Abstract
Sensitivity to cooling temperatures often becomes heightened in orofacial regions leading to orofacial cold allodynia/hyperalgesia after chronic trigeminal nerve injury. KCNQ2 channels are involved in controlling excitability of primary afferent neurons and thereby regulate sensory functions under both physiological and pathological conditions. In the present study, we sought to determine whether KCNQ2 channels in trigeminal nerves are involved in regulating orofacial operant behavioral responses to cooling stimulation. We also sought to examine whether chronic trigeminal nerve injury may alter KCNQ2 channel expression in trigeminal ganglions. Using the orofacial operant tests, animals show cold allodynia/hyperalgesia in orofacial regions following infraorbital nerve chronic constrictive injury (ION-CCI), which could be alleviated by subcutaneous administration of retigabine, a KCNQ2 activator. In contrast, subcutaneous administration of the KCNQ2 inhibitor XE991 directly elicits cold allodynia/hyperalgesia in sham animals. Using immunostaining, we show that KCNQ2 channels are primarily expressed in small-sized TG neurons. Interestingly, KCNQ2 channel expression becomes significantly upregulated in TG neurons following the ION-CCI. Our results suggest that KCNQ2 channels are involved in regulating orofacial cold sensitivity. Upregulation of KCNQ2 channels may be a compensatory change in attempting to limit injury-induced trigeminal hyperexcitability.
Keywords: trigeminal ganglion neurons, neuropathic pain, cold allodynia, cold hyperalgesia, KCNQ2 channels, retigabine
Introduction
Trigeminal neuropathic pain is a significant clinical issue due to its severity, location, and resistance to conventional treatment (Baron et al., 2010). Similar to neuropathic pain in other parts of the body, trigeminal neuropathic pain is often manifested with exaggerated painful sensations triggered by innocuous mechanical stimuli (mechanical allodynia) and also by innocuous or mild noxious cooling temperatures (cold allodynia/hyperalgesia). Cold allodynia/hyperalgesia is more difficult to manage in areas such as orofacial regions that are innervated by the trigeminal nervous system. This is because these regions normally directly expose to ambient temperatures, and covering these regions with clothing is not practical to avoid cold allodynia/hyperalgesia. A peripheral sensitization to cooling stimuli at primary afferent endings of the trigeminal nerves is believed to be an underlying mechanism of orofacial cold allodynia/hyperalgesia. Cooling stimuli at primary afferent endings are known to be primarily transduced by the cooling temperature sensor TRPM8 channels (McKemy et al., 2002, Peier et al., 2002). However, ion channels that are involved in regulating membrane excitability of the trigeminal nerves may also have a profound impact on the sense of cooling temperatures.
KCNQ2 channels are voltage-gated K+ channels expressed on the membranes of neurons in the CNS and the peripheral nervous system (PNS) (Brown and Passmore, 2009). In the PNS, functional KCNQ channels in dorsal root ganglion (DRG) neurons are thought to be mainly formed by KCNQ2 and KCNQ3 subunits in a heteromeric form (KCNQ2/3 channels), and these KCNQ2/3 channels are believed to underlie the M-type outward K+ currents (M-currents) recorded in DRG neurons (Passmore et al., 2003, Rose et al., 2011, Zheng et al., 2013). KCNQ2/3 channels have been shown to play a key role in regulating excitability of nociceptive DRG neurons (Brown and Passmore, 2009). Previous studies have shown that KCNQ2 and KCNQ3 subunits are down-regulated in nociceptive DRG neurons after nerve injury and in an animal model of a bone cancer (Rose et al., 2011, Duan et al., 2012, Zheng et al., 2013). These studies have further shown that KCNQ channel down-regulation is associated with the increase of the excitability of nociceptive DRG neurons (Duan et al., 2012, Zheng et al., 2013). In TG neurons, we have recently shown that KCNQ2 channels are expressed in nociceptive TG neurons including those that sense cooling temperatures, and we have also found that pharmacological inhibition of these channels leads to TG neuron hyperexcitability (Abd-Elsayed et al., 2015). More recently, we have found that KCNQ2 channels become down-regulated in rat TG neurons that innervate orofacial regions (the V2 branch of trigeminal nerves, or V2 TG) following oxaliplatin-induced trigeminal neuropathy (Ling et al., 2017). Furthermore, we have shown that systemic administration of retigabine can alleviate cold allodynia/hyperalgesia in animals following chronic trigeminal nerve injury or in animals treated with oxaliplatin (Abd-Elsayed et al., 2015). In the present study, we sought to determine whether KCNQ2 channels at peripheral endings of the trigeminal nerve play a role in regulating behavioral sensitivity to cooling temperatures. We also sought to determine whether there was a significant change in KCNQ2 channel expression in TG neurons following infraorbital nerve chronic constrictive injury.
