Abstract
To potentially identify proteins that interact (i.e. bind) and may contribute to mediate (−)-epicatechin (Epi) responses in endothelial cells we implemented the following strategy: 1) synthesis of novel Epi derivatives amenable to affinity column use, 2) in silico molecular docking studies of the novel derivatives on G protein-coupled estrogen receptor (GPER), 3) biological assessment of the derivatives on NO production, 4) implementation of an inmobilized Epi derivative affinity column and, 5) affinity column based isolation of Epi interacting proteins from endothelial cell protein extracts. For these purposes, the Epi phenol and C3 hydroxyl groups were chemically modified with propargyl or mesyl groups. Docking studies of the novel Epi derivatives on GPER conformers at 14 ns and 70 ns demostrated favorable thermodynamic interactions reaching the binding site. Cultures of bovine coronary artery endothelial cells (BCAEC) treated with Epi derivatives stimulated NO production via Ser1179 phosphorylation of eNOS, effects that were attenuated by the use of the GPER blocker, G15. Epi derivative affinity columns yielded multiple proteins from BCAEC. Proteins were electrophoretically separated and inmmunoblotting analysis revealed GPER as an Epi derivative binding protein. Altogether, these results validate the proposed strategy to potentially isolate and identify novel Epi receptors that may account for its biological activity.
Graphical Abstract
Flavonoids are an important class of widely distributed natural products that posses a diverse range of biological activities [1, 2]. Accumulating evidence indicates that the consumption of flavanol-rich foods such as those found in cacao-based products, protects against cardiometabolic diseases [3–5]. (−)-Epicatechin (Epi) is the main flavanol present in cacao seeds and its oral intake mimicks the beneficial vascular effects observed after the consumption of cocoa products [6, 7]. A proposed mechanism through which Epi mediates its vascular effects include the stimulation of nitric oxide (NO) production via endothelial NO synthase (eNOS) activation [8]. Evidence indicates that eNOS activation can occur secondary to the stimulation of cell surface receptors including those from the tyrosine kinase and G-protein-coupled receptor (GPCRs) families [9, 10]. Due to the healthy effects trigered by Epi, there is an increasing interest in elucidating the mechanisms by which this flavanol mediates its cardiometabolic protective effects [11, 12].
We recently demonstrated that Epi stimulates NO production through the involvement of the G-protein coupled estrogen (GPER) and epidermal growth factor receptors (EGFR) [13]. However, the use of selective blockers or receptor gene silencing approaches resulted in a partial blockade of Epi stimulated NO production. Thus, other cell membrane receptors are likely involved in mediating the effects of Epi and there is little knowledge about the identity of such structures. Interestingly, several studies have suggested that the biological properties of flavonoids are largely dependent on the availability of “free” phenol groups on their structure (Fig. 1) [14, 15].
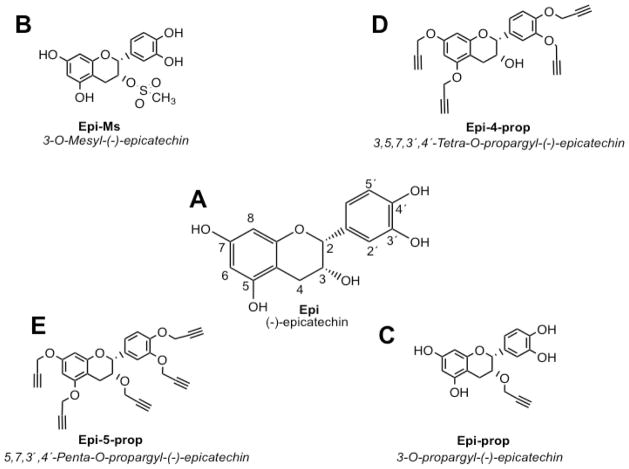
Fig. 1.
Structures of (A) −)-epicatechin (Epi) and its synthetized derivatives; Epi-5-prop (E), Epi-4-prop (D), Epi-Ms (B) and Epi-prop (C).
