Abstract
Objective
Attentional bias (AB) may be one mechanism contributing to the development and/or maintenance of disordered eating. AB has traditionally been measured using reaction time in response to a stimulus. Novel methods for AB measurement include eye tracking to measure visual fixation on a stimulus, and electroencephalography to measure brain activation in response to a stimulus. This systematic review summarizes, critiques, and integrates data on AB gathered using the above-mentioned methods in those with binge eating behaviors, including binge eating, loss of control eating, and bulimia nervosa.
Method
Literature searches on PubMed and PsycInfo were conducted using combinations of terms related to binge eating and biobehavioral AB paradigms. Studies using AB paradigms with three categories of stimuli were included: food, weight/shape, and threat. For studies reporting means and standard deviations of group bias scores, Hedges’ g effect sizes for group differences in AB were calculated.
Results
Fifty articles met inclusion criteria and were reviewed. Individuals who binge eat in the absence of compensatory behaviors show an increased AB to food cues, but few studies have examined such individuals’ AB toward weight/shape and threatening stimuli. Individuals with bulimia nervosa consistently show an increased AB to shape/weight cues and socially threatening stimuli, but findings for AB to food cues are mixed.
Discussion
While there are important research gaps, preliminary evidence suggests that the combination of AB to disorder-specific cues (i.e., food and weight/shape) and AB toward threat may be a potent contributor to binge eating. This conclusion underscores previous findings on the interaction between negative affect and AB to disorder-specific cues. Recommendations for future research are provided.
Keywords: binge eating, attentional bias, food, weight, shape, social threat
Binge eating is the hallmark behavior of binge-eating disorder (BED), bulimia nervosa (BN), and the binge-purge subtype of anorexia nervosa (AN-B/P; American Psychiatric Association, 2013). Even among individuals who do not meet diagnostic criteria for an eating disorder, the presence of binge and loss of control (LOC) eating confers risk for elevated eating disorder psychopathology, psychosocial distress, excess body weight, and clinical impairment (Tanofsky-Kraff, Yanovski, & Yanovski, 2011; Vannucci et al., 2013). Therefore, it is important to identify mechanisms that contribute to the etiology and/or maintenance of binge eating behaviors so that potentially modifiable risk factors can be identified for targeted interventions.
Attentional bias (AB) refers to the differential allocation of attention to particular stimuli and represents one facet of selective attention (Cisler & Koster, 2010). Maladaptive AB has been theorized to contribute to psychopathology (M. Field & Cox, 2008; Williams, Mathews, & MacLeod, 1996) and psychological problems such as eating disorders (Aspen, Darcy, & Lock, 2013), anxiety (Cisler & Koster, 2010), and substance abuse (M. Field & Cox, 2008; Franken, 2003). It is currently unknown if AB is triggered by an automatic response to the stimulus category (e.g., high-calorie food in general), or whether AB is evident when content directly related to participant’s fears, such as specific high-calorie foods, is presented (Pergamin-Hight, Naim, Bakermans-Kranenburg, van IJzendoorn, & Bar-Haim, 2015). Systematic reviews and meta-analyses have summarized the existing data concerning associations between eating disorders and biases in attention and information processing (Dobson & Dozois, 2004; Giel et al., 2011). Prior reviews have focused primarily on AB to food and body weight/shape in samples with full-syndrome BN and AN-B/P, and there is limited summative information on AB in binge/LOC eating or BED (Dobson & Dozois, 2004; Giel et al., 2011). While AB may be an implicit manifestation of risk factors for binge/LOC eating across the binge eating spectrum, such as preoccupation with food, eating, and weight/shape (A.E. Field et al., 2008; Stice, Presnell, & Spangler, 2002), it is conceivable that risk factors that are less content-specific but linked to LOC also manifest as AB. These include rumination about psychosocial stressors (Nolen-Hoeksema, Stice, Wade, & Bohon, 2007) and intolerance of aversive affective states such as anxiety (Werthmann, Jansen, & Roefs, 2015). However, previous reviews have not examined AB to anxiety-inducing stimuli (e.g., threat cues) among those with binge eating behaviors, and to date no existing AB review has encompassed the full spectrum of binge eating disturbances.
Attentional Bias Components
A number of terms are used to describe the AB components, given that AB involves distinct but related aspects of selective attention (Cisler & Koster, 2010; Ouimet, Gawronski, & Dozois, 2009). Facilitated attentional engagement is the speed with which attention is drawn towards a salient stimulus (e.g., preferred food) relative to non-salient stimuli. Difficulty with attentional disengagement is the degree to which a salient stimulus captures attention and impairs the shifting of attention away from that stimulus. By contrast, attentional avoidance is the preferential allocation of attention away from salient stimuli (Koster, Crombez, Verschuere, Van Damme, & Wiersema, 2006).
It has also been proposed that AB be conceptualized according to information processing theory stage (Shiffrin & Schneider, 1977). Specifically, automatic “bottom-up” cognitive processing is stimulus-driven and occurs without purpose or awareness, while strategic “top-down” cognitive processing is goal-oriented and dependent on conscious awareness (Cisler & Koster, 2010; Ouimet et al., 2009). Combining the two models, “bottom up” attentional engagement involves automatic and/or strategic processing stages, depending on the duration that attention is sustained (Ouimet et al., 2009). Disengagement and avoidance appear to be mediated primarily by strategic “top down” processing mechanisms (Cisler & Koster, 2010).
Attentional Bias Measurement and Paradigms
Attention can be measured via reaction time to a stimulus, visual fixation on a stimulus, or brain activation. There are several paradigms that measure reaction time to examine AB. The procedures of four AB paradigms originally developed for measuring AB using reaction time (emotional Stroop, visual probe task, spatial cueing, and visual search paradigm) are included in this review (see Table 1 for details). Emotional Stroop involves identifying the ink color of written words that are deemed either neutral or salient (Stroop, 1935; Williams et al., 1996). Slower reaction time indicates AB to disorder-salient stimuli relative to neutral stimuli. Emotional Stroop is designed to measure exclusively facilitated attention engagement. In the visual probe task, individuals react to a probe that replaces either a neutral or a salient stimulus. Faster reaction time during the trials in which the probe replaces the disorder-salient stimuli indicates AB to these stimuli relative to the neutral stimuli (M. Field & Cox, 2008; MacLeod, 1986). The visual probe task can measure facilitated attention engagement, difficulties with attention disengagement, and if administered at longer durations, attentional avoidance. In a spatial cueing task, individuals press a key corresponding to the location of a probe following the cue (Fox, Russo, Bowles, & Dutton, 2001; Posner, 1980). Faster reaction time on trials for which the disorder-salient cue predicts the location of the subsequent probe indicates greater facilitated attention engagement, compared to the neutral cue trials. Slower reaction time on trials for which the disorder-salient cue does not predict the location of the probe indicates difficulty with attention disengagement, relative to the neutral cue trials. The visual search task involves detecting an odd-one-out word in a matrix of either majority neutral or majority disorder-salient words, or detecting that all words are in the same category (Cisler, Bacon, & Williams, 2009; Smeets, Roefs, van Furth, & Jansen, 2008). Faster reaction time to detect salient target words indicates facilitated attention engagement relative to detecting that all words are neutral. Slower reaction time to detect neutral target word among disorder-salient words indicates attention disengagement difficulties relative to detecting that all words are neutral.
Table 1.
Attentional bias paradigms based on reaction time.
| Paradigm | Description | Outcome Measure | Attention Components Measured |
|---|---|---|---|
| Emotional Stroop (Ouimet et al., 2009; Stroop, 1935; Williams et al., 1996) | Participants are instructed to name aloud the ink color of written words that are deemed either neutral or salient. Cognitive interference means taking longer to name the ink color of disorder-salient words (e.g., “thighs”) than neutral words (e.g., “tower”). Disorder-salient content is assumed to serve as a distractor, limiting attention resources for the more deliberate task of naming the ink color of these words. | Reaction time Slower reaction time indicates AB to disorder-salient stimuli relative to neutral stimuli |
Facilitated attentional engagement |
| Visual Probe Task (M. Field & Cox, 2008; MacLeod, 1986; Ouimet et al., 2009) |
A fixation cross is presented to center participant’s gaze and then followed by paired images, typically one salient (e.g., pizza) and one neutral (e.g., clock). Immediately following the stimulus pair presentation, a probe appears on either the left or right region of the screen and participants must respond as quickly as possible. | Reaction time Faster reaction time during the trials in which the probe replaces the salient stimuli indicates AB to disorder-salient stimuli relative to neutral stimuli |
Facilitated attentional engagement at briefer durations (≤200 ms) Difficulties with attentional disengagement at intermediate durations (~500 ms) Attentional avoidance at longer durations (≥1000 ms), if measured |
| Spatial Cueing Task (Cisler et al., 2009; Fox et al., 2001; Posner, 1980) |
Participants fixate on a central point on the screen, then on one region of the screen where a directional cue is presented. Participants press a key corresponding to the location of a probe that appears following the cue. In the valid trials, the directional cue predicts the location of the subsequent probe. In the invalid trials, the directional cue does not accurately predict the location of the probe and thus draws attention away from it. | Reaction time Faster reaction time on valid salient cue trials indicates AB (facilitated attentional engagement) relative to valid neutral cue trials Slower reaction time on invalid salient cue trials indicates AB (difficulty with attentional disengagement) relative to invalid neutral cue trials |
Facilitated attention engagement: difference between salient and neutral cue reaction times on valid trials Difficulties with attentional disengagement: difference between salient and neutral cue reaction times on invalid trials |
| Visual Search (Cisler et al., 2009; Smeets et al., 2008) |
Participants are presented with a word matrix. Matrices may contain 20 words in the same category, 19 neutral words, and one salient “target” word, or 19 salient words and one neutral “target” word. Participants press a key to indicate whether the matrix contains an odd-one-out word or is entirely comprised of words within the same category. | Reaction time Faster reaction time to detect salient target words indicates AB (facilitated attentional engagement) relative to detecting that all words are neutral Slower reaction times to detect neutral target words within matrices of salient words indicate AB (attentional disengagement difficulties) relative to detecting that all words are neutral |
Facilitated attention engagement: faster detecting of salient words relative to neutral matrices Difficulties with attentional disengagement: slower detecting of neutral words in matrices with salient words |
Note: AB = attentional bias.
Two novel AB measurement methods that are not based on reaction time, eye tracking and electroencephalography (EEG), are also included in this review (see Table 2 for details). Eye tracking offers a direct, continuous measurement of AB (Werthmann et al., 2011). Facilitated attention engagement can be measured either through saccade latency (i.e., the time between target presentation and initial gaze shift) or initial gaze direction bias (i.e., the proportion of trials for which the initial gaze was oriented towards salient stimuli versus neutral stimuli). Difficulties with attention disengagement are indicated by overall gaze duration bias; that is, the difference in total dwell time on salient relative to neutral stimuli. EEG measures brain activity in response to stimuli exposure using event-related potentials (ERP) recorded from electrodes placed on the scalp (Blechert, Feige, Joos, Zeeck, & Tuschen-Caffier, 2011; Hajcak, MacNamara, & Olvet, 2010). Larger ERP amplitudes correspond to greater AB (Hajcak et al., 2010; Schupp, Flaisch, Stockburger, & Junghöfer, 2006). Positive and negative deflections at early (150 – 400 ms) and mid-ranges (400 – 800 ms) post stimulus presentation reflect attention engagement and disengagement processes. The positive slow wave ERP occurs from 1000–6000 ms, tapping into disengagement difficulties and avoidance (Schupp et al., 2006). Eye tracking and EEG allow people to view stimuli without interruption or time limitation, as is the case with reaction time tasks.
Table 2.
Novel attentional bias measurement methods.
| Method | Outcome Measure | Attention Components Measured |
|---|---|---|
| Eye Tracking (Werthmann et al., 2011) |
Saccade latency: the time between target presentation and initial gaze shift Initial gaze direction bias: the proportion of trials in which the initial gaze was oriented towards salient stimuli versus neutral stimuli Overall gaze duration bias: the difference in total dwell time on salient versus neutral stimuli |
Facilitated attentional engagement: measured via saccade latency and initial gaze direction bias Difficulty with attentional disengagement and attentional avoidance: measured via overall gaze duration bias |
| Electroencephalography (EEG) (Blechert et al., 2011; Hajcak et al., 2010) |
Event-related potentials (ERP): measures of brain activity in response to stimuli exposure, as recorded from electrodes placed on the scalp by EEG Early posterior negativity (EPP): ERP with a negative deflection that occurs approximately150–300 ms following stimulus presentation P300: ERP with a positive deflection which reflects brain activity approximately 300–400 ms following stimulus presentation Late positive potential (LPP): ERP that occurs approximately 400–800 ms after stimulus presentation Positive slow wave potential (PSW): ERP that occurs approximately 1000–6000 ms after stimulus presentation |
Larger ERP amplitudes correspond to greater AB Facilitated attentional engagement and difficulties with attentional disengagement: early posterior negativity (e.g., N2), P300, and late positive potential (LPP) Difficulties with attentional disengagement and attentional avoidance: late positive potential (LPP), positive slow wave potential (PSW) |
Note: AB = attentional bias; ERP = event-related potentials.