Materials and Methods
Male adult Sprague Dawley rats at the age of 6–10 weeks were used. Animal care and use conformed to National Institutes of Health guidelines for care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham. After 2 weeks of the pre-surgical adaptation training for orofacial operant behavioral test (see below), the rats were divided into sham and infraorbital nerve chronic constrictive injury (ION-CCI) groups. ION-CCI was created by unilateral ligation of the infraorbital nerves as described in our previous studies (Cha et al., 2012, Zuo et al., 2013). In brief, under anesthesia with ketamine/xylazine, a 2-cm curvilinear incision was made superior to the right orbital cavity. The infraorbital nerve was freed from the surrounding connective tissues and two ligatures were made approximately 5 mm apart with a 5–0 absorbable chromic gut suture Superion®. The incision was then closed with suture. The sham group had a similar surgery, but without any ligatures. After a 2-week healing period, the rats underwent a 2-week period of post-surgical adaption training of orofacial operant tests.
Orofacial operant tests were conducted on sham and ION-CCI animals as described in our previous studies (Cha et al., 2012). In brief, animals initially underwent 4 to 6 sessions of adaptation training in two weeks using the Ugo Basile Orofacial Stimulation Test System® (Comerio VA, Italy). For each training session, animals were first fasted for a 12-hour period. Each rat was then placed in a cage of Orofacial Stimulation Test System. The Orofacial Stimulation Test System had a drinking window for the rat head to enter and acquire a reward (30% sweetened condensed milk, Nestle Carnation®). The Orofacial Stimulation Test System also consisted of a thermal module with its temperature being set at 24oC for training sessions and at 17, 12, or 5oC for cold stimulation. An infrared beam was built in the drinking window to automatically detect and record the head accessing the milk. A training session was started by placing a rat in the cage. After the rat was given 10 minutes to familiarize itself with its environment, the drinking window was opened and the testing rat was subsequently timed for 10 minutes to allow drinking. After 2 weeks of training, procedures were performed on the animals to create ION-CCI group and sham group as described above. Subsequent orofacial operant tests were performed at different days for up to 6 weeks in the same manner as the adaptation training. In a set of experiments with sham animals the KCNQ2 blocker XE991 was subcutaneously injected to both sides of orofacial regions at the dose of 300 μg (30 μl) each side 28 to 42 days following sham surgery. In another set of experiments with ION-CCI animals, the KCNQ2 activator retigabine was administered subcutaneously to ipsilateral sides of orofacial regions at the doses of 300 μg (μl) 28 to 35 days following ION-CCI procedure. Orofacial operant tests were performed 60 min after XE991 or retigabine administration. The events of head assessing the milk were detected by the infrared beam. Orofacial operant behavioural parameters were the total contact time (measured by total time the infrared beam breaks) and total contact number (measured by total number of beam breaks) in a 10-min experimental session.
For immunostaining, TGs were harvested from bilateral ION-CCI and sham groups 28 to 30 days after the surgery procedures. We used bilateral procedures in this set of experiments to maximize the numbers of TGs that we could work with for immunostaining experiments. Immunostaining was performed in the same manner as described in our previous study (Ling et al., 2017). In brief, animals were fixed with 4% paraformaldehyde (PFA), and TGs were removed and cryoprotected in 30% sucrose for two nights. The TGs were then cut into 10 μm sections on a cryostat. The sections of TGs were thaw-mounted onto slides. For each slide, three sections from sham animals (control) and three sections from ION-CCI animals were mounted on the same slide. These sections were then encircled together with PAP Pen so that the two groups were paired in immunostaining. Immunostaining was conducted with a polyclonal rabbit anti-KCNQ2 antibody (1:2000 diluted with 5% normal goat serum in PBS; Abcam, Cambridge, MA, USA, incubated for 2 nights at 4°C) and a secondary antibody of goat anti-rabbit IgG conjugated with Alexa-594 (1:1000 in 5% normal goat serum in PBS, ThermoFisher Scientific, Waltham, MA USA). Slices after immunostaining were viewed under an upright fluorescent microscope and images under a 20× objective were captured using a CCD camera. KCNQ2-ir positive and total neurons in TG sections were visually identified and counted from the acquired fluorescent images.
Data were presented as Mean ± SEM, analysed by the unpaired Student’s t test or one-way AMoVA with the Tukey post hoc test, *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Orofacial operant tests performed at cooling temperatures showed differences in orofacial cold sensitivity between ION-CCI group and sham group (Figure 1A–D). In sham group, the total contact time in a 10-min testing session was 312 ± 13 s (n = 16) at 24°C, 246 ± 19 s (n = 15) at 17°C, 215 ± 17 s (n = 16) s at 12°C, and 171 ± 15 s (n = 12) s at 5°C. The total contact time in ION-CCI group was 154 ± 12 s (n = 11, P<0.05) at 12°C and 70 ± 12 s (n = 15, P<0.001) at 5°C, significantly shorter than that of sham group at the same cooling temperatures (Figure 1A, B&C). ION-CCI animals showed significantly higher total contact numbers at 24°C in comparison with sham group (Figure 1D). However, at cooling temperatures tested, there were no significant differences in total contact numbers between ION-CCI group and sham group.
