1988–2000, 1308 residents of Olmsted County developed a first lifetime deep vein thrombosis or pulmonary embolism; 5 (0.4%) had chronic thromboembolic pulmonary hypertension. After excluding the chronic thromboembolic pulmonary hypertension cases, the distribution of venous thromboembolism events by event type was 730 (56%) deep vein thrombosis alone and 573 (44%) pulmonary embolism with or without deep vein thrombosis; 725 (55.6%) were women. The mean ± SD age of the cases and matched controls (n=1494) was 65.2 ± 18.9 and 64.9 ± 18.8 years, respectively (Table 1). The overall mean ± SD duration of prior medical record documentation was 36.4 ± 20.8 and 36.3 ± 20.8 years, respectively, for cases and controls.
Table 1.
Demographic and Clinical Characteristics of Olmsted County Residents with Incident Venous Thromboembolism, 1988–2000, and Matched Resident Controls.
| Characteristic | Cases (n=1303) |
Controls (n=1494) |
|---|---|---|
| Patient Age ± SD, years | 65.2 ± 18.9 | 64.9 ± 18.8 |
| Female, n (%) | 725 (55.6) | 828 (55.4) |
| Body Mass Index ± SD, kg/m2 | 28.0 ± 7.0 | 26.6 ± 5.3 |
| Leg Paresis, n (%) | 92 (7.1) | 13 (0.9) |
| Trauma/Fracture, n (%) | 170 (13.0) | 39 (2.6) |
| Active Cancer, n (%) | 319 (24.5) | 47 (3.1) |
| Hospitalized for Surgery, n (%) | 342 (26.2) | 50 (3.3) |
| Hospitalized for Acute Medical Illness, n (%) | 347 (26.6) | 107 (7.2) |
| Nursing Home Confinement, n (%) | 171 (13.1) | 103 (6.9) |
| Superficial Vein Thrombosis, n (%) | 196 (15.0) | 83 (5.6) |
| Transvenous Catheter/Pacemaker, n (%) | 236 (18.1) | 51 (3.4) |
| Estrogen/Progesterone/Oral Contraceptives, n (%) | 221 (17.0) | 172 (11.5) |
| Chronic Lung Disease, n (%) | 267 (20.5) | 214 (14.3) |
| Congestive Heart Failure, n (%) | 238 (18.3) | 151 (10.1) |
| Ischemic Heart Disease, n (%) | 334 (25.6) | 284 (19.0) |
| Asthma, n (%) | 137 (10.5) | 111 (7.4) |
| Tobacco Smoking, n (%) | 682 (52.3) | 739 (49.5) |
| Liver Disease, n (%) | 14 (1.1) | 11 (0.7) |
| Chronic Renal Disease, n (%) | 44 (3.4) | 11 (0.7) |
| Diabetes Mellitus, n (%) | 164 (12.6) | 140 (9.4) |
| Pregnancy/Postpartum, n (%) | 25 (1.9) | 9 (0.6) |
Venous Thromboembolism Risk Factors
The number (%) of cases and controls with adjusting risk factors are shown in Table 1. A multivariable model of risk factors and potentially confounding variables was fit and included the usual list of venous thromboembolism risk factors (hospitalization with or without surgery, nursing home confinement, active cancer, trauma/fracture, and neurologic disease with leg paresis) and the additional venous thromboembolism risk factors of superficial thrombosis, body mass index, transvenous catheter/pacemaker, and estrogen/progesterone/oral contractive use (see Supplementary Table 1).
Univariate Analyses
Among the 1303 cases and 1494 controls, 513 (39.4%) and 189 (12.7%) had an infection in the previous 92 days, respectively (Table 2). Unadjusted for other venous thromboembolism risk factors, any infection increased the odds of venous thromboembolism by 4.5-fold (95%CI: 3.6, 5.5; p-value<0.0001) compared to no infection. Most infection sites were strongly associated with venous thromboembolism (Table 3). Antibiotic(s) and fever were associated, respectively, with 5.2- (95%CI: 4.2, 6.5; p<0.0001) and 14.5-fold (95%CI: 9.4, 22.4; p<0.0001) increased odds of venous thromboembolism. Odds ratios for individual infection sites compared to no infection and adjusted for all other infections ranged from 3.0 (bronchitis/upper respiratory tract) to 42.0 (systemic/blood stream; p-value ≤ 0.0001 for all but one location).
