Abstract
BACKGROUND
Cardiac allograft vasculopathy (CAV) has been associated with graft-infiltrating B cells, although their characteristics are still unclear. In this study we examined the frequency, localization and reactivity profile of graft-infiltrating B cells to determine their contribution to the pathophysiology of CAV.
METHODS
B cells, plasma cells and macrophages were examined by immunohistochemistry in 56 allografts with CAV, 49 native failed hearts and 25 autopsy specimens. A total of 102 B-cell clones were immortalized directly from the infiltrates of 3 fresh cardiac samples with CAV. Their secreted antibodies were assessed using enzyme-linked immunoassay and flow cytometry.
RESULTS
B-cell infiltration was observed around coronary arteries in 93% of allograft explants with CAV. Comparatively, intragraft B cells were less frequent and less dense in the intraventricular myocardium from where routine biopsies are obtained. Plasma cells and macrophages were also detected in 85% and 95% of explants, respectively. Remarkably, B-cell infiltrates were not associated with circulating donor-specific antibodies (DSA) or prior episodes of antibody-mediated rejection (AMR). Among all B-cell clones generated from 3 explants with CAV, a majority secreted natural antibodies reactive to multiple autoantigens and apoptotic cells, a characteristic of innate B cells.
CONCLUSIONS
Our study reveals a high frequency of infiltrating B cells around the coronary arteries of allografts with CAV, independent of DSA or AMR. These cells are enriched for innate B cells with a polyreactive profile. The findings shift the focus from conventional DSA-producing B cells to the potentially pathogenic polyreactive B cells in the development of clinical CAV.
Keywords: cardiac allograft vasculopathy, innate B cells, polyreactive B cells, graft-infiltrating B cells, autoantibodies
Cardiac allograft vasculopathy (CAV) is one of the leading causes of mortality after the first year of heart transplantation. CAV is a rapidly progressive form of atherosclerosis, characterized by intimal smooth muscle cell proliferation in the early stages, and by arterial occlusion, myocardial infarction and heart failure in later stages. The pathophysiological mechanisms of CAV are still poorly understood. T cells and natural killer (NK) cells have been implicated in this disease.1,2 Several studies have also established a strong association between CAV and graft-infiltrating B cells.3–5 As initially reported by Wehner et al, diffuse or nodular infiltrates of B cells are observed in chronically rejected human heart transplants.5 These infiltrates often include terminally differentiated plasma cells, suggesting the secretion of immunoglobulins in situ. An independent group corroborated these findings in a larger series of explanted cardiac allografts.4 In the latter study by Huibers et al, the investigators reported macrophages and T cells in addition to B cells harboring a memory phenotype in most graft infiltrates. Collectively, these observations support the view of an active and complex immune response, including T cells, B cells and macrophages, developing locally in close proximity to the CAV-affected vessels. It remains unclear whether these lymphocytic infiltrates are related to Quilty lesions commonly observed in endomyocardial biopsies.6 The specificity of the graft-infiltrating B cells is another important element required to appreciate their significance in the local immune response. Accordingly, our objectives were to further examine infiltrating B cells with regard to their location in cardiac allografts and determine their reactivity profile to uncover characteristics relevant to their function in situ and possible role in CAV.
Methods
Cardiac specimens
Tissue specimens from a total of 56 cardiac transplants were used in this study; 7 of these were fresh samples and the rest were tissue samples archived at the Columbia University Medical Center. The cardiac specimens were explanted during a second transplant procedure at Columbia University Medical Center, Massachusetts General Hospital (MGH) or Brigham and Women’s Hospital. The study was approved by all institutional review boards. The fresh tissues were obtained in the operating room and collected from different areas of the allografts, including the right coronary artery (RCA), left anterior descending coronary artery (LAD), circumflex coronary artery, the epicardium and endomyocardium.
Immunohistochemistry and immunofluorescence staining
Fresh graft tissue samples were fixed in 10% phosphate-buffered formalin (ThermoFisher Scientific, Waltham, MA) and embedded in paraffin blocks. Slides with 5-µm sections were heated for 1 hour at 60°C, deparaffinized, and heat-mediated antigen retrieval was performed using a solution of Diva Decloaker (Biocare Medical, Concord, CA) using a Decloaking Chamber (Biocare), followed by rinsing in Tris-buffered saline (TBS). Appropriate blocking steps (peroxidase and/or 1% bovine serum albumin in TBS) were performed. The following anti-human primary anti-bodies were used to stain the sections overnight at 4°C: CD20 (Clone L26, Dako, Glostrup, Denmark; or EP459Y, Abcam, Cambridge, UK); CD138 (Clone SP152 or B-A38, Abcam); CD68 (Clone KP1, Biocare); immunoglobulin M (IgM; Clone EPR5539-65-4, Abcam); and immunoglobulin G (IgG; Clone EPR4421, Abcam). PolyView immunohistochemistry (IHC) reagent (mouse–horseradish peroxidase) and PolyView IHC reagent (rabbit–alkaline phosphatase) or MultiView IHC reagent from Enzo Life Sciences (Farmingdale, NY) was used to perform IHC according to the manufacturer’s protocol. Tissue sections were counterstained for 30 seconds using hematoxylin (Sigma Co., St. Louis, MO) and lithium carbonate (bluing agent). Slides were then dehydrated through graded ethanol and xylene washes and then mounted with permanent mounting medium for IHC. The IHC slides were scanned using an automated slide scanner. SLIDEPATH software was used to analyze images and calculate the stained areas on the tissue sections. For the histologic grading of CAV explants, the following scheme was used: Grade 0 = no detectable CD20+ cells; Grade 1 = CD20+ B cells covering < 0.25% of the tissue area near the epicardial coronary artery (CA); Grade 2 = CD20+ B cells covering 0.25% to 0.5% of the tissue area near the epicardial CA; and Grade 3 = CD20+ B cells covering >0.5% of tissue area near the epicardial CA.