Figure 1. Orofacial operant test of cold sensitivity in sham and infraorbital nerve chronic constrictive injury (ION-CCI) groups.
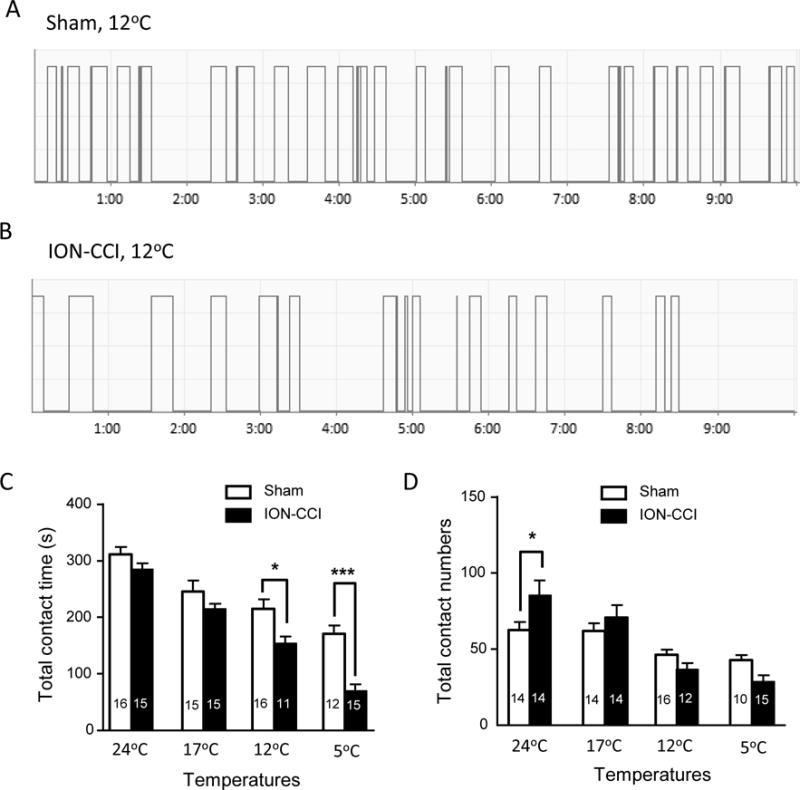
A) Sample trace of orofacial operant test at 12°C in a sham surgery rat. The trace shows contacts to cold stimulation module (12°C) by rat orofacial regions in a 10-min testing session. B) Similar to A except the operant test was performed in an ION-CCI rat. C&D) Summary data of total contact time (C) and total contact numbers (D) during orofacial operant tests at different temperatures. Open bars, sham group; closed bars, ION-CCI group. In both C and D, the number in each bar indicates the numbers of animals tested. Orofacial operant tests were performed between 14 to 28 days after ION-CCI or sham procedures. Data represent Mean ± SEM, *P < 0.05, ***P < 0.001, comparing between ION-CCI and sham groups.
XE991, a highly selective KCNQ2 channel blocker, was subcutaneously administered to orofacial regions of sham animals. This allowed us to determine whether KCNQ2 channels at peripheral trigeminal nerve endings were involved in controlling cold sensitivity of the skin in orofacial areas (Figure 2A–D). As tested at 12°C, the subcutaneous XE991 administration resulted in cold hyper-sensitivity as indicated by significantly shortening total contact time (Figure 2C). The total contact time was 222 ± 20 s (n = 7) in vehicle-injected group and was shortened to 136 ± 22 s (n = 7, P<0.05) in XE991-injected group (Figure 2C). The total contact numbers were not significantly different between the two groups (Figure 2D).
Figure 2. Subcutaneous administration of XE991 induces orofacial cold hypersensitivity.