We thus, implemented a rational strategy comprising the following steps: 1) synthesis of novel Epi derivatives (which relied on the introduction of mesyl or propargyl groups) that may be optimal for the generation of affinity columns and purification of Epi binding proteins, 2) in silico molecular docking of the novel Epi derivatives on a previously validated GPER platform, 3) in vitro analysis of the novel Epi derivatives on NO production and, 4) implementation of an inmobilized Epi derivative affinity column to isolate binding Epi proteins from endothelial cell protein extracts. Flavonoid effects appear to be structure-dependent and a major determinant factor is the presence of hydroxyl (i.e. phenols and alcohol groups) moeities [14, 15]. The esterification and alkylation of the hydroxyl groups are commonly used methods used to generate flavonoid derivatives. Using this strategy, others and we have synthesized novel flavanoid derivatives by targeting their phenol groups [16–19]. In this study, we modified the structure of Epi by targeting its phenol (3′,4′, 5 and 7 position) and alcohol (C-3 position) groups (Fig. 1A). For the synthesis of the derivatives, native Epi was used as a starting material. The detailed synthetic procedures used to obtain each Epi derivative are presented in supplementary data. As a first step, we introduced mesyl or propargyl group subtituents in the Epi molecule at the C-3 alcohol group in order to keep the four phenolic groups available (Fig. 1B and C respectively). We also alkylated the four phenol groups of Epi, and kept free the 3-alcohol group (Fig. 1D). Finally, we alkylated the four phenolic and the alcohol groups of Epi, which led to a molecule with no free hydroxyl groups (Fig. 1E). The resultant Epi derivatives were 3-O-mesyl-(−)-epicatechin (Epi-Ms), 5,7,3,4′-tetra-O-propargyl-(−)-epicatechin (Epi-4-prop), 3,5,7,3′,4′-penta-O-propargyl-(−)-epicatechin (Epi-5-prop), and 3-O-propargyl-(−)-epicatechin (Epi-prop).
On the other hand, to ascertain for their possible bioactivity and coupling to a known receptor, the novel Epi derivatives were evaluated in silico. For this purpose, molecular docking and dynamics studies were implemented as previously described (see supplementary data) [20].
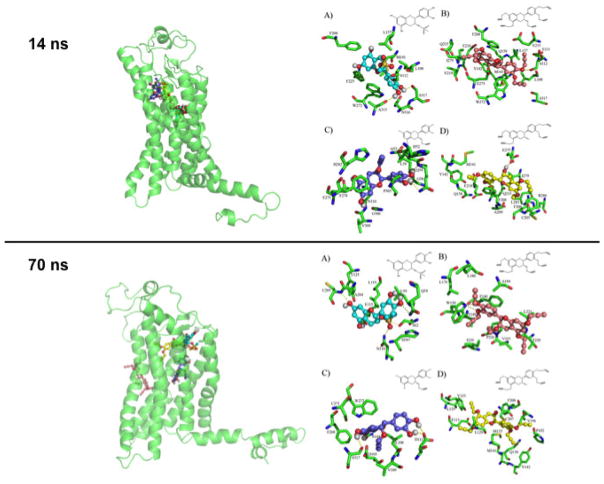
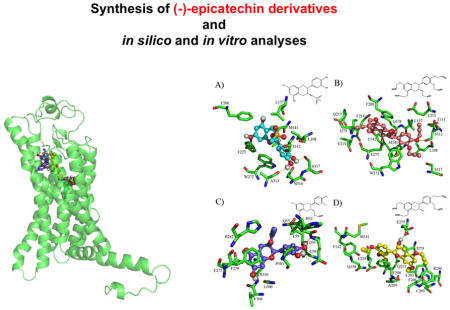
Docking results (Fig. 2) suggest that the interactions between Epi derivatives and GPER are energetically favorable and that the type of interactions generated are via hydrogen and Π–Π bonding. In general, the interactions of Epi derivatives on the GPER conformer at 14 ns are similar to those of Epi. In contrast, Epi derivatives docking results on GPER conformer at 70 ns evidenced different binding modes between Epi derivatives and Epi. In the 14 ns GPER conformer, Epi (ΔG= −7.9 kcal/mol) [13] and Epi-Ms (ΔG=−7.74 kcal/mol) reach some common aminoacid residues L137, M141 by hydrophobic interaction whereas under with F208, F223, W272 there are π-π interactions and with S317 and A313 there are hydrogend bonds, Epi-Ms also interacts with S112 residue via hydrogen bonds.