For immunofluorescence (IF), the following antibodies were used: CD163 (Abcam); CD206 (Clone 5C11, Abcam); inducible nitrous oxide synthase (iNOS; Enzo Life Sciences); and interleukin-10 (IL-10; Abcam). In addition, donkey anti-rabbit (AF488, Life Technologies, Carlsbad, CA) and donkey anti-mouse (NL557; R&D Systems) were used as secondary antibodies and applied for 45 minutes at room temperature. Hoechst 33342 and Prolong Anti-fade mountant (Life Technologies) were used for nuclear staining and mounting, respectively. IF was visualized using a fluorescence microscope (DMI 6000B, Leica).
Isolation and immortalization of B-cell clones
B cells were isolated from fresh, unfixed tissue fragments from 3 failed cardiac allografts with CAV explanted at time of retransplantation. Fragments of the explanted heart graft tissue were first dissociated mechanically, then digested for 45 minutes at 37°C in the presence of 1-mg/ml collagenase D (Roche, Penzberg, Germany) and 20 µg/ml of DNAse I (Roche) in RPMI 1640. Undigested fibrous material was removed by filtration through a cell strainer. Epstein–Barr virus (EBV)-transformed B-cell clones were generated by incubating total cell suspension with supernatant from EBV-producing B95-8 marmoset cells in the presence of the TLR9 agonist CpG 2006 at 2.5 µg/ml, as described elsewhere.7 Limiting dilution cloning was performed in 96-well plates by plating serial dilutions of graft-infiltrating cells on feeder cells consisting of 50,000 irradiated Jurkat cells per well in 96-well plates. Once immortalized, B-cell clones were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 4-mmol/liter glutamine, 1-mmol/liter sodium pyruvate, 10-mmol/liter hydroxyethylpiperazine ethanesulfonic acid (HEPES) and antibiotics. Cells growing in plates in which < 30% of wells contained growing cells were presumed clonal. Clonality was confirmed by molecular analysis of Ig heavy-chain transcripts.
Molecular analysis of Ig heavy-chain variable regions
Total RNA was extracted from immortalized clones using the Pure Link RNA Mini kit (Ambion, Invitrogen). A reverse transcriptase kit (Superscript III, Invitrogen) was used to generate cDNA. Variable regions of the Ig heavy chain were amplified by polymerase chain reaction (PCR) using 6 family-specific forward primers (VH1 to VH6)8 and a consensus heavy chain joining domain(JH) reverse primer.9 The PCR conditions were as follows: 95°C/5 min (95°C/30 seconds; 56°C/30 seconds; 72°C/30 seconds) × 35 cycles; 72°C/10 min. PCR products were sequenced using the corresponding heavy chain variable domain (VH) primer to confirm the clonality of the B cells. Sequencing was performed at the DNA core facility at MGH.
Enzyme-linked immunoassay
Antibody concentrations in culture supernatants were determined using an enzyme-linked immunoassay (ELISA) system (Bethyl Laboratories, Montgomery, TX). The reactivity of the monoclonal antibodies secreted by immortalized B-cell clones was determined by ELISA, as described elsewhere.10 In brief, immunosorbent microplates were coated overnight with lipopolysaccharide (LPS) from Escherichia coli (double-stranded DNA [dsDNA], Sigma-Aldrich, St. Louis, MO), cardiolipin (Sigma-Aldrich), human insulin (Sigma-Aldrich) or malondialdehyde–adducted bovine serum albumin (MDA-BSA). The MDA-BSA was prepared as previously described,11 by incubating acid-hydrolyzed 1,1,3,3-tetramethoxypropane (Sigma-Aldrich) with BSA. Briefly, 1-mol/liter 1,1,3,3-tetramethoxypropane was hydrolyzed in 96-mmol/liter HCl for 10 minutes at 37°C. The resulting MDA solution was neutralized with NaOH and the modification of 2 mg BSA with 0.2-mol/liter MDA was carried out for 3 hours at 37°C, followed by extensive dialysis against PBS at 4°C for 36 hours. Plates were blocked with TBS supplemented with 0.5% non-fat dry milk (TBS-milk) for 1 hour at room temperature (RT). Cell culture supernatants were diluted in TBS-milk and incubated for 2 hours at RT. Antibody binding was revealed with HRP-conjugated goat anti-human IgG or IgM (Jackson ImmunoResearch Laboratories, West Grove, PA), and developed using 3,3′,5,5′-tetramethylbenzidine (TMB; Life Technologies). Optical density was read at 450 nm.
