Abstract
Objective
Personalized Intervention for Depressed Patients with COPD (PID-C) is an intervention aiming to help patients adhere to their rehabilitation and care. This study tested the hypothesis that Problem Solving-Adherence (PSA) intervention, which integrates problem solving into adherence enhancement procedures, reduces dyspnea related disability more than PID-C. Exploratory analyses sought to identify patients with distinct dyspnea related disability trajectories and to compare their clinical profiles.
Design
Randomized controlled trial.
Setting
Acute inpatient rehabilitation and community.
Participants
101 diagnosed with COPD and major depression after screening 633 consecutive admissions for acute inpatient rehabilitation.
Intervention
14 sessions of PID-C vs. PSA over 26 weeks.
Measurements
Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ-M).
Results
The study hypothesis was not supported. Exploratory latent class growth modeling identified two distinct disability trajectories. Dyspnea related disability improved in 39% of patients and remained unchanged in the rest. Patients whose dyspnea related disability improved had more severe disability and less sense of control over their condition at baseline.
Conclusions
Improvement or no worsening of disability was noted in both treatment groups. This is a favorable course for depressed patients with a severe, deteriorating medical illness. PID-C is compatible with the expertise of clinicians working in community-based rehabilitation programs, and after further testing in the community, it can be integrated in the care of depressed COPD patients.
Keywords: Geriatric depression, COPD, Personalized intervention, clinical trial, dyspnea, disability
INTRODUCTION
Greying of the population is raising the number of people living with chronic, deteriorating illnesses. Many of them suffer from depression. According to the Center for Disease Control, 11.6% of Americans aged 65 years and older are diagnosed with chronic obstructive pulmonary disease (COPD) (1). One fourth of COPD patients have major depression and another large percentage have milder depressive syndromes (2). As in COPD, depression frequently afflicts older adults with common disabling conditions and complicates their care (3).
Depression comorbid with COPD typifies the health problems of aging persons living with chronic conditions and can serve as a model for development of interventions addressing their needs. Depression worsens overall medical burden, pulmonary health, and disability in COPD patients (4). A recent meta-analysis of more than 1.8 million participants showed that depression conferred a higher excess mortality risk in COPD than in other diseases. (5).
Antidepressants and psychotherapy may improve the depression outcomes of COPD patients, (6) but less than one-third of depressed COPD patients accept antidepressant drug treatment (7). Pulmonary rehabilitation can reduce disability and even ameliorate depressive symptoms of COPD patients (8). However, rehabilitation consists of strenuous exercises of muscle strengthening, breathing, and endurance. Adherence to pulmonary rehabilitation is problematic with only half of COPD patients engaging in walking exercises and using oxygen adequately (9). Depression reduces adherence in medical patients (8). Dyspnea related disability as well as the resignation of depression undermine adherence to pulmonary rehabilitation.
We developed a personalized intervention for depression and COPD (PID-C) targeting barriers to treatment adherence (10). Informed by the Theory of Reasoned Action, PID-C helps patients to weigh the inconveniences and benefits of treatment (11) with the goal to shift the balance in favor of treatment adherence. PID-C is administered by trained care managers who work with each patient and with the patient’s treatment team in order to increase adherence to rehabilitation exercises, use of oxygen, and medication prescribed by the patients’ own physicians. The PID-C care managers initially identify adherence barriers in individual patients. Then, they offer support and target these barriers with interventions such as correcting the misunderstanding of recommendations, misattribution of symptoms, hopelessness, dissatisfaction with treatment, and management of logistic obstacles. They also work with the patients’ physicians and their office personnel to reinforce the need for treatment adherence.
A randomized clinical trial showed that PID-C reduced depressive symptoms and dyspnea-related disability more than usual care (10). To further improve the efficacy of PID-C, we developed Problem Solving-Adherence (PSA), an intervention integrating problem solving into the adherence enhancement procedures of PID-C. PSA was based on the assumption that depressed, disabled COPD patients may lack problem solving skills needed for adhering to their regimens, as many of them have executive dysfunction as well as attention and memory deficits (12). Accordingly, PSA integrated problem solving techniques with adherence enhancement of PID-C since problem solving therapy has been found effective in reducing disability (13) in older adults with major depression and executive dysfunction. An earlier report showed that both PSA and PID-C had comparable efficacy in reducing depressive symptoms and signs in patients with major depression and severe COPD (14).
This report focuses on dyspnea related disability. Symptoms of depression, including helplessness, lack of energy, and reduced activity, may influence dyspnea related disability. However, dyspnea related disability is a central clinical expression of COPD and an important target for interventions aiming to improve treatment adherence. Accordingly, this study tests the hypothesis that PSA is more effective than PID-C in reducing dyspnea related disability over a period of 26 weeks. In addition to testing the above hypothesis, we sought to identify subgroups with distinct dyspnea related disability trajectories and examine their baseline clinical presentations.