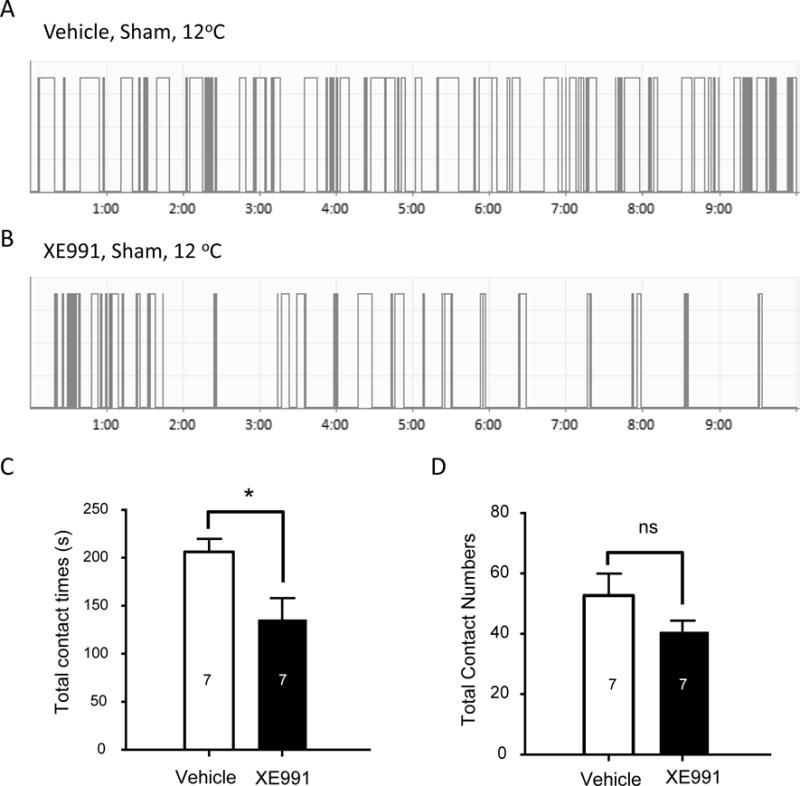
A&B) Two sample traces show contact events of orofacial operant tests at 12°C in a sham animal subcutaneously injected with vehicle (A) and a different sham animal subcutaneously injected with XE991 (B). C&D) Summary data of total contact time (C) and total contact numbers (D) during orofacial operant tests at 12°C. Open bar, vehicle-injected group; closed bar, XE991-injected group. In both C and D, the number in each bar indicates the numbers of animals tested. Orofacial operant tests were performed between 28 to 42 days after sham procedures. Data represent Mean ± SEM, *P < 0.05, comparing between XE991 and vehicle groups.
To further demonstrate that KCNQ2 channels at peripheral trigeminal nerve endings were involved in controlling cold sensitivity of the skin in orofacial areas, we subcutaneously administered either vehicle or the KCNQ2 channel activator retigabine into orofacial regions of ION-CCI animals and performed orofacial operant tests at 5°C. In ION-CCI group injected with vehicle, the total contact time was 62 ± 16 s (n = 6, Figure 3A&C). In contrast, in ION-CCI group injected with retigabine, the total contact time was 147 ± 16 s (n = 6, Figure 3B&C), significantly (P < 0.01) longer than that of vehicle-injected ION-CCI animals. We also examined effects of retigabine in sham group. The total contact time in sham group injected with retigabine was 185 ± 16 s (n = 7), and was not significantly different from vehicle-injected sham group (190 ± 17 s, n = 7). We examined total contact numbers in ION-CCI group following subcutaneous administration of either vehicle or retigabin. The total contact numbers were 39 ± 4 (n = 6) in retigabine-injected ION-CCI group, significantly more than that of vehicle-injected ION-CCI group (21 ± 3, n = 6). In sham group, there was no significant difference in total contact numbers between vehicle-injected group and retigabine-injected group.
Figure 3. Subcutaneous administration of retigabine attenuates cold hypersensitivity in orofacial regions of ION-CCI animals.
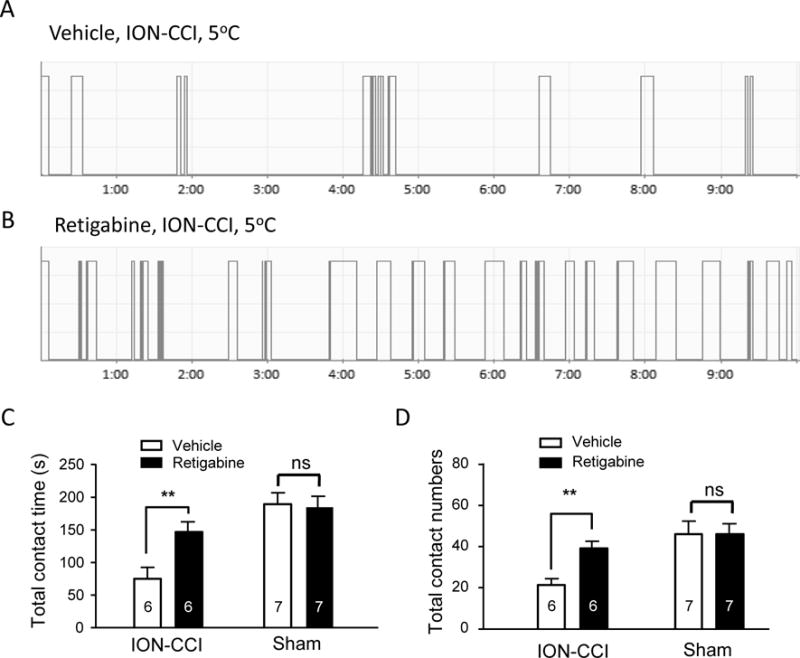
A&B) Two sample traces show contact events of orofacial operant tests at 5°C in a ION-CCI animal subcutaneously injected with vehicle (A) and a different ION-CCI animal subcutaneously injected with retigabine (B). C&D) Summary data of total contact time (C) and total contact numbers (D) during orofacial operant tests at 5°C. Open bar, vehicle-injected group; closed bar, retigabine-injected group. In both C and D, the number in each bar indicates the numbers of animals tested. Orofacial operant tests were performed between 28 to 35 days after ION-CCI or sham procedures. Data represent Mean ± SEM, **P < 0.01, comparing between retigabine and vehicle groups.