Assessment of reactivity to apoptotic cells
The reactivity of monoclonal antibodies secreted by immortalized B-cell clones to apoptotic cells was assessed by flow cytometry, as described elsewhere.12 In brief, human Jurkat T leukemia cells were exposed to ultraviolet (UV) light (240 × 10−3 J) to induce apoptosis using a UV crosslinker (Stratalinker 2400, Stratagene, La Jolla, CA). Apoptotic Jurkat T cells were incubated for 30 minutes at 37°C with 100 µl of IgM or IgG supernatant. After 2 washes in PBS at 4°C, samples were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-IgM or anti-IgG F(ab′)2 secondary antibodies, respectively (Invitrogen), for 30 minutes at 4°C. After 2 additional washes in PBS at 4°C, cells were acquired by flow cytometry (FACSCanto, BD Biosciences, San Jose, CA) after gating on apoptotic cells. FLOWJO software (FloJo LLC, Ashland, OR) was used to analyze the data.
Statistical analysis
Demographic and clinical variables were summarized using standard descriptive statistics and are expressed as median (with interquartile range) for skewed continuous variables and count (with percent) for categorical variables. Group comparisons were made using Fisher’s exact test or Kruskal–Wallis test, as appropriate. Multinomial logistic regression models were used to identify independent risk factors for increased B-cell score. p < 0.05 (2-tailed) was considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
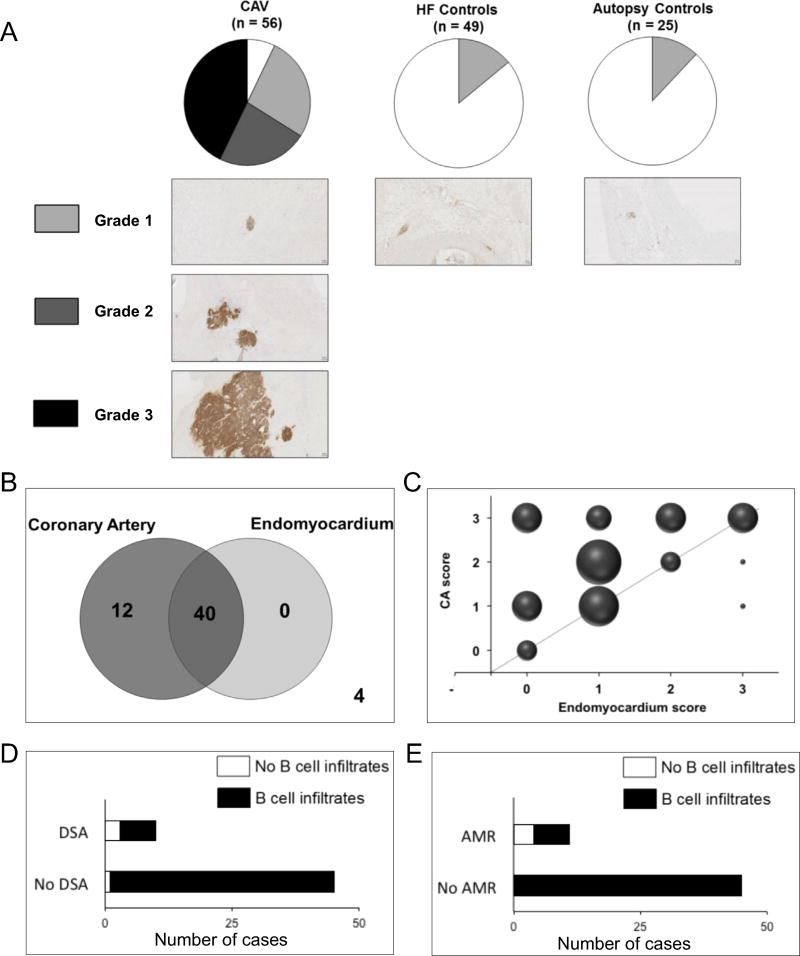
Tissues surrounding the CA as well as transmural epicardium to endomyocardium samples were obtained at 3 participating institutions from 56 cardiac allografts explanted at time of re-transplantation. These included 7 fresh cardiac allografts and 49 archived cardiac allograft specimens. Patients’ demographics are shown in Table 1. The presence of CAV was confirmed in all cases based on intimal thickening of intramural vessels. These specimens are henceforth referred to as CAV explants. Comparable tissue was also obtained from 49 hearts explanted during primary cardiac transplantation due to long-standing heart failure (HF) and 25 autopsied heart specimens from non-cardiac deaths as controls. All specimens were stained with immunoperoxidase using anti-CD20 antibodies to assess for B cells near the epicardial CA and the interventricular septum myocardium. To compare the intensity of B-cell infiltration, a histologic scoring method, with grades between 0 and 3, was devised. Tissue completely devoid of B cells was considered Grade 0 (white in Figure 1A). Grades 1, 2 and 3 corresponded to increasing degrees of B-cell infiltration (Figure 1A). B cells were found in almost all CAV explants, with a majority showing Grade 2 or 3 infiltration. In contrast, only a few of the controls had any detectable B cells infiltrating the collected tissue. These were all Grade 1. Multinomial logistic regression analysis did not reveal any significant association between individual risk factors (Table 1) and B-cell scores of CAV explants.
Table 1.