Table 2.
Infection Sites Among Olmsted County Residents with Incident Venous Thromboembolism, 1988–2000, and Matched Olmsted County Controls.
| Infection Site or Characteristic N (%) |
Cases (n=1303) |
Controls (n=1494) |
|---|---|---|
| Antibiotics | 495 (38.0) | 150 (10.0) |
| Fever | 294 (22.6) | 33 (2.2) |
| Any Infections | 513 (39.4) | 189 (12.7) |
| Genitourinary | 195 (15.0) | 45 (3.0) |
| Symptomatic Urinary Tract | 167 (12.8) | 38 (2.5) |
| Other Urinary Tract | 22 (1.7) | 0 (0.0) |
| Vaginal Cuff | 2 (0.2) | 0 (0.0) |
| Other Reproductive Tract | 8 (0.6) | 7 (0.5) |
| Lower Respiratory | 160 (12.3) | 28 (1.9) |
| Pneumonia | 153 (11.7) | 27 (1.8) |
| Other Lower Respiratory Tract | 10 (0.8) | 1 (0.1) |
| Sino-Upper Respiratory | 145 (11.1) | 83 (5.6) |
| Bronchitis | 45 (3.5) | 32 (2.1) |
| Mastoid | 5 (0.4) | 3 (0.2) |
| Oral | 28 (2.1) | 3 (0.2) |
| Sinusitis | 17 (1.3) | 17 (1.1) |
| Upper Respiratory Tract | 52 (4.0) | 29 (1.9) |
| Bronchitis and Upper Respiratory Tract | 89 (6.8) | 56 (3.8) |
| Other Eye/Ear/Nose/Throat/Mouth | 20 (1.5) | 11 (0.7) |
| Skin-Soft Tissue | 98 (7.5) | 43 (2.9) |
| Skin | 31 (2.4) | 18 (1.2) |
| Soft Tissue | 36 (2.8) | 20 (1.3) |
| Decubitus Ulcer | 13 (1.0) | 1 (0.1) |
| Breast Abscess or Mastitis | 0 (0.0) | 1 (0.1) |
| Other Skin-Soft Tissue | 5 (0.4) | 3 (0.2) |
| Superficial Incisional Site | 17 (1.3) | 2 (0.1) |
| Organ/Space Surgical Site | 7 (0.5) | 0 (0.0) |
| Other Surgical Site | 3 (0.2) | 1 (0.1) |
| Superficial Incisional Site, Organ/Space Surgical Site, and Other Surgical Site | 25 (1.9) | 2 (0.1) |
| Gastrointestinal System | 44 (3.4) | 11 (0.7) |
| Gastroenteritis | 6 (0.5) | 4 (0.3) |
| Gastrointestinal Tract | 6 (0.5) | 2 (0.1) |
| Intra-abdominal | 13 (1.0) | 1 (0.1) |
| Diverticulitis | 5 (0.4) | 2 (0.1) |
| Other Gastrointestinal System | 17 (1.3) | 2 (0.1) |
| Systemic/Bloodstream | 65 (5.0) | 2 (0.1) |
| Laboratory-confirmed Bloodstream | 43 (3.3) | 1 (0.1) |
| Clinical Sepsis | 11 (0.8) | 0 (0.0) |
| Other Bloodstream | 1 (0.1) | 0 (0.0) |
| Arterial or Venous | 16 (1.2) | 1 (0.1) |
| Endocarditis | 1 (0.1) | 0 (0.0) |
| Disseminated | 1 (0.1) | 0 (0.0) |
| Other | 39 (3.0) | 5 (0.3) |
| Osteomyelitis | 5 (0.4) | 1 (0.1) |
| Joint/Bursa | 3 (0.2) | 0 (0.0) |
| Space/Disc | 1 (0.1) | 0 (0.0) |
| Intracranial | 2 (0.2) | 0 (0.0) |
| Meningitis/Ventriculitis | 0 (0.0) | 1 (0.1) |
| Other Central Nervous System | 3 (0.2) | 0 (0.0) |
| Other Systemic | 1 (0.1) | 0 (0.0) |
| Other | 27 (2.1) | 3 (0.2) |
Table 3.