We examined whether there was a change in KCNQ2 channel expression in TG neurons following ION-CCI. Since orofacial regions are mainly innervated by V2 (maxillary) parts of TGs, we first focused on KCNQ2-ir in the V2 TG neurons (Figure 4). Strong KCNQ2 immunoreactivity (KCNQ2-ir) was observed mainly in small-sized V2 TG neurons of both sham animals (Figure 4A&C) and ION-CCI animals (Figure 4B&D). For sham group, the cell sizes (in diameters) of KCNQ2-ir V2 TG neurons were 21 μm (21.4 ± 0.4 μm, 210 KCNQ2-ir positive V2 TG neurons out of 2162 total V2 TG neurons, from total 24 TG sections of 8 rats, Figure 4C). Total V2 TG neurons of sham group showed broader cell size distribution with mean diameter of 27 μm (27.0 ± 0.2 μm, 2162 total neurons, Figure 4C). For ION-CCI group, KCNQ2-ir V2 TG neurons were also mostly small-sized neurons with mean diameter of 25 μm (25.0 ± 0.3 μm, 397 positive neurons out 2182 total neurons, from 24 TG sections of 8 rats, Figure 4D). Total V2 TG neurons in ION-CCI group displayed wider cell size distribution with mean diameter of 28 μm (27.5 ± 0.2 μm, 2182 total neurons, Figure 4D).
Figure 4. Up-regulation of KCNQ2 channel expression in V2 trigeminal ganglia following ION-CCI.
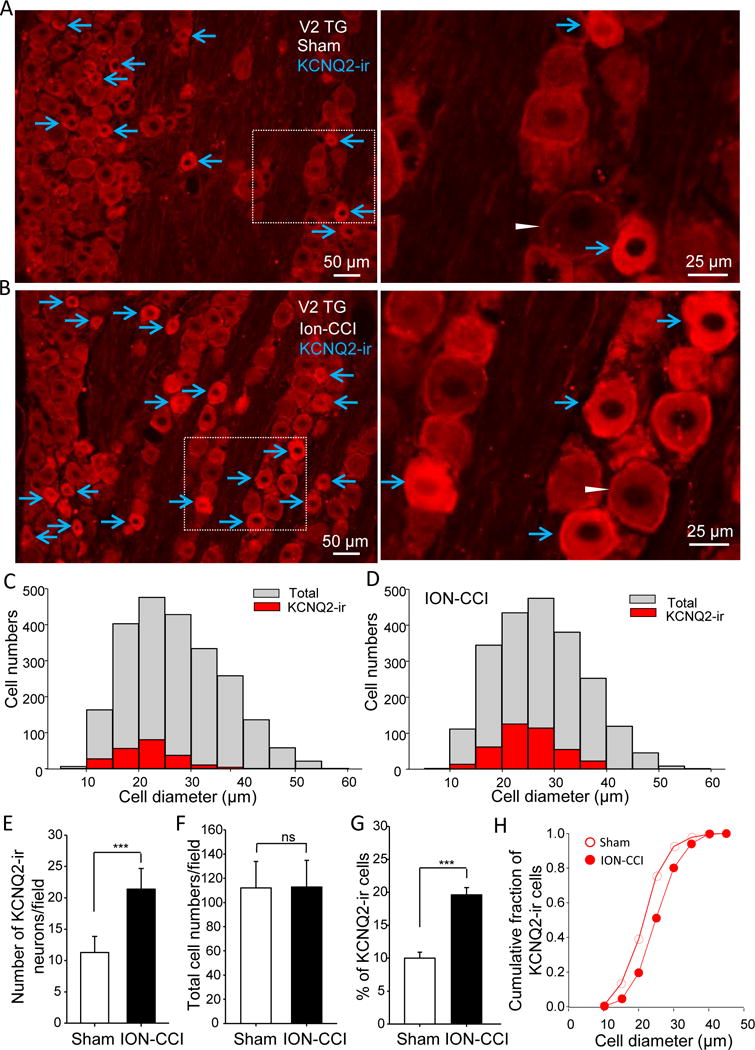
A) Right, image captured under a 20× objective shows KCNQ2 immunoreactivity (KCNQ2-ir) in V2 part of a TG section (V2 TG) from a sham rat. Left, enlarged image of the boxed region in A. B) Similar to A except the TG is harvested from an ION-CCI animal. In both A and B, arrows indicate KCNQ2-ir positive neurons, and an arrowhead in either A or B indicates a KCNQ2-r negative neuron. C) Overlay histograms of KCNQ2-ir positive (red bars) and total (gray bars) V2 TG neurons in sham group (8 rats). D) Overlay histograms of KCNQ2-ir positive (red bars) and total (gray bars) V2 TG neurons in ION-CCI group (8 rats). E–H) Summary data of the KCNQ2-ir positive cell numbers (E), total cell numbers (F), and percent of KCNQ2-ir positive V2 TG neurons (G). Open bars, sham group; closed bar, ION-CCI group. H). Cumulative fraction of KCNQ2-ir positive neurons plotted against their cell diameters. Open circles, sham (n = 210); closed circles, ION-CCI group (n = 397). TGs were harvested from sham or ION-CCI rats 28 days after sham or ION-CCI procedures. Data represent Mean ± SEM, ***P < 0.001, comparing with the data of control group.