Patient characteristics
| B-cell Score 0 | B-cell Score 1 | B-cell Score 2 | B-cell Score 3 | p-value | |
|---|---|---|---|---|---|
| N | 4 | 15 | 13 | 24 | |
| Male (%) | 0 (0) | 8 (53.3) | 11 (84.6) | 20 (83.3) | 0.003 |
| Age at retransplant (y) | 23 (10.0 to 33.5) | 39 (26 to 57) | 48 (28 to 57) | 45 (25.5 to 55.5) | 0.38 |
| Ischemic etiology (%) | 0 (0) | 2 (13.3) | 3 (23.1) | 6 (25.0) | 0.70 |
| Time from primary transplant (y) | 7 (2 to 18.5) | 12 (6 to 17) | 8 (6 to 13) | 8 (3.5 to 14) | 0.55 |
| MCS before to primary transplant (%) | 1 (25.0) | 2 (13.3) | 2 (15.4) | 6 (25.0) | 0.76 |
| ISHLT CAV on last angiogram (%) | 0.5 | ||||
| CAV 0 | 1 (25.0) | 0 (0) | 2 (15.4) | 4 (16.7) | |
| CAV 1 | 0 (0) | 0 (0) | 0 (0) | 2 (8.3) | |
| CAV 2 | 0 (0) | 1 (6.7) | 0 (0) | 2 (8.3) | |
| CAV 3 | 3 (75.0) | 14 (93.3) | 11 (84.6) | 16 (66.7) | |
| PRA > 10% at primary transplant (%) | 2 (50.0) | 2 (13.3) | 3 (23.1) | 2 (8.7) | 0.18 |
| DSA before retransplanta (%) | 3 (75.0) | 3 (25.0) | 3 (23.1) | 1 (4.4) | 0.01 |
| Previous cellular rejection (%) | 2 (50.0) | 11 (73.3) | 11 (84.6) | 16 (66.7) | 0.48 |
| Prior AMR (%) | 4 (100) | 3 (20.0) | 3 (23.1) | 1 (4.2) | 0.0005 |
| BMI at retransplant | 20.0 (18.2 to 23.6) | 26.9 (22.3 to 30.1) | 24.7 (22.7 to 26.6) | 25.8 (23.1 to 31.0) | 0.22 |
| Hyptertension (%) | 1 (25.0) | 6 (40.0) | 6 (46.2) | 10 (41.7) | 0.98 |
| Diabetes (%) | 0 (0) | 2 (13.3) | 3 (23.1) | 6 (25.0) | 0.7 |
| Dyslipidemia (%) | 0 (0) | 4 (26.7) | 3 (23.1) | 11 (45.8) | 0.24 |
| Use of statin (%) | 2 (50.0) | 10 (66.7) | 8 (61.5) | 17 (70.1) | 0.86 |
| Calcineurin inhibitor (%) | 0.04 | ||||
| Tacrolimus | 4 (100) | 9 (60.0) | 6 (46.2) | 7 (29.2) | |
| Cyclosporine | 0 (0) | 6 (40.0) | 7 (53.8) | 17 (70.8) | |
| Anti-metabolite (%) | 0.26 | ||||
| Mycophenolate mofetil | 3 (75.0) | 13 (86.7) | 12 (92.3) | 21 (87.5) | |
| Azathioprine | 1 (25.0) | 2 (13.3) | 0 (0) | 3 (12.5) |
Data expressed as number, number (%) or number (range). AMR, antibody-mediated rejection; BMI, body mass index; DSA, donor-specific antibodies; MCS, mechanical circulatory support; ISHLT, International Society for Heart and Lung Transplantation; PRA, panel-reactive antibodies.
Within 1 year of retransplant (N = 55). DSA information was unavailable for 1 patient
Figure 1. B-cell infiltrates in cardiac allografts and markers of humoral alloimmunity.
(A) Pie charts represent the frequency of B-cell infiltrates of varying grades in cardiac explants with CAV, failed hearts explanted during primary heart transplantation (HF controls), and autopsy controls. Representative images depicting the range of CD20+ B-cell (brown) infiltration observed in each group are shown below the pie charts. (B) Venn diagram representation of the distribution of CAV specimens with or without B cells near the coronary arteries and in the endomyocardium. The “4” outside the diagrams in the lower right corner indicates that 4 samples did not show B-cell infiltrates. (C) Bubble plot representation of the comparison between B-cell infiltrate grades around coronary arteries and in the endomyocardium in paired samples. The size of the bubbles reflects the number of cases (p = 0.002, Fisher’s exact test). (D, E) Frequency of cardiac allograft explants with evidence of B-cell infiltrates obtained from patients with or without circulating DSA (D), or with or without history of AMR (E).
Endocardial biopsies are routinely collected from the interventricular septum as part of post-transplant monitoring. Dense lymphocytic infiltrates are commonly observed in the biopsy tissue, where they are called Quilty lesions. We used the paired tissue fragments obtained from different locales from the 56 CAV explants to compare the level of B-cell infiltration around the epicardial CA and in the interventricular septum myocardium. Forty specimens showed B-cell infiltrates in both locales, whereas 12 cases only showed B cells near the arteries (Figure 1B). Moreover, we found that the extent of B-cell infiltration in or around CA exceeded that seen in the endomyocardium (p = 0.002, Fisher’s exact test; Figure 1C). We next investigated the association between B-cell infiltrates and evidence of humoral alloimmunity, including circulating DSA and previous episodes of AMR in the CAV group. As illustrated in Figure 1D and E, the majority of patients did not have DSA at the time or during the year that preceded retransplantation (45 of 55, with DSA information unavailable for 1 patient), and had not experienced AMR (45 of 56). Consequently, there was no positive correlation between the presence of intragraft B-cell infiltrates and either DSA or history of AMR. However, we observed a negative correlation between B-cell infiltrates and DSA (p = 0.01) or AMR (p = 0.0005; Table 1) in this patient series.