Univariate Analysis of Individual Infection Sites* on the Risk for Venous Thromboembolism Among Olmsted County Residents, 1988–2000; all Remaining Infection Sites combined, separately for each analysis†.
| Unadjusted | Adjusted w/ Main Venous Thromboembolism Risk Factors‡ |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infection | Remaining Infections | Infection | Remaining Infections | |||||||||
| Variable | OR | 95% CI | P- value |
OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Genitourinary | 6.80 | (4.74, 9.75) | <.0001 | 3.68 | (2.91, 4.65) | <.0001 | 2.54 | (1.54, 4.18) | 0.0003 | 2.31 | (1.67, 3.20) | <.0001 |
| Symptomatic Urinary Tract | 6.92 | (4.69, 10.20) | <.0001 | 3.81 | (3.03, 4.79) | <.0001 | 2.76 | (1.60, 4.77) | 0.0003 | 2.27 | (1.66, 3.12) | <.0001 |
| Lower Respiratory | 9.14 | (5.83, 14.33) | <.0001 | 3.63 | (2.90, 4.54) | <.0001 | 4.33 | (2.43, 7.72) | 0.0000 | 2.01 | (1.47, 2.76) | <.0001 |
| Pneumonia | 9.14 | (5.77, 14.47) | <.0001 | 3.67 | (2.94, 4.59) | <.0001 | 4.25 | (2.35, 7.71) | 0.0000 | 2.05 | (1.49, 2.81) | <.0001 |
| Sino-Upper Respiratory | 2.99 | (2.21, 4.04) | <.0001 | 5.57 | (4.32, 7.18) | <.0001 | 2.05 | (1.37, 3.08) | 0.0006 | 2.62 | (1.83, 3.76) | <.0001 |
| Bronchitis | 2.62 | (1.61, 4.28) | 0.0001 | 4.78 | (3.84, 5.95) | <.0001 | 1.96 | (1.03, 3.72) | 0.0391 | 2.45 | (1.79, 3.34) | <.0001 |
| Oral | 13.83 | (4.11, 46.62) | <.0001 | 4.27 | (3.47, 5.27) | <.0001 | 9.42 | (2.06, 43.02) | 0.0038 | 2.27 | (1.69, 3.06) | <.0001 |
| Bronchitis and Upper Respiratory Tract | 2.85 | (1.99, 4.10) | <.0001 | 5.07 | (4.03, 6.39) | <.0001 | 2.05 | (1.26, 3.33) | 0.0040 | 2.50 | (1.80, 3.46) | <.0001 |
| Skin-Soft Tissue | 3.80 | (2.58, 5.59) | <.0001 | 4.63 | (3.70, 5.81) | <.0001 | 1.77 | (1.00, 3.12) | 0.0491 | 2.52 | (1.84, 3.45) | <.0001 |
| Soft Tissue | 2.95 | (1.66, 5.25) | 0.0002 | 4.64 | (3.74, 5.76) | <.0001 | 0.72 | (0.33, 1.57) | 0.4061 | 2.65 | (1.95, 3.59) | <.0001 |
| Superficial Incisional Site, Organ/Space Surgical Site, and Other Surgical Site | 20.17 | (4.67, 87.16) | 0.0001 | 4.27 | (3.47, 5.26) | <.0001 | 6.19 | (0.74, 51.70) | 0.0923 | 2.34 | (1.74, 3.14) | <.0001 |
| Gastrointestinal | 6.49 | (3.25, 12.96) | <.0001 | 4.33 | (3.50, 5.34) | <.0001 | 2.19 | (0.86, 5.57) | 0.1013 | 2.38 | (1.77, 3.20) | <.0001 |
| Intra-abdominal | 20.35 | (2.57, 160.82) | 0.0043 | 4.39 | (3.54, 5.36) | <.0001 | 9.85 | (0.78, 123.97) | 0.0767 | 2.33 | (1.74, 3.13) | <.0001 |
| Systemic / Blood Stream | 41.97 | (10.19, 172.89) | <.0001 | 3.92 | (3.17, 4.84) | <.0001 | 16.02 | (3.25, 79.02) | 0.0007 | 2.17 | (1.61, 2.92) | <.0001 |
| Laboratory-confirmed Bloodstream | 52.47 | (7.17, 384.19) | 0.0001 | 4.08 | (3.31, 5.03) | <.0001 | 31.27 | (3.58, 273.47) | 0.0019 | 2.19 | (1.62, 2.94) | <.0001 |
| Other | 10.24 | (3.96, 26.50) | <.0001 | 4.24 | (3.44, 5.24) | <.0001 | 5.90 | (1.60, 21.75) | 0.0076 | 2.27 | (1.69, 3.06) | <.0001 |
Adjusting risk factors are the following: Age, Leg Paresis, Trauma/Fracture, Active Cancer, Hospitalized for Surgery, Hospitalized for Acute Medical Illness, Nursing Home Confinement, Superficial Vein Thrombosis, BMI, Transvenous Catheter/Pacemaker, and Estrogen/Progesterone/Oral Contraceptives.