METHOD
Participants
The methods of this study have been reported elsewhere (14). Briefly, participants were recruited from consecutive additions to an acute rehabilitation hospital and all signed consent approved by the Weill Cornell IRB. Inclusion criteria were: 1) COPD diagnosed by a pulmonologist according to the American Thoracic Society Guidelines (15); 2) unipolar major depression by SCID-R and DSM-IV (16, 17); and 3) a score of 20 or greater on 24-item Hamilton Depression Rating Scale (HAM-D) (18). Exclusion criteria were: 1) DSM-IV diagnoses other than unipolar major depression; 2) Mini Mental State Examination (19) score of 23 or lower; and 3) intent or plan to attempt suicide in the near future. Depressed patients with anxiety disorders other than obsessive compulsive disorder were included.
Assessment
Trained research assistants, blind to treatment assignment and hypotheses assessed all participants. The outcome variable of this study was dyspnea-related disability quantified with the Pulmonary Functional Status and Dyspnea Questionnaire–Modified (PFSDQ-M), a 40-item scale for COPD patients assessing dyspnea during the performance of ten activities, each rated on an 11-point scale ranging from 0=no impairment to 10=very severe impairment. The PFSDQ-M has a total score based on the sum of three subscales: Change in Activities (10 items), Dyspnea with Activities (15 items) and Fatigue with Activities (15 items). The PFSDQ-M has high test-retest reliability and internal consistency (20). Its score has significant correlations with FEV1, FEV1 (% predicted), FVC (% predicted), FEV1/FVC (%) and PO2 (20, 21). The PFSDQ-M was assessed at baseline, discharge from the rehabilitation hospital, and at 10th, 14th, and 26th week. The subjects of this study were asked to complete the PFSDQ-M and received help from research assistants when they had difficulties.
Global disability was assessed with the World Health Organization Disability Assessment Schedule II 12-item [WHODAS-II-12 (22)]. Depressive symptoms were rated with the HAM-D. Medical burden was quantified with the Charlson Comorbidity Index [CCI (23)]. Anxiety [Generalized Anxiety Disorder 7-item Scale (24)], neuroticism [NEO-PI (25)], social support [Duke Social Support (26)] and self-efficacy [Liverpool Scale (27)] were assessed at entry (28–33). Interviews by research assistants were conducted prior to therapy sessions.
Treatment
Hospital rehabilitation has an average length of stay of about 2.5 weeks. Participants were randomized to PID-C or PSA in 5-subject blocks using random numbers. The clinical team was blind to randomization.
Intervention and Assessment Protocol
Interventions were offered by master’s level social workers and consisted of 14 sessions of PID-C or PSA administered over 26 weeks. The first 2 sessions were offered during the hospitalization. During these sessions, care managers introduced the rationale of treatment, assessed barriers to treatment adherence and needs, and formed a personalized plan. The subsequent 8 sessions were administered weekly after discharge at the participants’ homes or the therapists’ offices depending on the participants’ ability to travel. Four additional monthly sessions intended to reinforce the skills and behaviors imparted during the first 10 weekly sessions. All sessions were audiotaped and 10% were randomly selected for evaluation of treatment fidelity sessions and were evaluated with the PID-C and PSA Adherence Scales (Composite scores: 1: very poor, 2: poor: 3: satisfactory, 4: good, 5: excellent). Treatment fidelity ratings were performed by an experienced psychologist, not a member of the research team, trained in problem solving therapy, PID-C and PSA.
PID-C
PID-C consisted of in-person sessions of approximately 45 minute duration. When needed, therapists interacted with the participants’ physicians by telephone. The PID-C Manual (Appendix) guided care managers on how to evaluate barriers to adherence to physicians’ recommendations in individual patients and plans to address them.(34) When caregivers participated in the patients’ care, the care managers interacted with them using the same guidelines with those for patient participants. The care managers informed the participants’ physicians of any changes in the patients’ status and any problems with adherence.
PSA
PSA integrates the PID-C approach to adherence barriers with development of problem solving skills. The first problems to target were related to adherence to treatment recommendations. These problems (e.g. misunderstanding, limited information) were addressed with education and direct instruction. Problems related to hopelessness, helplessness and fatigue interfering with exercise and activities, social isolation and neglect of important relationships were addressed with problem solving skill development using the following steps: 1. Selection and problem definition; 2. Establishment of achievable goals; 3. Generation of alternative solutions; 4. Application of the decision making process; 5. Evaluation and selection of solutions; 6. Implementation of the preferred solution; and 7. Evaluation of outcome. PSA therapists used exercises that relied on multi-modal presentation of material, and repetition of the taught material during the session, i.e. “Say-it, show-it, do-it”.(35) They encouraged patients to focus on the process of addressing the problem at hand, so that they could use the same approach in subsequent problems.