We compared numbers of KCNQ2-ir V2 TG neurons between sham and ION-CCI groups (Figure 4E). In the sham group, the numbers of KCNQ2-ir V2 TG neurons were 11 per section (11.3 ± 2.5, 24 TG sections of 8 rats, n = 8). In ION-CCI group, the numbers of KCNQ2-ir positive V2 TG neurons were 21 per section (21.4 ± 3.3, 24 TG sections of 8 rats, n = 8), significantly more than that of the sham group (P < 0.001, Figure 4E). We examined the numbers of total V2 TG neurons on each section of sham and ION-CCI groups. The cell numbers were 112 per section (112.0 ± 21.8, 24 TG sections of 8 rats, n = 8) in ION-CCI group, not significantly different from sham group (112.8 ± 21.9 per section, 24 TG sections of 8 rats, n = 8, Figure 4F). Percentages of KCNQ2-ir positive V2 TG neurons were compared between sham and ION-CCI groups (Figure 4G). In the sham group with a total of 2162 V2 TG neurons examined, about 10% (10.0 ± 0.9%, 24 TG sections of 8 rats) of them were KCNQ2-ir positive. In ION-CCI group with a total of 2182 V2 TG neurons examined, KCNQ2-ir positive V2 TG neurons were about 20% (19.6 ± 1.1%, 24 TG sections of 8 rats), significantly more than that of the sham group (P < 0.001, Figure 4G). Cumulative histogram of KCNQ2-ir cell sizes showed a right shift to relatively larger cell diameters following ION-CCI (25.0 ± 0.3 μm, 397 positive neurons) in comparison with sham group (21.4 ± 0.4 μm, 210 positive neurons, P < 0.001, Figure 4H).
We examined KCNQ2-ir in TG neurons that innervate ophthalmic (V1) areas. In sham group, the numbers of KCNQ2-ir V1 TG neurons per field were 8.0 ± 0.9 (23 sections of 8 rats, n = 8, Figure 5A). In ION-CCI group, the numbers of KCNQ2-ir V1 TG neurons per field increased to 11.3 ± 0.7 (23 sections of 8 rats, n = 8, Figure 5A), significantly more than that of sham group (P<0.001). The total V1 TG neuron numbers were not significantly different between the sham and ION-CCI groups (Figure 5B). Consistent with the increases in KCNQ2-ir V1 TG neurons in ION-CCI group, the percentage of KCNQ2-ir V1 TG neurons was significantly higher in ION-CCI group (17.8 ± 1.1, 23 sections of 8 rats, n = 8, Figure 5C) than in sham group (12.2 ± 1.0, 23 sections of 8 rats, n = 8, Figure 5C). We also examined KCNQ2-ir in TG neurons that innervate mandibular (V3) areas. Similar to V1 TG neurons, numbers of KCNQ2-ir V3 TG neurons (13.4 ± 1.0, 12 sections of 6 rats, n = 6, Figure 5D) in ION-CCI group were significantly more than that of sham group (7.2 ± 0.8, 12 sections of 4 rats, n = 4, Figure 5D). There was no significant difference in total V3 TG neuron numbers between sham (n = 4 rats) and ION-CCI (n = 6 rats) groups (Figure 5E). The percentage of KCNQ2-ir V3 TG neurons was significantly higher in ION-CCI group (14.0 ± 0.4, 12 sections of 6 rats, n = 6, Figure 5C) than in sham group (7.4 ± 1.0, 12 sections of 4 rats, n = 4, Figure 5F).
Figure 5. Up-regulation of KCNQ2 channel expression in V1 and V3 trigeminal ganglia following ION-CCI.