All infections from table 2 with <1% of subjects were excluded from this analysis along with a couple which were essentially repeated variables.
No infection is the referent group
Multivariate Analysis
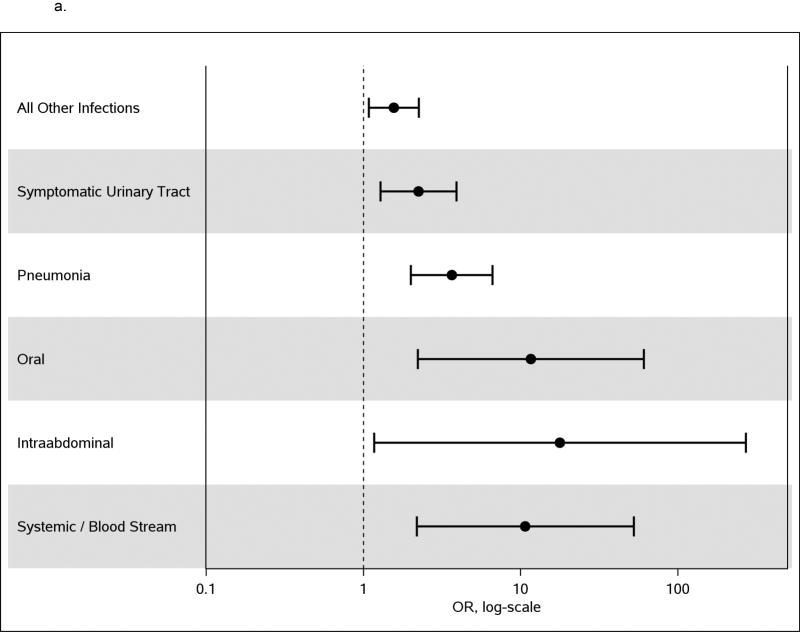
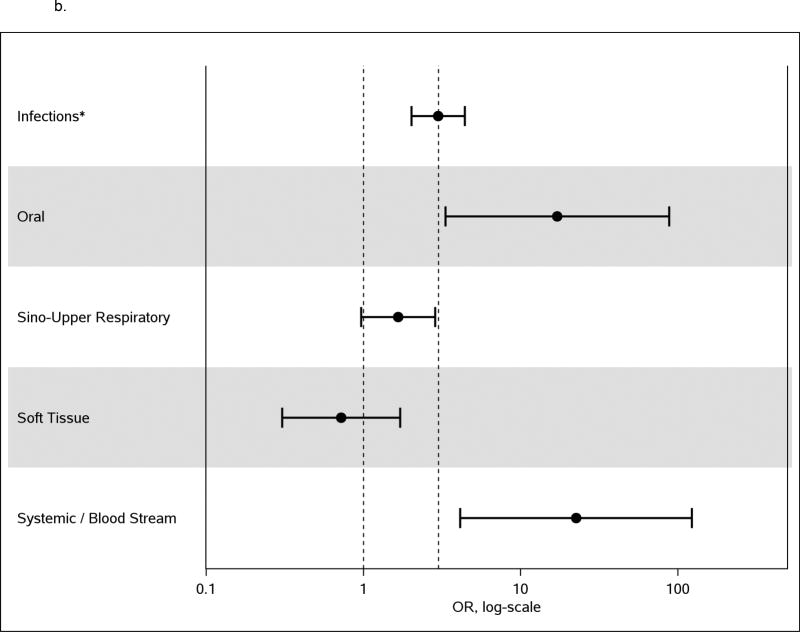
Simultaneously adjusting for all previously established venous thromboembolism risk factors as well as those variables significant at p<0.01, the odds of venous thromboembolism due to any infection was 2.4-fold higher than no infection (95% CI: 1.8, 3.2; p<0.0001; Supplemental Table 1). In the forward stepwise model adjusting for all previously established venous thromboembolism risk factors, symptomatic urinary tract infection, pneumonia, oral infection, systemic/blood stream infection and intra-abdominal infection came into the model. Rerunning the model with all other infections combined into one variable and adjusting for all previously established venous thromboembolism risk factors, the odds of venous thromboembolism were 2.24-fold (95%CI: 1.29, 3.91, p=0.004) higher in those with symptomatic urinary tract infection, 3.64-fold (95%CI: 2.00, 6.63; p<0.0001) higher in those with pneumonia, 11.61-fold (95%CI: 2.22, 60.82; p=0.004) higher in those with oral infection, 10.69-fold (95%CI: 2.18, 52.35; p=0.004) higher in those with systemic/blood stream infection, and 17.8-fold (95%CI: 1.17, 269.7; p=0.04) higher in those with intra-abdominal infection than in those with no infection. The odds of venous thromboembolism for all remaining infections combined were 1.56-fold (95%CI: 1.08, 2.25; p=0.017) higher than those with no infection (Table 4 and Figure 1a). In the forward regression model adjusting for all previously established venous thromboembolism risk factors, forcing the global infection variable into the model (yes to any infection in any location in the prior 92 days) and allowing other infections to compete for entry, having any infection had a 3.0-fold increased odds of venous thromboembolism compared to no infection. In this model, sino-upper respiratory infection and soft tissue infection showed a lower risk of