Training and Fidelity
Care managers studied the PID-C and the PSA Manual and readings on COPD and depression. They attended a one-day workshop conducted by PJR, in which they role played typical sessions. Care managers treated 3 practice cases of each treatment, which were audiotaped and rated by PJR. More practice cases were used as needed until therapists received an average PID-C Adherence Scale and PAS Adherence score of 4 for each case in 3 consecutive cases. Clinician investigators offered weekly supervision during the trial. During the trial, the average composite score of 10% of randomly selected audiotaped sessions in the PID-C Adherence Scale was 4.67 (SD: 0.55) and in the PSA Adherence Scale 4.29 (SD: 0.69), i.e. “good” to “excellent” range.
Data Analysis
We compared the profiles of repeated dyspnea related disability scores (PFSDQ-M) of participants treated with PID-C or PSA using mixed-effects models to account for repeated measurements over time. They included time-trend parameter(s), treatment group, and time by treatment interaction as fixed effect and participant-specific random intercept. To identify subgroups with distinct dyspnea related disability trajectories, we employed latent class growth modeling (LCGM). We used the entire sample and tested separate models with one, two, and three distinct trajectories, using the censored normal distribution (36). First, we estimated the trajectory coefficients, calculated their 95% Bootstrap-t confidence interval in 1000 bootstrap samples, and selected the order (constant, linear, or non-linear, i.e. quadratic, cubic or quartic) of each trajectory using a significance level of α=0.05. We, then, tested the derived model against a model with one less trajectory using the change in Bayesian Information Criterion (BIC) as an approximation to the log Bayes factor (37) (2ΔBIC > 2). The average posterior probabilities of group membership were used as a measure of internal reliability for each trajectory. Values greater than .70 to .80 suggest that the trajectories classify groups with similar patterns of change separately from groups with different patterns of change.(36)
RESULTS
We screened 633 consecutively admitted pulmonary patients. Of these, 147 met criteria and signed consent. Their flow into the study (CONSORT diagram) has been reported earlier (14) and can be found in the Appendix. Among randomized participants (N=101), the mean inpatient stay was 19.6 days (SD=17.1). There were no significant differences in demographic and clinical characteristics among participants assigned to PID-C or PSA. There were no differences in overall rates of attrition between the two arms at 26 weeks (χ2=0.0714, df=1, p=0.7893). The demographic and clinical characteristics of participants who exited the trial for reasons other than death were statistically indistinguishable from those who remained in the trial.
Comparison of Interventions
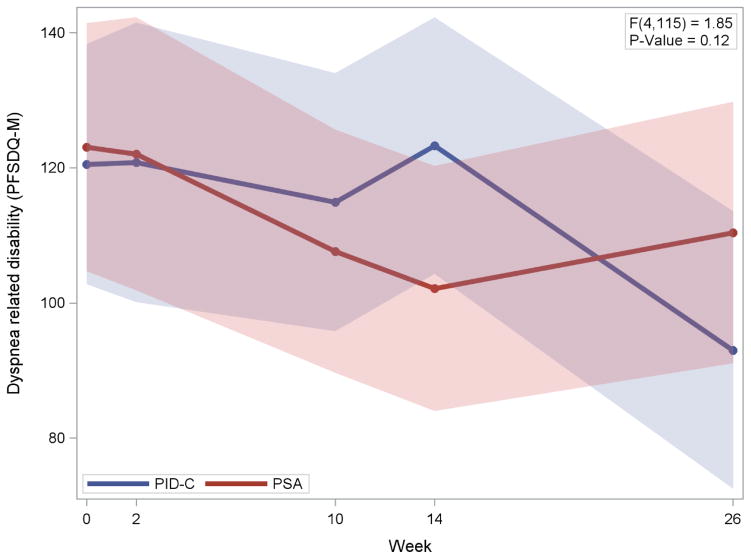
Dyspnea related disability (PFSDQ-M) was assessed at baseline, discharge, and weeks 10, 14 and 26; the mean (SD) values for PID-C were 122.1 (74.2), 126.4 (76.3), 98.6 (69.2), 107. 7 (77.4), 81.5 (58.8) respectively and for PSA 125.4 (74.5), 130.4 (79.6), 105.8 (66.0), 102.2 (63.5), and 116.7 (72.1). There was within group change in each of the two treatment groups and in the combined sample. In the PID-C group, dyspnea related disability improved with time (t38=−2.49, p = 0.02) by an estimated 0.65 points per week (95% CI: 0.12,1.18). Dyspnea related disability also improved with time in PSA participants (t40=−2.04, p < 0.05) by an estimated 0.63 points per week (95% CI: 0.01,1.25). Similarly in the combined sample of both treatment groups, dyspnea related disability improved with time (t79=−3.03, p = 0.003) by an estimated 0.62 points per week (95% CI: 0.21,1.03).