Theory and Current Study
Several theoretical models have been proposed to explain the role of AB within current conceptualizations of eating disorders (Williamson, White, York-Crowe, & Stewart, 2004). Although there are subtle differences across models (Williamson et al., 2004), all propose that disorder-salient stimuli (e.g., images of thin models for individuals with BN) elicit AB toward these cues, precipitating increased negative affect. Similarly, the interpersonal theory posits that individuals engage in binge eating behaviors to cope with interpersonal distress (Rieger et al., 2010), emphasizing the potential importance of social evaluative threats in binge/LOC eating. Escape theory (Heatherton & Baumeister, 1991; Williamson et al., 2004) proposes urges to avoid or escape aversive negative affect trigger binge eating. Alternative AB models of binge eating behaviors focus on the role of appetitive motivation and incentive salience mechanisms (Berridge, 2009). These models suggest that conditioned palatable food cues elicit AB toward those stimuli, resulting in exacerbated cravings and a compulsive motivation to eat, resulting in binge eating episodes as individuals seek the anticipated food reward and alleviation of cravings. However, no review has critically evaluated the existing literature across binge eating behaviors, potentially informing these theoretical models.
Therefore, this systematic review will provide a summary of the available empirical evidence examining the associations between AB and binge eating behaviors, including binge eating, loss of control eating, and bulimia nervosa. The mechanisms that underlie ABs will be examined by considering differences in stimuli, samples, and methodologies across studies, as well as the components of selective attention. Effect sizes are reported for the available studies. A meta-analysis is not included because, with the exception of the Stroop task, which has been analyzed using meta-analytic reviews in the past (Dobson & Dozois, 2004), the other paradigms in this review have been used in a relatively small number of studies and with significant methodological differences. Research recommendations are offered and implications of the findings are discussed in the context of prevention and treatment approaches.
Methods
Literature Search and Study Selection
Literature searches on PubMed and PsychInfo were conducted using the following terms: binge eating, bulimia nervosa, anorexia nervosa, binge-purge, eating disorder*, emotional eating, disinhibited eating, attention* bias, cognitive bias, selective attention, Stroop, dot probe, visual probe, eye movement*, eye tracking, visual search, event-related potential, food*, body*, *weight, shape, and threat*. (The asterisk is a wildcard boolean search operator; if the asterisk follows the word, it will search any term that begins with the root of the word truncated by the asterisk. If the asterisk precedes the word, it will search any term that ends with the word following the asterisk.) The searches were performed in July 2017. Articles were evaluated for inclusion/exclusion criteria by the co-first author (LS). The first author conferred with co-first author (MS) regarding any articles for which inclusion was unclear. Additionally, cross-references from eligible studies and previous review articles were evaluated for potential inclusion. Studies had to be published in English in peer-reviewed journals, include samples that reported binge eating behaviors, and used an AB paradigm of interest. All included studies evaluated standardized visual AB stimuli that have been purported as salient to binge eating behaviors, specifically cues related to food, body weight/shape, and social evaluation (Aspen et al., 2013; Brooks, Prince, Stahl, Campbell, & Treasure, 2011).
Data Abstraction and Analysis
The following information was extracted from each included study: demographics [sex, age, and body mass index (BMI)] for the overall sample and comparison groups, qualitative and quantitative characteristics of the AB paradigm employed, example stimuli within each stimulus category, AB task performance by group, and correlations between AB task performance and measures of binge eating behavior. If a study included samples unrelated to this review (e.g., participants with AN), only data pertinent to binge eating were included.
To calculate effect sizes for differences between comparison groups, the mean and standard deviation of the bias score was extracted for each group. If scores were not available, the authors of the papers were contacted for additional information. When possible, Hedges’ g was calculated for each finding. Following previous reviews (e.g., Hofmann, Sawyer, Witt, & Oh, 2010), effect sizes were interpreted using Cohen’s (1988) guidelines of small (0.2), medium (0.5), and large (0.8).
Results
Study Characteristics
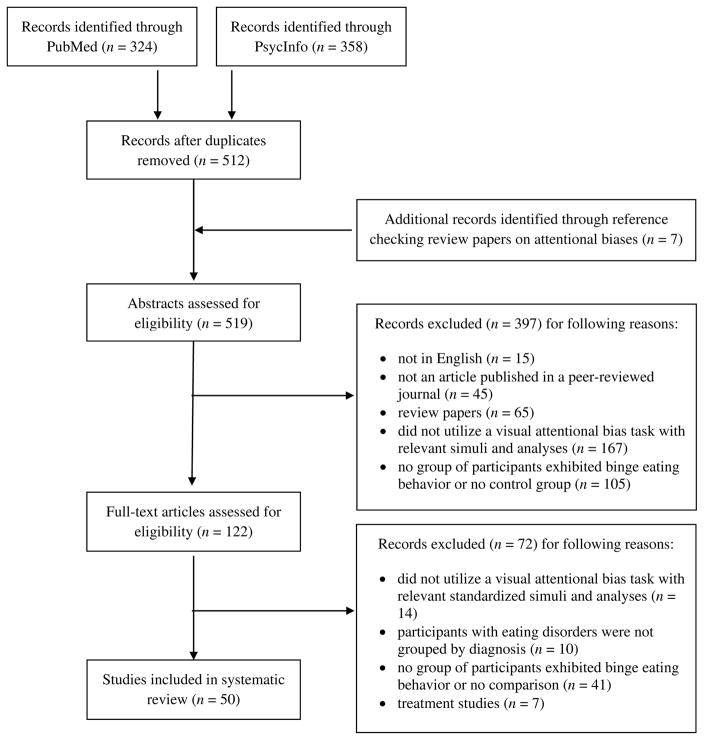
From the 519 articles identified, 50 articles met inclusion criteria (Figure 1). Studies were excluded if they 1) were not in English (n=15); 2) were not an article published in a peer-reviewed journal (n=45); 3) were review papers (n=65); 4) did not utilize a visual AB task with relevant stimuli and analyses (n=181); 5) did not include at least one group of participants exhibiting binge eating behaviors and a comparison group or analysis (n=146); 6) did not separate participants with eating disorders into distinct groups by binge eating behavior (n =10); or 7) did not report data on group differences or correlations in the AB paradigms in a non-treatment condition (n=7). In studies that included individuals with AN, findings were only considered if they pertained to individuals with the binge-purge subtype.
Figure 1.
Study Inclusion Flow. Date last searched: July 9, 2017.
All included studies had a cross-sectional design. Most studies included clinical samples of BN (n=30). The rest included participants diagnosed with BED, AN-B/P, or individuals with sub-threshold binge eating behavior. Studies measured AB using the emotional Stroop (n=28), visual probe task (n=3), spatial cueing task (n=3), visual search paradigm (n=3), eye tracking (n=8), and electroencephalography (n=6).
Emotional Stroop
The Stroop was the most commonly used AB paradigm (Table 3). Of the 28 studies, 18 used food stimuli, 15 weight/shape stimuli, and eight threat stimuli. Additionally, five used stimuli across a range of BN disorder-related words (e.g., weight/shape and food cues), without distinguishing specific categories. All studies examined female participants.
Table 3.
Attentional biases as measured by the Emotional Stroop.
| Study | Sample | Groups | Paradigm | Findings |
|---|---|---|---|---|
| Albery et al. (2016) | 88 adults from the United Kingdom 100% female Mage = 30.4 y† |
BN (n = 45; Age = 28.9±10.2)† HC (n = 43; Age = 31.9±10.6)† |
Stimuli
Instructions: respond to the color of the word (red, blue, yellow, green) by pressing the appropriately colored key on a keyboard |
Interference for food (vs. neutral) words
|
| Ben-Tovim and Walker (1991) | 93 adults and adolescents from Australia 100% Female Mage = 17.6 y MBMI = 21.4 kg/m2 |
BN (n = 27; Age = 26.8±9.1 y; BMI = 23.3±4.6 kg/m2) HC High-DFT (n = 29; Age = 13.6±1.1 y; BMI = 21.8±5.7 kg/m2) HC Low-DFT (n = 37; Age = 14.0±1.3 y; BMI = 19.7±3.47 kg/m2) |
Stimuli
Instructions: Name ink color of words as quickly as possible and do not correct any mistakes. |
Interference for food (vs. neutral) words
|
| Ben-Tovim et al. (1989) | 57 adults from Australia 100% Female Mage = 24.2 y MBMI = 21.7 kg/m2 |
BN (n = 19; Age = 26.9±10 y; BMI = 22.6±4.7 kg/m2) HC (n = 38; Age = 22.8±4.5 y; BMI = 21.3±2.5 kg/m2) |
Stimuli
Instructions: Name ink color of words as quickly as possible and do not correct any mistakes. |
Interference for food (vs. neutral) words
|
| Black et al. (1997) | 45 adults from the United States 100% female Mage = 22.1 y MBMI = 22.7 kg/m2 |
BN (n = 16; Age = 23.8; BMI = 23.0) HC-Res (n = 16; Age =20.6; BMI = 23.7) HC (n = 13; Age = 21.9; BMI = 21.2) |
Stimuli
Instructions: Say the color of the words aloud and press the corresponding color key as quickly as possible. |
Interference for food (vs. control) words
|
| Cooper, Anastasiades, and Fairburn (1992) | 54 adults from the United Kingdom 100% Female Mage = 23.6 y MBMI = 21.5 kg/m2 |
BN (n = 36; Age = 24.3±6.2 y; BMI = 21.8±2.2 kg/m2) HC (n = 18; Age = 22.1±3.5 y; BMI = 20.9±1.5 kg/m2) |
Stimuli
Instructions: Name ink colors of words as quickly as possible and immediately correct any mistakes. |
Interference for target (vs. neutral) words |
| Cooper and Fairburn (1993) | 75 adults from the United Kingdom 100% female Mage = 24.2 y MBMI = 22.3 kg/m2 |
No groups; all participants diagnosed with BN |
Stimuli
Instructions: Name ink color of words as quickly as possible and immediately correct any mistakes. |
Interference for target (vs. control) words
|
| Cooper and Fairburn (1992) | 36 individuals from the United Kingdom 100% female† |
BN (n = 12)† Dieting HC (n = 12)† Non-dieting HC (n = 12)† |
Stimuli
Instructions: Name ink color of words as quickly as possible and immediately correct any mistakes. |
Interference for target (vs. control) words
|
| Cooper and Todd (1997) | 30 individuals from the United Kingdom 100% female† |
BN (n = 12)† HC (n = 18)† |
Stimuli
Instructions: Name ink color of words as quickly as possible and immediately correct any mistakes. |
Interference for eating-related (vs. control) words
|
| Davidson and Wright (2002) | 35 adults from the United Kingdom 100% female Mage = 25.2 y MBMI = 21.1 kg/m2 |
BN (n = 17; Age = 25.5±6.4 y; BMI = 21.2±3.2 kg/m2) HC (n = 18; Age = 24.9±6.1 y; BMI = 21.1±2.0 kg/m2) |
Stimuli
Instructions: Press one of four buttons to identify the ink color of words as quickly and accurately as possible. |
Interference for food (vs. neutral) words
|
| Fairburn, Cooper, Cooper, McKenna, and Anastasiades (1991) | 74 adults from the United Kingdom 100% female Mage = 20.4 y MBMI = 21.5 kg/m2 |
BN (n = 24; Age = 21.3±3.8 y; BMI = 22.6±3.1 kg/m2) HC (n = 50; Age = 20.0±1.1 y; BMI = 20.9±1.6 kg/m2) |
Stimuli
Instructions: Name ink color of words as quickly as possible and immediately correct any mistakes. |
Interference for target (vs. control) words
|
| Flynn and McNally (1999) | 58 adults from the US 100% female Mage = 28.5 y† |
BN (n = 15; Age = 27.1±7.0 y)† Short-term Rec-BN (n = 15; Age = 28.2±6.6 y)† Long-term Rec-BN (n = 15; Age = 35.5±7.0 y)† HC (n = 13; Age = 22.3±4.7 y)† |
Stimuli
Instructions: Name the colors of the words as quickly as possible while ignoring their meanings. Note: prior to Emotional Stroop task, participants completed a cognitive task to activate concerns about body weight.) |
Interference for food (vs. office supply) words
|
