Abstract
Background
Neuroscience research in brain development and disorders can benefit from an in vivo animal model that portrays normal white matter (WM) development trajectories and has a sufficiently large cerebrum for imaging with human MRI scanners and protocols.
New method
Twelve three-month-old Sinclair™ miniature pigs (Sus scrofa domestica) were longitudinally evaluated during adolescent development using advanced diffusion weighted imaging (DWI) focused on cerebral WM. Animals had three MRI scans every 23.95 ± 3.73 days using a 3-T scanner. The DWI imaging protocol closely modeled advanced human structural protocols and consisted of fifteen b-shells (b = 0–3500 s/mm2) with 32-directions/shell. DWI data were analyzed using diffusion kurtosis and bi-exponential modeling that provided measurements that included fractional anisotropy (FA), radial kurtosis, kurtosis anisotropy (KA), axial kurtosis, tortuosity, and permeability-diffusivity index (PDI).
Results
Significant longitudinal effects of brain development were observed for whole-brain average FA, KA, and PDI (all p < 0.001). There were expected regional differences in trends, with corpus callosum fibers showing the highest rate of change.
Comparison with existing method(s)
Pigs have a large, gyrencephalic brain that can be studied using clinical MRI scanners/protocols. Pigs are less complex than non-human primates thus satisfying the “replacement” principle of animal research.
Conclusions
Longitudinal effects were observed for whole-brain and regional diffusion measurements. The changes in diffusion measurements were interepreted as evidence for ongoing myelination and maturation of cerebral WM. Corpus callosum and superficial cortical WM showed the expected higher rates of change, mirroring results in humans.
Keywords: Diffusion weighted imaging, White matter, Miniature swine, Adolescent brain development
1. Introduction
Adolescence to early adulthood is a crucial period for human brain development that is associated with acquisition of higher cognitive functions and emergence of neuropsychiatric illnesses (Rapoport et al., 2005a; Casey et al., 2007; Kalia, 2008). Magnetic resonance imaging (MRI) based brain assessments have become the primary approach for studying human brain development, and there is a need for an animal model that allows equivalent neuroimaging to be complemented with invasive neuroscience techniques. However, the established animal models for brain development, including laboratory rodents and ferrets (Jacob, 1999; Bryda, 2013; Rosenthal and Brown, 2007; Hardouin and Nagy, 2000; Armstrong et al., 1991; Welker, 1990) and non-human primates (Goodman and Check, 2002; Bontrop, 2001; Torres et al., 2010; Patterson and Carrion, 2005; VandeBerg and Williams-Blangero, 1997), have significant limitations or challenges. Rats and mice have a lissencephalic cortex and their brains are different from human in both appearance and structural proportionality. Ferrets do have a gyrencephalic cortex, but their cerebral gyrification process differs from humans and occurs after birth (Smart and McSherry, 1986; Kochunov et al., 2010b). Further, the small brain volume of both rodents and ferrets necessitates the use of dedicated high-field, small-bore animal MRI scanners and special imaging protocols, which substantially reduces the potential for translational research. Non-human primates have a cerebral development pattern comparable to humans and can be studied using a human MRI scanner (Armstrong et al., 1991; Kochunov et al., 2010b; Armstrong et al., 1995; Pillay and Manger, 2007; Kochunov et al., 2011a); however, non-human primates are expensive and have ethical constraints that limit their availability for basic neuroscience research. Here, we tested the miniature pig (Sus scrofa domestica), as an animal model for translational neuroimaging studies of adolescent brain development that overcomes most of the above limitations.
Human brain development includes two critical periods separated in time: primary gyrification and primary myelination (Kochunov et al., 2010, 2009a). The primary gyrification starts during the third trimester and is completed by birth. During this period, the cortical folding coupled with cortical surface area expansion and the differentiation/branching of axons and dendrites, leads to the creation of a gyrencephalic cortex (Xu et al., 2010; Bayly et al., 2014). Abnormalities in cortical folding are generally caused by genetic disorders and result in reduced cognitive ability (Bayly et al., 2014; Pang et al., 2008; Nordahl et al., 2007; Hardan et al., 2004). Primary cerebral myelination begins soon after birth and continues until early adulthood (Flechsig, 1901a). It is a highly heterochronic process (Dubois et al., 2012; Kulikova et al., 2015; Dean et al., 2016, 2015, 2017; Lebel and Beaulieu, 2011; Lebel et al., 2012; Kinney et al., 1988; Barkovich et al., 1988) with motor and sensory WM bundles become fully myelinated during childhood (Lebel et al., 2008; Eluvathingal et al., 2007; Bonekamp et al., 2007; Ashtari et al., 2007; Barnea-Goraly et al., 2005; Schmithorst et al., 2002; Mukherjee et al., 2001). Myelinated axons in the brain’s WM support its functionality by propagating electric signal transmissions through saltatory conductance (Hildebrand et al., 1993; Susuki, 2013; Miller et al., 2013). Thus, the myelination level impacts the propagation speed of the action potential (Lutz et al., 2005; Ashe and Georgopoulos, 1994; Bartzokis et al., 2010). In adolescence, changes in the fronto-cortical and fronto-striatal circuits lead to the development of higher cognitive skills (Bartzokis et al., 2010; Bartzokis, 2004; Bartzokis et al., 1999; Bastos Leite et al., 2004; Kochunov et al., 2010a; Kochunov et al., 2009b; Prins et al., 2004; Schiavone et al., 2009). Adolescence is also a peak period for the development of neuropsychiatric disorders, such as schizophrenia, and when reduced inhibition and excessive reward seeking and risky behaviors may lead to life-long substance use disorders (Weinberger and Lipska, 1995; Lewis and Levitt, 2002; Rapoport et al., 2012, 2005b; Murray et al., 1992; McGorry et al., 2011). Myelination of associative WM tracts improves connectivity of the intra-cortical connectivity networks and maximizes information processing speed and energy efficiency (Hildebrand et al., 1993; Harris and Attwell, 2012; Vaishnavi et al., 2010; Lee et al., 2012; Wen and Chklovskii, 2005; Laughlin and Sejnowski, 2003).
The miniature pig is commonly utilized in translational research pertaining to the cardiovascular, digestive, and integumentary systems (Stricker-Krongrad et al., 2016; Swindle et al., 2012; Schomberg et al., 2016) due to anatomical, physiological, and genetic similarities to humans (Swindle et al., 2012; Schomberg et al., 2016; Wernersson et al., 2005). The intermediate size of miniature pigs allows for easier housing (Stricker-Krongrad et al., 2016), and they are significantly less expensive than non-human primates (Schomberg et al., 2016; Coors et al., 2010). Miniature pigs are intelligent, have a mild temperament, and are amendable for training and cognitive research (Stricker-Krongrad et al., 2016; Schomberg et al., 2016). Miniature pigs have a fully gyrencephalic brain, undergo primary gyrification in-utero and as adults have an equal ratio of cerebral white and gray matter (Fig. 1), comparable to that in humans (Swindle et al., 2012; Schomberg et al., 2016; Conrad et al., 2012; Ostergaard et al., 1998). Further, miniature pigs and humans have analogous brain development, including similar myelination and brain white matter growth patterns (Sauleau et al., 2009).
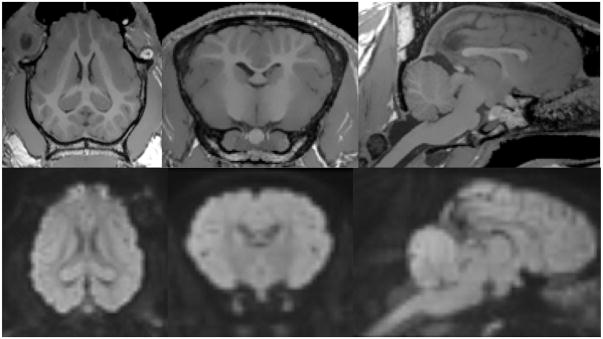
Fig. 1.