Linear mixed model analysis showed no significant differences in dyspnea related disability between PID-C and PSA during the period extending from baseline to 26 weeks (time × treatment: F4,115=1.85, p=0.12) (Figure 1) indicating that the study hypothesis was not supported. Similarly, there was no difference in the course of overall disability (WHODAS) between PID-C and PSA (F4,128=0.28, p=0.8911).
Figure 1.
Dyspnea Related Disability in Participants with Major Depression and COPD Randomly Assigned to Personalized Intervention for Depression and COPD (PID-C) and Problem Solving-Adherence (PSA).
Legend to Figure 1. Mixed effects model consisting of time-trend parameter(s), treatment group, and time by treatment interaction as fixed effect and participant-specific random intercept.
PFSDQ-M: Pulmonary Functional Status and Dyspnea Questionnaire-Modified
During the 26 weeks of the study, 14% (7/50) of PID-C and 13.7% (7/51) of PSA participants died. There were no significant differences in death between the treatment groups (χ2=0.0016, df=1, p=0.9682). Participants who died during the 26-week trial were older than those who remained alive in the entire group (mean years (SD): 76.93 (8.16) vs. 70.97 (9.07); t=−2.31, df=99, p=0.023) and in the PID-C treatment arm (mean years (SD): 79.86 (4.88) vs. 71.14 (9.47); t=−2.37, df=48, p=0.022), but not in the PSA arm (mean age (SD): 74.0(10.02) vs. 70.8 (8.77); t=−0.88, df=49, p=0.382). There were no significant differences in any clinical characteristics between those who died and those who remained alive during the 26 week trial.
Trajectories of Dyspnea
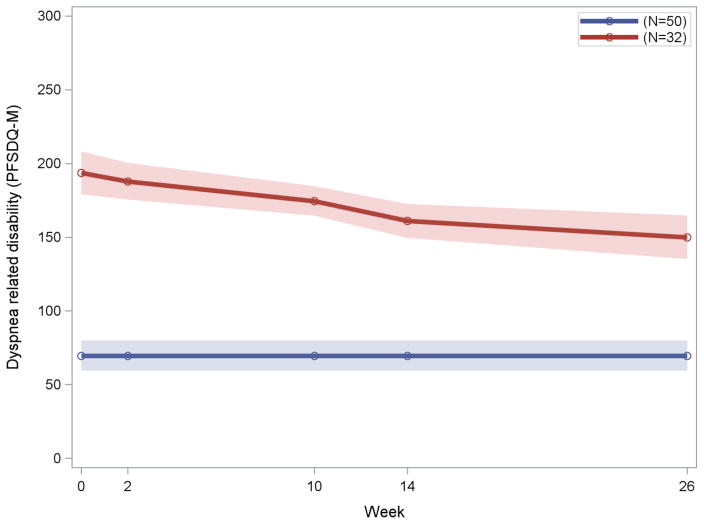
Exploratory LCGM analysis sought to identify distinct trajectories of dyspnea related disability in the entire sample. Participants who had at least one follow-up assessment (N=82) had two distinct trajectories (2ΔBIC=136.44) (Figure 2), an improving trajectory with a decline of dyspnea related disability and a stable trajectory with no change of dyspnea related disability over time. The average posterior probabilities for membership in the improving and stable trajectories were 0.97 for each group. Thirty two of the 82 participants had an improving trajectory with a linear trend over the course of 26 weeks (estimate of slope = −1.25, 95% Bootstrap CI=−1.72,−0.80) while 50 had a stable trajectory showing no evidence of change over time (estimate of intercept = 68.13, 95% Bootstrap CI: 61.58–74.65). In the group with the improving trajectory, dyspnea related disability began to decline during the hospitalization and continued to decline during the rest of the treatment phase (26th week). In contrast, the stable trajectory had no change in dyspnea throughout the 26 weeks of observation. Approximately 53% (17/32) of participants with the improving dyspnea related disability trajectory were treated with PSA and the remaining 47% (15/32) received PID-C. In the stable trajectory, 52% (26/50) were treated with PSA and the remaining 48% (24/50) received PID-C. There was no significant difference in the distribution of treatments in the two dyspnea related disability trajectories (χ2 = 0.01, df=1, p=0.92).