| Formea and Burns (1996) | 58 university students from the United Kingdom 100% female Mage = 19.0 y† |
Participants grouped on BULIT-R and BDI BN, diagnosed on BULIT-R (n = 22; Age = 19.8±3.2 y)† Non-BN depressed (n = 11; Age = 19.0±2.6 y)† Non-BN non-depressed (n = 25; Age = 18.4±1.0 y)† |
Stimuli
Instructions: Name ink color of words as quickly and as accurately as possible. |
Interference for target (vs. control) words |
| Harrison et al. (2010) | 140 adults from the United Kingdom 100% female Mage = 28.1 y MBMI = 21.4 kg/m2 |
BN (n = 50; Age = 27.5±8.8 y; BMI = 21.0±2.4 kg/m2) HC (n = 90; Age = 28.5±9.9 y; BMI = 21.6±1.9 kg/m2) |
Stimuli
|
Interference for face (vs. chair) images
|
| Johansson et al. (2008) | 78 adults from Sweden 100% female Mage = 24.4 y MBMI = 21.9 kg/m2 |
BN (n = 20; Age = 24.6±3.9 y; BMI =21.3±2.2 kg/m2) Sub-BN (n = 27; Age = 24.9±4.5 y; BMI =22.4±3.8 kg/m2) HC (n = 31; Age = 23.8±2.6 y; BMI =21.8±2.6 kg/m2) |
Stimuli
Instructions: Ignore meaning of the words and focus on colors in which they are presented. Instructed to click the box on the screen representing ink color of each word as quickly as possible. |
Interference for high calorie food (vs. neutral) words
|
| Jones-Chesters et al. (1998) | 32 adults from the United Kingdom 100% female Mage = 25.9 y MBMI = 22.9 kg/m2 |
BN (n = 16; Age = 25.3±7.7 y; BMI =23.8±3.0 kg/m2) HC (n = 16; Age = 26.6±7.5 y; BMI =22.1±2.8 kg/m2) |
Stimuli
Instructions: Name out loud the colors in which the words are displayed as quickly and accurately as possible, ignoring the meaning of the words. |
Interference for weight/shape (vs. control) words
|
| Kanakam et al. (2013) | 74 older adolescent and adult twins from the United Kingdom 100% female† |
Lifetime BN (n = 26)† Non-BN co-twins (n = 6)† HC twins (n = 42)† |
Stimuli
Instructions: Name the color of the picture you see as quickly as possible. |
Interference for social (vs. non-social) images
|
| Lee et al. (2017) | 39 adults from South Korea 100% female Mage = 23.5 y MBMI = 22.5 kg/m2 |
BED (n = 13; Age = 23.6±2.6 y; BMI = 25.6±3.8 kg/m2) BN (n = 12; Age = 23.7±2.2 y; BMI = 21.5±2.2 kg/m2) HC (n = 14; Age = 23.3±2.2 y; BMI = 20.4±2.6 kg/m2) |
Stimuli
Instructions: using right hand, press a red key when the cue and word matched and a green key when they did not match |
Interference for food (vs. neutral) images
|
| Léonard et al. (1997) | 74 adults from France 100% female Mage = 25.7 y MBMI = 19.1 kg/m2 |
AN-BP (n = 25; Age = 25.6±5.6 y; BMI = 16.5±1.5 kg/m2) BN (n =20; Age = 26.2±5.2y; BMI = 20.8±2.3 kg/m2) HC (n = 29; Age = 25.4± 6.1y; BMI = 20.2±2.1 kg/m2) |
Stimuli
Instructions: Name ink color of words as quickly as possible. |
Interference for eating (vs. control) words
|
| Lokken et al. (2006) | 90 adults from the US 100% Female Mage = 19.3 y MBMI = 22.6 kg/m2 |
HC and Sub-BN grouped on BULIT-R BN (n = 30; Age = 19.1±1.4 y; BMI = 22.1±4.3 kg/m2) Sub-BN (n = 30; Age = 19.4±1.2 y; BMI = 23.4±4.7 kg/m2) HC (n = 30; Age = 19.5±1.0 y; BMI = 22.2±2.5 kg/m2) |
Stimuli
Instructions: Name ink color of words as quickly as possible and immediately correct any mistakes. |
Interference for eating (vs. neutral) words
|
| Lovell et al. (1997) | 68 adults from the United Kingdom 100% female Mage = 27.0 y MBMI = 22.8 kg/m2 |
BN (n =24; Age = 26.9±11.1y; BMI = 21.8±3.5kg/m2) Rec-BN (n =11; Age = 34.4±10.5 y; BMI = 24.6±4.8 kg/m2) HC (n = 33; Age = 24.7±8.1 y; BMI = 22.9±3.6 kg/m2) |
Stimuli
Instructions: Name ink color of words as quickly and accurately as possible and immediately correct any mistakes. |
Interference for food (vs. control) words
|
| McManus et al. (1996) | 60 individuals from the United Kingdom 100% female Mage = 22.5 y† |
BN/AN-BP (n = 30; Age = 22.5±5.0y) HC (n = 30; Age = 22.5±3.8y, BMI = 22.0) |
Stimuli
Instructions: Ignore the words and name the color of the ink in which they are presented. |
Interference for sociotropy threat (vs. control) words
|
| Perpiñá et al. (1993) | 46 adults from the United Kingdom 100% female Mage = 26.7 y MBMI = 24.4 kg/m2 |
BN (n = 14; Age = 26.4±4.9 y; BMI = 26.6±8.3 kg/m2) HC (n = 32; Age = 26.9±6.0 y; BMI = 23.4±3.5 kg/m2) |
Stimuli
|
Interference for food (vs. control) words
|
| Perpiña et al. (1998) | 28 adults from the United Kingdom 100% female Mage = 28.8 y MBMI = 21.1 kg/m2 |
BN (n = 10; Age = 27.8±8.2 y; BMI =21.1±2.2 kg/m2) HC (n = 18; Age = 29.3±9.7 y; BMI =21.1±2.7 kg/m2) |
Stimuli
|
Interference for food (vs. control) words
|
| Rofey et al. (2004) | 165 adults from the US 100% female Mage = 19.2 y† |
No groups; all participants completed the BULIT-R and Positive and Negative Affect Scale |
Stimuli
Instructions: Pay attention to the meaning of each word and, as quickly and accurately as possible, press a key corresponding to the color of each word. |
Interference for food (vs. neutral) words |
| Ruiz et al. (2008) | 62 older adolescents and young adults from Mexico 100% female† |
In-patient BN (n = 26)† HC (n = 36)† |
Stimuli
|
Interference for positive food words
|
| Seddon and Waller (2000) | 80 adults from the United Kingdom 100% female Mage = 21.7 y MBMI = 21.7 kg/m2 |
Participants completed the BITE Younger adults (n = 48; Age = 19.7±1.0 y; BMI = 21.3±2.4 kg/m2) Older adults (n = 32; Age = 24.7±5.0 y; BMI = 22.2±2.2 kg/m2) |
Stimuli
Instructions: Press a button corresponding to the color in which the word was displayed. |
Interference for negative (vs. control) words
|
| Waller and Ruddock (1995) | 60 adults from the United Kingdom 100% female Mage = 24.6 y MBMI = 22.1 kg/m2 |
BN (n = 30; Age = 25.0±6.5 y; BMI = 22.4±3.1 kg/m2) HC (n = 30; Age = 24.2±6.1 y; BMI = 21.7±2.1 kg/m2) |
Stimuli
Instructions: Name the colors in which the words are printed as quickly as possible, ignoring the words themselves, and immediately correct any mistakes. |
Interference for food (vs. control) words
|
| Waller et al. (1996) | 80 older adolescents and adults from the United Kingdom 100% female Mage = 18.7 y MBMI = 20.9 kg/m2 |
Participants completed the EDI High-BN† (n = 20) Low-BN† (n = 60) |
Stimuli
|
Interference for self-directed ego threat (vs. control) words
|
Note: Studies are organized alphabetically. Interference in all cases above refers to slower color-naming time for target (vs. control) words. Clinical diagnoses are based on DSM criteria (e.g. for BN) unless noted otherwise.
No further demographics available.
Effect sizes not available.
Abbreviations: M = mean; BMI = body mass index; NS = non-significant; BED = binge eating disorder; BN = bulimia nervosa; Sub-BN = subclinical bulimia nervosa; AN-BP = anorexia nervosa, binge/purge type; HC = healthy control; Rec = recovered; ITI = intertrial interval; EAT = Eating Attitudes Test; BULIT-R = Bulimia Test-Revised; BDI = Beck Depression Inventory; BITE = Bulimic Investigatory Test; EDI = Eating Disorders Inventory; Res = DEBQ, restraint subscale; DFT = drive for thinness.
Among studies using the Stroop task with food-related cues, the majority of studies found no significant differences in AB between women with BN and healthy controls (Black, Wilson, Labouvie, & Heffernan, 1997; Davidson & Wright, 2002; Flynn & McNally, 1999; Johansson, Carlbring, Ghaderi, & Andersson, 2008; Lee, Namkoong, & Jung, 2017; Lovell, Williams, & Hill, 1997; Perpiñá, Hemsley, Treasure, & de Silva, 1993; Perpiña, Leonard, Treasure, Bond, & Banos, 1998; Ruiz, de Leon, & Diaz, 2008; Waller & Ruddock, 1995), and six studies indicated that women with BN had greater AB to food cues compared to healthy controls (Ben-Tovim & Walker, 1991; Ben-Tovim, Walker, Fok, & Yap, 1989; Cooper & Todd, 1997; Jones-Chesters, Monsell, & Cooper, 1998; Léonard, Divac, Bichindaritz, Rouer-Saporta, & Samuel-Lajeunesse, 1997; Lokken, Marx, & Ferraro, 2006). Further, all but one of the available effect sizes across both significant and non-significant findings (Black et al., 1997) indicated greater AB to food in women with BN. No differences were found in AB to food cues between women with BN vs. AN-B/P (Léonard et al., 1997) or between women with threshold vs. subthreshold BN (Lokken et al., 2006).
The majority of Stroop task paradigms with body weight/shape cues found that women with BN had greater AB relative to controls (Davidson & Wright, 2002; Flynn & McNally, 1999; Johansson et al., 2008; Jones-Chesters et al., 1998; Ruiz et al., 2008) (Ben-Tovim & Walker, 1991; Ben-Tovim et al., 1989; Cooper & Todd, 1997; Lokken et al., 2006; Lovell et al., 1997; Perpiñá et al., 1993), with all available effect sizes indicating greater AB to weight/shape cues in women with BN compared to healthy controls. Women with clinical BN exhibited greater AB than those with recovered/subthreshold BN (Flynn & McNally, 1999; Lokken et al., 2006; Lovell et al., 1997) and women with recovered/subthreshold BN did not differ from healthy controls in AB to weight/shape cues (Lovell et al., 1997).
The majority of studies using the Stroop task with threat-related cues found that adolescent and adult women with binge eating and/or BN behaviors had greater AB to threat-related cues compared to healthy controls. Greater AB was associated with higher binge frequency in one study (McManus, Waller, & Chadwick, 1996); however, another study found no differences in AB to threat cues between women with BN, recovered BN, or healthy controls (Lovell et al., 1997). Some studies suggest that findings may be contingent upon the frequency or type of threat cue used (e.g., self-directed ego threat words, angry faces) or sample characteristics (Harrison, Sullivan, Tchanturia, & Treasure, 2010; Jones-Chesters et al., 1998; Kanakam, Krug, Raoult, Collier, & Treasure, 2013; Seddon & Waller, 2000; Waller, Watkins, Shuck, & McManus, 1996). For instance, self-directed ego threat words were associated with AB in a non-clinical sample of women who endorsed high BN symptomatology compared to those with low or no symptoms, but there were no differences for sociotropy threat, autonomy threat, physical threat, or ego threat from others words (Waller et al., 1996). Additionally, the effect may depend on whether words or images are used (Harrison et al., 2010; Kanakam et al., 2013). Additionally, some studies examined clinical samples while others used convenience samples with high BN symptomatology, thus the effects may be heterogeneous based on the sample characteristics.
In summary, facilitated attentional engagement with food, weight/shape, and threat cues appears to be greater in women with BN compared to control female participants when using the emotional Stroop. Women who engage in binge eating in the absence of compensatory behaviors appear to exhibit greater facilitated attentional engagement with threat cues compared to control female participants but research is lacking using the Stroop with food and weight/shape cues.