infection than other infections (sino upper respiratory infection had an increase of only 1.7 fold higher than no infection and soft tissue infection was no different from no infection). Oral infection and systemic/blood stream infection showed a significantly higher odds of venous thromboembolism than the global infection variable (Figure 1b).
Table 4.
Multivariate Analysis of Infection Sites on the Risk of Venous Thromboembolism Among Olmsted County Residents, 1988–2000, Adjusting for the Listed Venous Thromboembolism Risk Factors.
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Patient Age | 1.31 | (1.00, 1.70) | 0.0476 |
| Body Mass Index (kg/m2) | 1.07 | (1.05, 1.10) | <.0001 |
| Neurologic Disease | 6.59 | (2.89, 15.07) | <.0001 |
| Trauma/Fracture | 3.39 | (2.00, 5.76) | <.0001 |
| Active Cancer | 10.96 | (6.99, 17.21) | <.0001 |
| Community Hospitalization with Surgery | 7.73 | (4.89, 12.21) | <.0001 |
| Community Hospitalization without Surgery | 3.49 | (2.38, 5.11) | <.0001 |
| Nursing Home | 2.15 | (1.33, 3.46) | 0.0017 |
| Superficial Vein Thrombosis | 3.59 | (2.43, 5.29) | <.0001 |
| Transvenous Catheter/Pacemaker | 1.58 | (0.94, 2.65) | 0.0841 |
| Estrogen/Progesterone/Oral Contraceptives | 2.14 | (1.45, 3.16) | 0.0001 |
| All Other Infections† | 1.56 | (1.08, 2.25) | 0.0167 |
| Symptomatic Urinary Tract† | 2.24 | (1.29, 3.91) | 0.0044 |
| Pneumonia† | 3.64 | (2.00, 6.63) | <.0001 |
| Oral† | 11.61 | (2.22, 60.82) | 0.0037 |
| Intra-abdominal† | 17.77 | (1.17, 269.69) | 0.0381 |
| Systemic/Blood Stream† | 10.69 | (2.18, 52.35) | 0.0035 |
No infection is the referent group
Figure 1.
a. Multivariate analysis of infection sites as risk factors for venous thromboembolism among Olmsted County residents, 1988–2000, adjusting for common venous thromboembolism risk factors. Reference group for infection is ‘No infection’.
b. Multivariate analysis of any infection as a risk factor for venous thromboembolism among Olmsted County residents, 1988–2000, adjusting for common venous thromboembolism risk factors, with the incremental effect of specific infection assessed compared to ‘any infection’ effect.