T1-weighted and DWI-weighted (average across all b-value) images collected in a live pig. T1-weighted image demonstrates fully gyrified cortex with excellent gray matter/white matter contrast. DWI-weighted image demonstrates excellent resolution and lack of shape distortion artifacts.
We used quantitative diffusion weighted imaging (DWI) for longitudinal assessment of changes in cerebral WM in the adolescent-age miniature pigs. Diffusion tensor imaging (DTI) is the most commonly used DWI approach that describes the Gaussian properties of the diffusion displacement distribution of water in the brain by fitting a mono-exponential function to the weighted diffusion signal decay at a low “diffusion weighting” (b-value ≤ 1000 s/mm2). The choice of a lower b-value range makes DTI sensitive to water molecules with high diffusivities. Neuroimaging studies in humans have mapped the normal developmental trajectory of cerebral WM using DTI’s fractional anisotropy (FA) of water diffusion (Kochunov et al., 2012; Flechsig, 1901b; Yakovlev and Lecours, 1967; Kochunov et al., 2011b; Gao et al., 2009; Ben Bashat et al., 2005). FA is also a valuable phenotype in neuropsychiatric disorders. Reduced FA has been found in adolescents with high clinical risk for development of psychosis (Karlsgodt et al., 2009, 2012, 2015), schizophrenia (Wright et al., 2015; Nazeri et al., 2013; Perez-Iglesias et al., 2010; Friedman et al., 2008; Alba-Ferrara and de Erausquin, 2013; Phillips et al., 2012; Kubicki et al., 2007; Glahn et al., 2013; Ellison-Wright and Bullmore, 2009), and substance use disorders (Acheson et al., 2015, 2014a,b). FA is a simple and empirical parameter but it lacks biological specificity and that makes interpretation of FA findings difficult (Alba-Ferrara and de Erausquin, 2013; Pierpaoli and Basser, 1996). Moreover, the water diffusion signal is non-Gaussian, and at higher b-values, DTI fails to approximate the non-mono-exponential decay of diffusion signal that is caused by the restriction of diffusion by cellular membranes (Jensen and Helpern, 2010; Sukstanskii et al., 2003, 2004). We supplemented DTI metrics with two DWI analysis methods: diffusion kurtosis imaging (DKI) (Jensen et al., 2005a) and permeability-diffusivity (PD) (Kochunov et al., 2013). DKI and PD models overcome the limitations of DTI and characterize the sources of the non-Gaussian distribution of diffusion signals.
In the present study, we hypothesized that the diffusion signal from white matter (WM) in the miniature pigs can serve as a good animal model for adolescent to young adult brain development in humans. Specifically, we used adolescent aged miniature pigs to (1) study the sensitivity of the different DWI measurements to adolescent development in whole-brain WM and (2) explore the WM regional heterochronicity to assess the different developmental trajectories in various brain regions.
2. Methods
2.1. Animal subjects
Twelve female Sinclair™ miniature swine underwent three MRI scans every 23.95 ± 3.73 days. Female swine were chosen to facilitate bladder catherization for the prolonged duration MRI. All remained free of disease throughout the duration of the study. The average ages for MRI #1, #2, and #3 were 102.58 ± 16.91 days, 123.58 ± 17.10 days, and 149.5 ± 15.96 days, respectively. The sample was age-selected to correspond with human adolescence/early adulthood (age 10–19 years). The pigs are considered late pre-pubescent for MRI #1 and sexually mature by MRI #3. Four of the twelve animals were transferred into another study prior to MRI #3 and one was missing DKI data on MRI #3. The local Institutional Animal Care and Use Committee reviewed and approved all animal protocols. All animals were housed in the 59th Clinical Research Division vivarium of Joint Base San Antonio Lackland Air Force Base (AFB), TX.
2.2. Imaging protocol
All imaging was performed using a Siemens 3-T Verio scanner with a multi-channel knee coil at the Wilford Hall Ambulatory Surgical Center, 59th Medical Wing, Joint Base San Antonio-Lackland AFB, TX. A human knee coil (inner diameter ~ 180 mm) provided an excellent coverage (field of view ~200 mm) for the brain and a comfortable fit for the animals placed in prone position. It provided a better signal-to-noise ratio (SNR) than volumetric coils for human brain imaging, however, SNR may likely be improved by the use of custom RF coils optimized for miniature pig anatomy. General anesthesia in the form of isoflurane was administered with an MRI-compatible machine for the 3-h scan duration. Metabolic and fluid status was continuously monitored and no subjects displayed signs of distress. Pigs were imaged in the prone position.
2.3. Diffusion weighted imaging and data processing
The DWI protocol was developed based on q-space protocols for in vivo mapping of water diffusion in the brain (Wu et al., 2011; Clark et al., 2002). This protocol consisted of 15 shells of b-values (b = 0, 250, 500, 600, 700, 800, 900, 1000, 1250, 1500, 1750, 2000, 2500, 3000, 3500 s/mm2; diffusion gradient duration = 47 ms, diffusion gradient separation = 54 ms). Thirty-two isotropically distributed diffusion weighted directions were collected per shell, including sixteen b = 0 images. Three dummy scans preceded the data collection to establish the steady state. The highest b-value (b = 3500 s/mm2) was chosen because the signal-to-noise ratio (SNR) for the corpus callosum in the diffusion weighted image (SNR = 6.9 ± 0.7) measured during protocol development approached the empirically selected lower limit of SNR = 5.0. The b-values and the number of directions per shell were chosen for improved fit of the bi-exponential model and SNR (Jones et al., 1999). The imaging data were collected using a single-shot, echo-planar, single refocusing spin-echo, T2-weighted sequence with a spatial resolution of 1.72 × 1.72 × 1.70 mm and 40 slices prescribed in axial orientation for complete brain coverage. The sequence was repeated with the reversal of the phase encoding gradients for correction of spatial distortions and improvement in SNR (Fig. 2). In addition to DWI, high-resolution (0.5-mm isotropic) T1-weighted structural data were collected.
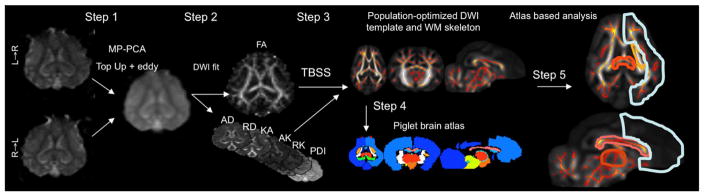
Fig. 2.
Analysis of DWI data. DWI data collected with reversal of phase-encoding gradients was denoised using MP-PCA filtering and corrected for shape distortions using TopUp and eddy current corrections (Step 1). In step 2, DWI model parameters were estimated including Fractional Anisotropy (FA) and Axial and Radial Diffusivity (AD and RD), Kurtosis Anisotropy (KA), Axial and Radial Kurtosis (AK and RK) and Permeability Diffusivity Index (PDI). Tract-based spatial statistics (TBSS) approach was used to warp individual FA images to a population-optimized FA atlas and project the center of the major WM tracts on the population-wide skeleton (Step 3). The other DWI parameters were projected on the skeleton based on FA projection maps for each subject (Step3). A piglet-based brain atlas was used to register the DWI brain template (Step 4). The overlap between regions of interests provided by the atlas and the skeleton of the cerebral white matter was used to calculate the regional average DWI parameters (Step 5).