Figure 2.
Distinct trajectories of Dyspnea Related Disability of Patients with Major Depression and Severe COPD Randomly Assigned to PID-C or PSA (N=82)
Legend to Figure 2: Latent class growth modelling (LCGM) identified two subgroups with distinct dyspnea related disability (PFSDQ-M)
PFSDQ-M: Pulmonary Functional Status and Dyspnea Questionnaire-Modified
Severity of depression at baseline did not distinguish the two dyspnea related disability trajectories. Similarly, anxiety, overall cognitive impairment, executive functions, social support, blood gases, overall medical burden, gender, age, and education, were statistically indistinguishable in participants with the two dyspnea trajectories. However at baseline, participants with an improving trajectory had greater dyspnea related disability (PFSDQ-M) and overall disability (WHODAS-12) than participants with a stable trajectory (Table 1). Those with improving trajectories also had lower scores in the control subscale of the self-efficacy scale at baseline. The interactions of the control subscale with the PFSDQ-M (Wald χ2=2.65, df=1, p=0.104) and with the WHODAS-12 (Wald χ2=2.55, df=1, p=0.110) scores were not significantly associated with membership to the two trajectories of dyspnea related disability.
Table 1.
Demographic and Clinical Characteristics of 82 Older Patients with Major Depression and COPD followed for 26 weeks after discharge from an Inpatient Rehabilitation Service.
| Variable | PFSDQ-M Trajectory | χ2 | df | Holm adjusted p value | |||
|---|---|---|---|---|---|---|---|
| Stable N=50 |
Improving N=32 |
||||||
| N | % | N | % | ||||
| Female | 35 | 70 | 22 | 68.75 | 0.0144 | 1 | 1 |
| PSA Group | 26 | 52 | 17 | 53.13 | 0.0099 | 1 | 1 |
| Mean | SD | Mean | SD | t* | df | Holm adjusted p value | |
| Age (years) | 72.06 | 9.74 | 71.56 | 8.39 | 0.24 | 80 | 1 |
| Education (years) | 13.62 | 3.6 | 13.91 | 2.48 | −0.39 | 80 | 1 |
| HAM-D | 24.02 | 4.07 | 24.41 | 3.57 | −0.44 | 79 | 1 |
| MADRS PFSDQ-M | 22.96 | 5.28 | 21.07 | 4.91 | 1.59 | 77 | 1 |
| TOTAL | 73.02 | 34.54 | 193.82 | 47.08 | −13.14 | 77 | <.0001 |
| Change in Activities | 26.17 | 14.67 | 64.73 | 15.63 | −11.12 | 77 | <.0001 |
| Dyspnea with Activities | 22.24 | 12.49 | 63.81 | 22.74 | −10.47 | 77 | <.0001 |
| Fatigue with Activities | 24.61 | 13.43 | 65.28 | 16.63 | −11.96 | 77 | <.0001 |
| CCI | 3.64 | 2.8 | 3.56 | 2.14 | 0.13 | 77 | 1 |
| MMSE | 27.69 | 1.94 | 27.19 | 1.87 | 1.12 | 77 | 1 |
| DRS-IP | 32.89 | 4.15 | 32.21 | 3.9 | 0.7 | 73 | 1 |
| Stroop Word-Color | 22.59 | 8.3 | 23.45 | 11.33 | −0.35 | 61 | 1 |
| GAD-7 | 8.02 | 5.66 | 7.81 | 5.41 | 0.17 | 77 | 1 |
| NEO | 11.58 | 5.53 | 12.66 | 4.97 | −0.89 | 80 | 1 |
| Liverpool Self-Efficacy | |||||||
| Control | 15.06 | 2.43 | 12.81 | 2.79 | 3.86 | 80 | 0.0055 |
| Personal Agency | 12.4 | 1.93 | 11.34 | 2.15 | 2.31 | 80 | 0.4928 |
| WHODAS | 29.63 | 5.72 | 36.97 | 7.6 | −4.91 | 78 | 0.0001 |
| SF-12 | |||||||
| Mental Composite Score (MCS) | 41.12 | 10.51 | 33.54 | 13.21 | 2.83 | 77 | 0.1365 |
| Physical Composite Score (PCS) | 32.05 | 9.12 | 27.27 | 7.34 | 2.44 | 77 | 0.3737 |
| Blood Gases | |||||||
| FIO2 | 21.53 | 2.21 | 22.38 | 3.41 | −1.18 | 58 | 1 |
| PCO2 | 46.67 | 13.24 | 48.62 | 7.22 | −0.67 | 57 | 1 |
| pH | 7.47 | 0.03 | 7.46 | 0.03 | 0.74 | 57 | 1 |
| PO2 | 57.79 | 13.38 | 60.35 | 14.82 | −0.7 | 57 | 1 |
| Duke Social Support | |||||||
| Social Network | 3.71 | 3.73 | 3.66 | 3.1 | 0.07 | 79 | 1 |
| Social Interaction | 6.08 | 2.01 | 5.69 | 2.39 | 0.8 | 79 | 1 |
| Instrumental Support | 8.67 | 2.58 | 8.59 | 2.89 | 0.12 | 78 | 1 |
| Subjective Support | 17.86 | 2.84 | 18.56 | 2.45 | −1.15 | 79 | 1 |
Two Independent Sample t-test
Note: HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery-Åsberg Depression Rating Scale; PFSDQ-M: Pulmonary Functional Status & Dyspnea Questionnaire-Modified; CCI: Charlson Comorbidity Index; MMSE: Mini Mental State Examination; DRS-IP: Dementia Rating Scale-Initiation/Perseveration; GAD-7: Generalized Anxiety Disorder 7-item Scale; NEO: Neuroticism; WHODAS II-12: World Health Organization Disability Assessment Schedule II 12-item.