*Adjusted for infections listed
DISCUSSION
Infection promotes thrombosis through endothelial injury, tissue factor-induced activation of the procoagulant pathway, down-regulation of the endogenous anticoagulant pathway, and inhibition of fibrinolysis.19–22 Venous thrombosis has been linked to neutrophil activation and release of neutrophil extracellular traps23–27, which promote initiation of platelet adhesion, activation and aggregation through the P-selectin mediated pathway.28, 29 In this study, we found that compared to no infection, overall infection and infection site were risk factors for venous thromboembolism after adjusting for all other known venous thromboembolism risk factors; the highest magnitude of risk was imparted by intra-abdominal infection (OR=18) followed by oral infection (OR=12), systemic blood stream infection (OR=11), lower respiratory infection such as pneumonia (OR=3.6), and symptomatic urinary tract infection (OR=2.2), respectively. The remaining infections, combined, contributed to a 1.6-fold increased risk of venous thromboembolism, adjusting for the other risk factors. Oral infection was a significant independent risk factor for venous thromboembolism compared to no infection and adjusting for other risk factors and for other infections (OR=11.6), and had significantly higher risk (p=0.03) than those other infections.
Our findings are consistent with previous reports.9,7,8,30,31,32 In a case-crossover study using data from the Health and Retirement Study and Medicare, 1991–2007, infection within the 90-day period before hospitalization was associated with a 2.9-fold increased risk of hospitalization for venous thromboembolism.9 In a large population-based, case-control (Multiple Environmental and Genetic Assessment [MEGA] of risk factors for venous thrombosis) study, pneumonia was associated with a 5.0-fold increased risk of venous thrombosis within 1 year of infection.32 In a self-controlled case-series study using data from a United Kingdom general practice database, urinary and respiratory tract infections were associated with significantly increased risks for deep vein thrombosis.8 In a case-control study using data from a different United Kingdom general practice database, respiratory and urinary tract infection were significantly and marginally associated with deep vein thrombosis and pulmonary embolism, respectively.31 In a population-based study from Denmark, respiratory tract, urinary tract and intra-abdominal infection were associated with 4.9-, 1.7- and 2.4-fold increased risks of venous thromboembolism, respectively, adjusted for other venous thromboembolism risk factors.8 In this same study, septicemia was associated with a 3.6-fold increased venous thromboembolism risk. Finally, a nationwide population-based cohort study from Taiwan found pneumococcal pneumonia was associated with 2- and 1.8-fold increased risks for pulmonary embolism and deep vein thrombosis, respectively.30
We were surprised to find an independent association of oral infection with venous thromboembolism; to our knowledge, this finding is novel. We noted the wide confidence intervals around the OR point estimate, indicating that the magnitude of venous thromboembolism risk associated with oral infections could be considerably smaller (or larger) than the point estimate. As we had no good explanation for this finding, we again reviewed the medical records of all cases and controls with an oral infection. Two of the three controls had dental abscess as their only identifiable potential venous thromboembolism risk factor; the third control had dental infection, CHF, and was hospitalized for CHF exacerbation within 92 days of the index date. Of the 28 cases with oral infection, 21 had oral candidiasis (four had concurrent oral HSV infection), six had dental infection/ abscess, and one had sublingual salivary gland infection. All 28 cases with oral infection had one or more additional identifiable venous thromboembolism risk factors within 92 days of the event date, including hospitalization for surgery (n=6) or medical illness (n=17), nursing home stay (n=2), active cancer (n=12), undergoing chemotherapy (n=7), autoimmune disease (n=5), pregnancy (n=1), oral contraceptive pill use (n=1), stroke (n=1), long road trip >6 hours (n=1), and additional infections (n=23). Although we adjusted for the above covariates in the multivariate analysis, we cannot exclude residual confounding as an explanation for the observed association between venous thromboembolism and oral infection. Oral candidiasis comprised 75% of oral infections among venous thromboembolism cases. Oral candidiasis is a potential marker for patient debility which may be a venous thromboembolism risk factor not captured by the other covariates we tested.