Visual Probe Task
Of the three visual probe studies in participants with binge eating behaviors (Table 4), two examined AB to food-related cues and one examined AB to threat-related cues. No study has utilized weight/shape cues. In a sample of children and adolescents (Shank et al., 2015), youth who engaged in objective and subjective binge eating episodes did not differ on AB for high- or low-palatable foods in analyses adjusted for age, sex, race, and BMI-z score. While higher frequency of binge eating (combined subjective and objective) was associated with greater AB toward high-palatable foods, the effect was small and the relationship was attenuated after adjusting for body composition (Shank et al., 2015). In a primarily adult female sample, individuals with binge eating demonstrated greater difficulties with attentional disengagement from food cues, but there were no differences between healthy controls in facilitated attentional engagement or avoidance (Deluchi, Costa, Friedman, Gonçalves, & Bizarro, 2017). In a study using the visual probe task with threat cues (Cardi, Di Matteo, Corfield, & Treasure, 2013), participants were presented images of male and female faces with accepting, rejecting, or neutral expressions. Those with lifetime BN (both currently ill and recovered groups) demonstrated an AB toward rejecting faces, but attentional avoidance of accepting faces. The opposite pattern was observed in healthy controls, and the magnitude of the group difference was small-to-medium.
Table 4.
Attentional biases as measured by the visual probe task.
| Study | Sample | Groups | Paradigm | Findings |
|---|---|---|---|---|
| Cardi et al. (2013) | 76 adults and older adolescents from the United Kingdom 100% female† |
Lifetime BN (n = 26; ncurrent: n = 17, npast: n = 9)† HC (n = 50)† |
Stimuli
Instructions: Press button to classify probe as vertical or horizontal dots. |
AB for rejecting (vs. paired neutral) face images
|
| Deluchi et al. (2017) | 42 adults from Brazil 95.2% female Mage = 47.0 y MBMI = 48.1 kg/m2 |
Participants completed the BES High-BES (n = 19; BMI = 47.85 kg/m2)† Low-BES (n = 23; BMI = 48.24)† |
Stimuli
Instructions: Press up or down arrows on computer keypad to indicate direction of target arrow as quickly and accurately as possible. |
AB for unhealthy food (vs. neutral objects)
|
| Shank et al. (2015) | 76 children and adolescents from the United States 86.8% female Mage = 14.5 y MBMI-z = 1.71 |
LOC (n = 47; Age = 13.8±2.4 y; BMI-z = 2.0±0.4) HC (n = 29; Age = 15.6±1.6 y; BMI-z = 1.2±0.8) |
Stimuli
Instructions: Press button to classify probe as left or right arrow. |
AB for high (vs. paired low) palatable food images
|
Note: Studies are organized alphabetically. Clinical diagnoses are based on DSM criteria (e.g. for BN) unless noted otherwise.
No further demographics available.
Effect sizes not available.
Abbreviations: M = mean; NS = non-significant; AB = attentional bias; BN = bulimia nervosa; BED = binge-eating disorder; HC = healthy controls; BES = Binge Eating Scale; BMI = body mass index; BMI-z: body mass index based on age-, sex-, and country-specific BMI reference data; LOC = loss of control.
Overall, there are few studies using the visual probe task in individuals with binge eating behaviors. Those who binge eat exhibit greater difficulties with attentional disengagement from, but not greater facilitated attention toward food cues compared to controls. Individuals with BN exhibit greater facilitated attention toward socially threatening stimuli and greater attentional avoidance of socially non-threatening stimuli compared to controls.
Spatial Cueing Task
Three studies examined AB using a modified spatial cueing paradigm (Table 5), two that used food-related cues and one that used threat-related cues. No study has utilized weight/shape cues. Women with BED demonstrated greater facilitated attention toward food— but no difference in attentional disengagement from food— compared to healthy controls; this effect was of medium magnitude (Schmitz, Naumann, Trentowska, & Svaldi, 2014). Among healthy-weight college women, those with binge eating demonstrated more difficulty disengaging from high-calorie food images, but not low-calorie food images, compared to women without binge eating (Lyu, Zheng, & Jackson, 2016). In a nonclinical sample of female undergraduates, greater disengagement difficulties for ego-threatening images (attractive female faces) were correlated with higher BN symptom frequency (Maner et al., 2006). No effect was present for attractive male faces.
Table 5.
Attentional biases as measured by the spatial cueing task.
| Study | Sample | Groups | Paradigm | Findings |
|---|---|---|---|---|
| Lyu et al. (2016) | 64 adult undergraduate students from China 100% female Mage = 21.5 y MBMI = 20.0 kg/m2 |
Healthy weight sub-BED (n = 33; Age = 21.5±1.4 y; BMI = 20.4±2.3 kg/m2) HC (n = 31; Age = 21.4±1.5 y; BMI = 19.6±2.1 kg/m2) |
Stimuli
Instructions: Indicate left or right location of the dot by pressing ‘A’ or ‘L’ respectively, on standard computer keyboard. |
AB for high-calorie foods
|
| Maner et al. (2006) | 66 adults from the United States 100% female Mage = 18.5 y† |
Participants completed EDI High EDI-BN (n = 6)† Low EDI-BN (n = 60)† |
Spatial cueing stimuli
Instructions: Following a single cue image in one quadrant, press “a” or “k” key to classify probe as a circle or square. Only analyzed invalid trials, in which probe appeared in a different location from the stimulus. |
AB for attractive female faces (vs. mean reaction time across all stimuli)
|
| Schmitz et al. (2014) | 60 adults from Germany 100% female Mage = 44.0 y MBMI = 33.4 kg/m2 |
BED-OW (n = 27; Age = 46.0±14.5 y; BMI = 34.7±5.1 kg/m2) HC-OW (n = 33; Age = 42.4±13.5 y; BMI = 32.4±6.4 kg/m2) |
Spatial cueing stimuli
Instructions: Following a single cue image, press left or right button to indicate position of subsequent dot probe, while ignoring question mark probe. |
AB for food (vs. neutral) cues
|
Note: Studies are organized alphabetically. Clinical diagnoses are based on DSM criteria (e.g. for BN) unless noted otherwise.
No further demographics available.
Effect sizes not available.
Abbreviations: M = mean; NS = non-significant; HC = healthy controls; OW = overweight; BMI = body mass index; BED = binge eating disorder; sub = subthreshold; EDI = Eating Disorder Inventory; BN = bulimia nervosa.
In summary, findings using the spatial cueing task are mixed with some evidence for greater facilitated attention (clinical sample) and some evidence for greater difficulties with attentional disengagement (non-clinical sample) in women who binge eat. This discrepancy may be due to a clinical vs. non-clinical sample. Women with BN exhibit difficulties with attentional disengagement from socially threatening stimuli.
Visual Search Paradigm
Three studies (Meyer, Waller, & Watson, 2000; Waller, Quinton, & Watson, 1995) used a visual search task, one with food-related cues and two with threat-related cues (Table 6). No study has utilized weight/shape cues. In a primarily female sample of German adolescents, those with BED demonstrated greater facilitated attentional engagement to food cues compared to healthy controls; this was a medium-size effect (Schmidt, Lüthold, Kittel, Tetzlaff, & Hilbert, 2016). Both studies using threat-related cues examined the relationship between BN symptoms and AB to threatening words in nonclinical female undergraduates. One (Waller et al., 1995) found that increased BN symptoms were associated with greater AB to threat words; this effect was small. The second study (Meyer et al., 2000) found that BN symptoms were associated with greater difficulties in attentional disengagement (with a small effect size), but were not associated with facilitated attention.
Table 6.
Attentional biases as measured by the visual search paradigm.
| Study | Sample | Groups | Paradigm | Findings |
|---|---|---|---|---|
| Meyer et al. (2000) | 50 adults from the United Kingdom 100% Female Mage = 21.5 y† |
No groups; all participants completed the EDI |
Stimuli
Instructions: Following (threat or neutral) target word presentation, indicate whether the word is present or absent from the subsequent 4 × 4 word matrix. The matrix included the target word half of the time among otherwise neutral words. |
Response time for threat (vs. neutral) words present in the matrices
|
| Schmidt et al. (2016) | 50 adolescents from Germany 88% Female Mage = 14.7 y MBMI-z = 1.8 kg/m2 |
BED (n = 25; Age = 14.7±2.9 y; BMI-z = 1.8±0.8) HC (n = 25; Age = 15.3±2.4 y; BMI-z = 1.8±0.8) |
Stimuli
Instructions: press the right button if there was an image of a different category and press the left button if there were only images of the same category |
Response time for food (vs. non-food) images present in the matrices
|
| Waller et al. (1995) | 100 adults from the United Kingdom 100% Female Mage = 22.0 y MBMI = 21.5 kg/m2 |
Participants completed EAT-26 High EAT-26-BN (n = 26)† Low EAT-26-BN (n = 74)† |
Stimuli
Instructions: Following (threat or neutral) target word presentation, indicate whether the word is present or absent from the subsequent 4 × 4 word matrix. The matrix included the target word half of the time among otherwise neutral words. |
Response time for threat (vs. neutral) words present in the matrices
|
Note: Studies are organized alphabetically. Clinical diagnoses are based on DSM criteria (e.g. for BED) unless noted otherwise.
No further demographics available.
Effect sizes not available.
Abbreviations: M = mean; NS = non-significant; AB = attentional bias; BN = bulimia nervosa; EAT-26 = Eating Attitudes Test; EDI = Eating Disorder Inventory; BMI = body mass index; BMI-z: body mass index based on age-, sex-, and country-specific BMI reference data; BED = binge eating disorder; HC = healthy control
Taken together, there are few studies using the visual search paradigm with individual with disorders of binge eating. Those who binge eat exhibit greater facilitated attention toward food cues. Findings on AB to social threat in individuals with BN are mixed, with some evidence for greater facilitated attention and some evidence for greater difficulties with attentional disengagement compared to controls.
Eye Tracking
Eight eye tracking studies evaluated AB in connection with binge eating behavior (Table 7). Of these, four used food-related cues and four used weight/shape-related cues. No study used threat-related cues in an eye tracking paradigm. For the studies using food cues, the findings are heterogeneous. In one study (Schag et al., 2013), all participants showed greater facilitated attention toward food (vs. neutral) images; however, women with overweight/obesity and BED had greater difficulties with disengaging from food stimuli than weight-matched and non-overweight control groups. These effects were of medium magnitude. In another study (Popien, Frayn, von Ranson, & Sears, 2015), compared to controls, those with binge eating showed greater facilitated attention toward and more difficulty with attentional disengagement from food cues. The third study (Kim, Kim, & Lee, 2016) examined AB to high- and low-calorie food (vs. non-food) images in female undergraduates with “BN tendencies” compared to a control group. The BN group exhibited greater facilitated attention toward high-calorie food cues compared to the other cues, as well as attentional avoidance for the high-calorie food versus non-food images. Finally, in a primarily female sample of German adolescents, those with BED demonstrated greater difficulties with attentional disengagement from food cues, compared to healthy controls (Schmidt et al., 2016). This was a medium size effect. There were no group differences in facilitated attentional engagement (Schmidt et al., 2016).
Table 7.
Attentional biases as measured by eye tracking paradigms.