Thermal noise in the data was reduced by applying the Marchenko Pastour Principle Component Analysis (MPPCA) denoising technique that exploits the inherent redundancy in diffusion MRI data (Veraart et al., 2016a,c,b). Thereafter, the data was corrected for Gibbs ringing (Kellner et al., 2016), Rician bias (Gudbjartsson and Patz, 1995), geometric eddy current and EPI distortions, and subject motion (Andersson and Sotiropoulos, 2016, 2015; Andersson et al., 2003; Smith et al., 2004).
2.3.1. DKI model calculations
DKI is a model-independent diffusion signal representation that extends conventional DTI by the addition of the kurtosis term to account for non-Gaussian behavior of the diffusion signal (Eq. (1), Fig. S1, see supplement) (Jensen and Helpern, 2010; Jensen et al., 2005b; Lu et al., 2006). Both the diffusion and kurtosis tensors can be derived from the second-order expansion of the multi b-shell diffusion-weighted signal decay as function of the b-value:
| (1) |
with gi the ith component of gradient direction g and S(0) the nondiffusion-weighted signal intensity. Dij is the ijth element of the fully symmetric diffusion tensor D, characterized by six independent elements and Wijkl denotes an element of the diffusion kurtosis tensor W, which is fully parameterized by 15 independent elements. The DKI model was fit based on the reduced set of diffusion values (b up to 2500s/mm2) to minimize approximation errors O(b3), yet to capture the non-Gaussian diffusion effect that become apparent at intermediate b-values.
During the model fit, multivariate regression was used to estimate the eigenvalues for the diffusion (L1,2,3) and kurtosis (K1,2,3) tensors. The three eigenvalues for diffusion tensor were converted to axial (L|), radial, (L⊥), and FA. Similarly, the three eigenvalues of the kurtosis tensor were converted to axial (K|), radial, (K⊥), and kurtosis anisotropy (KA) values (Poot et al., 2010)
2.3.2. Permeability-diffusivity model calculations
The PD model is based on the bi-exponential fit of diffusion decay (Eqs. (2) and (3)) (Kochunov et al., 2013):
| (2) |
| (3) |
Here S(b) is a diffusion weighted signal for a given b value, averaged across all directions. Mu is the compartmental fraction of the signal that comes from unrestricted diffusion, and (1–Mu) is the signal from the restricted diffusing compartment. Du and Dr are the diffusivity of the unrestricted and restricted compartments, respectively. The expansion was fit based on the full set of diffusion values (0–3500 s/mm2); using the following initial fit values: Mu = 0.5,. Du = 0.1 and Dr = 0.01 mm2/s.
2.3.3. Global and regional diffusion measurements
All diffusion measurements were extracted using tract-based spatial statistics protocol by combining ENIGMA-DTI protocol (Jahanshad et al., 2013) with the piglet MRI brain atlas (Conrad et al., 2014). Briefly, T1-brain images were registered to the pig brain atlas using a 12-parameter transformation and the transformation was used to reslice FA images for individual brains. Next, a “minimal deformation target” was created based on FA images from the participating animals as previously described (Jahanshad et al., 2013). The data were then processed using FSL’s tract-based spatial statistics (TBSS; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS) analytic method (Smith et al., 2006) modified to project individual FA values on the hand-segmented pig skeleton mask. After extracting the skeletonized WM, FA values corresponding to the middle of major WM tracts, based on the local maximum in the direction normal to the skeleton, were projected onto the WM skeleton. This analysis was repeated for images of the DKI and PD index (PDI) parameters using the non-FA option of the pipeline. This option produced skeletonized WM images of corresponding non-FA values. Following projection, regions of interest, derived from the pig brain atlas, were transferred to extract the mean values for the full skeleton and average values for major brain areas, including the corpus callosum (CC) and superficial WM. Whole-brain averaged diffusion parameters were obtained by averaging the entire skeleton. Overall, the entire DWI imaging and DKI/PDI modeling approaches essentially remained the same as human DWI protocols (Kochunov et al., 2016a) to maximize the translational potential of the miniature pig model.
2.4. Statistical analyses
2.4.1. Regression analysis
To assess longitudinal trends in the diffusion measurements, we used a generalized linear mixed effects (GLME) model. The GLME model partitions the variance in the measurements from each subject into the fixed (predictor) effects that are observed on the group level and a random set of latent, non-independent effects that are individual to each subject. The normality of the distribution of the diffusion parameters were verified using Kolmogorov-Smirnov and Shapiro-Wilk statistics (all p > 0.2) Modeling longitudinal measurements using GLME models allows the results of the study to be more representative by accounting for non-independent individual variance. The GLME model is more flexible than repeated ANOVA analyses because it does not require the same number of measurements for each subject. The GLME modeling used age as the fixed predictor for the diffusion measurements and a random effect variable (ε) to account for individualized variance for each animal. This model is shown in Eq. (4):
| (4) |
where Pi,j is the diffusion parameter (FA, KA, AK, AD, RD, or PDI) for the “jth” pig at the age (Agei,j) in days since birth of the “ith” imaging session, A is a constant, β is the regression coefficient, and αi,j is a mixed effect coefficient.
GLME modeling was performed in R (R-Development-Core-Team., 2009) using the nonlinear mixed effect model library (nlme) (Pinheiro et al., 2012). The model accounts for variance between subjects by determining both the fixed and random effects. Linearity between the diffusion measurements and age is established before outputting the standardized regression coefficients (β) and standard errors.
For the regional measurements, Bonferroni correction set statistical significance at p ≤ 0.007 to reduce type 1 errors associated with multiple (N = 7 brain regions) comparisons; p-values between 0.008 and 0.05 were deemed suggestively significant and were also discussed.
3. Results
3.1. Whole-brain longitudinal trends
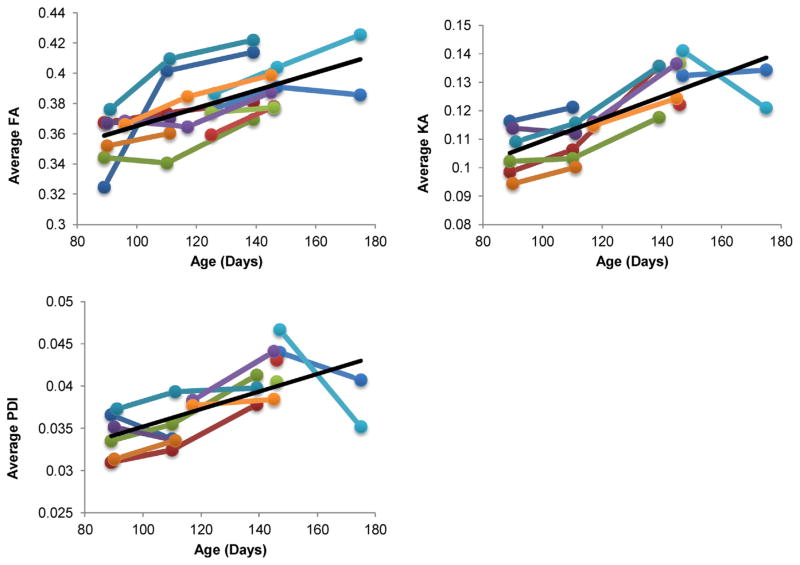
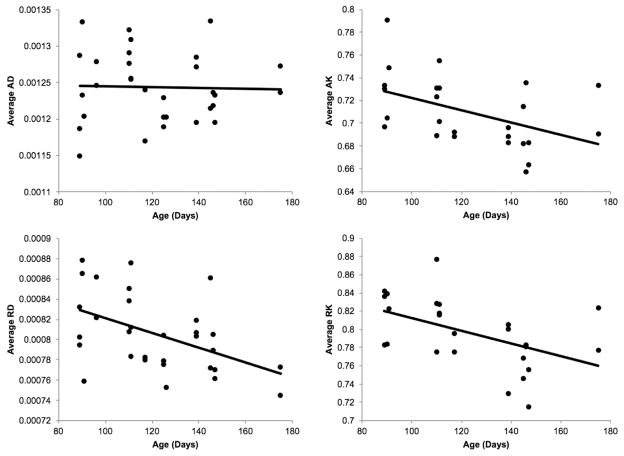
Significant longitudinal effects of age were observed for FA, KA and PDI (all p < 0.001) (Table 1, Fig. 3). All three diffusion measurements showed consistent longitudinal rise with age, except for one animal (Fig. 3). Significant decreases with age were observed for axial kurtosis (AK) and radial kurtosis (RK) (p = 0.027 and 0.025, respectively) (Fig. 4). The radial diffusivity showed a negative trend but it was not significant (Fig. S2). The average FA was significantly and positively correlated with both KA and PDI (r = 0.59 and 0.40, p < 0.001). KA and PDI were also significantly correlated (r = 0.81, p < 10−8).
