Abstract
Background & Aims
Patients who receive a colonoscopy from a physician with a low adenoma detection rate are at higher risk of subsequent colorectal cancer. It is unclear what drives the variation across physicians in adenoma detection rate. We describe physician characteristics associated with higher ADR.
Methods
In this retrospective cohort study, a natural language processing system was used to analyze all outpatient colonoscopy examinations and their associated pathology reports from October 2013 to September 2015 for adults 40 and older across physicians from four diverse health systems. Physician performance on adenoma detection rate was risk adjusted for differences in patient population and procedure indication. Our sample included 201 physicians performing at least 30 colonoscopy examinations during the study period, totaling 104,618 colonoscopy examinations.
Results
The mean ADR was 33.2% (range 6.3%-58.7%). Higher ADR was seen among female physicians (4.2 percentage points higher than men, p=0.020), gastroenterologists (9.4 percentage points higher than vs non-gastroenterologists, p<0.001), and physicians with ≤9 years since their residency completion (6.0 percentage points higher than physicians who have had 27 to 51 years of practice, p=0.004).
Conclusions
Gastroenterologists, female physicians, and more recently trained physicians had higher performance in adenoma detection.
Introduction & Background
Colorectal cancer screening that leads to the identification and removal of premalignant adenomatous polyps reduces cancer incidence and mortality.(1-5) Colonoscopy is used both for primary colorectal cancer screening and for follow up testing after a positive fecal test, CT colonography, or sigmoidoscopy. Over 14 million colonoscopies are performed yearly in the United States.(6)
The benefit of colonoscopy in screening for colorectal cancer is limited by lower quality colonoscopy examinations. A common metric of colonoscopy quality is the adenoma detection rate (ADR), which is the percentage of colonoscopy examinations in which an adenoma is found. There is considerable variation in ADR across physicians (7-11) and a physician's ADR is associated with a patient's subsequent colorectal cancer risk.(12) For example, a prior U.S. study of Kaiser Permanente found that patients of physicians in the highest quintile of ADR had a 52% lower rate of interval cancer, colorectal cancer identified in the subsequent to 10 years, compared with patients of physicians in the lowest quintile of ADR.(8)
The factors driving variation in physician ADR remain unclear. Physician characteristics such as specialty training and volume of cases may play a role,(13, 14) but the relationship between these characteristics and quality is not always consistent.(15, 16) Differences in physician performance may also be partially driven by differences in the patient population they treat.(17-20)
Given that prior interventions to improve ADR have been largely unsuccessful,(21) we sought to examine the physician characteristics associated with higher performance to better inform future efforts to improve quality. We measured ADR across multiple health care systems using natural language processing (NLP), a technique from the field of computer science in which software “reads” free text, to automate abstracting the necessary data from both colonoscopy and pathology reports.
Methods
Study Sites
Four health care sites were selected to achieve variation in geography and financial incentive for colonoscopy performance Kaiser Permanente Washington (KPW, formerly Group Health Cooperative) is a staff-model health maintenance organization based in Washington State with 18 gastroenterologists on staff. Central Illinois Endoscopy (CIE) is a private endoscopy center with 11 gastroenterologists in Peoria, Illinois. The University of North Carolina (UNC) is an academic center with 53 gastroenterologists. UPMC is based in Western Pennsylvania with 46 gastroenterologists in its 3 primarily academic hospitals and 73 gastroenterologists in affiliate hospitals and private practices. Each site either used endoscopy specific software (eg, ProVation MD, ProVation Medical, Minneapolis, Minn) or their own EHR systems and locally customized note templates to create the colonoscopy reports. Some reports were also dictated. When we obtained the data from each site we agreed to not publicly report the mean performance at the individual health systems.
Sample of colonoscopy and pathology reports
We analyzed all outpatient colonoscopies and their associated pathology reports from October 1, 2013 to September 30, 2015 for adults 40 and older. We excluded inpatient colonoscopies, procedures for younger adults and children, and colonoscopy on patients with inflammatory bowel disease. Consistent with prior work,(12) we further limited our sample to physicians performing ≥30 examinations over the 2-year period to ensure a sufficient sample size to evaluate a physician's quality. At each site, a software program (De-ID or MITRE MIST(22)) stripped the reports of patient identifiers, and the reports were sent to the University of Pittsburgh for analysis.