There were no differences in mortality between the two dyspnea related disability trajectories. Approximately 15.6% (5/32) of participants with an improving trajectory and 12% (6/50) of participant with a stable trajectory died during the 26 weeks of the study (χ2=0.2207, df=1, p=0.6385).
DISCUSSION
This study tested the hypothesis that an intervention integrating problem solving techniques with adherence enhancement procedures (PSA) reduces dyspnea related disability more than a structured adherence enhancement intervention (PID-C) in depressed COPD patients. This hypothesis was not supported. Regardless of intervention, dyspnea related disability improved gradually over 26 weeks in 39 percent of depressed COPD patients. In the remainder 61 percent, dyspnea related disability was essentially unchanged during the same period. Absence of worsening of dyspnea related disability over a period of 26 weeks can be viewed as a favorable outcome since the course of severe COPD is one of deterioration and the frequency of intervention sessions decreased after the 10th week. Documenting that the two interventions were followed by improvement or no worsening of disability is encouraging in patients with a severe deteriorating condition and medical comorbidity, evidenced by a mortality rate of 14% over 26 weeks.
It is unclear why the integration of problem solving techniques with adherence enhancement procedures in PSA did not further improve the course of dyspnea related disability. It is possible that inadequate statistical power prevented us to identify a difference between PID-C and PSA. Another explanation may be that adherence to rehabilitation regimen is critical for improving or not worsening dyspnea related disability, which is the most pressing problem of severely ill COPD patients. Both PID-C and PSA target adherence to rehabilitation directly. The adherence enhancement component of these interventions might have been sufficient to improve dyspnea related disability in these very sick patients.
An important difference between patients whose dyspnea related disability improved from those whose disability remained unchanged was the severity of dyspnea related disability at baseline. Participants with an improving trajectory of dyspnea related disability had 2.7 times greater disability scores at study entry than participants whose dyspnea related disability remained unchanged. This difference was reflected in each domain of PFSDQ-M, i.e. Change in activities, dyspnea with activities, and fatigue with activities. Consistent with the PFSDQ-M findings, was the high overall disability (WHODAS-12) experienced at baseline by the group whose dyspnea related disability had an improving course. We note that patients with a stable course of dyspnea related disability had clinically significant disability at baseline indicated by an average score of 73.02 (SD: 34.54), well above zero (no change in activities or in dyspnea or fatigue with activities).
Patients with the improving dyspnea related disability course had a lower sense of control over their disability compared to patients whose disability remained unchanged. This is understandable since this group had a severe dyspnea related disability limiting what they could actually do. Moreover, facing progressive disability has been shown to weaken the sense of control (38). Enrichment of PID-C with control enhancement interventions may be a reasonable next step that has the potential to improve its efficacy, especially in depressed patients with severe COPD, who lack of control of their condition. Enhancement of sense of control and self-efficacy has been shown to reduce disability and improve quality of life in patients with a variety of disorders (39). Self-management programs aiming to improve self-efficacy led to better health outcomes in patients with chronic diseases (40).
This study has several limitations including the administration of both interventions by the same therapists, the small sample, the absent of a no-intervention comparison group, and the high number of participants who exited prior to randomization. Assignment of the same therapists to both interventions reduces the need to introduce the impact of therapists in group comparisons and increases the power to detect differences in this rather small sample of a difficult to recruit population. However, administration of both interventions by the same therapists may contaminate the interventions. Arguably, the high fidelity of therapists to each intervention manuals suggests that contamination was minimal and may not have had an appreciable impact on the results. A no-intervention comparison group was not feasible in this very sick group of patients. A usual care comparison group could have shown whether the interventions were superior to usual care and examine if the course of dyspnea related disability was influenced by the interventions. We note, however, that an earlier study using similar selection criteria for depressed COPD patients from the same rehabilitation hospital demonstrated that PID-C led to a greater decline in depressive symptoms, and less disability than usual care over 28 weeks and 6 months after the last session (10). One third of qualified patients exited before randomization, mainly because of increased medical burden or mortality. Thus, this study’s findings are pertinent to less severely ill populations.