Table 1.
Fixed factor regression results (beta ± standard error (SE) and p-values).
| Measurement | βAge ± SE | p-value |
|---|---|---|
| DTI Model | ||
| FA | 6.22E-04 ± 1.18E-04 | 1.46E-05 |
| AD | 2.52E-07 ± 3.50E-07 | 0.54 |
| RD | −4.13E-07 ± 2.29E-07 | 0.24 |
| DKI Model | ||
| KA | 3.89E-04 ± 6.91E-05 | 1.28E-05 |
| AK | −5.21E-04 ± 2.19E-04 | 0.027 |
| RK | −6.26E-04 ± 2.60E-04 | 0.025 |
| PDI Model | ||
| PDI | 1.04E-04 ± 2.49E-05 | 3.71E-04 |
Note: Bolded values indicate significance.
Fig. 3.
Average whole-brain Fractional Anisotropy (FA), Kurtosis Anisotropy (KA), and Permeability-diffusivity index (PDI) showed significant longitudinal effects of age. The fitted regresson line is shown in black. The average whole-brain diffusion trajectory for each individual pig is shown in color.
Fig. 4.
Average whole-brain AD, RD, AK, and RK diffusion measures versus age (days). AK and RK showed suggestive significance with age (p = 0.02). The fitted regression line is indicated in black.
3.2. Regional longitudinal trends
Regional effects of aging were assessed using diffusion metrics obtained for six brain areas from the piglet brain atlas (Conrad et al., 2014): CC, superficial WM, parahippocampal WM, midbrain, thalamic radiation (TR), and internal/external capsule (I/EC) (Table 2). Measures from the left and right hemispheres of the same structure were averaged, as there was no evidence of significant lateralization.
Table 2.
Regional WM regression results for the fixed factors (beta ± standard error (SE) and p-values).
| Region | FA βAge ± SE (p-value) |
AD βAge ± SE (p-value) |
RD βAge ± SE (p-value) |
KA βAge ± SE (p-value) |
AK βAge ± SE (p-value) |
RK βAge ± SE (p-value) |
PDI βAge ± SE (p-value) |
|---|---|---|---|---|---|---|---|
| Corpus Callosum | 7.59E-04 ± 1.46E-04 (2.28E-05) | −2.97E-07 ± 9.78E-07 (0.79) | −1.22E-06 ± 7.32E-07 (0.24) | 4.36E-04 ± 9.61E-05 (2.10E-04) | −4.24E-04 ± 2.90E-04 (0.16) | −5.37E-04 ± 2.36E-04 (0.04) | 1.48E-04 ± 2.13E-05 (5.35E-07) |
| Superficial WM | 5.71E-04 ± 1.12E-04 (2.33E-05) | 3.77E-07 ± 2.91E-07 (0.33) | −1.31E-07 ± 1.78E-07 (0.58) | 2.68E-04 ± 7.17E-05 (1.13E-03) | −5.33E-04 ± 2.19E-04 (0.02) | −4.17E-04 ± 2.48E-04 (0.11) | 8.87E-05 ± 2.51E-05 (1.87E-03) |
| Fornix/Parahippocampal WM | 1.60E-04 ± 1.89E-04 (0.40) | −8.01E-07 ± 7.53E-07 (0.39) | −5.96E-07 ± 5.25E-07 (0.31) | 1.57E-04 ± 1.52E-04 (0.31) | 1.30E-04 ± 3.14E-04 (0.68) | 3.70E-04 ± 3.17E-04 (0.26) | 3.43E-05 ± 3.30E-05 (0.31) |
| Midbrain | 7.96E-04 ± 1.41E-04 (4.19E-06) | 2.50E-07 ± 6.31E-07 (0.73) | −7.42E-07 ± 5.00E-07 (0.20) | 4.83E-04 ± 1.36E-04 (1.73E-03) | −5.47E-04 ± 2.39E-04 (0.03) | −1.68E-03 ± 3.50E-04 (7.47E-05) | 1.60E-04 ± 4.10E-05 (7.03E-04) |
| Thalamic Radiation | 4.32E-04 ± 1.03E-04 (2.45E-04) | 1.40E-07 ± 3.50E-07 (0.72) | −3.16E-07 ± 2.86E-07 (0.38) | 8.32E-04 ± 1.32E-04 (1.96E-06) | −9.31E-04 ± 3.17E-04 (0.01) | −1.46E-03 ± 3.85E-04 (9.54E-04) | 2.18E-04 ± 4.38E-05 (4.96E-05) |
| Internal/External Capsules | 8.32E-04 ± 1.45E-04 (6.13E-06) | 8.27E-07 ± 3.66E-07 (0.14) | −4.74E-07 ± 2.23E-07 (0.19) | 6.28E-04 ± 1.90E-04 (3.20E-03) | −7.34E-04 ± 2.89E-04 (0.02) | 7.79E-05 ± 4.23E-04 (0.86) | 5.57E-05 ± 2.83E-05 (0.06) |
Note: Bolded values indicate significance and italicized values indicate suggestive (.007 < p ≤ .05) significance after correcting for multiple (N = 7 brain regions of interest) comparisons.
Significant longitudinal changes in DWI parameters were observed for all brain regions except for the parahippocampal WM which showed no age effects on any of the diffusion measurements. FA and KA increased in all regions except for the parahippocampal WM, and PDI significantly increased in the CC, superficial WM, midbrain, and TR. RK significantly decreased in the midbrain and TR. The PDI increase in the corpus callosum was the most robust effect observed (p = 6.7 10−7), followed by the rise in KA in the TR (p = 1.4 10−6) and the rise in FA in the I/EC (p = 5.6 10−6). Except for AK and RK, the diffusion metrics increased with age.
4. Discussion
A longitudinal imaging protocol was used to assess changes in WM integrity during normal adolescent maturation in miniature pigs. The trends of WM DWI measurements were similar to these observed during the period of adolescence and young adulthood in humans (Kochunov et al., 2012; Grinberg et al., 2017; Howroyd et al., 2016; Peter et al., 2016). In addition to the rise in DTI-FA values, we observed significant changes in two DWI parameters that describe the non-Gaussian diffusion properties KA and PDI. We interpreted these changes based on findings in humans as evidence for ongoing structural maturation of cerebral WM. Also, analogous to humans, the highest rates of change were observed in the CC and superficial WM, suggesting heterochronicity of WM development in pigs (Kochunov et al., 2012, 2011b).