Fig. 2.
GPER 3D model docked with (−)-epicatechin (Epi) derivatives. GPER 3D model at 14 ns docked with (A) Epi-Ms, (B) Epi-5-prop, (C) Epi-prop and (D) Epi-4-prop (upper panel right). Ligands superimposed into the binding site of 14 ns GPER conformer (upper panel left). GPER 3D model at 70 ns docked with (A) Epi-Ms, (B) Epi-5-prop, (C) Epi-prop and (D) Epi-4-prop (bottom panel right). Ligands superimposed into the binding site of 70 ns GPER conformer (bottom panel left).
Epi-5-prop (ΔG=−9.2 kcal/mol) reaches the aminoacid residues L108 and L137 by hydrophobic interactions and W272, F208 and Y142 by π-π interactions and with S112 and Q138 under hydrogen bonds (Fig. 2 upper panel). Epi-prop shows a ΔG=− 8.05 kcal/mol and makes π-π interactions with F278 and hydrogen bonds with N310. Epi-4-prop (ΔG=−8.68 kcal/mol) reaches the aminoacid residues Q138, E218, Q215, E275 by hydrogen bonds; Y142, F208 and F206 by π-π interactions and; R286 by a π-cation interaction.
In contrast, using GPER conformer at 70 ns, docking analyses demonstrate that Epi derivatives interact with aminoacid residues distinct than those observed with Epi derivatives at 14 ns (Fig. 1 bottom panel). Epi-Ms establishes hydrogend bonds with N310, S62, Q54, E115 and C205 and π-π interactions with Y123. Epi-5-prop makes hydrogen bonds with P226, T220 and W150; π-π interactions with F146, W150 and; hydrophobic interactions with L221, V225, F146 and L176. Epi-prop, establishes hydrogen bonds with S317, D111 and D105; π-π interactions with F268 and W272 and; hydrophobic interactions with L108. Epi-4-prop recognized Q138 and C207 using hydrogen bonds. Additionally, this Epi derivative established π-π interactions with F208 and Y123 as well as hydrophobic interactions with L129, V196 and M133. Using both GPER conformers, docking modeling estimates that Epi-4-prop and Epi-5-prop interact with more aminoacid residues than Epi-prop and Epi. We therefore propose, that the alkyne groups enable additional interactions with aminoacid residues of the GPER binding pocket, which contributes to a higher binding free energy when compared to the natural flavanol.
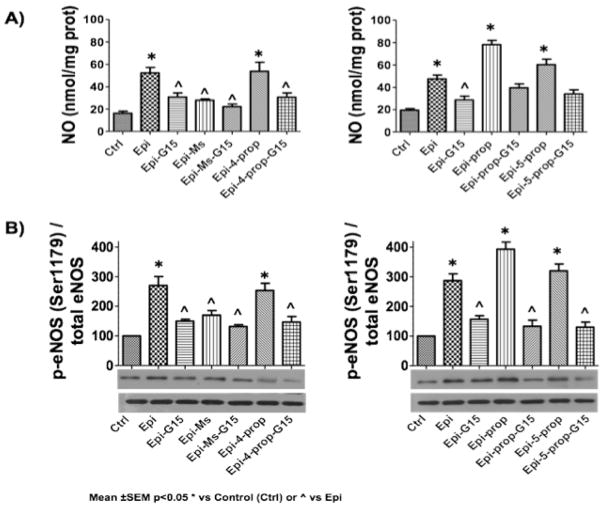
Once the derivatives were synthesized and pre-validated in silico, we tested their in vitro efficacy using the GPER expressing bovine coronary artery endothelial cells (BCAEC). Our primary endpoint was to evaluate the capacity of derivatives to stimulate eNOS/NO pathway activation (Fig. 3). Cells were stimulated for 10 minutes by either 1 μM Epi (used as a positive control) or 1 μM of each Epi derivative. Nitrates/nitrites levels in the supernatant and immunodetection of phosphorylated (at Ser1179 residue) eNOS (p-eNOS by Western blots) were measured as a surrogate of NO synthesis and eNOS activation, respectively (Fig. 3A). To explore the participation of GPER the antagosist G15 was used. Cells were pre-incubated during 30 min with 1 μM of G15, a GPER inhibitor. Results demonstrate that as predicted by in silico modeling, the derivatives were able to stimulate to different degrees NO production (Fig. 3A left and rigth panels) via eNOS activation (Fig. 3B left and rigth panels) and that effects were notably attenuated by the GPER antagonist G15 (Table 1).