In sum, we failed to find evidence that integration of problem solving strategies with adherence enhancement procedures improves the course of dyspnea related disability more than a simpler personalized intervention focusing on adherence to rehabilitation and medical care in depressed COPD patients. Regardless of intervention, 39 percent of depressed COPD patients had an improvement in dyspnea related disability over 26 weeks, while disability remained essentially stable in the rest. Improvement or no worsening of disability is a favorable outcome in patients with a severe, deteriorating medical illness. These findings favor PID-C, a simple to administer, personalized adherence enhancement intervention. The efficacy of PID-C will need to be further tested in large community based studies. If shown that it can be taught with fidelity to bachelor and masters-level rehabilitation clinicians and found effective, it can be integrated in the care of COPD patients.
Supplementary Material
Highlights.
Both PID-C and PSA led to improvement or no worsening of dyspnea related disability in patients with major depression and severe COPD.
Exploratory latent class growth modeling identified two distinct dyspnea related disability trajectories.
Dyspnea related disability improved in 39% of patients and remained unchanged in the rest.
Patients whose dyspnea related disability improved had severe disability and weak sense of control over their condition at baseline.
PID-C is compatible with the expertise of clinicians working in community-based rehabilitation programs and can become part of the care of depressed COPD patients.
Acknowledgments
Funding/Support: NIMH grants R01 MH076829, P50 MH113838, T32 MH019132 and by the Sanchez Foundation. Dr. Novitch is partially supported by a grant from the Will Rogers Institute.
Footnotes
Conflicts of Interest: Dr. Alexopoulos serves at the speakers’ bureaus of Takeda, Lundbeck, Otsuka, and Sunovion. No other authors report conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC. Chronic Obstructive Pulmonary Disease Among Adults — United States. Washington, DC: CDC; 2011. [Google Scholar]
- 2.Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127:1205–1211. doi: 10.1378/chest.127.4.1205. [DOI] [PubMed] [Google Scholar]
- 3.Katon JW. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues in Clinical Neuroscience. 2011;13:7–23. doi: 10.31887/DCNS.2011.13.1/wkaton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jennings JH, Digiovine B, Obeid D, et al. The association between depressive symptoms and acute exacerbations of COPD. Lung. 2009;187:128–135. doi: 10.1007/s00408-009-9135-9. [DOI] [PubMed] [Google Scholar]
- 5.Cuijpers P, Vogelzangs N, Twisk J, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. The American journal of psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- 6.Jordan N, Lee TA, Valenstein M, et al. Effect of depression care on outcomes in COPD patients with depression. Chest. 2009;135:626–632. doi: 10.1378/chest.08-0839. [DOI] [PubMed] [Google Scholar]
- 7.Yohannes AM, Connolly MJ, Baldwin RC. A feasibility study of antidepressant drug therapy in depressed elderly patients with chronic obstructive pulmonary disease. Int J Geriatr Psychiatry. 2001;16:451–454. doi: 10.1002/gps.461. [DOI] [PubMed] [Google Scholar]
- 8.Alexopoulos GS, Sirey JA, Raue PJ, et al. Outcomes of depressed patients undergoing inpatient pulmonary rehabilitation. Am J Geriatr Psychiatry. 2006;14:466–475. doi: 10.1097/01.JGP.0000199381.98971.d1. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan RM, Toshima M, Atkins CJ, et al. Adherence to prescribed regimens for patients with chronic obstructive pulmonary disease. In: Shumaker SA, Schron EB, Ockene JK, editors. The Handbook of Health Behavior and Change. New York: Springer Publishing Co; 1990. [Google Scholar]
- 10.Alexopoulos GS, Kiosses DN, Sirey JA, et al. Personalised intervention for people with depression and severe COPD. The British journal of psychiatry : the journal of mental science. 2013;202:235–236. doi: 10.1192/bjp.bp.112.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajzden I. The directive influence of attitudes on health behavior. In: Gollwitzer PM, Bargh JA, editors. The psychology of action:linking cognition and motivation to behavior. New York: The Guilford Press; 1996. [Google Scholar]
- 12.Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. The European respiratory journal. 2010;35:913–922. doi: 10.1183/09031936.00125109. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Archives of general psychiatry. 2011;68:33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Sirey JA, Banerjee S, et al. Two Behavioral Interventions for Patients with Major Depression and Severe COPD. Am J Geriatr Psychiatry. 2016;24:964–974. doi: 10.1016/j.jagp.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77–121. [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured clinical interview for DSM-IV - patient version (SCID-P) Washington: American Psychiatric Press; 1995. [Google Scholar]