We are developing the miniature pig adolescent WM development model to complement existing small animal laboratory models including rats (Crum et al., 2017; Hansen et al., 2017; Lentz et al., 2014; Petrenko et al., 2017) and ferrets (Das and Takahashi, 2017; Hutchinson et al., 2016; Knutsen et al., 2010; Schwerin et al., 2017). Large, gyrencephalic brains of miniature pigs can be studied using a human MRI scanner and imaging protocols developed for human subjects (Kochunov et al., 2013, 2014). The lower costs and shorter development span for the miniature pig model compares favorably with the non-human primate research models of brain development (Kochunov et al., 2010b, 2011a; Kohama et al., 2012; Li et al., 2017; Sakai et al., 2017a,b). Miniature pigs are sexually mature by six months of age, while it may take 4–5 years for non-human primates to reach that developmental landmark (Onyango et al., 2013; Abee, 2017). The gestational period for miniature pigs is likewise shorter, lasting only 16 weeks (McAnulty et al., 2017) compared to 38 weeks for non-human primates (Onyango et al., 2013; Abee, 2017). Mice have a shorter (three week) gestational period and are sexually mature between 5 and 8 weeks of age (Lambert, 2007). However, weaning of mice at 3 weeks of age shortens the opportunity for pre-/adolescent development research to 2–5 weeks. Miniature pigs are weaned at 6–8 weeks of age and therefore, longer, but still compressed, development period posits a miniature pig as a valuable research model for adolescent development.
We observed significant increases in FA in pigs, similar to these reported during adolescent development in humans. FA is a non-specific index that reflects the anisotropy of the Gaussian diffusivity of the fast diffusing water pool (Kochunov et al., 2013). The non-specific nature of this index renders interpretation of changes in FA speculative. For example, increases in FA values may be due to increased myelination of WM microstructures and can be considered an indirect measurement of myelin level (Budde et al., 2007; Madler et al., 2008; Song et al., 2003, 2005). A decrease in RD, a parameter that reflects water movement perpendicular to the fiber axis, reinforces this interpretation. In pigs, RD showed a decreasing trend with age, but was not statistically significant due to higher variance in this measurement. However, FA is also sensitive to reorganization of white matter fibers including changes in axon diameter, axonal density, and/or membrane permeability (Beaulieu, 2002; Jones and Cercignani, 2010; Jones et al., 2013; Takahashi et al., 2002).
Advancements in DWI technology provide for a noninvasive, quantitative analysis of non-Gaussian diffusion indexes to portray developmental trajectories that correspond to changes in the slow diffusing pool of water that are sensitive to membrane permeability, axonal growth, and maturation (Lebel et al., 2008; Barnea-Goraly et al., 2005; Kochunov et al., 2013). Overall, our findings are suggestive of cerebral WM maturation and are consistent with the study performed by Grinberg and colleagues, who showed significant changes in Gaussian and non-Gaussian DWI parameters with age in adolescent humans (Grinberg et al., 2017). The DTI-FA, DKI-KA and PDI rose significantly with age in pigs, similar to the trends observed in humans (Grinberg et al., 2017). The DTI, DKI and PD techniques provide convenient mathematical representation of the signal but make no specific assumptions regarding the underlying biophysical phenomena. The derived diffusion metrics should primarily be discussed in a phenomenological way, without necessarily referring to specific biological postulations of the model features, but taking advantage of the measurements in the extended range of b-values and bi-exponential fits (Grinberg et al., 2011). The robust and significant changes in the FA, KA and PDI during maturation encourage basic neuroscience and biophysics efforts to identify the water diffusion mechanisms governing these DWI signal.
Analysis of regional diffusion trends suggested that pigs may have a heterochronic WM development pattern. In humans, WM development is highly heterochronic (Dubois et al., 2012; Kulikova et al., 2015; Dean et al., 2016, 2015, 2017; Lebel and Beaulieu, 2011; Lebel et al., 2012; Kinney et al., 1988; Barkovich et al., 1988). The motor and sensory WM bundles in humans become fully myelinated during childhood (Lebel et al., 2008; Eluvathingal et al., 2007; Bonekamp et al., 2007; Ashtari et al., 2007; Barnea-Goraly et al., 2005; Schmithorst et al., 2002; Mukherjee et al., 2001). In contrast, the associative WM tracts develop into the third and fourth decades of life (Barkovich et al., 1988; Lebel et al., 2008; Eluvathingal et al., 2007; Bonekamp et al., 2007; Ashtari et al., 2007; Barnea-Goraly et al., 2005; Schmithorst et al., 2002; Mukherjee et al., 2001), well past the average age of onset for schizophrenia and other neuropsychiatric disorders (Flechsig, 1901a; Yakovlev and Lecours, 1967; Kochunov and Hong, 2014; Kochunov et al., 2016b).
There are few DTI studies on normal longitudinal white matter development in mice (Zhang et al., 2006; Larvaron et al., 2007). Small brain size and limited spatial resolution prevents visualization of WM tracts and DTI imaging in mice is susceptible to partial volume effects of GM/WM tissue and cerebral spinal fluid (Larvaron et al., 2007; Wu et al., 2013). Only limited DTI research was conducted in ferrets due to similar limitations (Hutchinson et al., 2016; Kroenke et al., 2009; Bock et al., 2010; Tao et al., 2012; Olavarria et al., 2012; Hutchinson et al., 2017).
There are several limitations in this pilot study. First, an all-female pig population was used due to ease of bladder catherization. Prior studies have established significant brain maturation differences between the sexes, including development rates and brain-volume differences (Eluvathingal et al., 2007; Luders et al., 2005; Allen et al., 2003; Durston et al., 2001). This study is also limited by a small number of animals and the use of a coarse, piglet brain atlas. Investigations with a larger sample size would allow for timing comparisons amongst the regional trajectory rates. Future development should also include a detailed atlas of cerebral WM tracts in pigs that is validated with histological measurements to map the brain development trends from birth to maturity.
5. Conclusion
We demonstrated the applicability of an advanced DWI protocol in pigs and showed agreement in trends and heterochronicity between adolescent development in pigs and humans. The fully gyrencephalic brain and prolonged WM maturation make the miniature pig a more accurate model of human development than small laboratory animals, and low costs and a shorter period of cerebral development make it a good alternative to non-human primate. A limitation of all animal models is the ability to translate research findings to the humans and the use of animal-specific MRI scanners and sequences may delay translation. The benefit of a miniature pig model is that human scanners/protocols can be used for collecting neuroimaging indices in an animal model that permits basic neuroscience research. We demonstrated the potential use of miniature pigs as an animal model for neuroimaging studies of brain development. Additional applications of this model may include traumatic brain/diffuse axonal injury, neuroinflammation and other neuroimaging translational research studies that require large gyrencephalic animal models.
Supplementary Material
HIGHLIGHTS.
An advanced diffusion weigted imaging protocol was used to map adolescent brain development in miniture pig.
Longitudinal effects of aging were readily observed for whole-brain and regional diffusion metrics.
Regional diffusion metrics showed the expected heterochronic developmental patterns.
Acknowledgments
Funding
This work was supported by U.S. Air Force Surgeon General I-11-10 and I-11-44711HPW FA8650-10-D -6052 0048.
The authors wish to thank Ms. Elaine “Sandy” Kawano, U.S. Air Force School of Aerospace Medicine, 711th Human Performance Wing, Wright-Patterson AFB, OH, for scientific editorial assistance and Dr. Jisuk Park, 59th Medical Wing, Joint Base San Antonio-Lackland, TX, for statistical assistance.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Air Force, the Department of Defense, or the U.S. Government.
This work was supported by U.S. Air Force Surgeon General I-11-10 and I-11-44 711HPW FA8650-10-D -6052 0048. This work was also supported by NIH grants: R01EB015611, R01MH094520, R01MH096263 and P50 MH103222.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jneumeth.2017.12.017.
Footnotes
Conflict of interest
Authors declare no conflict of interest.