Fig. 3.
Effects of (−)-epicatechin (Epi) and Epi derivatives on eNOS/NO pathway activation. (A) Epi derivatives induced nitric oxide (NO) production and (B) endothelial nitric oxide synthase (eNOS) activation (phosphorylation at Ser-1179 [p-eNOS]) in bovine coronary artery endothelial cells (BCAEC). All compounds were tested at 1 μM. Control was arbritarily set to 1 and values are reported as mean ± SEM, p<0.05 * vs control or ^ vs Epi (n=5 per group).
Table 1.
Quantification of the effects of (−)-epicatechin (Epi) and Epi derivatives on eNOS/NO pathway activation in BCAEC.
| Compound | +G15 | +G15 | ||
|---|---|---|---|---|
| NO production (%) | p-eNOS activation (%) | NO production (%)b | p-eNOS activation (%)b | |
|
| ||||
| Epia | 100.0 | 100.0 | 60.0 | 55.6 |
| Epi-Ms | 54.5 | 64.8 | 41.8 | 53.0 |
| Epi-5-prop | 101.8 | 92.6 | 60.0 | 54.8 |
| Epi-4-prop | 107.3 | 111.1 | 63.6 | 57.4 |
| Epi-prop | 141.8 | 137.0 | 70.9 | 59.3 |
Epi levels were arbritarily set to 100% and values of derivatives are adjusted to Epi values and expressed as percentages.
For GPER blocking, cells were pre-incubated with G15 (1 μM) for 30 minutes before the addition of 1 μM of ligands.
Noteworthy, Epi-4-prop, Epi-5-prop induced an effect comparable to Epi, suggesting that the presence of “free” phenolic groups might not be crucial for the stimulation of eNOS/NO pathway[14]. On the other hand, Epi-Ms, which possesses a mesylate group at C-3, stimulated to a lesser extent the eNOS/NO pathway vs. Epi (Fig. 3 and Table 1). Interestingly, although Epi-prop also possesses a subtituent at the C-3 alcohol group, displayed increased efficacy on NO/eNOS activation pathway endpoints when compared to Epi-Ms and Epi. These results suggest that the modification of the C-3 alcohol group in Epi molecule increase the efficacy of the flavanol to stimulate the eNOS pathway. It may be possible that the introduction of the hydrophilic mesylate group in the Epi molecule affects its ability to interact with GPER present on the cell membrane, where it has been localized [13]. Interestingly, Epi-Ms also denoted the lowest free binding energy in docking studies, suggesting a close relationship between our in silico and in vitro data. In addition, all other derivatives showed higher free binding energy values vs. Epi that was also accompanied by higher efficacy on eNOS/NO activation endpoints by derivatives.