Physician Characteristics
To characterize the age, years of practice, and training of physicians, we linked each physician to the Doximity database, a database that has been used in prior research.(23-25) The data are compiled using the National Plan and Provider Enumeration System, National Provider Identifier Registry, self-registered members, and collaborating hospitals and medical schools. Years of practice was measured between 2014 and the year of residency completion. To categorize physician years in practice, we stratified physicians into quartiles, based on the distribution of the sample (≤9 years, 10-18 years, 19-26 years, and 27-51 years).
Extracting Relevant Information from Colonoscopy and Pathology Reports
Relevant data from the colonoscopy and pathology reports were extracted using an NLP system.(26) Details of the development and validation of this tool are reported elsewhere.(11, 26, 27) The NLP system was validated in a sample of 2127 colonoscopy and associated pathology reports which were analyzed both by the NLP system and manually abstracted.(27) The NLP system achieved a high level of accuracy, with accuracy scores of 0.87-0.99 for relevant data fields.(27) See Appendix 1 for additional details on the variables extracted by the NLP system.
Measuring adenoma detection rate
Our primary measure was ADR, defined as the proportion of all colonoscopies where any adenoma, serrated lesion or carcinoma was identified. We focused on ADR as it is the only validated colonoscopy quality measure. Although we recognize serrated polyps have a different biology than adenomas,(28, 29) in practice they are treated similarly in terms of recommended surveillance examination follow-up.(30) In sensitivity analyses we measured ADR where we did not include serrated lesions or carcinomas in the definition (Supplementary Table 1).
To better understand the associations between physician characteristics and adenoma detection, we also examined proximal and distal ADR. Proximal colon was defined as proximal to the splenic flexure or >50 cm from anal verge; distal colon was defined as from the anus to the splenic flexure, or ≤50 cm from anal verge.
We chose not to focus on certain physician quality measures (withdrawal time, cecal intubation rates, adequacy of preparation), because they were inconsistently recorded in the colonoscopy reports and variation in physician performance appeared driven primarily by whether the relevant data was recorded versus variation in performance. We conducted a sensitivity analysis using the outcome of advanced adenoma detection rate. A colonoscopy was classified as having an advanced adenoma if there was (1) a polyp with villous or dysplastic changes, (2) a carcinoma, or (3) an adenoma detected and a polyp greater than or equal to 10 mm.
We included all colonoscopies in our denominator. Prior work has highlighted there is little consistency in prior research on which colonoscopy examinations are included when measuring ADR (based on quality of preparation, complete procedures, screening colonoscopies) and varying exclusion criteria has little impact on a physician's ADR relative ranking.(31) Because gastroenterology specialty societies have advocated for minimum quality thresholds (ADR of 30% in men, 20% in women) for screening colonoscopy examinations,(32) we conducted a sensitivity analysis in which we limited our sample to colonoscopies with a screening indication (Supplementary Tables 2 and 3). We also measure the fraction of physicians who met these specialty society thresholds based on screening colonoscopies alone.
Risk Adjustment
Prior research has found that both patient factors and the indication for the colonoscopy are associated with the likelihood of identifying an adenoma. To facilitate comparison of ADR across physicians who care for different patient populations,(33) we used risk-adjustment. We did this using two different methods. In the first method, we generated a risk-adjusted ADR for each physician using an observed to expected ratio which is the methodology used in many national quality measurement efforts (eg, readmission rates and mortality elsewhere in Medicare).(34) This allowed us to describe the variation in ADR across physicians controlling for differences in patient characteristics. Details of this method for risk adjustment are in Appendix 2.
In the second method of risk adjustment we used a multivariable regression model. Our unit of analysis was the colonoscopy and the predictor variables were patient's age, sex, and colonoscopy indication and physician's sex, years in practice (quartiles), training, and volume of procedures over the 2 years (also stratified into quartiles of the sample). Standard errors were clustered at the physician level to account for multiple colonoscopies performed by the same physician. The odds ratios that we report for this analysis can be interpreted as a measure of the association of a given variable with the likelihood of detecting an adenoma in a colonoscopy, controlling for other variables in the model.
Analyses
We present descriptive results characterizing the sample as well as the detection of adenomas, proximal adenomas and distal adenomas by patient characteristics. To understand whether certain physician characteristics are driving this variation, we used a multivariable linear regression model, with the physician as the unit of the analysis, to describe the relationship between physician predictors (gender, specialty, years in practice and colonoscopy volume) and the outcomes of risk-adjusted ADR, proximal ADR and distal ADR.