References
- Abee CR. Nonhuman Primates in Biomedical Research: Biology and Management. Elsevier; 2017. 2012. [Google Scholar]
- Acheson A, et al. Combining diffusion tensor imaging and magnetic resonance spectroscopy to study reduced frontal white matter integrity in youths with family histories of substance use disorders. Hum Brain Mapp. 2014a;35(12):5877–5887. doi: 10.1002/hbm.22591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, et al. Assessment of whole brain white matter integrity in youths and young adults with a family history of substance-use disorders. Hum Brain Mapp. 2014b;35(11):5401–5413. doi: 10.1002/hbm.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A, et al. Striatal activity and reduced white matter increase frontal activity in youths with family histories of alcohol and other substance-use disorders performing a go/no-go task. Brain Behav. 2015;5(7):e00352. doi: 10.1002/brb3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alba-Ferrara LM, de Erausquin GA. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front Integr Neurosci. 2013;7:9. doi: 10.3389/fnint.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, et al. Sexual dimorphism and asymmetries in the gray-white composition of the human cerebrum. Neuroimage. 2003;18(4):880–894. doi: 10.1016/s1053-8119(03)00034-x. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Sotiropoulos SN. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage. 2015;122:166–176. doi: 10.1016/j.neuroimage.2015.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016;125:1063–1078. doi: 10.1016/j.neuroimage.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20(2):870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Armstrong E, et al. Cortical gyrification in the rhesus monkey: a test of the mechanical folding hypothesis. Cereb Cortex. 1991;1(5):426–432. doi: 10.1093/cercor/1.5.426. [DOI] [PubMed] [Google Scholar]
- Armstrong E, et al. The ontogeny of human gyrification. Cereb Cortex. 1995;5(1):56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex. 1994;4(6):590–600. doi: 10.1093/cercor/4.6.590. [DOI] [PubMed] [Google Scholar]
- Ashtari M, et al. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. Neuroimage. 2007;35(2):501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ, et al. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1 Pt 1):173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, et al. MRI evaluation of brain iron in earlier- and later-onset Parkinson’s disease and normal subjects. Magn Reson Imaging. 1999;17(2):213–222. doi: 10.1016/s0730-725x(98)00155-6. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010 Sep;31(9):1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. http://dx.doi.org/10.1016/j.neurobiolaging.2008.08.015, Epub 2008 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Bastos Leite AJ, Scheltens P, Barkhof F. Pathological aging of the brain: an overview. Top Magn Reson Imaging. 2004;15(6):369–389. doi: 10.1097/01.rmr.0000168070.90113.dc. [DOI] [PubMed] [Google Scholar]
- Bayly PV, Taber LA, Kroenke CD. Mechanical forces in cerebral cortical folding: a review of measurements and models. J Mech Behav Biomed Mater. 2014;29:568–581. doi: 10.1016/j.jmbbm.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system –a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Ben Bashat D, et al. Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging. 2005;21(5):503–511. doi: 10.1002/jmri.20281. [DOI] [PubMed] [Google Scholar]
- Bock AS, et al. Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret. Front Syst Neurosci. 2010;4:149. doi: 10.3389/fnsys.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp D, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontrop RE. Non-human primates: essential partners in biomedical research. Immunol Rev. 2001;183:5–9. doi: 10.1034/j.1600-065x.2001.1830101.x. [DOI] [PubMed] [Google Scholar]
- Bryda EC. The Mighty Mouse: the impact of rodents on advances in biomedical research. Mo Med. 2013;110(3):207–211. [PMC free article] [PubMed] [Google Scholar]
- Budde MD, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57(4):688–695. doi: 10.1002/mrm.21200. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Nigg JT, Durston S. New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Curr Opin Neurol. 2007;20(2):119–124. doi: 10.1097/WCO.0b013e3280a02f78. [DOI] [PubMed] [Google Scholar]
- Clark CA, Hedehus M, Moseley ME. In vivo mapping of the fast and slow diffusion tensors in human brain. Magn Reson Med. 2002;47(4):623–628. doi: 10.1002/mrm.10118. [DOI] [PubMed] [Google Scholar]
- Conrad MS, Dilger RN, Johnson RW. Brain growth of the domestic pig (Sus scrofa) from 2 to 24 weeks of age: a longitudinal MRI study. Dev Neurosci. 2012;34(4):291–298. doi: 10.1159/000339311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad MS, et al. An in vivo three-dimensional magnetic resonance imaging-based averaged brain collection of the neonatal piglet (Sus scrofa) PLoS One. 2014;9(9):e107650. doi: 10.1371/journal.pone.0107650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coors ME, et al. The ethics of using transgenic non-human primates to study what makes us human. Nat Rev Genet. 2010;11(9):658–662. doi: 10.1038/nrg2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum WR, et al. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: a longitudinal in vivo MRI study. Brain Behav Immun. 2017;63:50–59. doi: 10.1016/j.bbi.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Takahashi E. Characterization of white matter tracts by diffusion MR tractography in cat and ferret that have similar gyral patterns. Cereb Cortex. 2017:1–10. doi: 10.1093/cercor/bhx048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, 3rd, et al. Characterizing longitudinal white matter development during early childhood. Brain Struct Funct. 2015;220(4):1921–1933. doi: 10.1007/s00429-014-0763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, 3rd, et al. Mapping an index of the myelin g-ratio in infants using magnetic resonance imaging. Neuroimage. 2016;132:225–237. doi: 10.1016/j.neuroimage.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DC, 3rd, et al. Mapping white matter microstructure in the one month human brain. Sci Rep. 2017:9759. doi: 10.1038/s41598-017-09915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, et al. Brain development of infant and MRI by diffusion tensor imaging. Neurophysiol Clin. 2012;42(1–2):1–9. doi: 10.1016/j.neucli.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Durston S, et al. Anatomical MRI of the developing human brain: what have we learned? J Am Acad Child Adolesc Psychiatry. 2001;40(9):1012–1020. doi: 10.1097/00004583-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, et al. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17(12):2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. Developmental (myelogenetic) localisation of the cerebral cortex in the human. Lancet. 1901a;158:1027–1030. [Google Scholar]
- Flechsig P. Developmental (myelogenetic) localization of the cerebral cortex in the human subject. Lancet. 1901b;158(4077):1027–1030. [Google Scholar]
- Friedman JI, et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165(8):1024–1032. doi: 10.1176/appi.ajp.2008.07101640. [DOI] [PubMed] [Google Scholar]
- Gao W, et al. Temporal and spatial development of axonal maturation and myelination of white matter in the developing brain. AJNR Am J Neuroradiol. 2009;30(2):290–296. doi: 10.3174/ajnr.A1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, et al. Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging. Proc Natl Acad Sci U S A. 2013;110(47):19006–19011. doi: 10.1073/pnas.1313735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S, Check E. The great primate debate. Nature. 2002;417(6890):684–687. doi: 10.1038/417684a. [DOI] [PubMed] [Google Scholar]
- Grinberg F, et al. Non-Gaussian diffusion in human brain tissue at high b-factors as examined by a combined diffusion kurtosis and biexponential diffusion tensor analysis. Neuroimage. 2011;57(3):1087–1102. doi: 10.1016/j.neuroimage.2011.04.050. [DOI] [PubMed] [Google Scholar]
- Grinberg F, et al. Diffusion kurtosis metrics as biomarkers of microstructural development: a comparative study of a group of children and a group of adults. Neuroimage. 2017;144(Pt A):12–22. doi: 10.1016/j.neuroimage.2016.08.033. [DOI] [PubMed] [Google Scholar]
- Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995;34(6):910–914. doi: 10.1002/mrm.1910340618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen B, et al. White matter biomarkers from fast protocols using axially symmetric diffusion kurtosis imaging. NMR Biomed. 2017;30(9) doi: 10.1002/nbm.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, et al. Increased frontal cortical folding in autism: a preliminary MRI study. Psychiatry Res. 2004;131(3):263–268. doi: 10.1016/j.pscychresns.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Hardouin SN, Nagy A. Mouse models for human disease. Clin Genet. 2000;57(4):237–244. doi: 10.1034/j.1399-0004.2000.570401.x. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32(1):356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C, et al. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40(3):319–384. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- Howroyd PC, Peter B, de Rijk E. Review of sexual maturity in the minipig. Toxicol Pathol. 2016;44(4):607–611. doi: 10.1177/0192623316642881. [DOI] [PubMed] [Google Scholar]
- Hutchinson EB, et al. Quantitative MRI and DTI abnormalities during the acute period following CCI in the ferret. Shock. 2016;46(3 Suppl 1):167–176. doi: 10.1097/SHK.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson EB, et al. Population based MRI and DTI templates of the adult ferret brain and tools for voxelwise analysis. Neuroimage. 2017;152:575–589. doi: 10.1016/j.neuroimage.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob HJ. Functional genomics and rat models. Genome Res. 1999;9(11):1013–1016. doi: 10.1101/gr.9.11.1013. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, et al. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JH, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005a;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jensen JH, et al. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005b;53(6):1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. NMR Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med. 1999;42(3):515–525. [PubMed] [Google Scholar]
- Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kalia M. Brain development: anatomy, connectivity, adaptive plasticity, and toxicity. Metabolism. 2008;57(Suppl 2):S2–5. doi: 10.1016/j.metabol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, et al. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66(6):562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, et al. The relationship of developmental changes in white matter to the onset of psychosis. Curr Pharm Des. 2012;18(4):422–433. doi: 10.2174/138161212799316073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, et al. The accumbofrontal tract: diffusion tensor imaging characterization and developmental change from childhood to adulthood. Hum Brain Mapp. 2015;36(12):4954–4963. doi: 10.1002/hbm.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner E, et al. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76(5):1574–1581. doi: 10.1002/mrm.26054. [DOI] [PubMed] [Google Scholar]
- Kinney HC, et al. Sequence of central nervous system myelination in human infancy: II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- Knutsen AK, et al. A new method to measure cortical growth in the developing brain. J Biomech Eng. 2010;132(10):101004. doi: 10.1115/1.4002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40(4):721–728. doi: 10.1093/schbul/sbu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Genetics of primary cerebral gyrification: heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. Neuroimage. 2009a;15(53):1126–1132. doi: 10.1016/j.neuroimage.2009.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Can structural MRI cerebral health markers track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp. 2009b;30(8):2581–2594. doi: 10.1002/hbm.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010a January ;49(2):1190–1199. doi: 10.1016/j.neuroimage.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Mapping primary gyrogenesis during fetal development in primate brains: high-resolution in utero structural MRI of fetal brain development in pregnant baboons. Front Neurosci. 2010b;4:20. doi: 10.3389/fnins.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Fetal brain during a binge drinking episode: a dynamic susceptibility contrast MRI fetal brain perfusion study. Neuroreport. 2011a;21(10):716–721. doi: 10.1097/WNR.0b013e32833b5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Fractional anisotropy of cerebral white matter and thickness of cortical gray matter across the lifespan. Neuroimage. 2011b;58(1):41–49. doi: 10.1016/j.neuroimage.2011.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2012;33(1):9–20. doi: 10.1016/j.neurobiolaging.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Chiappelli J, Hong LE. Permeability-diffusivity modeling vs: fractional anisotropy on white matter integrity assessment and application in schizophrenia. Neuroimage Clin. 2013;3:18–26. doi: 10.1016/j.nicl.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Multimodal white matter imaging to investigate reduced fractional anisotropy and its age-related decline in schizophrenia. Psychiatry Res. 2014;223(2):148–156. doi: 10.1016/j.pscychresns.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, et al. Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci U S A. 2016a;113(47):13504–13509. doi: 10.1073/pnas.1608246113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Ganjgahi H, Winkler A, Kelly S, Shukla DK, Du X, Jahanshad N, Rowland L, Sampath H, Patel B, O’Donnell P, Xie Z, Paciga SA, Schubert CR, Chen J, Zhang G, Thompson PM, Nichols TE, Hong LE. Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 2016b December ;37(12):4673–4688. doi: 10.1002/hbm.23336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama SG, Rosene DL, Sherman LS. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age (Dordr) 2012;34(5):1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CD, et al. Regional patterns of cerebral cortical differentiation determined by diffusion tensor MRI. Cereb Cortex. 2009;19(12):2916–2929. doi: 10.1093/cercor/bhp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulikova S, et al. Multi-parametric evaluation of the white matter maturation. Brain Struct Funct. 2015;220(6):3657–3672. doi: 10.1007/s00429-014-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert R. Breeding Strategies for Maintaining Colonies of Laboratory Mice. The Jackson Laboratory; 2007. [Google Scholar]
- Larvaron P, et al. In vivo analysis of the post-natal development of normal mouse brain by DTI. NMR Biomed. 2007;20(4):413–421. doi: 10.1002/nbm.1082. [DOI] [PubMed] [Google Scholar]
- Laughlin SB, Sejnowski TJ. Communication in neuronal networks. Science. 2003;301(5641):1870–1874. doi: 10.1126/science.1089662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lebel C, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, et al. Diffusion tensor and volumetric magnetic resonance measures as biomarkers of brain damage in a small animal model of HIV. PLoS One. 2014;9(8):e105752. doi: 10.1371/journal.pone.0105752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Li Q, et al. Chronic ketamine exposure causes white matter microstructural abnormalities in adolescent cynomolgus monkeys. Front Neurosci. 2017;11:285. doi: 10.3389/fnins.2017.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, et al. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006;19(2):236–247. doi: 10.1002/nbm.1020. [DOI] [PubMed] [Google Scholar]
- Luders E, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26(2):493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Lutz K, et al. Asymmetry of cortical activation during maximum and convenient tapping speed. Neurosci Lett. 2005;373(1):61–66. doi: 10.1016/j.neulet.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Madler B, et al. Is diffusion anisotropy an accurate monitor of myelination? Correlation of multicomponent T2 relaxation and diffusion tensor anisotropy in human brain. Magn Reson Imaging. 2008;26(7):874–888. doi: 10.1016/j.mri.2008.01.047. [DOI] [PubMed] [Google Scholar]
- McAnulty PA, et al. 2017 The Minipig in Biomedical Research. Taylor & Francis; 2011. [Google Scholar]
- McGorry PD, et al. Age of onset and timing of treatment for mental and substance use disorders: implications for preventive intervention strategies and models of care. Curr Opin Psychiatry. 2011;24(4):301–306. doi: 10.1097/YCO.0b013e3283477a09. [DOI] [PubMed] [Google Scholar]
- Miller DJ, et al. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci U S A. 2013;109(41):16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, et al. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221(2):349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Murray RM, et al. A neurodevelopmental approach to the classification of schizophrenia. Schizophr Bull. 1992;18(2):319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- Nazeri A, et al. Alterations of superficial white matter in schizophrenia and relationship to cognitive performance. Neuropsychopharmacology. 2013;38(10):1954–1962. doi: 10.1038/npp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, et al. Cortical folding abnormalities in autism revealed by surface-based morphometry. J Neurosci. 2007;27(43):11725–11735. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olavarria JF, et al. Deafferentation-induced plasticity of visual callosal connections: predicting critical periods and analyzing cortical abnormalities using diffusion tensor imaging. Neural Plast. 2012;2012:250196. doi: 10.1155/2012/250196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango PO, et al. Puberty and dispersal in a wild primate population. Horm Behav. 2013;64(2):240–249. doi: 10.1016/j.yhbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, et al. Absolute cerebral blood flow and blood volume measured by magnetic resonance imaging bolus tracking: comparison with positron emission tomography values. J Cereb Blood Flow Metab. 1998;18(4):425–432. doi: 10.1097/00004647-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Pang T, Atefy R, Sheen V. Malformations of cortical development. Neurologist. 2008;14(3):181–191. doi: 10.1097/NRL.0b013e31816606b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JL, Carrion R., Jr Demand for nonhuman primate resources in the age of biodefense. ILAR J. 2005;46(1):15–22. doi: 10.1093/ilar.46.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Iglesias R, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Peter B, et al. Sexual maturation in the female gottingen minipig. Toxicol Pathol. 2016;44(3):482–485. doi: 10.1177/0192623315621413. [DOI] [PubMed] [Google Scholar]
- Petrenko V, et al. Multimodal MRI imaging of apoptosis-Triggered microstructural alterations in the postnatal cerebral cortex. Cereb Cortex. 2017:1–14. doi: 10.1093/cercor/bhw420. [DOI] [PubMed] [Google Scholar]
- Phillips KA, et al. Genetic contributions to the midsagittal area of the corpus callosum. Twin Res Hum Genet. 2012;15(3):315–323. doi: 10.1017/thg.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Pillay P, Manger PR. Order-specific quantitative patterns of cortical gyrification. Eur J Neurosci. 2007;25(9):2705–2712. doi: 10.1111/j.1460-9568.2007.05524.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, et al. The Nlme Package: Linear and Nonlinear Mixed Effects Models, R Version 3. 2012. [Google Scholar]
- Poot DH, et al. Optimal experimental design for diffusion kurtosis imaging. IEEE Trans Med Imaging. 2010;29(3):819–829. doi: 10.1109/TMI.2009.2037915. [DOI] [PubMed] [Google Scholar]
- Prins ND, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61(10):1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- R-Development-Core-Team. R: A Language and Environment for Statistical Computing. 2009 Available from: http://www.R-project.org.