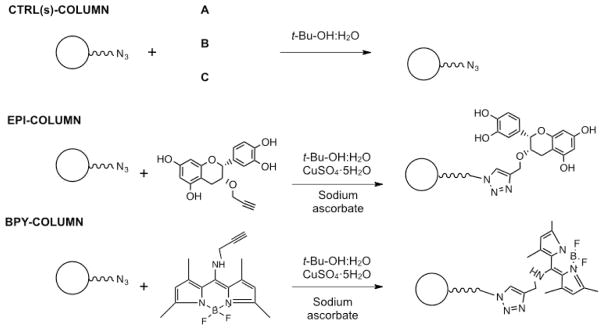
Our in vitro and docking results indicate that GPER is capable of interacting and be activated by Epi and its derivatives. However, other receptors are also likely involved as G15 only partially inhibited eNOS activation and NO production. In order to explore this possibility, we implemented affinity chromatography. This approach is based on the immobilization of the molecule of interest to a solid support as a means to isolate and eventually characterize specific binding proteins including receptors. However, the immobilization of ligands to a solid support may alter the biological properties of the free ligand. Therefore, we implemented an affinity column (Epi-based column) using Epi-prop. The assumption is that this molecule as per its enhanced in vitro bioactivity and free in silico binding energy retains intact, the core bioactive elements of Epi. For this purpose, Epi-prop was covalently immobilized by the use of Click chemistry to commercially available agarose azide beads, which were then packed into a minicolumn [21, 22] (see supplementary data). To achieve this goal, we took advantage of the propargyl substituent at the C-3 alcohol group in Epi-prop that reacts selectivively with organic azides groups such as those present on agarose azide beads, leaving free the Epi phenol groups. The specific method used for the synthesis of the affinity columns are described in detail in supplementary data. As controls, we generated three different agarose columns, A) a column where all reactants except copper (II) sulfate pentahydrate were added, B) a column where all reactants except sodium ascorbate were added and, C) a column where all reactants except Epi-prop were ommited in the reaction (Fig. 4). Reagents on the column surfaces were removed by exhaustive washing using EDTA. These columns were used to identify possible nonspecific protein binding to the matrix. The experimental column was obtained adding all the reactants to the agarose beads. The binding of Epi-prop to the agarose-azide beads was determined indirectly by fluorescence and quantification of Epi-prop recovered after column washings. An external calibration curve using known concentration solutions of Epi-prop served to extrapolate the fluorescence levels measured in the column washings. Results indicated that 86.6% of added Epi-prop to the agarose-azide beads was attached (data not shown).
Fig. 4.
Scheme depicting the generation of the affinity columns by the use of Click chemistry. Three control columns (CTRL(s)-COLUMN) were fabricated by the addition of all components except (A) Epi-prop, (B) copper(II) sulfate pentahydrate and sodium ascobate and, (C) Epi-prop, copper(II) sulfate pentahydrate and sodium ascorbate.
Moreover, to test the feasibility of the Click chemistry reaction, agarose-azide beads were treated with an alkyne-fluorescein (excitation/emission peak at 400/460 nm, [BPY]) reactive under the same conditions employed in the reaction with Epi-prop. The BPY was successfully attached to the agarose beads (~80%), as indicated by the fluorescence levels noted under ultraviolet light (Fig. 4) and the fluorescent quantification levels (using an external calibration cruve) of BPY recovered in continuous washings (data not shown).
Once implemented, the ability of the affinity Epi-column to isolate protein targets was evaluated using protein extracts from BCAEC. Proteins (0.8–1.0 mg) were loaded into control columns and Epi-Column (incubated overnight at 4 °C with mild tumbling) and columns were then washed thoroughly with at least 5 volumes of wash buffer (5 mM EDTA, 0.5% SDS, 250 mM NaCl in 1X PBS pH 7.5), 1X PBS (20% in wash buffer) and 1X PBS, or until no detectable protein was found in the eludates. Attached proteins were eluted using PBS (1X) buffer containing 8 M urea. Eludates containing isolated proteins were concentrated using centrifugal filters (Amicon Ultra 10K), and samples were separated using SDS-PAGE gel electrophoresis and proteins stained using Coomassie-Blue. Consistent bands were evidenced in the elution fractions of Epi-column (data not shown). The control columns eludates were analyzed under the same conditions in parallel. However, no bands were observed when stained with Coomassie-Blue (data not shown.) suggesting specific binding to the experimental Epi-column.
SDS-PAGE proteins derived from Epi-column were transferred onto PVDF membranes. Western blot analysis revealed the presence of a 42-kDa protein that was reactive to a GPER antibody (Fig. 5). These findings are in agreement with our previous published data using BCAEC to determine the role of GPER in mediating the effects of Epi on eNOS activation [13]. In such study, using siRNA and chemical blockers approaches, we validated GPER as a likely receptor candidate for Epi in endothelial cells.