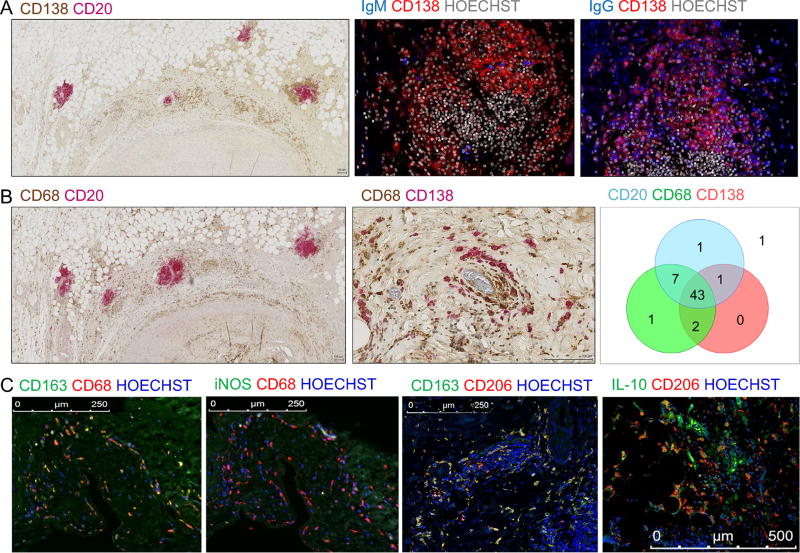
Among all CAV explants, 85% showed the presence of CD138+ plasma cells associated with CD20+ B-cell infiltrates detected around coronary arteries (representative example shown in Figure 2A, left panel). Using IF staining, we further demonstrated the presence of antibody-producing plasma cells in situ. The majority of these plasma cells secreted IgG with only sporadic IgM-secreting cells being detectable in graft infiltrates (Figure 2A, middle and right panels). In addition to CD20+ B cells and fully differentiated CD138+ plasma cells, 95% of CAV explants contained CD68+ macrophages. These were almost invariably in close contact with B cells and plasma cells (Figure 2B, left and middle panels). A summary distribution of CD20+ B cells, plasma cells and macrophages in all CAV explants is reported as a Venn diagram in Figure 2B (right panel). A significant fraction of infiltrating macrophages displayed a CD68+ CD163+ CD206+ M2 phenotype, but lacked the expression of M1 marker iNOS (Figure 2C). Remarkably, a large proportion of these macrophages also produced IL-10 (example shown in Figure 2C, right panel).
Figure 2. Plasma cells, B cells and macrophages in cardiac allografts with CAV.
(A) Left panel: Representative image of a tissue section from a CAV patient stained for CD20+ B cells (pink) and CD138+ plasma cells (brown). Middle and right panels: Immunofluorescence staining of consecutive formalin-fixed paraffin-embedded (FFPE) tissue sections from a representative CAV sample showing plasma cell–rich infiltrates adjacent to the coronary artery of a heart explant with CAV. CD138+ plasma cells (red) co-stained for secreted IgM or IgG (blue). Nuclei are shown in gray. (B) Representative staining of cardiac explant sections with CAV showing CD68+ macrophages (brown) and CD20+ B cells (pink, left panel) or CD138+ plasma cells (pink, middle panel). Right panel: Venn diagram representation of the type of infiltrates observed in the 56 CAV explants: B cell; plasma cell; and macrophage. The number of specimens with evidence of infiltrate is indicated for each cell type. One unique sample had no infiltration. (C) Left and middle left panel: Representative immunofluorescence staining of consecutive FFPE tissue sections of a cardiac explant with CAV for M2 macrophage marker CD163 (left) or M1 marker iNOS (middle left) in combination with CD68. Middle right panel: Immunofluorescence staining of an FFPE tissue section of a cardiac explant with CAV for M2 macrophage markers CD206 and CD163. Right panel: Representative immunofluorescence staining of an FFPE tissue section of a cardiac explant with CAV for IL-10 and M2 macrophage marker CD206.