| Study | Sample | Groups | Paradigm | Findings |
|---|---|---|---|---|
| Blechert et al. (2010) | 39 adults from Germany 100% Female Mage = 27.0 y MBMI = 21.5 kg/m2 |
BN (n = 18; Age = 26.9±8.4 y; BMI = 22.9±3.4 kg/m2) HC (n = 21; Age = 27.1±4.8 y; BMI = 20.3±2.1 kg/m2) |
Stimuli
Metric: saccade latency Instructions: Only upon presentation of green or blue frame surrounding photos (one color denoted targets), move eyes from central fixation cross to target photo as quickly as possible, then return gaze to fixation cross. |
AB for self-target (vs. other-target) |
| Blechert et al. (2009) | 42 adults from Germany 100% Female Mage = 26.5 y MBMI = 21.4 kg/m2 |
BN (n = 20; Age = 26.6±7.7 y; BMI = 22.6±3.4 kg/m2) HC (n = 22; Age = 26.5±4.7 y; BMI = 20.3±2.24 kg/m2) |
Stimuli
Metrics: gaze dwell time bias; saccade latency; initial saccade direction Instructions: Slides consist of your own body photo in the middle and other body photos to the left and right. Look at the pictures. You will have to evaluate them later. |
AB for self image
|
| Kim et al. (2016) | 86 adult undergraduates from Korea 100% Female Mage = 21.2 y† |
Split by high and low scores on a Korean version of BULIT-R High BULIT-R (n = 38; Age = 21.5±2.0 y)† Low BULIT-R (n = 38; Age = 21.1±2.0 y)† |
Stimuli:
Metrics: fixation latency; dwell time; percentage dwell time for 0–1000, 1000–2000, 2000–3000, and 3000–4000 ms intervals Instructions: Fixate eyes freely |
AB towards high-calorie food (vs. low-calorie food)
|
| Popien et al. (2015) | 57 adults from Canada 75% Female Mage = 21.7 y MBMI = 23.1 kg/m2 |
BE (n = 27; Age = 21.6±3.4 y; BMI = 24.4±4.9 kg/m2) HC (n = 30; Age = 21.8±2.2 y; BMI = 22.0±3.4 kg/m2) |
Stimuli
Metrics: percent total gaze dwell time on food items (calculated for each 2000 ms stimulus presentation interval) and time to first fixation for food items Instructions: Look freely at the image throughout the presentation, as if watching a slide show. |
AB for high-calorie food AB for low-calorie food AB for high- and low-calorie food (in images that contained both types of food) |
| Schag et al. (2013) | 70 adults from Germany 100% Female Mage = 39.7 y MBMI = 31.2 kg/m2 |
OW/OB-BED (n = 24; Age = 39.7±11.7 y; BMI = 35.4±5.6 kg/m2) OW/OB-HC (n = 24; Age = 39.9±12.6 y; BMI = 35.4±5.4 kg/m2) HC (n = 22; Age = 39.4±11.8 y; BMI = 22.5±1.6 kg/m2) |
Stimuli
Metrics: percent total gaze dwell time and initial fixations on each stimulus type Instructions: Freely explore the image pairs as if watching TV. |
AB for food (vs. nonfood) cues
|
| Schmidt et al. (2016) | 50 adolescents from Germany 88% Female Mage = 14.7 y MBMI-z = 1.8 kg/m2 |
BED (n = 25; Age = 14.7±2.9 y; BMI-z = 1.8±0.8) HC (n = 25; Age = 15.3±2.4 y; BMI-z = 1.8±0.8) |
Stimuli
Metrics: percent initial gaze direction and gaze duration Instructions: freely explore pairs of food and non-food images on the computer screen as if watching television |
AB for food (vs. nonfood) cues
|
| Svaldi et al. (2012) | 46 adults from Germany 100% Female Mage = 40.3 y MBMI = 33.8 kg/m2 |
BE (n = 23; BMI = 37.7±6.9 kg/m2)† HC-OW (n = 23; BMI = 29.8±3.9 kg/m2)† |
Stimuli
Metrics: frequency of fixation and gaze dwell time for first (265 ms) and second (570 ms) fixations Instructions: Paired self and control images were presented side-by-side. During the cue condition, participants were told on which side the self image would appear. In no cue condition, participants did not know where the image would appear. |
AB for self body
|
| Svaldi et al. (2011) | 44 adults from Germany 100% Female Mage = 44.2 y MBMI = 35.1 kg/m2 |
BE (n = 26; BMI = 38.7±8.2 kg/m2)† HC-OW (n = 18; BMI = 30.0±3.8 kg/m2)† |
Stimuli
Metrics: gaze dwell time and frequency of fixations on specific body parts Instructions: Watch images of yourself and a control person. After task, identify ugliest and most beautiful body parts of your own image and the control body image. |
AB for ugliest self body part
|
Note: Studies are organized alphabetically. Clinical diagnoses are based on DSM criteria (e.g. for BN) unless noted otherwise.
No further demographics available.
Effect sizes not available.
Abbreviations: M = mean; NS = non-significant; AB = attentional bias; BN = bulimia nervosa; AN-BP: anorexia nervosa, binge eating/purging type; HC = healthy controls; BED = binge eating disorder; BE = binge eating; OW = overweight; OB = obese; BMI = body mass index; BULIT-R = Bulimia Test-Revised.
Four studies from the same research group (Blechert, Ansorge, & Tuschen-Caffier, 2010; Blechert, Nickert, Caffier, & Tuschen-Caffier, 2009; Svaldi, Caffier, & Tuschen-Caffier, 2011, 2012) compared women on AB for self- and other-body photographs. One (Blechert et al., 2010) found no group differences between individuals with BN and healthy controls, while another study found women with BN exhibited greater difficulty in disengagement from the lower-BMI other-body image only and greater avoidance of the higher-BMI other-body image only (Blechert et al., 2009). Women with binge eating but without compensatory behavior exhibited greater attentional engagement toward and greater difficulties with disengaging from the self-body images compared to women without binge eating (Svaldi et al., 2012). In the second study (Svaldi et al., 2011), those with binge eating demonstrated greater difficulties disengaging from the ugliest self-body part relative to controls. The effect size was medium, but the finding was attenuated after adjusting for body composition.
In summary, it appears that those who binge eat consistently exhibit difficulties with attentional disengagement from food cues in studies using eye tracking. Those with BN appear to exhibit greater facilitated attention toward as well as attentional avoidance of food-related cues, although this finding is based on only one study. There is some evidence from eye tracking studies that those with BN have greater difficulty with disengaging from low-BMI images of other people, and that they intentionally avoid high-BMI images of other people. Those who binge eat appear to have difficulties with disengaging from images depicting themselves.
EEG
Six studies assessed attention processing with event-related potentials (ERPs) in participants with binge eating behaviors, of which four examined AB to palatable food cues, one used weight/shape cues, and two used threat cues (Table 8). Women with BN exhibited greater attentional engagement toward all food images vs. non-food images (Blechert et al., 2011). Women without BN showed greater attentional engagement toward high-calorie food vs. low-calorie food and non-food images (Blechert et al., 2011). Women with both overweight and BED had greater attentional engagement toward and greater disengagement difficulties from high-calorie (vs. low-calorie) food images compared to controls with overweight, with a large effect size (Svaldi, Tuschen-Caffier, Peyk, & Blechert, 2010). However, a sample of Spanish women with BN or BED did not exhibit greater facilitated attentional engagement or difficulties with attentional disengagement compared to healthy controls (Wolz et al., 2017). Among female university students, no relationship was observed between binge eating and attentional engagement or disengagement processes (Sarlo, Ubel, Leutgeb, & Schienle, 2013).
Table 8.
Attentional biases as measured by electroencephalography.
| Study | Sample | Groups | Paradigm | Findings |
|---|---|---|---|---|
| Blechert et al. (2011) | 54 adults from Germany 100% Female Mage = 26.2 y MBMI = 21.5 kg/m2 |
BN (n = 22; Age = 26.1±7.5 y; BMI = 22.6±3.2 kg/m2) HC (n = 32; Age = 26.2±5.0 y; BMI = 20.7±2.4 kg/m2) |
Stimuli
ERPs measured: EPN (220–310 ms) Instructions: Watch images during rapid serial visual presentation task, with images presented from each category in a pseudorandom order. |
AB for food (vs. neutral) images
|
| Kuhnpast et al. (2012) | 26 adults from Germany 100% Female Mage = 25 y MBMI = 21.7 kg/m2 |
BN (n = 13; Age = 24.6±7.1 y; BMI = 21.7±3.7 kg/m2) HC (n =13; Age = 25.4±3.2 y; BMI = 21.6±2.0 kg/m2) |
Stimuli
ERPs measured: N170 (120–180 ms); N2 (190–260 ms); P3 (270–500 ms) Instructions: Attend to stimuli very closely. |
AB for angry face images (vs. low level baseline)
|
| Mai et al. (2015) | 40 adults from Germany 100% Female Mage = 24.6 y MBMI = 21.3 kg/m2 |
BN (n = 20; Age = 24.6±7.5 y; BMI = 20.7±3.4 kg/m2) HC (n = 20; Age = 24.6±3.3 y; BMI = 21.8±2.6 kg/m2) |
Stimuli
ERPs measured: N170 (150–220 ms); P2 (180–270 ms); N2 (250–350 ms); P3 (350–450 ms); PSW (500–900 ms) Instructions: Attentively watch the pictures and avoid exploratory eye movements and eye blinks. |
AB for overweight (vs. healthy weight) body stimuli
|
| Sarlo et al. (2013) | 33 adults from Austria 100% Female Mage = 22.5 y MBMI = 21.4 kg/m2 |
No groups; all participants completed EDI |
Stimuli
ERPs measured: P300 (280–400 ms); LPP1 (500–1000 ms) and LPP2 (1000–1500 ms) Instructions: View each picture, understand its content, and allow yourself to experience any emotional or appetitive response to the image. Rate current appetite and arousal. |
AB for food images (vs. low level baseline)
|
| Svaldi et al. (2010) | 44 adults from Germany 100% Female Mage = 39.7 y MBMI = 33.5 kg/m2 |
BED (n = 22; Age = 41.9±13.9 y; BMI = 36.5±6.9 kg/m2) HC (n = 22; Age = 37.5±13.6 y; BMI = 30.5±3.9 kg/m2) |
Stimuli
ERPs measured: LPP (500–800 ms); PSW (1000–6000 ms) Instructions: Passively watch images (viewing section) and then watch images that you will have to taste later (availability section). |
AB for high (vs. low) calorie food cues
|
| Wolz et al. (2017) | 39 adults from Spain 100% female Mage = 32.4 y MBMI = 26.5 kg/m2 |
BE (n = 19; Age = 35.0±9.6 y; BMI = 31.2±10.5 kg/m2) HC (n = 20; Age = 30.0±9.0 y; BMI = 22.0±2.8 kg/m2) |
Stimuli
ERPs measured: N2 (180–350 ms); LPP (300–1000 ms) Instructions: No information provided in manuscript. |
AB for chocolate (vs. neutral) cues |
Note: Studies are organized alphabetically. Clinical diagnoses are based on DSM criteria (e.g. for BN) unless noted otherwise.
No further demographics available.
Effect sizes not available.
Abbreviations: M = mean; NS = non-significant; AB = attentional bias; BED = binge-eating disorder; BN = bulimia nervosa; HC = healthy control; BMI = body mass index; BE = binge eating; EDI = Eating Disorder Inventory; ERP = event-related potential; EPN = early posterior negativity (negative deflection at 200–300 ms); LPP = late positive potential (positive deflection that lasts up to 6000 ms); LPP1 = late positive potential at 500–1000 ms; LPP2 = late positive potential at 1000–1500 ms; N2 = negative deflection at 190–260 ms); N170 = ERP associated with processing of facial features (negative deflection at 140–220 ms); P2 = positive deflection at 180–200 ms); P3 = positive deflection at 300 ms; P300 = positive deflection at 300–500 ms) PSW = positive slow wave (positive deflection at 1000 – 6000 ms)
In the study utilizing weight/shape cues, women with and without BN (Mai et al., 2015) viewed photographs of other women’s bodies. Participants with BN showed greater attentional engagement toward overweight (vs. healthy weight) body stimuli compared to women without BN. However, no group differences were observed in attentional disengagement.
In one study using threat cues (Kuhnpast, Gramann, & Pollatos, 2012), individuals viewed male and female face images with angry, fearful, happy and neutral expressions. Women with BN had more difficulties than controls with categorizing angry faces in the early presentation intervals, suggesting emotion recognition deficits at the facilitated attentional engagement stage. Women with BN exhibited greater attentional disengagement difficulties across all face types relative to controls. The second study (Blechert et al., 2011) found no group difference in AB to pleasant or unpleasant (vs. neutral) images. Taken together, EEG studies using food cues present mixed evidence for facilitated attentional engagement and disengagement difficulties in women with binge eating behaviors. In contrast to the evidence from eye tracking studies, the EEG study suggests that women with BN exhibit facilitated attentional engagement (as opposed to attentional avoidance) toward stimuli representing overweight. The EEG studies using threat cues are inconclusive and while one suggests attentional disengagement difficulties in women with BN, the other found no such effect.
Discussion
This review aimed to systematically review findings on AB to food cues, weight and shape cues, and threatening cues in individuals who engage in binge eating behaviors. Fifty articles met the review criteria, of which 30 focused on AB to food cues, 20 to weight/shape-related stimuli, and 13 to threatening cues. All included studies used a cross-sectional design.
Food Cues
The relationship between AB to food cues and binge eating behaviors appears contingent on the task employed and specific population studied. The evidence to date indicates that women with full-syndrome or subthreshold BN may demonstrate greater facilitated attentional engagement to food cues compared to healthy controls, although not all studies found that effect. Few studies examined attentional disengagement and avoidance in relation to food cues in women with BN so conclusions cannot be drawn at this time. Those with BN showed increased attentional engagement to all foods versus increased attention only to palatable foods in the control participants (Blechert et al., 2011), which may be reflective of chronic caloric deprivation associated with disordered eating. Of note, in studies that used samples with subthreshold or recovered BN, no AB was detected. It may be that for BN, AB serves as a maintenance factor for the disorder, or potentially an artifact of the illness secondary to dietary restriction. It is also conceivable that as a result of binge eating behaviors over time, the frontostriatal neurocircuitry becomes less responsive to rewarding properties of food, consistent with the incentive salience theory (Berridge & Robinson, 1998).