Acute infection is a component of one inpatient venous thromboembolism risk prediction model (the Padua prediction score);33 our findings regarding infection site-specific venous thromboembolism risk may allow further refinement of such risk prediction models. Future studies are required to assess the utility of venous thromboembolism prophylaxis among outpatients with high venous thromboembolism-risk infections.
Our study has several important strengths. Due to the unique features of the REP, our study avoids referral bias and other potential distortions of including a too healthy population. All venous thromboembolism cases met strict criteria for objectively-diagnosed acute deep vein thrombosis and/or pulmonary embolism and we confirmed that controls did not have venous thromboembolism based on direct review of their source documents (i.e., imaging, surgical and autopsy reports) rather than depending on administrative codes. We included the entire spectrum of venous thromboembolism disease occurring in the community, including persons with rapidly fatal and chronic care facility (e.g., nursing home) venous thromboembolism events who did not reach the hospital. Patients with infection within 92 days prior to the index date were confirmed by reviewing the medical records. We adjusted for all potential venous thromboembolism risk factors when testing for association between patients with infection and venous thromboembolism, and we included all infections sites with a prevalence >5% in the multivariable modeling.
Our study also has important limitations. Due to the difficulty in dating the onset of an infection, we were unable to test the association of different durations of infection (within the preceding 92 days) with venous thromboembolism. The age-, sex- and racial distribution of Olmsted County is similar to that for Minnesota, the upper mid-west, and the U.S. white population; however, residents of Olmsted County exhibit higher median income and education level compared to these geographic regions.6–8 While no single geographic area is representative of all others, the under-representation of minorities may compromise the generalizability of our findings to different racial and ethnic groups.
In conclusion, infection is an independent risk factor for venous thromboembolism. Venous thromboembolism risk can be further stratified by infection site.
Supplementary Material
Clinical Significance.
Compared to no infection, overall infection (OR=2.4) and infection site were independent risk factors for venous thromboembolism.
The highest venous thromboembolism-risk infections were intra-abdominal (OR=18), oral (OR=12), systemic blood stream infection (OR=11), lower respiratory (e.g., pneumonia; OR=3.6), and urinary tract (OR=2.2).
Infection and infection sites are independent risk factors for venous thromboembolism and should be considered as potential indications for venous thromboembolism prophylaxis.
Acknowledgments
Research reported in this publication was supported in part by grants from the National Heart Lung and Blood Institute under Award Numbers R01HL66216 and K12HL83797 to JAH, and was made possible by the Rochester Epidemiology Project (Award Number R01AG034676 of the National Institute on Aging, National Institutes of Health). Research support also was provided by Mayo Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None. All authors had access to the data and a role in writing the manuscript.
References
- 1.Heit JA. The epidemiology of venous thromboembolism in the community. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:370–372. doi: 10.1161/ATVBAHA.108.162545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yusuf HR, Tsai J, Atrash HK, Boulet SL, Grosse SD. Venous thromboembolism in adult hospitalizations – United States, 2007–2009. MMWR. 2012;61:401–404. [PubMed] [Google Scholar]
- 3.Heit JA, Crusan DJ, Ashrani AA, Petterson TM, Bailey KR. Venous thromboembolism attack rates among recently hospitalized vs. community residents: a population-based cohort study. Blood. 2017 in submission. [Google Scholar]
- 4.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Predictors of survival after deep vein thrombosis and pulmonary embolism: a population-based, cohort study. Archives of Internal Medicine. 1999;159:445–453. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 5.Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Archives of Internal Medicine. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, Schulman S, Murad H. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2_suppl):e195Se226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–9. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt M, Horvath-Puho E, Thomsen RW, Smeeth L, Sørensen HT. Acute infections and venous thromboembolism. J Intern Med. 2012;271:608–18. doi: 10.1111/j.1365-2796.2011.