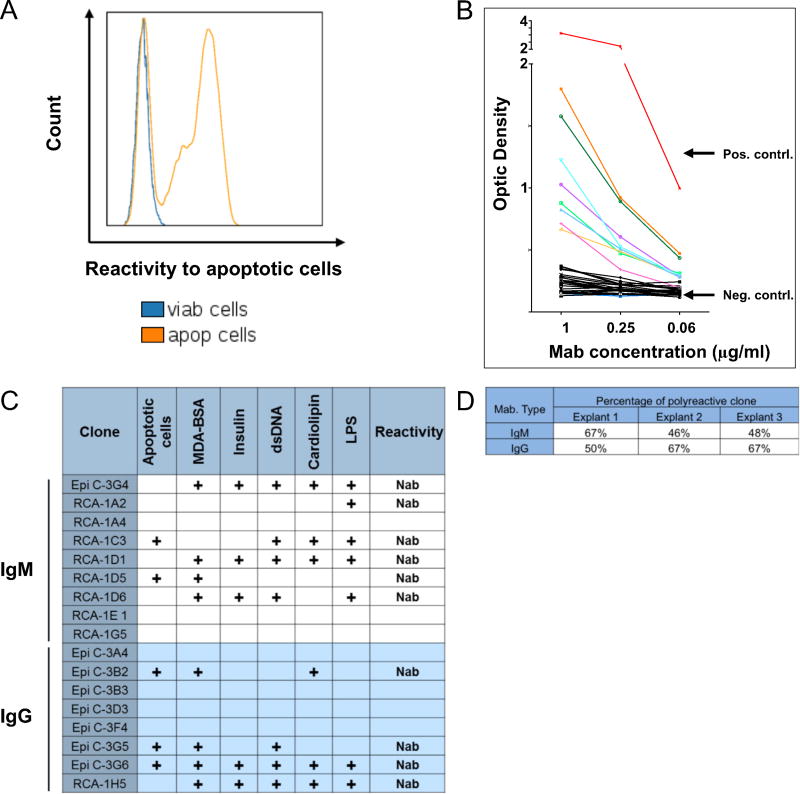
To identify the reactivity profile of graft-infiltrating B cells, we isolated these cells directly from fresh, unfixed tissue collected from 3 explanted allografts. None of the corresponding patients had a history of AMR or circulating DSA. IHC staining revealed B-cell infiltrates with scores of 3, 1 and 3 for explants 1, 2 and 3 respectively. After isolation, infiltrating B cells were immortalized in vitro by infection with EBV, as described elsewhere.7 Different tissue fragments were collected for each allograft specimen, corresponding to distinct locations in the explanted hearts, as described in the Methods section. A total of 17, 34 and 51 clones were generated from CAV explants 1, 2 and 3, respectively. The immortalized clones secreted either IgG or IgM in culture. The reactivity of the secreted monoclonal antibodies (MAbs) to HLA was first assessed by Luminex using HLA-coated beads. None of the immortalized clones’ MAbs reacted to HLA Class I or II (data not shown). We next assessed MAb reactivity to apoptotic cells using flow cytometry. A majority of these antibodies reacted to apoptotic Jurkat cells but not to viable Jurkat cells, as depicted in Figure 3A for a representative clone derived from CAV explant 3. We also tested the reactivity of all antibodies to several self-antigens by ELISA. These auto-antigens included insulin, dsDNA, LPS, cardiolipin and MDA-BSA as a generic oxidized epitope. Figure 3B reports the reactivity of CAV explant 2 IgM MAbs toward insulin as an example. Results showed a predominance of polyreactive clonal immunoglobulins, that is, reactive to multiple self-antigens and/or apoptotic cells. Polyreactivity and reactivity to apoptotic cells or oxidized antigens are characteristics of natural antibodies. The reactivity profiles for all immortalized clones are presented in Figure 3C for CAV explant 1 and in Tables S1 and S2 (refer to Supplementary Material available online at www.jhltonline.org) for CAV explant 2 and CAV explant 3, respectively. A summary of the frequency of natural antibody-producing B-cell clones in the 3 CAV explants is presented in Figure 3D.
Figure 3. Reactivity profiling of cardiac allograft–infiltrating B-cell clones.
(A) Reactivity of a representative monoclonal antibody secreted by a graft-infiltrating B-cell clone derived from CAV explant 3, to viable (blue line) and apoptotic Jurkat cells (orange line). (B) Reactivity of 28 IgM MAbs secreted by graft-infiltrating B-cell clones derived for CAV explant 2 to insulin measured by ELISA. The reactivity of negative and positive control antibodies is shown in red and blue, respectively. (C) Summary of the reactivity profile for all B-cell clones derived from CAV explant 1. (D) Summary of the frequency of natural antibody-producing clones among B cells derived from CAV explants 1, 2 and 3.
Discussion
B-cell infiltrates are frequent in cardiac allografts,13 yet their biologic significance is still uncertain. Herein, we have examined representative infiltrates in tissue specimens collected from 56 cardiac allograft explants with ongoing CAV. We primarily focused our investigations on the location of the B cells in the graft, their relation to previous episodes of AMR and circulating DSA, and, last, their reactivity profiles. Our studies revealed several unrecognized characteristics of infiltrating B cells that have implications for our understanding of their function and possible contribution to graft rejection.
CAV is a complication affecting primarily the coronary arteries. Unfortunately, immune cells recruited in the tissue surrounding the CA cannot be detected through standard post-transplant immunomonitoring. Biopsies routinely used to follow transplant recipients are obtained from the endomyocardium of the right ventricle, away from the epicardial CA affected by CAV. Immune cells are commonly observed in the biopsied myocardial tissue, sometimes organized in structures reported as Quilty lesions.6 However, their correspondence to similar infiltrates in the vicinity of epicardial coronary arteries has not been fully evaluated. Wehner et al5 and Huibers et al4 used tissue fragments from explanted cardiac grafts or autopsy specimens to detect B cells around the CA, but they did not report on infiltrates in the endomyocardium of the same grafts. To compare the breadth of B-cell infiltrates in the 2 locales, we used paired specimens obtained from the same grafts from the epicardium surrounding the CA and the endomyocardial tissue. We have shown that B cells are more frequent near the arteries than in the endocardium. The density of the infiltrates was also more pronounced in the tissue surrounding the CA. These findings suggest a proximal-to-distal stepwise recruitment of lymphocytes, starting around the epicardial CA and progressing toward the ventricular myocardium. Histologic features seen in routine biopsies may therefore not accurately reflect the local immune responses elicited around the vascular targets of CAV. Cases in which infiltrates are restricted to the proximal sites are undetectable using myocardial biopsies. In contrast, our results indicate that cardiac grafts with detectable infiltrates in myocardial biopsies always have similar or even denser infiltrates near the CA. This discrepancy between the 2 locales may in part explain why Quilty lesions have been inconsistently associated with complications and graft failure.14 Because all explanted specimens showed evidence of CAV, we could not assess the presence of B cells in cardiac allografts without this complication. For this reason, we cannot rule out the possibility that B cells gradually infiltrate the tissue surrounding coronary arteries at an early stage, that is, before CAV is clinically detected.