All analyses were conducted in Stata version 13.1. This study was approved by the IRB at Harvard Medical School.
Results
Across 104,618 colonoscopy examinations the largest fraction of colonoscopies were performed on adults aged 50 to 59 (36.3%) (Table 1). Screening colonoscopy made up 44.9% of all examinations.
Table 1. Patient characteristics in study cohort.
| Characteristic | All colonoscopies | Screening colonoscopies | ||
|---|---|---|---|---|
| n | % | n | % | |
| Total | 104,618 | - | 46,930 | 44.87 |
|
| ||||
| Site | ||||
| Central Illinois Endoscopy | 12,116 | 11.6 | 5,992 | 12.8 |
| Kaiser Permanente Washington | 10,875 | 10.4 | 3,476 | 7.4 |
| University of North Carolina | 16,641 | 15.9 | 7,893 | 16.8 |
| UPMC | 64,986 | 62.1 | 29,572 | 63.0 |
|
| ||||
| Sex | ||||
| Male | 48,065 | 45.9 | 20,982 | 44.7 |
| Female | 55,325 | 52.9 | 25,356 | 54.0 |
| Missing | 1,229 | 1.2 | 595 | 1.3 |
|
| ||||
| Age | ||||
| 40-492 | 8,485 | 8.1 | 2,300 | 4.9 |
| 50-59 | 37,936 | 36.3 | 23,359 | 49.8 |
| 60-69 | 35,121 | 33.6 | 15,213 | 32.4 |
| 70 and over | 22,814 | 21.8 | 6,015 | 12.8 |
| Missing | 263 | 0.3 | 46 | 0.1 |
|
| ||||
| Race1 | ||||
| White | 62,295 | 76.3 | 27,850 | 74.3 |
| Black | 8,342 | 10.2 | 4,172 | 11.1 |
| Other | 6,455 | 5.6 | 3,153 | 8.4 |
| Missing | 4,535 | 7.9 | 2,290 | 6.1 |
|
| ||||
| Insurance | ||||
| Medicare | 38,065 | 37.6 | 12,386 | 27.3 |
| Private | 56,639 | 56.0 | 29,790 | 65.7 |
| Medicaid | 5,451 | 5.4 | 2,592 | 5.7 |
| Other | 1,030 | 1.0 | 546 | 1.2 |
|
| ||||
| Indication | ||||
| Screening, no family history | 36,989 | 35.4 | 36,989 | 78.8 |
| Screening, with family history3 | 9,944 | 9.5 | 9,944 | 21.2 |
| Surveillance | 32,974 | 31.5 | - | - |
| Diagnostic | 22,013 | 21.0 | - | - |
| Missing | 2,698 | 2.6 | - | - |
|
| ||||
| Fraction with a pathology report | ||||
| Yes | 64,688 | 61.8 | 25,948 | 55.3 |
| No | 39,930 | 38.2 | 20,985 | 44.7 |
Notes:
Race data not available for 2 of the 4 clinical sites and therefore was not used in other analyses.
Patients are classified as having a family history if they have any relative with a history of colorectal cancer or an adenoma.
Among 40- to 49-year-olds with screening colonoscopies, 68% were with family history and 32% were without family history
The 201 physicians were predominantly male (81.6%) and trained as gastroenterologists (85.6%) (Table 2). Non-gastroenterologists were general or colorectal surgeons (n=26), family practice physicians (n=2), and the sample included one thoracic surgeon. Physicians' years in practice ranged from 3 to 51 years. The maximum number of colonoscopies performed by a physician over the 2-year period was 2,654. Compared with all gastroenterologists nationally, the 201 physicians in our sample were more likely with be female (18.4% vs 14.8%,) and to have ≤9 years of practice (26.4% vs 19.2%) (Appendix 3).