The majority of studies support the notion that individuals who engage in binge eating behaviors without compensatory behaviors exhibit AB toward food, both in the automatic facilitated attentional engagement and purposeful attentional disengagement stages. Specifically, food cues capture attention of those with binge eating behaviors faster than controls, and more time is spent attending to palatable foods, suggesting difficulties with attentional disengagement. This tendency potentially reflects the powerful reward responses and decreased inhibitory control when processing food cues, following the approach-approach pattern (Leehr et al., 2016). Decreased inhibitory control and increased impulsivity, both documented in individuals who binge eat (Mobbs, Iglesias, Golay, & Van der Linden, 2011; Schag et al., 2013), may prevent them from engaging in effective down-regulation of processing of these cues. However, this theory requires testing.
Weight- and Shape-Related Cues
Adults with BN or subthreshold BN exhibit greater facilitated attentional engagement toward weight/shape cues. Evidence also suggests that in the strategic “top down” processing stages, these individuals exhibit avoidance of images of heavier persons and bias towards images of leaner figures. Generally, individuals with BN or subthreshold BN appear to orient toward negative weight/shape stimuli more rapidly than controls, then disengage from these cues, following an approach-avoidance pattern of processing (Lang, Bradley, & Cuthbert, 1997). Overvaluation of weight/shape is a clinical symptom of BN, thus AB manifesting as more rapid orienting toward these cues may be an example of “selective automatic processing” of disorder-related cues (Ben-Tovim et al., 1989; Channon, Hemsley, & de Silva, 1988). The evidence for AB to weight/shape cues in those who binge eat without compensatory behavior is limited and inconclusive, and there is limited support for AB toward dissatisfying own body parts among individuals with binge eating. The finding that those with BN have AB toward other’s lean bodies, while those with binge eating alone show AB toward their own bodies might represent an automatic process that distinguishes these two “subtypes.” Both the BN “subtype” and the binge eating only “subtype” exhibit body dissatisfaction. However, those with BN may have a tendency to compare their bodies with others’ bodies that they perceive as more attractive, while those with binge eating only may be more prone to fixate on their own bodies, which they perceive as unattractive. The finding that individuals with BN purposefully avoid high BMI bodies may suggest that while both “subtypes” may exhibit body image distortion, those with BN are more prone to it. Taken together, these findings suggest biases towards weight/shape cues may be a consequence and/or a maintenance factor of the disorder aspects related to dissatisfaction with shape and weight. However, it is unclear whether such an AB is a risk factor for development of binge eating behaviors.
Threatening Cues
Studies across tasks demonstrate greater facilitated engagement toward threatening cues in women with BN and subthreshold BN. Women with subthreshold BN process threatening cues more slowly than healthy controls, suggesting that they exhibit greater cognitive avoidance of these cues, consistent with the information processing theory (Foa & Kozak, 1986). Additionally, women with BN have increased difficulties in recognizing— and devote more cognitive resources to evaluating— negative emotions (Kuhnpast et al., 2012). Exposure to threatening cues potentiates AB to palatable food in women who endorse emotional-eating tendencies (Blechert, Goltsche, Herbert, & Wilhelm, 2014), suggesting an interaction between affective states and incentive salience of palatable foods.
Taken together, these findings lend support for both the approach-avoidance and escape theories of binge eating in women with BN. Following automatic attentional engagement to threatening cues, individuals then more reflectively engage in avoidance processes, which may facilitate a strong desire to eat or be a manifestation of dietary restraint. It is possible that AB to threatening cues represents a general latent vulnerability to binge eating behaviors characterized by escape from awareness. AB to threat may distinguish those motivated to binge eat by negative reinforcement mechanisms (i.e., escape from negative emotional experiences) from those motivated by positive reinforcement mechanisms (i.e., rewarding properties of food). However, no study has examined AB to threatening cues in individuals who engage in binge eating in the absence of compensatory behaviors, thus it will be important to assess AB to threat in these individuals.
Integration of AB Types in Disorders of Binge Eating
It appears that the intersection of AB to disorder-specific cues (i.e., food and weight/shape cues) combined with AB toward threat may be a particularly potent combination of vulnerabilities leading to LOC eating. Several studies demonstrate an interaction between negative affective states and AB toward food or weight/shape cues (Blechert, Goltsche, et al., 2014; Hepworth, Mogg, Brignell, & Bradley, 2010; Rofey, Corcoran, & Tran, 2004). AB to threatening stimuli also consistently distinguishes those with BN from healthy controls.
Several theories could account for this interaction. The cognitive depletion model proposes that self-regulation is a limited resource and may become exhausted when an individual attempts to regulate response to multiple stimuli (Baumeister & Heatherton, 1996). For example, if in response to bottom-up automatic facilitated attention toward threatening cues, an individual engages in top-down regulatory attentional processes aimed at avoiding such cues (consistent with the approach-avoidance model), then putatively there are fewer cognitive resources available to engage in top-down regulation of processing of food cues. For individuals with BN behaviors, it has been proposed that weight/shape stimuli are the equivalent of threatening cues, given the social evaluative salience that they carry for individuals with weight/shape concerns (Williamson, Muller, Reas, & Thaw, 1999). Therefore, they may contribute to depletion of cognitive regulation in response to food cues in those with BN behaviors. The cognitive depletion model has, however, been criticized and the findings from a large replication study have suggested that the effects of the purported mental fatigue on self-control are much smaller than previously reported (Hagger et al., 2016).
It is also conceivable that threatening cues acquire salience over time through associative learning and become conditioned stimuli that precipitate craving and subsequent binge eating. This process would be analogous to the negative reinforcement model of addictive behaviors wherein individuals seek out drugs to cope with negative affect (Higgins, Heil, & Lussier, 2004) and is supported by findings using ecological momentary assessment among those with BN (Smyth et al., 2007). Another analogous model from the addiction literature is the compulsivity model in which binge eating is motivated primarily by the hedonic properties of food (Davis & Carter, 2009). AB to palatable food may represent sensitivity to rewarding foods, reflecting another vulnerability toward binge eating. Therefore, processes involved in AB may reflect two subtypes for development and maintenance of binge eating. One subtype involves the negative reinforcement path, in which binge eating is an escape mechanism for coping with negative affect and is captured by AB to threatening stimuli. The other subtype involves the positive reinforcement path, in which binge eating develops as a result of oversensitivity to rewarding properties of food and is captured by AB to food. More research is needed to clarify whether these attention processes map onto these proposed behavioral models.
There is some preliminary evidence to suggest that AB toward weight/shape cues may function differently in those with BN versus binge eating without compensatory behaviors. Those with BN engage with low BMI weight/shape cues more rapidly, and disengage from high BMI cues more rapidly, while those with binge eating without compensatory behaviors appear to engage with their own self-perceived unattractive body parts more rapidly. This may suggest that those with BN are more likely to be dissatisfied with their bodies due to social comparisons with other individuals whom they purportedly perceive as more attractive, while those with binge eating alone experience body dissatisfaction due to being focused on their own perceived unattractiveness. More research is needed to clarify these processes, particularly with samples who binge eat without compensatory behaviors.
Recommendations for Future Research
As previously suggested, AB may be an endophenotype of various disorders involving binge eating and as such merits further study. AB has been a treatment target in other fields (e.g., anxiety disorders), and AB modification programs have been found to be effective in reducing AB and associated psychopathology (Linetzky, Pergamin-Hight, Pine, & Bar-Haim, 2015) Therefore, further study of AB may contribute to improving existing treatments and to the development of more precise treatments for disorders related to binge eating. Future research directions are discussed below.
Cross-Diagnostic Research
Although the field of AB research is rapidly growing, most research related to binge eating has been conducted in individuals with BN. Thus, there is a need to continue to study these processes in those who binge eat without compensatory behaviors, as these individuals appear to exhibit distinct patterns of AB from those with eating disorders characterized by weight-control behaviors. It is conceivable that AB represents an endophenotype for development or maintenance of binge eating behaviors. Thus, elucidating the genetic and neural underpinnings of different forms of AB across binge eating subtypes will be important. Furthermore, examining physiological markers such as cortisol, appetite hormones, and insulin sensitivity in conjunction with cognitive markers, such as AB and executive function, may provide a more complete understanding of distinct binge eating behavioral phenotypes. These biomarkers in individuals with certain types of AB (e.g., AB to palatable food vs. threatening stimuli) may more precisely identify those who binge eat in response to stress versus hedonic cues. It is also possible that these biomarkers moderate the relationship between AB and binge eating subtypes. Additionally, neuroimaging studies may clarify whether different types of AB reflect distinct subtypes of individuals prone to binge eating (i.e., reward-driven positive reinforcement vs. avoidance-driven negative reinforcement) by identifying different neural circuitries associated with these biases (e.g., frontostriatal circuitry for the reward-based, approach-approach subtype versus the corticolimbic circuitry for the approach-avoidance subtype). The investigation of AB as it relates to binge eating would also be enriched by examining the influences of personality traits, impulsivity, and the role of family and culture in individuals’ susceptibility to these various biases. This line of inquiry would likely facilitate more personalized treatments tailored to the needs and circumstances of individual patients, and clarify additional moderators of the effect of AB on binge eating.
Longitudinal Research
All included studies are cross-sectional. There is a critical need to conduct longitudinal studies examining the association of AB with adverse outcomes, such as the development of obesity, binge eating and/or compensatory behaviors. Cross-sectionally, some data support a link between AB and obesity (Roefs & Jansen, 2002; Stoeckel et al., 2008) and disordered eating (Aspen et al., 2013), but longitudinal studies would elucidate the potential mechanisms and trajectories of these phenomena. As excessive weight gain is best prevented when addressed before adulthood (Stice, Shaw, & Marti, 2006) and disordered eating behaviors typically manifest in adolescence (Smink, Van Hoeken, & Hoek, 2012), studies in youth are needed to explore the role of AB in these processes. The addiction literature has reported that longer duration of alcohol use dependence is associated with greater AB (Loeber et al., 2009), and given the normative development of motivational and emotional circuitry across childhood and adolescence— such as increases in inhibitory control, ability to focus attention with age, and emotional volatility (Eisenberg, Smith, & Spinrad, 2011) — youth may exhibit differential patterns of AB than adults. If AB becomes more pronounced over time, intervening to reduce biases early in development may hold greater potential for prevention of binge eating and excess weight gain.
Moderating Factors
Laboratory studies examining the moderating influence of subjective state variables on AB would help clarify the role and stability of AB to food, weight/shape, and threatening cues. Some (Nijs, Franken, & Muris, 2010; Werthmann et al., 2011), but not all (Nijs, Muris, Euser, & Franken, 2010), studies found moderating effects of craving and hunger on AB to food cues in individuals with overweight. More research is needed to clarify these preliminary results. Examining the effects of momentary stress on AB may shed light on eating in response to negative emotions. AB studies included in this review did not employ experimental mood induction paradigms. However, given the variety of mood inductions employed in extant studies, the moderating effects of the type of stressor (e.g., social evaluative stress in the Trier Social Stress Test vs. sad mood induction in the personalized mood induction) on AB in individuals who engage in binge eating warrants exploration. As noted, social evaluative stress may be particularly relevant in eliciting binge eating behavior (Rieger et al., 2010). Thus, investigating social stressors versus other non-social types of stressors may be particularly informative. Inhibitory control deficits have also been implicated in binge eating behaviors (Mobbs et al., 2011; Schag et al., 2013), thus measures of inhibitory control should be included as moderators of the relationship between AB and eating behavior. Finally, it will be important to integrate these moderators to study the interactions between stress, stress reactivity, inhibitory control and palatable food cues, as these phenomena rarely occur in isolation.
Gender should also be considered as a moderator of the relationship between AB and binge eating behavior. Most of the reviewed studies were conducted with females, thus there is a large gap in knowledge regarding these processes among males who engage in binge eating behaviors. Given that nearly a third of individuals with BED are males (Smink et al., 2012), it is essential to include men in future studies to examine the moderating effects of sex. Finally, the moderating effects of culture on response to salient cues may also be examined. Although AB tasks have been employed by research groups internationally (e.g., United States, Canada, Germany and South Korea), there are few considerations given to the salience of specific food cues or socially threatening stimuli across cultures. It will be important to consider the cultural relevance of the cues used in studies across the globe and with diverse populations.
Task Standardization
In order to draw more definitive conclusions about the role of AB in binge eating behaviors, the field would benefit from a more homogeneous approach to measuring AB. For instance, in visual probe tasks, the images are presented at varying latencies (between 200 and 2000 ms), potentially tapping into different attentional processes of attention engagement and sustained attention, but are often interpreted similarly. In interpretation of results, it is important to consider the varying aspects of the tasks and how they may be influencing results. Moreover, not all studies provided enough information to calculate between-group effect sizes. Although we contacted authors for this information, a sizeable number of effect sizes were still unavailable. Such missing data limits the ability to examine effects across studies. Future research should consider presenting findings in a standardized manner and including effect sizes when possible.