- 17.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision) 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 18.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Lareau SC, Meek PM, Roos PJ. Development and testing of the modified version of the pulmonary functional status and dyspnea questionnaire (PFSDQ-M) Heart Lung. 1998;27:159–168. doi: 10.1016/s0147-9563(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 21.Lareau SC, Meek PM, Roos PJ. Additional testing of the modified pulmonary functional status and dyspnea questionnaire (PFSDQ-M): ease of use, stability, reliability, and validity. Am J Crit Care Med. 1997;155:A722. [Google Scholar]
- 22.Epping-Jordan JA, Ustun TB. The WHODAS-II: leveling the playing field for all disorders. WHO Mental Health Bulletin. 2000;6:5–6. [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer RL, Kroenke K, Williams JW, et al. A brief measure for assessing generalized anxiety disorder: The gad-7. Archives of Internal Medicine. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 25.Costa PT, McCrae RR. The NEO personality inventory. Manual, Odessa, FL: Psychological Assessment Resources; 1985. [Google Scholar]
- 26.Koenig HG, Westlund RE, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics. 1993;34:61–69. doi: 10.1016/S0033-3182(93)71928-3. [DOI] [PubMed] [Google Scholar]
- 27.Airlie J, Baker GA, Smith SJ, et al. Measuring the impact of multiple sclerosis on psychosocial functioning: the development of a new self-efficacy scale. Clinical rehabilitation. 2001;15:259–265. doi: 10.1191/026921501668362643. [DOI] [PubMed] [Google Scholar]
- 28.Andreescu C, Lenze EJ. Anxiety – depression comorbidity – bête noire or quick fix? The British journal of psychiatry : the journal of mental science. 2012;200:179–181. doi: 10.1192/bjp.bp.111.097022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi M, Shirayama Y, Muneoka K, et al. Low Openness on the Revised NEO Personality Inventory as a Risk Factor for Treatment-Resistant Depression. PLoS ONE. 2013;8:e71964. doi: 10.1371/journal.pone.0071964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadimpati S, Zale EL, Hooten MW, et al. Associations between Neuroticism and Depression in Relation to Catastrophizing and Pain-Related Anxiety in Chronic Pain Patients. PLoS ONE. 2015;10:e0126351. doi: 10.1371/journal.pone.0126351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCathie HC, Spence SH, Tate RL. Adjustment to chronic obstructive pulmonary disease: the importance of psychological factors. The European respiratory journal. 2002;19:47–53. doi: 10.1183/09031936.02.00240702. [DOI] [PubMed] [Google Scholar]
- 32.Kohler CL, Fish L, Greene PG. The relationship of perceived self-efficacy to quality of life in chronic obstructive pulmonary disease. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2002;21:610–614. doi: 10.1037//0278-6133.21.6.610. [DOI] [PubMed] [Google Scholar]
- 33.Marino P, Sirey JA, Raue PJ, et al. Impact of social support and self-efficacy on functioning in depressed older adults with chronic obstructive pulmonary disease. International Journal of Chronic Obstructive Pulmonary Disease. 2008;3:713–718. doi: 10.2147/copd.s2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sirey JA, Raue PJ, Alexopoulos GS. An intervention to improve depression care in older adults with COPD. Int J Geriatr Psychiatry. 2007;22:154–159. doi: 10.1002/gps.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson LW, Davies R, Gallagher D, et al. Cognitive therapy with older adults. Clinical Gerontologist. 1986;5:245–279. [Google Scholar]
- 36.Andruff H, Carraro N, Thompson A, et al. Latent class growth modelling: a tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5:11–24. [Google Scholar]
- 37.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological methods & research. 2001;29:374–393. [Google Scholar]
- 38.Macleod L, Macleod G. Control cognitions and psychological disturbance in people with contrasting physically disabling conditions. Disability and rehabilitation. 1998;20:448–456. doi: 10.3109/09638289809166109. [DOI] [PubMed] [Google Scholar]
- 39.Schuz B, Wurm S, Warner LM, et al. Self-efficacy and multiple illness representations in older adults: a multilevel approach. Psychology & health. 2012;27:13–29. doi: 10.1080/08870446.2010.541908. [DOI] [PubMed] [Google Scholar]
- 40.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Medical care. 2001;39:1217–1223. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.