B cells infiltrating cardiac grafts are often assumed to reflect the development of an allospecific adaptive immune response in situ, even though evidence to support this concept is virtually non-existent. The only findings suggesting a link between intragraft B cells and donor-specific antibodies come from a recent report by Huibers et al.15 In their study, antibodies eluted directly from the graft tissue showed some level of reactivity to HLA, including donor antigens. Our own study does not confirm this observation. None of the 102 clones we generated from 3 separate cardiac graft specimens with CAV reacted to HLA molecules as assessed by Luminex. However, a majority of these clones secreted natural antibodies reactive to multiple autoantigens, oxidized epitopes and apoptotic cells. This reactivity profile is characteristic of innate B cells. It remains possible that some infiltrating B-cell clones are specific to donor HLA. However, these do not appear to represent a dominant component within the infiltrates.
Circulating autoantibodies have been reported in cardiac transplant recipients and are often associated with rejection.16,17 Cardiac myosin and vimentin appear to be common targets in these humoral responses.18–22 Our lab has previously observed an association between natural antibodies and kidney graft rejection.12,23 The level of circulating natural antibodies has also been shown to be dramatically increased after ventricular assist device implantation in heart failure patients.11 Studies in mice and humans also reported the association and pathogenic properties of antibodies recognizing LG3, a fragment of perlecan, exposed on apoptotic cells.24 The present study reveals for the first time the prevalence of innate B-cell clones within human cardiac graft infiltrates amidst active CAV. The reason for such enrichment of innate B-cell clones in graft infiltrates compared with the peripheral blood is uncertain. CAV is accompanied by apoptosis of graft endothelial cells that can be detected by the presence of apoptotic bodies or microparticles in the serum.25 Sensing apoptotic cells is an important attribute of innate immunity, including innate B cells. Antigenic structures associated with apoptotic bodies, such as oxidized lipids, or, more generally, the release of damage-associated molecular patterns (DAMPs), may largely contribute to the recruitment of innate B cells and their local retention. It is also plausible that myocardial damage in the donor graft pre-transplant, due to ischemia or other causes, could contribute to the recruitment of the B cells. A larger study would be required to establish this causal link.
In cynomolgus monkeys, anti-CD20 monoclonal antibody was successfully utilized to deplete B cells in recipients of heterotopic heart transplantation and decreased the incidence of DSA formation, as well as the intensity of CAV relative to control groups.26 In humans, several groups, including ours, have reported an association between CAV and AMR.27,28 These studies suggested a causal relation between the 2 complications. More specifically, it is often assumed that CAV develops as a consequence of AMR. In the present study we have shown prominent B-cell infiltration in allografts with active signs of CAV, further supporting the involvement of B-cell immunity. However, the absence of circulating DSA or previous episodes of AMR in the majority of these patients suggests that intragraft B cells exert a function unrelated to allospecific immunity in the context of CAV. CD20+ B cells were almost always observed in association with terminally differentiated CD138+ plasma cells around the epicardial CA. From this observation, 2 main functions can be considered for infiltrating B cells. First, they can serve as antigen-presenting cells (APC) and activate T cells, which are also abundant in graft infiltrates observed around the CA. This function was highlighted in a recent report by Zeng et al, demonstrating the requirement for Class II expression on B cells in CAV progression in a mouse transplantation model.29 B cells were found to provoke CAV by supporting T-cell responses in an antibody-independent fashion, through their role as APC. It is important to note, however, that B cells are allospecific in this model. The prevalence of innate B-cell clones rather than allospecific clones observed through our experiments suggests a different mechanism in human CAV. The polyreactive nature of the B-cell receptor (BCR) expressed by these cells may broaden the range of antigens captured in situ, resulting in local autoimmunity. Second, the presence of plasma cells points to functions mediated by antibodies produced in situ. Their reactivity to apoptotic debris and damaged self may contribute to the build-up of fibrotic tissue through the polarization of neighboring macrophages toward an M2 phenotype and their secretion of IL-10. IL-10 is a well-known anti-inflammatory cytokine. However, prolonged exposure to IL-10 is also implicated in fibrosis, which could be relevant to CAV pathogenesis.30 These 2 distinct functions of intragraft B cells are not mutually exclusive. The importance of innate immunity in mechanisms of acute and chronic rejection of solid-organ grafts is increasingly being recognized. The present study adds another cell type to this complex arm of the immune system. Although innate B cells had been known for years, their implication in allograft rejection had not been reported. Future investigations are now warranted to elucidate their exact function in relation to other immune cells recruited locally in the graft environment.
In brief, our study has revealed the unexpected predominance of innate B-cells within immune infiltrates bordering epicardial CA in human CAV. The reactivity of these cells toward apoptotic cells and damaged self-associated determinants is in sharp contrast to the assumption that B cells recruited in situ are allospecific. These findings cast a new light on the local immune response associated with CAV after heart transplantation. Targeting these cells, irrespective of circulating DSA or biopsy-proven signs of AMR, may impact disease prognosis, especially at an early stage when lesions are still reversible.
Supplementary Material
Acknowledgments
This work was supported by grants from the Roche Organ Transplant Research Foundation (ROTRF), the National Institutes of Health (R01-AI116814, T32-HL-007854-21 to K.J.C., S10RR027050 for flow cytometry instrumentation) and Enduring Hearts (fellowship grant to S.B.S.).