It is also important to consider the type of cues used within tasks. Some studies used word-based cues, while others used picture-based cues. It is possible that the magnitude of the AB effect may differ depending on the type of cue. However, there is also a lack of standardization in the cues used within each cue type, which may contribute to inconsistent findings. For example, among Stroop tasks, different studies used various categories of words as threatening stimuli, such as adolescent social context words, threatening words, and negative emotion words. Similarly, food images used across studies vary in color, size, palatability, and content, which may introduce error in measurement of AB to food cues (Blechert, Meule, Busch, & Ohla, 2014). One group studying AB has introduced a database of standardized images collected from researchers globally (Blechert, Meule, et al., 2014). Using standardized images is an important step toward producing results that are comparable across studies, in order for future reviews to conduct meaningful meta-analyses.
Alternatively, it may also be informative to customize the AB tasks as content-specific by using cues that are most salient to each individual. This would allow for studying the intensity of response based on the salience of the cues, which could have implications for interventions aimed at altering attention to these cues. Finally, honing our understanding and use of the innovative methodologies of eye tracking and EEG would allow for examining AB to more ecologically valid complex scenes of food and people (Popien et al., 2015). These methodologies may also eliminate some drawbacks of reaction-time based tasks, such as the time limitation which interferes with assessing avoidance processes. The use of these innovative methods may address the issues of task standardization present in reaction-time research.
Conclusions
Individuals with binge eating appear to exhibit AB, although there is significant heterogeneity in study samples, grouping variables, and AB tasks. AB to food cues may represent oversensitivity to rewarding properties of food, making it a vulnerability and maintenance factor for binge eating. AB to weight/shape cues may be a maintenance factor for binge eating alone and with compensatory behaviors but may function differently in BN vs. binge eating alone groups; however, this requires further study. AB to threatening stimuli warrants study in individuals who engage in binge eating without compensatory behaviors to determine whether it is a common underlying feature across the binge eating spectrum. Longitudinal research should examine whether AB is a risk factor for developing disorders of binge eating. Ultimately, AB may be an important factor to target for intervention and prevention of binge eating behaviors.
Acknowledgments
Research Support: NIH Intramural Research Program Grant 1ZIAHD000641 (to J. Yanovski) from NICHD with supplemental funding from the NIH Bench to Bedside Program; USUHS: R072300415 (to M. Tanofsky-Kraff and A. Waters).
Footnotes
Disclosures: J.A. Yanovski is a Commissioned Officer in the United States Public Health Service (PHS). Disclaimer: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS, USUHS, or the United States Department of Defense (DoD).
References
- Albery IP, Wilcockson T, Frings D, Moss AC, Caselli G, Spada MM. Examining the relationship between selective attentional bias for food-and body-related stimuli and purging behaviour in bulimia nervosa. Appetite. 2016;107:208–212. doi: 10.1016/j.appet.2016.08.006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition. Washington, DC: American Psychiatric Association, Inc; 2013. [Google Scholar]
- Aspen V, Darcy AM, Lock J. A review of attention biases in women with eating disorders. Cognition & Emotion. 2013;27(5):820–838. doi: 10.1080/02699931.2012.749777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychological Inquiry. 1996;7(1):1–15. [Google Scholar]
- Ben-Tovim DI, Walker MK. Further evidence for the Stroop Test as a quantitative measure of psychopathology in eating disorders. International Journal of Eating Disorders. 1991;10(5):609–613. [Google Scholar]
- Ben-Tovim DI, Walker MK, Fok D, Yap E. An adaptation of the Stroop test for measuring shape and food concerns in eating disorders: a quantitative measure of psychopathology? International Journal of Eating Disorders. 1989;8(6):681–687. [Google Scholar]
- Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiology & Behavior. 2009;97(5):537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Black C, Wilson G, Labouvie E, Heffernan K. Selective Processing of Eating Disorder Relevant Stimuli: Does the Stroop Test Provide an Objective Measure of Bulimia Nervosa? International Journal of Eating Disorders. 1997;22(3):329–333. doi: 10.1002/(sici)1098-108x(199711)22:3<329::aid-eat13>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Blechert J, Ansorge U, Tuschen-Caffier B. A Body-Related Dot-Probe Task Reveals Distinct Attentional Patterns for Bulimia Nervosa and Anorexia Nervosa. Journal of Abnormal Psychology. 2010;119(3):575–585. doi: 10.1037/a0019531.supp. [DOI] [PubMed] [Google Scholar]
- Blechert J, Feige B, Joos A, Zeeck A, Tuschen-Caffier B. Electrocortical processing of food and emotional pictures in anorexia nervosa and bulimia nervosa. Psychosomatic Medicine. 2011;73(5):415–421. doi: 10.1097/PSY.0b013e318211b871. [DOI] [PubMed] [Google Scholar]
- Blechert J, Goltsche JE, Herbert BM, Wilhelm FH. Eat your troubles away: electrocortical and experiential correlates of food image processing are related to emotional eating style and emotional state. Biological Psychology. 2014;96:94–101. doi: 10.1016/j.biopsycho.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Blechert J, Meule A, Busch NA, Ohla K. Food-pics: an image database for experimental research on eating and appetite. Frontiers in Psychology. 2014;5(617):1–10. doi: 10.3389/fpsyg.2014.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Nickert T, Caffier D, Tuschen-Caffier B. Social comparison and its relation to body dissatisfaction in bulimia nervosa: evidence from eye movements. Psychosomatic Medicine. 2009;71(8):907–912. doi: 10.1097/PSY.0b013e3181b4434d. [DOI] [PubMed] [Google Scholar]
- Brooks S, Prince A, Stahl D, Campbell IC, Treasure J. A systematic review and meta-analysis of cognitive bias to food stimuli in people with disordered eating behaviour. Clinical Psychology Review. 2011;31(1):37–51. doi: 10.1016/j.cpr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Cardi V, Di Matteo R, Corfield F, Treasure J. Social reward and rejection sensitivity in eating disorders: an investigation of attentional bias and early experiences. The World Journal of Biological Psychiatry. 2013;14(8):622–633. doi: 10.3109/15622975.2012.665479. [DOI] [PubMed] [Google Scholar]
- Channon S, Hemsley D, de Silva P. Selective processing of food words in anorexia nervosa. British Journal of Clinical Psychology. 1988;27(3):259–260. doi: 10.1111/j.2044-8260.1988.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: A critical review. Cognitive Therapy and Research. 2009;33(2):221–234. doi: 10.1007/s10608-007-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cooper MJ, Anastasiades P, Fairburn C. Selective Processing of Eating-, Shape-, and Weight-Related Words in Persons with Bulimia Nervosa. Journal of Abnormal Psychology. 1992;101(2):352–355. doi: 10.1037//0021-843x.101.2.352. [DOI] [PubMed] [Google Scholar]
- Cooper MJ, Fairburn C. Selective Processing of Eating, Weight and Shape Related Words in Patients with Eating Disorders and Dieters. British Journal of Clinical Psychology. 1992;36(2):279–281. doi: 10.1111/j.2044-8260.1992.tb01007.x. [DOI] [PubMed] [Google Scholar]
- Cooper MJ, Fairburn C. Demographic and Clinical Correlates of Selective Information Processing in Patients with Bulimia Nervosa. International Journal of Eating Disorders. 1993;13(1):109–116. doi: 10.1002/1098-108x(199301)13:1<109::aid-eat2260130113>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Cooper MJ, Todd G. Selective Processing of Three Types of Stimuli in Eating Disorders. British Journal of Clinical Psychology. 1997;36(2):279–281. doi: 10.1111/j.2044-8260.1997.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Davidson E, Wright P. Selective processing of weight- and shape-related words in bulimia nervosa: Use of a computerised Stroop test. Eating Behaviors. 2002;3(3):261–273. doi: 10.1016/s1471-0153(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite. 2009;53(1):1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Deluchi M, Costa FS, Friedman R, Gonçalves R, Bizarro L. Attentional bias to unhealthy food in individuals with severe obesity and binge eating. Appetite. 2017;108:471–476. doi: 10.1016/j.appet.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Dozois DJA. Attentional biases in eating disorders: A meta-analytic review of Stroop performance. Clinical Psychology Review. 2004;23(8):1001–1022. doi: 10.1016/j.cpr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Smith CL, Spinrad TL. Effortful Control: Relations with Emotion Regulation, Adjustment, and Socialization in Childhood. In: Vohs KD, Baumeister RF, editors. Handbook of Self-Regulation: Research, Theory, and Applications. New York, NY: Guilford Press; 2011. [Google Scholar]
- Fairburn CG, Cooper P, Cooper M, McKenna F, Anastasiades P. Selective information processing in bulimia nervosa. International Journal of Eating Disorders. 1991;10(4):415–422. [Google Scholar]
- Field AE, Javaras KM, Aneja P, Kitos N, Camargo CA, Jr, Taylor CB, Laird NM. Family, peer, and media predictors of becoming eating disordered. Archives of Pediatrics & Adolescent Medicine. 2008;162(6):574–579. doi: 10.1001/archpedi.162.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97(1):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Flynn SV, McNally RJ. Do Disorder-Relevant Cognitive Biases Endure in Recovered Bulimics? Behavior Therapy. 1999;30(4):541–553. doi: 10.1016/S0005-7894(99)80024-0. [DOI] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99(1):20–35. [PubMed] [Google Scholar]
- Formea GM, Burns GL. Selective processing of food, weight, and body-shape words in nonpatient women with bulimia nervosa: interference on the Stroop task. Journal of Psychopathology and Behavioral Assessment. 1996;18(2):105–118. [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Franken IH. Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(4):563–579. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Giel KE, Teufel M, Friederich HC, Hautzinger M, Enck P, Zipfel S. Processing of pictorial food stimuli in patients with eating disorders--a systematic review. International Journal of Eating Disorders. 2011;44(2):105–117. doi: 10.1002/eat.20785. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Chatzisarantis NL, Alberts H, Anggono CO, Batailler C, Birt AR, … Bruyneel S. A multilab preregistered replication of the ego-depletion effect. Perspectives on Psychological Science. 2016;11(4):546–573. doi: 10.1177/1745691616652873. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Harrison A, Sullivan S, Tchanturia K, Treasure J. Emotional functioning in eating disorders: attentional bias, emotion recognition and emotion regulation. Psychological Medicine. 2010;40(11):1887–1897. doi: 10.1017/S0033291710000036. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychological Bulletin. 1991;110(1):86–108. doi: 10.1037/0033-2909.110.1.86. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=1991-33164-001&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- Hepworth R, Mogg K, Brignell C, Bradley BP. Negative mood increases selective attention to food cues and subjective appetite. Appetite. 2010;54(1):134–142. doi: 10.1016/j.appet.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annual Review of Psychology. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Carlbring P, Ghaderi A, Andersson G. Emotional Stroop via Internet among individuals with eating disorders. Scandinavian Journal of Psychology. 2008;49(1):69–76. doi: 10.1111/j.1467-9450.2007.00606.x. [DOI] [PubMed] [Google Scholar]
- Jones-Chesters M, Monsell S, Cooper P. The Disorder-Salient Stroop Effect as a Measure of Psychopathology in Eating Disorders. International Journal of Eating Disorders. 1998;24(1):65–82. doi: 10.1002/(sici)1098-108x(199807)24:1<65::aid-eat6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kanakam N, Krug I, Raoult C, Collier D, Treasure J. Social and emotional processing as a behavioural endophenotype in eating disorders: a pilot investigation in twins. European Eating Disorders Review. 2013;21(4):294–307. doi: 10.1002/erv.2232. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim K, Lee JH. Time Course of Visual Attention to High-Calorie Virtual Food in Individuals with Bulimic Tendencies. Cyberpsychology, Behavior, and Social Networking. 2016;19(1):28–33. doi: 10.1089/cyber.2015.0090. [DOI] [PubMed] [Google Scholar]
- Koster EH, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: Facilitated engagement, impaired disengagement, and attentional avoidance. Behaviour Research and Therapy. 2006;44(12):1757–1771. doi: 10.1016/j.brat.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Kuhnpast N, Gramann K, Pollatos O. Electrophysiologic evidence for multilevel deficits in emotional face processing in patients with bulimia nervosa. Psychosomatic Medicine. 2012;74(7):736–744. doi: 10.1097/PSY.0b013e31825ca15a. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban MF, editors. Attention and orienting: Sensory and motivational processes. Hilldale, NY: Erlbaum; 1997. [Google Scholar]