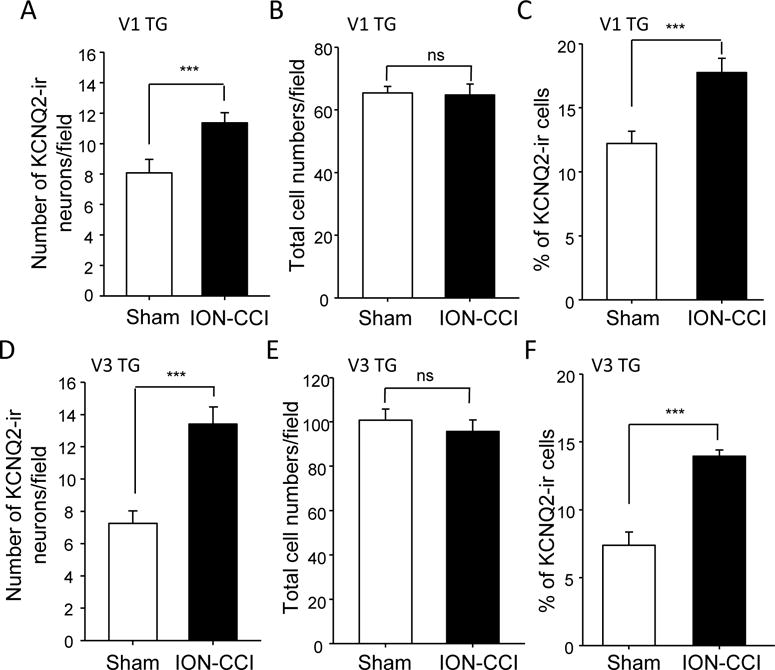
A–C) Summary data of the KCNQ2-ir positive V1 TG neuron numbers (A), total V1 TG neuron numbers (B), and percent of KCNQ2-ir positive V1 TG neurons (C). Open bars, sham group; closed bar, ION-CCI group. D–F) Summary data of KCNQ2-ir positive V3 TG neuron numbers (D), total V3 TG neuron numbers (E), and percent of KCNQ2-ir positive V3 TG neurons (F). From A to F, open bars, sham group; closed bar, ION-CCI group. TGs were harvested from sham or ION-CCI rats on day 28 after sham or ION-CCI procedures. Data represent Mean ± SEM, ***P < 0.001, comparing with the data of control group.
Discussion
In the present study, we used the orofacial operant test to determine effects of subcutaneous administration of KCNQ2 blocker XE991 and KCNQ2 activator retigabine on orofacial cold sensitivity. We show that XE991 directly enhances orofacial cold sensitivity in sham animals and retigabine attenuates cold hyper-sensitivity in ION-CCI group. We used XE991 in the present study because it is highly selective to KCNQ2 channels. Our previous study has shown that subcutaneous administration of linopirdine, a structurally different type of KCNQ2 channel blocker (Blackburn-Munro et al., 2005), enhances orofacial behavioral cold sensitivity (Abd-Elsayed et al., 2015). Thus, the present study with XE991 confirms our previous study with linopirdine. In contrast to the KCNQ2 channel blockers, we show in the present study that subcutaneous administration of retigabine attenuates orofacial cold hyper-sensitivity in our ION-CCI animal model. Previously we have shown that systemic administration (i.p. injection) of retigabine alleviates cold allodynia/hyperalgesia in ION-CCI rats (Abd-Elsayed et al., 2015). However, the action of systemic retigabine administration can be at multiple sites including the CNS (Du et al., 2017). The present study with orofacial subcutaneous administration of retigabine preferentially targeted KCNQ2 channels at the local peripheral endings of trigeminal nerves. Together with the results of subcutaneous administration of both XE991 and retigabine, our results support the idea that KCNQ2 channels at the peripheral endings of trigeminal nerves are involved in controlling orofacial cold sensitivity and can be therapeutic targets for trigeminal neuropathic pain manifested with cold allodynia/hyperalgesia. This idea is also consistent with other studies showing that peripheral KCNQ2 channels control excitability of nociceptive spinal afferent fibers (Passmore et al., 2003, Lang et al., 2008, Du et al., 2017).
Our present study with ION-CCI model shows significant up-regulation of KCNQ2 channels in TG neurons with V2 TG neurons showing most prominent upregulation. This is an unexpected finding since we have recently shown that orofacial cold allodynia/hyperalgesia is associated with a down-regulation of KCNQ2 channels in V2 TG neurons in a model of trigeminal neuropathy induced by the chemotherapy drug oxaliplatin (Ling et al., 2017). Other previous studies have also shown KCNQ2 down-regulation in DRG neurons following partial sciatic nerve ligation (Rose et al., 2011), axotomy (Cisneros et al., 2015), and in a rat model of bone cancer pain (Zheng et al., 2013). KCNQ2 channel down-regulation is thought to be a potential mechanism underlying thermal and mechanical allodynia/hyperalgesia seen in these animal models (Du et al., 2017). A down-regulation of KCNQ2 expression triggered by the partial sciatic nerve ligation in rat DRG neurons has been found to be dependent on repressor element 1-silencing transcription factor (Rose et al., 2011). The discrepancy between the present study and previous studies are currently not clear. However, a previous study has shown that expression of KCNQ2/3 channels in hippocampal neurons undergoes transcriptional up-regulation in an activity dependent manner in a rodent model of epilepsy (Zhang and Shapiro, 2012). The up-regulation is shown to be involved in activation of calcineurin and nuclear factor of activated T cell (NFAT) transcription factors, and is orchestrated by A kinase-anchoring protein 79/150 and requires Ca2+ influx through L-type Ca2+ channels (Zhang and Shapiro, 2012). This raises a possibility that the upregulation of KCNQ2 channels seen in TG neurons of the present study may be also due to an activity dependent transcriptional regulation.