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose.
Supplementary data associated with this article can be found in the online version at www.jhltonline.org.
References
- 1.Arora S, Gullestad L. The challenge of allograft vasculopathy in cardiac transplantation. Curr Opin Organ Transplant. 2014;19:508–14. doi: 10.1097/MOT.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 2.Jansen MA, Otten HG, de Weger RA, et al. Immunological and fibrotic mechanisms in cardiac allograft vasculopathy. Transplantation. 2015;99:2467–75. doi: 10.1097/TP.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 3.Gareau A, Hirsch GM, Lee TD, et al. Contribution of B cells and antibody to cardiac allograft vasculopathy. Transplantation. 2009;88:470–7. doi: 10.1097/TP.0b013e3181b076cc. [DOI] [PubMed] [Google Scholar]
- 4.Huibers MM, Gareau AJ, Vink A, et al. The composition of ectopic lymphoid structures suggests involvement of a local immune response in cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34:734–45. doi: 10.1016/j.healun.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Wehner JR, Fox-Talbot K, Halushka MK, et al. B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation. 2010;89:1141–8. doi: 10.1097/TP.0b013e3181d3f271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Porcheray F, DeVito J, Helou Y, et al. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12:2088–97. doi: 10.1111/j.1600-6143.2012.04053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell MJ, Zelenetz AD, Levy S, et al. Use of family specific leader region primers for PCR amplification of the human heavy chain variable region gene repertoire. Mol Immunol. 1992;29:193–203. doi: 10.1016/0161-5890(92)90100-c. [DOI] [PubMed] [Google Scholar]
- 9.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 10.Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 11.See SB, Clerkin KJ, Kennel PJ, et al. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant. 2017;36:862–70. doi: 10.1016/j.healun.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcheray F, Fraser JW, Gao B, et al. Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant. 2013;13:2590–600. doi: 10.1111/ajt.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin WM, 3rd, Halushka MK, Valujskikh A, et al. B cells in cardiac transplants: from clinical questions to experimental models. Sem Immunol. 2012;24:122–30. doi: 10.1016/j.smim.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marboe CC, Billingham M, Eisen H, et al. Nodular endocardial infiltrates (Quilty lesions) cause significant variability in diagnosis of ISHLT Grade 2 and 3A rejection in cardiac allograft recipients. J Heart Lung Transplant. 2005;24(suppl):S219–26. doi: 10.1016/j.healun.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Huibers MM, Gareau AJ, Beerthuijzen JM, et al. Donor-specific antibodies are produced locally in ectopic lymphoid structures in cardiac allografts. Am J Transplant. 2017;17:246–54. doi: 10.1111/ajt.13969. [DOI] [PubMed] [Google Scholar]
- 16.Bharat A, Mohanakumar T. Immune responses to tissue-restricted nonmajor histocompatibility complex antigens in allograft rejection. J Immunol Res. 2017;2017:6312514. doi: 10.1155/2017/6312514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Win TS, Pettigrew GJ. Humoral autoimmunity and transplant vasculopathy: when allo is not enough. Transplantation. 2010;90:113–20. doi: 10.1097/TP.0b013e3181e25a59. [DOI] [PubMed] [Google Scholar]
- 18.Fedoseyeva EV, Kishimoto K, Rolls HK, et al. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169:1168–74. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 19.Fedoseyeva EV, Zhang F, Orr PL, et al. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–42. [PubMed] [Google Scholar]
- 20.Kalache S, Dinavahi R, Pinney S, et al. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187:1023–30. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Marquez A, Aguilera I, Blanco RM, et al. Positive association of anticytoskeletal endothelial cell antibodies and cardiac allograft rejection. Hum Immunol. 2008;69:143–8. doi: 10.1016/j.humimm.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 23.Gao B, Moore C, Porcheray F, et al. Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant. 2014;14:1581–91. doi: 10.1111/ajt.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardinal H, Dieude M, Brassard N, et al. Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant. 2013;13:861–74. doi: 10.1111/ajt.12168. [DOI] [PubMed] [Google Scholar]
- 25.Singh N, van Craeyveld E, Tjwa M, et al. Circulating apoptotic endothelial cells and apoptotic endothelial microparticles independently predict the presence of cardiac allograft vasculopathy. J Am Coll Cardiol. 2012;60:324–31. doi: 10.1016/j.jacc.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 26.Kelishadi SS, Azimzadeh AM, Zhang T, et al. Preemptive CD20+ B cell depletion attenuates cardiac allograft vasculopathy in cyclosporine-treated monkeys. J Clin Invest. 2010;120:1275–84. doi: 10.1172/JCI41861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clerkin KJ, Restaino SW, Zorn E, et al. The effect of timing and graft dysfunction on survival and cardiac allograft vasculopathy in antibody-mediated rejection. J Heart Lung Transplant. 2016;35:1059–66. doi: 10.1016/j.healun.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loupy A, Cazes A, Guillemain R, et al. Very late heart transplant rejection is associated with microvascular injury, complement deposition and progression to cardiac allograft vasculopathy. Am J Transplant. 2011;11:1478–87. doi: 10.1111/j.1600-6143.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- 29.Zeng Q, Ng YH, Singh T, et al. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest. 2014;124:1052–6. doi: 10.1172/JCI70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017;24:e12305. doi: 10.1111/micc.12305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.