Fig. 5.
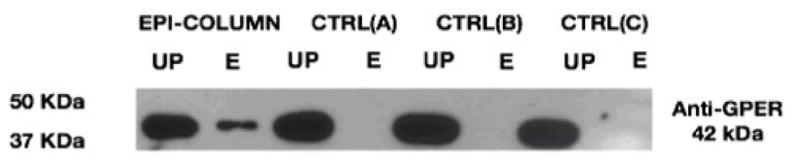
Identification of GPER in isolated proteins from the affinity chromatography columns. Lysates from endothelial cells were loaded into the (−)-epicatechin (Epi) affinity column (EPI-COLUMN) and control columns (CTRL(A), CTRL(B) and CTRL(C)). Western blot analysis using a monoclonal GPER antibody on proteins separated by affinity columns. UP: Unbound proteins fraction, E: Elution fraction.
In summary, four new Epi derivatives were synthesized by modifying Epi at either its phenol or C-3 alcohol groups in order to assess the potential contribution of such positions on Epi biological effects. Our limited SAR data suggest that propargyl substitutions at the 3,5,7,3′,4′ phenols or C-3 alcohol positions on Epi molecule do not impair its capacity to stimulate eNOS. In fact, it appears that substitutions with hydrophilic groups (i.e. mesylate) at the C-3 alcohol in the Epi molecule reduces its in vitro efficacy, which may be secondary to an increase in polarity that could affect its ability to interact with cell membrane targets. Also, we demonstrate that the Epi-prop derivative possesses a favorable thermodynamic interaction using a tridimensional model of GPER that is validated in vitro by noting an improved efficacy on eNOS/NO activation vs. Epi. The chemical blockade of GPER and affinity chromatography columns with immobilized Epi-prop confirmed the role of this receptor in mediating the effects of Epi.
Based on our in vitro results where the antagonist G15 only partially blocks the responses and on the presence of several proteins interacting with the Epi-column, the posibility of more receptors or effectors participating on Epi-induced effects is highly likely. More work is necessary to identify and characterize these molecules.
Supplementary Material
Acknowledgments
V. Sarmiento acknowledges support from CONACyT in the form of graduate scholarships. Dr. Villarreal was supported by NIH DK98717 and Dr. Ceballos by a Conacyt # 253769 grant. We thank CONACyT for ITT NMR and HRMS facilities (Grants INFR-2011-3-173395 and INFR- 2012-01-187686).
We would like to thank Dr. IA Rivera for the generous donation of the Alkyne-fluorescein (BPY) and Dr. A Ochoa for allowing to use his lab during this research both from Centro de Graduados e Investigación en Química del Instituto Tecnológico de Tijuana.
Abbreviations
- Epi
(−)-Epicatechin
- eNOS
endothelial nitric oxide synthase
- NO
nitric oxide
- GPER
G-protein coupled estrogen receptor
- BCAEC
bovine coronary artery endothelial cells
- GPCRs
G-protein coupled receptors
- EPI-COLUMN
affinity column with Epi covalently bound
- Epi-4-prop
3,5,7,3′,4′-Penta-O-propargyl-(−)-epicatechin
- Epi-Ms
3-O-Mesyl-(−)-epicatechin
- Epi-5-prop
5,7,3′,4′-Tetra-O-propargyl-(−)-epicatechin
- Epi-prop
3-O-propargyl-(−)-epicatechin
- G15
GPER antagonist
- BPY
5,5-difluoro-1,3,7,9-tetramethyl-N-(prop-2-yn-1-yl)-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1′-f][1,3,2]diazaborinin-10-amine
- SAR
structure-activity relationship
Footnotes
Disclosures
Dr. Villarreal is a co-founder and stockholder of Cardero Therapeutics Inc. and Dr. Ceballos is a stockholder.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arts ICW, van de Putte B, Hollman PCH. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. Journal of Agricultural and Food Chemistry. 2000;48(5):1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 2.Pan M-H, Lai C-S, Ho C-T. Anti-inflammatory activity of natural dietary flavonoids. Food & function. 2010;1(1):15–31. doi: 10.1039/c0fo00103a. [DOI] [PubMed] [Google Scholar]
- 3.Buijsse B, et al. Cocoa intake, blood pressure, and cardiovascular mortality: The zutphen elderly study. Archives of Internal Medicine. 2006;166(4):411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 4.Buijsse B, et al. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. European Heart Journal. 2010;31(13):1616–1623. doi: 10.1093/eurheartj/ehq068. [DOI] [PubMed] [Google Scholar]