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers MAM, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of hospitalization for venous thromboembolism. Circulation. 2012;125:2092–9. doi: 10.1161/CIRCULATIONAHA.111.084467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leibson CL, Petterson TM, Smith CY, Bailey KR, Ashrani AA, Heit JA. Venous thromboembolism in nursing home residents: role of selected risk factors. J Am Geriatr. 2012;60(9):1728–23. doi: 10.1111/j.1532-5415.2012.04100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Archives of Internal Medicine. 2000;160(6):809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 12.Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ., 3rd Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arteriosclerosis, Thrombosis, and Vascular Biology logy. 2009;29(9):1399–405. doi: 10.1161/ATVBAHA.109.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurland LT, Molgaard CA. The patient record in epidemiology. Scientific American. 1981;245(4):54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 14.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Archives of Internal Med. 1998;158(6):585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 15.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Archives of Internal Medicine. 2000;160(6):809–15. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 16.Heit JA, Leibson CL, Ashrani AA, Petterson TM, Bailey KR, Melton LJ., 3rd Is diabetes mellitus an independent risk factor for venous thromboembolism?: a population-based case-control study. Arteriosclerosis, Thrombosis, and Vascular Biology logy. 2009;29(9):1399–405. doi: 10.1161/ATVBAHA.109.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thrombosis Research. 2010;126(5):373–8. doi: 10.1016/j.thromres.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infection in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010 Feb;38(2 Suppl):S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 20.Mosad E, Elsayh KI, Eltayeb AA. Tissue factor pathway inhibitor and P-selectin as markers of sepsis-induced non-overt disseminated intravascular coagulopathy. Clin Appl Thromb Hemost. 2011 Feb;17(1):80–87. doi: 10.1177/1076029609344981. [DOI] [PubMed] [Google Scholar]
- 21.Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. 2008 Jul;34(5):459–468. doi: 10.1055/s-0028-1092876. [DOI] [PubMed] [Google Scholar]
- 22.Ahamed J, Niessen F, Kurokawa T, et al. Regulation of macrophage procoagulant responses by the tissue factor cytoplasmic domain in endotoxemia. Blood. 2007 Jun 15;109(12):5251–5259. doi: 10.1182/blood-2006-10-051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz JA, Fuchs TA, Jackson TO, et al. Plasma DNA is Elevated in Patients with Deep Vein Thrombosis. J Vasc Surg Venous Lymphat Disord. 2013 Oct 01;1(4) doi: 10.1016/j.jvsv.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimball AS, Obi AT, Diaz JA, Henke PK. The Emerging Role of NETs in Venous Thrombosis and Immunothrombosis. Front Immunol. 2016;7:236. doi: 10.3389/fimmu.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savchenko AS, Martinod K, Seidman MA, et al. Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J Thromb Haemost. 2014 Jun;12(6):860–870. doi: 10.1111/jth.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012 Aug;32(8):1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010 Sep 07;107(36):15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coleman DM, Wakefield TW. Biomarkers for the diagnosis of deep vein thrombosis. Expert Opin Med Diagn. 2012 Jul;6(4):253–257. doi: 10.1517/17530059.2012.692674. [DOI] [PubMed] [Google Scholar]
- 29.Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015 Jul 09;126(2):242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YG, Lin TY, Huang WY, Lin CL, Dai MS, Kao CH. Association between pneumococcal pneumonia and venous thromboembolism in hospitalized patients: A nationwide population-based study. Respirology. 2015 Jul;20(5):799–804. doi: 10.1111/resp.12501. [DOI] [PubMed] [Google Scholar]
- 31.Clayton TC, Gaskin M, Meade TW. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice database. International J Epidemiol. 2011;40:819–27. doi: 10.1093/ije/dyr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribeiro DD, Lijfering WM, Van Hylckama Vlieg A, Rosendaal FR, Cannegieter SC. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost. 2012 Jun;10(6):1179–82. doi: 10.1111/j.1538-7836.2012.04732.x. [DOI] [PubMed] [Google Scholar]
- 33.Vardi M, Ghanem-Zoubi NO, Zidan R, Yurin V, Bitterman H. Venous thromboembolism and the utility of the Padua Prediction Score in patients with sepsis admitted to internal medicine departments. J Thromb Haemost. 2013;11:467–473. doi: 10.1111/jth.12108. doi. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.