Table 2. Physicians characteristics in study cohort (n=201).
| N | % | |
|---|---|---|
|
| ||
| Site | ||
| Central Illinois Endoscopy | 11 | 5.5 |
| Kaiser Permanente Washington | 18 | 9.0 |
| University of North Carolina | 53 | 26.4 |
| University of Pittsburgh Medical Center | 119 | 59.2 |
|
| ||
| Sex | ||
| Male | 164 | 81.6 |
| Female | 37 | 18.4 |
|
| ||
| Primary specialty | ||
| Gastroenterology | 172 | 85.6 |
| Other | 29 | 14.4 |
|
| ||
| Years in practice | ||
| ≤9 | 53 | 26.4 |
| 10-18 | 49 | 24.4 |
| 19-26 | 51 | 25.4 |
| 27-51 | 48 | 23.9 |
|
| ||
| Number of colonoscopies performed over 2 year period | ||
| 30-115 | 51 | 25.4 |
| 116-278 | 50 | 24.9 |
| 279-771 | 50 | 24.9 |
| 772-2654 | 50 | 24.9 |
There was a higher likelihood of identifying an adenoma (any, distal, proximal) among older patients and men (Table 3). Physicians were most likely to identify an adenoma in surveillance colonoscopies.
Table 3. Detection of pre-cancerous lesions used in quality metrics by patient characteristics (unadjusted).
| Number of Colonoscopies (n) | Adenoma Detection Rate (%) | Proximal ADR (%) | Distal ADR (%) | |
|---|---|---|---|---|
|
| ||||
| Overall | 104,619 | 35.0 | 24.9 | 12.7 |
|
| ||||
| Sex | ||||
| Female | 55,325 | 29.9 | 21.0 | 10.4 |
| Male | 48,065 | 40.9 | 29.4 | 15.3 |
|
| ||||
| Age | ||||
| 40-49 | 8,652 | 22.1 | 13.4 | 9.1 |
| 50-59 | 37,936 | 31.5 | 21.0 | 12.2 |
| 60-69 | 35,121 | 37.5 | 27.5 | 13.0 |
| 70+ | 22,814 | 42.3 | 17.9 | 14.4 |
|
| ||||
| Indication | ||||
| Screening, no family history | 36,989 | 32.0 | 22.0 | 12.3 |
| Screening, with family history | 9,944 | 30.5 | 20.9 | 11.2 |
| Surveillance | 32,947 | 45.3 | 34.3 | 14.9 |
| Diagnostic | 22,013 | 28.1 | 18.7 | 11.2 |
| Missing | 2,698 | 24.6 | 17.1 | 8.6 |
Note: Adenomas include conventional adenomas, serrated polyps and carcinomas.
Variation in ADR across physicians
There was considerable variation in ADR across physicians after controlling for differences in patient population (Figure 1). The means and range of risk-adjusted physician performance were 33.2% (6.3 to 58.7%) for ADR, 22.7% (3.3% to 45.8%) for proximal ADR and 13.7% (1.7% to 36.4%) for distal ADR. The coefficient of variation, a measure of variability, was lowest for overall ADR (31.7) and was slightly higher for distal ADR (42.1) than proximal ADR (40.3) (Figure 1). Similar levels of variation were observed when the sample was limited to screening colonoscopies (Supplementary Table 3).
Figure 1.
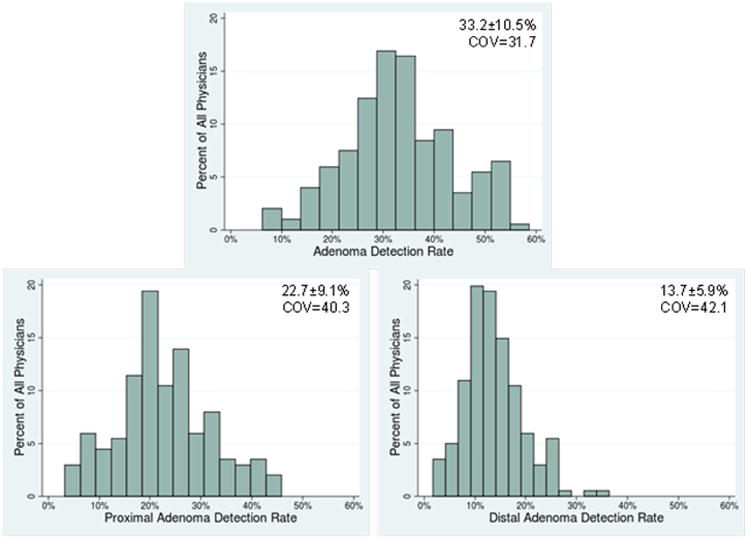
Distribution of Physician Performance across Quality Measures (Mean ± standard deviation along with coefficient of variation shown in each panel)
Notes: (1) All outcomes are for n=201 physicians. Physician performance was risk-adjusted for differences in patient populations
(2) COV = coefficient of variation
Of the 201 physicians in our sample, 112 (56%) met both the screening colonoscopy targets set by American Society for Gastrointestinal Endoscopy of 20% ADR for women and 30% ADR for men; an additional 47 (23%) met the threshold for one sex but not the other.