Our results of KCNQ2 expression indicate that cold allodynia/hyperalgesia is not due to a down-regulation of KCNQ2 channels in our ION-CCI models. Upregulation of KCNQ2 TG neurons shown in our study may be a compensatory change to restrict over excitability of TG neurons following chronic constrictive trigeminal nerve injury. We have recently shown that M channels mediated by KCNQ2 channels are involved in setting resting membrane potential and rheobase in cold-sensing TG nociceptors (Abd-Elsayed et al., 2015) and also in most neurons that are sensitive to cold but are not primarily cold-sensing (Kanda and Gu, 2017). We have also shown that M currents are significantly inhibited by cooling temperatures (Kanda and Gu, 2017). This raises a possibility that the suppression of KCNQ2 channels by cooling temperatures may contribute to enhanced TG neuron excitability and cold allodynia/hyperalgesia in our ION-CCI animals. Thus, potentiation of KCNQ2 channels with their activators may provide an ideal approach to alleviate cold allodynia/hyperalgesia in chronic trigeminal neuropathy.
Highlights.
Cold allodynia/hyperalgesia was induced by chronic trigeminal nerve injury.
The neuropathic pain could be alleviated by subcutaneous injection of retigabine.
KCNQ2 channels were primarily expressed in small-sized trigeminal ganglion neurons.
KCNQ2 channel expression was upregulated following chronic trigeminal nerve injury.
Acknowledgments
This study was supported by NIH grants DE018661 and DE023090 to J.G.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abd-Elsayed AA, Ikeda R, Jia Z, Ling J, Zuo X, Li M, Gu JG. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain. 2015;11:45. doi: 10.1186/s12990-015-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha M, Kohan KJ, Zuo X, Ling JX, Gu JG. Assessment of chronic trigeminal neuropathic pain by the orofacial operant test in rats. Behav Brain Res. 2012;234:82–90. doi: 10.1016/j.bbr.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisneros E, Roza C, Jackson N, Lopez-Garcia JA. A New Regulatory Mechanism for Kv7.2 Protein During Neuropathy: Enhanced Transport from the Soma to Axonal Terminals of Injured Sensory Neurons. Front Cell Neurosci. 2015;9:470. doi: 10.3389/fncel.2015.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Gao H, Jaffe D, Zhang H, Gamper N. M-type K+ channels in peripheral nociceptive pathways. Br J Pharmacol. 2017 doi: 10.1111/bph.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan KZ, Xu Q, Zhang XM, Zhao ZQ, Mei YA, Zhang YQ. Targeting A-type K(+) channels in primary sensory neurons for bone cancer pain in a rat model. Pain. 2012;153:562–574. doi: 10.1016/j.pain.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Lang PM, Fleckenstein J, Passmore GM, Brown DA, Grafe P. Retigabine reduces the excitability of unmyelinated peripheral human axons. Neuropharmacology. 2008;54:1271–1278. doi: 10.1016/j.neuropharm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Ling J, Erol F, Viatchenko-Karpinski V, Kanda H, Gu JG. [EXPRESS] Orofacial neuropathic pain induced by oxaliplatin: downregulation of KCNQ2 channels in V2 trigeminal ganglion neurons and treatment by the KCNQ2 channel potentiator retigabine. Mol Pain. 2017;13 doi: 10.1177/1744806917724715. 1744806917724715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Passmore GM, Selyanko AA, Mistry M, Al-Qatari M, Marsh SJ, Matthews EA, Dickenson AH, Brown TA, Burbidge SA, Main M, Brown DA. KCNQ/M currents in sensory neurons: significance for pain therapy. J Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Rose K, Ooi L, Dalle C, Robertson B, Wood IC, Gamper N. Transcriptional repression of the M channel subunit Kv7.2 in chronic nerve injury. Pain. 2011;152:742–754. doi: 10.1016/j.pain.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shapiro MS. Activity-dependent transcriptional regulation of M-Type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron. 2012;76:1133–1146. doi: 10.1016/j.neuron.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Fang D, Liu M, Cai J, Wan Y, Han JS, Xing GG. Suppression of KCNQ/M (Kv7) potassium channels in dorsal root ganglion neurons contributes to the development of bone cancer pain in a rat model. Pain. 2013;154:434–448. doi: 10.1016/j.pain.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Zuo X, Ling JX, Xu GY, Gu JG. operant behavioral responses to orofacial cold stimuli in rats with chronic constrictive trigeminal nerve injury: effects of menthol and capsazepine. Mol Pain. 2013;9:28. doi: 10.1186/1744-8069-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]