- Rapoport JL, Addington A, Frangou S. The neurodevelopmental model of schizophrenia: what can very early onset cases tell us? Curr Psychiatry Rep. 2005a;7(2):81–82. doi: 10.1007/s11920-005-0001-z. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, et al. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005b;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat Cell Biol. 2007;9(9):993–999. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- Sakai T, Komaki Y, Hata J, Okahara J, Okahara N, Inoue T, Mikami A, Matsui M, Oishi K, Sasaki E, Okano H. Elucidation of developmental patterns of marmoset corpus callosum through a comparative MRI in marmosets, chimpanzees, and humans. Neurosci Res. 2017a;122(September):25–34. doi: 10.1016/j.neures.2017.04.001. http://dx.doi.org/10.1016/j.neures.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Sakai T, Mikami A, Suzuki J, Miyabe-Nishiwaki T, Matsui M, Tomonaga M, Hamada Y, Matsuzawa T, Okano H, Oishi K. Developmental trajectory of the corpus callosum from infancy to the juvenile stage: Comparative MRI between chimpanzees and humans. PLoS One. 2017b Jun;12(6):e0179624. doi: 10.1371/journal.pone.0179624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauleau P, et al. The pig model in brain imaging and neurosurgery. Animal. 2009;3(8):1138–1151. doi: 10.1017/S1751731109004649. [DOI] [PubMed] [Google Scholar]
- Schiavone F, et al. Imaging age-related cognitive decline: a comparison of diffusion tensor and magnetization transfer MRI. J Magn Reson Imaging. 2009;29(1):23–30. doi: 10.1002/jmri.21572. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, et al. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomberg DT, et al. Miniature swine for preclinical modeling of complexities of human disease for translational scientific discovery and accelerated development of therapies and medical devices. Toxicol Pathol. 2016;44(3):299–314. doi: 10.1177/0192623315618292. [DOI] [PubMed] [Google Scholar]
- Schwerin SC, et al. Establishing the ferret as a gyrencephalic animal model of traumatic brain injury: optimization of controlled cortical impact procedures. J Neurosci Methods. 2017;285:82–96. doi: 10.1016/j.jneumeth.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, McSherry GM. Gyrus formation in the cerebral cortex in the ferret. I. Description of the external changes. J Anat. 1986;146:141–152. [PMC free article] [PubMed] [Google Scholar]
- Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Shoemake CR, Bouchard GF. The miniature swine as a model in experimental and translational medicine. Toxicol Pathol. 2016;44(4):612–623. doi: 10.1177/0192623316641784. [DOI] [PubMed] [Google Scholar]
- Sukstanskii AL, Ackerman JJ, Yablonskiy DA. Effects of barrier-induced nuclear spin magnetization inhomogeneities on diffusion-attenuated MR signal. Magn Reson Med. 2003;50(4):735–742. doi: 10.1002/mrm.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukstanskii AL, Yablonskiy DA, Ackerman JJ. Effects of permeable boundaries on the diffusion-attenuated MR signal: insights from a one-dimensional model. J Magn Reson. 2004;170(1):56–66. doi: 10.1016/j.jmr.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Susuki K. Node of Ranvier disruption as a cause of neurological diseases. ASN Neuro. 2013;5(3):209–219. doi: 10.1042/AN20130025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle MM, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012;49(2):344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- Takahashi M, et al. Magnetic resonance microimaging of intraaxonal water diffusion in live excised lamprey spinal cord. Proc Natl Acad Sci U S A. 2002;99(25):16192–16196. doi: 10.1073/pnas.252249999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JD, et al. Histopathologic correlation with diffusion tensor imaging after chronic hypoxia in the immature ferret. Pediatr Res. 2012;71(2):192–198. doi: 10.1038/pr.2011.32. [DOI] [PubMed] [Google Scholar]
- Torres LB, et al. The use of new world primates for biomedical research: an overview of the last four decades. Am J Primatol. 2010;72(12):1055–1061. doi: 10.1002/ajp.20864. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, et al. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J Med Primatol. 1997;26(3):113–119. doi: 10.1111/j.1600-0684.1997.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Veraart J, et al. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016a;142:394–406. doi: 10.1016/j.neuroimage.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, et al. Gibbs ringing in diffusion MRI. Magn Reson Med. 2016b;76(1):301–314. doi: 10.1002/mrm.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magn Reson Med. 2016c;76(5):1582–1593. doi: 10.1002/mrm.26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16(2):87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Welker W. Why does cerebral cortex fissure and fold? A review of determinants of gyri and sulci. In: Jones EG, Peters A, editors. Cerebral Cortex. Vol. 8B: Comparative Structure and Evolution of Cerebral Cortex, Part II. Springer; New York: 1990. pp. 3–136. [Google Scholar]
- Wen Q, Chklovskii DB. Segregation of the brain into gray and white matter: a design minimizing conduction delays. PLoS Comput Biol. 2005;1(7):e78. doi: 10.1371/journal.pcbi.0010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson R, et al. Pigs in sequence space: a 0: 66X coverage pig genome survey based on shotgun sequencing. BMC Genom. 2005;6:70. doi: 10.1186/1471-2164-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SN, et al. Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum Brain Mapp. 2015;36(10):3793–3804. doi: 10.1002/hbm.22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, et al. Age- and gender-related changes in the normal human brain using hybrid diffusion imaging (HYDI) Neuroimage. 2011;54(3):1840–1853. doi: 10.1016/j.neuroimage.2010.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, et al. In vivo high-resolution diffusion tensor imaging of the mouse brain. Neuroimage. 2013;83:18–26. doi: 10.1016/j.neuroimage.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, et al. Axons pull on the brain, but tension does not drive cortical folding. J Biomech Eng. 2010;132(7):071013. doi: 10.1115/1.4001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours A-R. In: The Myelogenetic Cycles of Regional Maturation of the Brain, in Regional Development of the Brain in Early Life. Minkowski A, editor. Blackwell Scientific Publications; Oxford: 1967. pp. 3–65. [Google Scholar]
- Zhang J, et al. Characterization of mouse brain and its development using diffusion tensor imaging and computational techniques. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2252–2255. doi: 10.1109/IEMBS.2006.259388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.