- Lee JE, Namkoong K, Jung YC. Impaired prefrontal cognitive control over interference by food images in binge-eating disorder and bulimia nervosa. Neuroscience Letters. 2017;651:95–101. doi: 10.1016/j.neulet.2017.04.054. [DOI] [PubMed] [Google Scholar]
- Leehr EJ, Schag K, Brinkmann A, Ehlis AC, Fallgatter AJ, Zipfel S, … Dresler T. Alleged Approach-Avoidance Conflict for Food Stimuli in Binge Eating Disorder. PLoS One. 2016;11(4):e0152271. doi: 10.1371/journal.pone.0152271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard T, Divac S, Bichindaritz I, Rouer-Saporta S, Samuel-Lajeunesse B. Selective Processing of Eating and Body Words in Restricting-Type Anorexics, Binge-Eating-Type Anorexics, Bulimics, and Control Subjects. Eating and Weight Disorders. 1997;2(1):17–23. doi: 10.1007/BF03339945. [DOI] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and anxiety. 2015;32(6):383–391. doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- Loeber S, Vollstädt - Klein S, Von Der Goltz C, Flor H, Mann K, Kiefer F. Clinical study: attentional bias in alcohol - dependent patients: the role of chronicity and executive functioning. Addiction Biology. 2009;14(2):194–203. doi: 10.1111/j.1369-1600.2009.00146.x. [DOI] [PubMed] [Google Scholar]
- Lokken KL, Marx HM, Ferraro FR. Severity of Bulimic Symptoms is the Best Predictor of Interference on an Emotional Stroop Paradigm. Eating and Weight Disorders. 2006;11(1):38–44. doi: 10.1007/BF03327742. [DOI] [PubMed] [Google Scholar]
- Lovell DM, Williams JM, Hill AB. Selective processing of shape-related words in women with eating disorders, and those who have recovered. British Journal of Clinical Psychology. 1997;36(3):421–432. doi: 10.1111/j.2044-8260.1997.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Lyu Z, Zheng P, Jackson T. Attention Disengagement Difficulties among Average Weight Women Who Binge Eat. European Eating Disorders Review. 2016;24(4):286–293. doi: 10.1002/erv.2438. [DOI] [PubMed] [Google Scholar]
- MacLeod CMA, Tata P. Attentional Bias in Emotional Disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mai S, Gramann K, Herbert BM, Friederich HC, Warschburger P, Pollatos O. Electrophysiological evidence for an attentional bias in processing body stimuli in bulimia nervosa. Biological Psychology. 2015;108:105–114. doi: 10.1016/j.biopsycho.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Maner JK, Holm-Denoma JM, Van Orden KA, Gailliot MT, Gordon KH, Joiner TE., Jr Evidence for attentional bias in women exhibiting bulimotypic symptoms. International Journal of Eating Disorders. 2006;39(1):55–61. doi: 10.1002/eat.20222. [DOI] [PubMed] [Google Scholar]
- McManus F, Waller G, Chadwick P. Biases in the Processing of Different Forms of Threat in Bulimic and Comparison Women. The Journal of Nervous and Mental Disease. 1996;184(9):547–554. doi: 10.1097/00005053-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Meyer C, Waller G, Watson D. Cognitive avoidance and bulimic psychopathology: The relevance of temporal factors in a nonclinical population. International Journal of Eating Disorders. 2000;27(4):405–410. doi: 10.1002/(sici)1098-108x(200005)27:4<405::aid-eat4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Mobbs O, Iglesias K, Golay A, Van der Linden M. Cognitive deficits in obese persons with and without binge eating disorder. Investigation using a mental flexibility task. Appetite. 2011;57(1):263–271. doi: 10.1016/j.appet.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Nijs IMT, Franken IHA, Muris P. Food-related Stroop interference in obese and normal-weight individuals: Behavioral and electrophysiological indices. Eating Behaviors. 2010;11(4):258–265. doi: 10.1016/j.eatbeh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Nijs IMT, Muris P, Euser AS, Franken IHA. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54:243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Stice E, Wade E, Bohon C. Reciprocal relations between rumination and bulimic, substance abuse, and depressive symptoms in female adolescents. Journal of Abnormal Psychology. 2007;116(1):198–207. doi: 10.1037/0021-843X.116.1.198. [DOI] [PubMed] [Google Scholar]
- Ouimet AJ, Gawronski B, Dozois DJA. Cognitive vulnerability to anxiety: A review and an integrative model. Clinical Psychology Review. 2009;29(6):459–470. doi: 10.1016/j.cpr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Naim R, Bakermans-Kranenburg MJ, van IJzendoorn MH, Bar-Haim Y. Content specificity of attention bias to threat in anxiety disorders: a meta-analysis. Clinical Psychology Review. 2015;35:10–18. doi: 10.1016/j.cpr.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Perpiñá C, Hemsley D, Treasure J, de Silva P. Is the selective information processing of food and body words specific to patients with eating disorders? International Journal of Eating Disorders. 1993;14(3):359–366. doi: 10.1002/1098-108x(199311)14:3<359::aid-eat2260140314>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Perpiña C, Leonard T, Treasure J, Bond A, Banos R. Selective processing of food- and body-related information and autonomic arousal in patients with eating disorders. The Spanish Journal of Psychology. 1998;1(1):3–10. [Google Scholar]
- Popien A, Frayn M, von Ranson KM, Sears CR. Eye gaze tracking reveals heightened attention to food in adults with binge eating when viewing images of real-world scenes. Appetite. 2015;91:233–240. doi: 10.1016/j.appet.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rieger E, Van Buren DJ, Bishop M, Tanofsky-Kraff M, Welch R, Wilfley DE. An eating disorder-specific model of interpersonal psychotherapy (IPT-ED): Causal pathways and treatment implications. Clinical Psychology Review. 2010;30(4):400–410. doi: 10.1016/j.cpr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Roefs A, Jansen A. Implicit and explicit attitudes toward high-fat foods in obesity. Journal of Abnormal Psychology. 2002;111(3):517–521. doi: 10.1037//0021-843x.111.3.517. [DOI] [PubMed] [Google Scholar]
- Rofey DL, Corcoran KJ, Tran GQ. Bulimic symptoms and mood predict food relevant Stroop interference in women with troubled eating patterns. Eating Behaviors. 2004;5(1):35–45. doi: 10.1016/s1471-0153(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Ruiz EJC, de Leon MDEP, Diaz JMM. Neuropsychological evaluation in patients with eating disorders. Salud Mental. 2008;31:441–446. [Google Scholar]
- Sarlo M, Ubel S, Leutgeb V, Schienle A. Cognitive reappraisal fails when attempting to reduce the appetitive value of food: an ERP study. Biological Psychology. 2013;94(3):507–512. doi: 10.1016/j.biopsycho.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Schag K, Teufel M, Junne F, Preissl H, Hautzinger M, Zipfel S, Giel KE. Impulsivity in binge eating disorder: food cues elicit increased reward responses and disinhibition. PLoS One. 2013;8(10):e76542. doi: 10.1371/journal.pone.0076542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Lüthold P, Kittel R, Tetzlaff A, Hilbert A. Visual attentional bias for food in adolescents with binge-eating disorder. Journal of Psychiatric research. 2016 doi: 10.1016/j.jpsychires.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Naumann E, Trentowska M, Svaldi J. Attentional bias for food cues in binge eating disorder. Appetite. 2014;80:70–80. doi: 10.1016/j.appet.2014.04.023. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: event-related brain potential studies. In: Anders GEMJJKS, Wildgruber D, editors. Progress in Brain Research. Vol. 156. Elsevier; 2006. pp. 31–51. [DOI] [PubMed] [Google Scholar]
- Seddon K, Waller G. Emotional Processing and Bulimic Psychopathology: Age as a Factor Among Nonclinical Women. International Journal of Eating Disorders. 2000;28(4):364–369. doi: 10.1002/1098-108x(200012)28:4<364::aid-eat3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Shank LM, Tanofsky-Kraff M, Nelson EE, Shomaker LB, Ranzenhofer LM, Hannallah LM, … Yanovski JA. Attentional bias to food cues in youth with loss of control eating. Appetite. 2015;87:68–75. doi: 10.1016/j.appet.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending and a general theory. Psychological Review. 1977;84(2):127–190. [Google Scholar]
- Smeets E, Roefs A, van Furth E, Jansen A. Attentional bias for body and food in eating disorders: increased distraction, speeded detection, or both? Behaviour Research and Therapy. 2008;46(2):229–238. doi: 10.1016/j.brat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Smink FR, Van Hoeken D, Hoek HW. Epidemiology of eating disorders: incidence, prevalence and mortality rates. Current Psychiatry Reports. 2012;14(4):406–414. doi: 10.1007/s11920-012-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JM, Wonderlich SA, Heron KE, Sliwinski MJ, Crosby RD, Mitchell JE, Engel SG. Daily and momentary mood and stress are associated with binge eating and vomiting in bulimia nervosa patients in the natural environment. Journal of Consulting and Clinical Psychology. 2007;75(4):629–638. doi: 10.1037/0022-006X.75.4.629. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: A 2-year prospective investigation. Health Psychology. 2002;21(2):131–138. [PubMed] [Google Scholar]
- Stice E, Shaw H, Marti CN. A meta-analytic review of obesity prevention programs for children and adolescents: The skinny on interventions that work. Psychological Bulletin. 2006;132(5):667–691. doi: 10.1037/0033-2909.132.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JF. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General. 1935;8(6):643–662. [Google Scholar]
- Svaldi J, Caffier D, Tuschen-Caffier B. Attention to ugly body parts is increased in women with binge eating disorder. Psychotherapy and Psychosomatics. 2011;80(3):186–188. doi: 10.1159/000317538. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Caffier D, Tuschen-Caffier B. Automatic and intentional processing of body pictures in binge eating disorder. Psychotherapy and Psychosomatics. 2012;81(1):52–53. doi: 10.1159/000329110. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Tuschen-Caffier B, Peyk P, Blechert J. Information processing of food pictures in binge eating disorder. Appetite. 2010;55(3):685–694. doi: 10.1016/j.appet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Yanovski JA. Loss of control over eating in children and adolescents. In: Striegel-Moore RH, Wonderlich SA, Walsh BT, Mitchell JE, editors. Developing an evidence-based classification of eating disorders: Scientific findings for DSM-5. Washington, DC: American Psychiatric Association Press; 2011. pp. 221–236. [Google Scholar]
- Vannucci A, Theim KR, Kass AE, Trockel M, Genkin B, Rizk M, … Aspen V. What constitutes clinically significant binge eating? Association between binge features and clinical validators in college-age women. International Journal of Eating Disorders. 2013;46(3):226–232. doi: 10.1002/eat.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller G, Quinton S, Watson D. Processing of threat-related information by women with bulimic eating attitudes. International Journal of Eating Disorders. 1995;18(2):189–193. doi: 10.1002/1098-108x(199509)18:2<189::aid-eat2260180212>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Waller G, Ruddock A. Information-processing correlates of reported sexual abuse in eating-disordered and comparison women. Child Abuse & Neglect. 1995;19(6):745–759. doi: 10.1016/0145-2134(95)00032-4. [DOI] [PubMed] [Google Scholar]
- Waller G, Watkins H, Shuck V, McManus F. Bulimic psychopathology and attentional biases to ego threats among non-eating disordered women. International Journal of Eating Disorders. 1996;20(2):169–176. doi: 10.1002/(SICI)1098-108X(199609)20:2<169::AID-EAT7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Werthmann J, Jansen A, Roefs A. Worry or craving? A selective review of evidence for food-related attention biases in obese individuals, eating-disorder patients, restrained eaters and healthy samples. The Proceedings of the Nutrition Society. 2015;74(2):99–114. doi: 10.1017/S0029665114001451. [DOI] [PubMed] [Google Scholar]
- Werthmann J, Roefs A, Nederkoorn C, Mogg K, Bradley BP, Jansen A. Can (not) take my eyes off it: Attention bias for food in overweight participants. Health Psychology. 2011;30(5):561–569. doi: 10.1037/a0024291. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120(1):3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Williamson DA, Muller SL, Reas DL, Thaw JM. Cognitive Bias in Eating Disorders: Implications for Theory and Treatment. Behavior Modification. 1999;23(4):556–577. doi: 10.1177/0145445599234003. [DOI] [PubMed] [Google Scholar]
- Williamson DA, White MA, York-Crowe E, Stewart TM. Cognitive-behavioral theories of eating disorders. Behavior Modification. 2004;28(6):711–738. doi: 10.1177/0145445503259853. [DOI] [PubMed] [Google Scholar]
- Wolz I, Sauvaget A, Granero R, Mestre-Bach G, Baño M, Martín-Romera V, … Roefs A. Subjective craving and event-related brain response to olfactory and visual chocolate cues in binge-eating and healthy individuals. Scientific Reports. 2017;7:41736. doi: 10.1038/srep41736. [DOI] [PMC free article] [PubMed] [Google Scholar]