- 5.Mostofsky E, et al. <span hwp:id=“article-title-1” class=“article-title”>Chocolate Intake and Incidence of Heart Failure</span><span hwp:id=“article-title-42” class=“sub-article-title”>Clinical Perspective</span>. A Population-Based Prospective Study of Middle-Aged and Elderly Women. 2010;3(5):612–616. doi: 10.1161/CIRCHEARTFAILURE.110.944025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeter H, et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(4):1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottaviani JI, et al. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Radical Biology and Medicine. 2011;50(2):237–244. doi: 10.1016/j.freeradbiomed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-Sanchez I, et al. (−)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55(6):1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dudzinski DM, Michel T. Life history of eNOS: Partners and pathways. Cardiovascular Research. 2007;75(2):247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panneerselvam M, et al. Dark chocolate receptors: epicatechin-induced cardiac protection is dependent on δ-opioid receptor stimulation. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299(5):H1604–H1609. doi: 10.1152/ajpheart.00073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreno-Ulloa A, et al. Cell membrane mediated (−)-epicatechin effects on upstream endothelial cell signaling: evidence for a surface receptor. Bioorganic & medicinal chemistry letters. 2014;24(12):2749–2752. doi: 10.1016/j.bmcl.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Ulloa A, et al. The effects of (−)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER) Pharmacological research. 2015;100:309–320. doi: 10.1016/j.phrs.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaya J, et al. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry. 2003;62(1):89–99. doi: 10.1016/s0031-9422(02)00445-4. [DOI] [PubMed] [Google Scholar]
- 15.Garg R, Kurup A, Hansch C. Comparative QSAR: On the Toxicology of the Phenolic OH Moiety. Critical Reviews in Toxicology. 2001;31(2):223–245. doi: 10.1080/20014091111686. [DOI] [PubMed] [Google Scholar]
- 16.SPENCER JP, et al. Epicatechin and its in vivo metabolite. 3′-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochemical Journal. 2001;354(3):493–500. doi: 10.1042/0264-6021:3540493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uesato S, et al. Inhibitory effects of 3-O-acyl-(+)-catechins on Epstein-Barr virus activation. Chemical and pharmaceutical bulletin. 2003;51(12):1448–1450. doi: 10.1248/cpb.51.1448. [DOI] [PubMed] [Google Scholar]
- 18.Park KD, et al. Anticancer activity of 3-O-acyl and alkyl-(−)-epicatechin derivatives. Bioorganic & medicinal chemistry letters. 2004;14(20):5189–5192. doi: 10.1016/j.bmcl.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Ulloa A, et al. Effects of (−)-epicatechin and derivatives on nitric oxide mediated induction of mitochondrial proteins. Bioorganic & medicinal chemistry letters. 2013;23(15):4441–4446. doi: 10.1016/j.bmcl.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méndez-Luna D, et al. Deciphering the GPER/GPR30-agonist and antagonists interactions using molecular modeling studies, molecular dynamics, and docking simulations. Journal of Biomolecular Structure and Dynamics. 2015;33(10):2161–2172. doi: 10.1080/07391102.2014.994102. [DOI] [PubMed] [Google Scholar]
- 21.Nwe K, Brechbiel MW. Growing applications of “click chemistry” for bioconjugation in contemporary biomedical research. Cancer Biotherapy and Radiopharmaceuticals. 2009;24(3):289–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anseth KS, Klok HA. Click Chemistry in Biomaterials, Nanomedicine, and Drug Delivery. Biomacromolecules. 2016;17(1):1–3. doi: 10.1021/acs.biomac.5b01660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.