The median physician withdrawal time at the clinical site where we have these data was 10.9 minutes (25th percentile 9.0 minutes, 75th percentile 11.9 minutes). We observe little correlation between risk-adjusted ADR and physician withdrawal time (r=0.34, p=0.92).
Physician characteristics associated with higher ADR
In our multivariate logistic regression controlling for patient age, sex and colonoscopy indication, an adenoma was more likely to be identified if the endoscopist was female (OR, 1.26; 95% CI, 1.004 - 1.59), trained in gastroenterology (OR, 1.71; 95% CI, 1.38 -2.12), or had 9 or fewer years of practice since residency (OR, 1.45; 95% CI, 1.16 - 1.82 vs physicians with 27-51 years of practice) (Table 4; see Appendix 4 for unadjusted results).
Table 4. Association between detection of cancer precursors and physician characteristics.
| N | Adenoma Detected OR (95% CI) | Proximal Adenoma Detected OR (95% CI) | Distal Adenoma Detected OR (95% CI) | |
|---|---|---|---|---|
|
|
||||
| Physician Sex | ||||
| Female | 37 | 1.26 (1.004, 1.59) | 1.19 (0.97, 1.46) | 1.25 (1.02, 1.52) |
| Male | 164 | Ref | Ref | Ref |
| Primary specialty | ||||
| Gastroenterology | 172 | 1.71 (1.38, 2.12) | 2.03 (1.57, 2.62) | 1.28 (1.07, 1.53) |
| Other | 29 | Ref | Ref | Ref |
| Years in Practice | ||||
| ≤9 | 53 | 1.45 (1.16, 1.82) | 1.55 (1.22, 1.97) | 1.24 (1.004, 1.52) |
| 10-18 | 49 | 1.27 (0.99, 1.63) | 1.24 (0.97, 1.59) | 1.02 (0.79, 1.31) |
| 19-26 | 51 | 1.18 (0.95, 1.48) | 1.25 (0.995, 1.58) | 1.05 (0.82, 1.36) |
| 27-51 | 48 | Ref | Ref | Ref |
| Colonoscopies performed2 | ||||
| 30-115 | 51 | Ref | Ref | Ref |
| 116-278 | 50 | 0.90 (0.74, 1.09) | 0.93 (0.76, 1.15) | 0.81 (0.63, 1.03) |
| 279-771 | 50 | 0.88 (0.72, 1.08) | 0.89 (0.71, 1.11) | 0.74 (0.58, 0.94) |
| 772-2654 | 50 | 1.08 (0.88, 1.31) | 1.12 (0.92, 1.37) | 0.84 (0.66, 1.07) |
Notes:
Multivariable models include patient age, sex, colonoscopy indication, physician years in practice, gender, specialty, and volume. Standard errors are clustered at the physician level.
Volume of colonoscopies performed is measured over two-year period
Similar associations were observed between proximal and distal ADR and physician characteristics. Female physicians were more likely than male physicians to identify distal adenomas (OR, 1.25; 95% CI, 1.02 - 1.52) (Table 4). For gastroenterologists and more recent graduates from residency, the magnitude of the association was greater for proximal adenomas than distal adenomas. Gastroenterologists were more likely to identify both proximal (OR, 2.03; 95% CI, 1.57 - 2.62) and distal adenomas (OR, 1.28; 95% CI, 1.07 - 1.53). Physicians with 9 or fewer years of practice since residency competition were also more likely to identify proximal (OR, 1.55; 95% CI, 1.22 - 1.97) and distal adenomas (OR, 1.24; 95% CI, 1.0004 - 1.52) compared with physicians with 27 to 51 years of practice. There was no consistent relationship between volume of colonoscopies and adenoma detection.
To better illustrate the magnitude of these odds ratios, we calculated the percentage point differences in ADR across physician characteristics using a multivariable linear regression model. Consistent with the results presented above, higher ADR was seen among female physicians (3.8 percentage points higher than male physicians, p=0.04), gastroenterologists (9.6 percentage points higher than non-gastroenterologists, p<0.001), and physicians with ≤9 years of practice (6.0 percentage points higher than physicians with 27-51 years of practice, p=0.004) (Figure 2).
Figure 2.
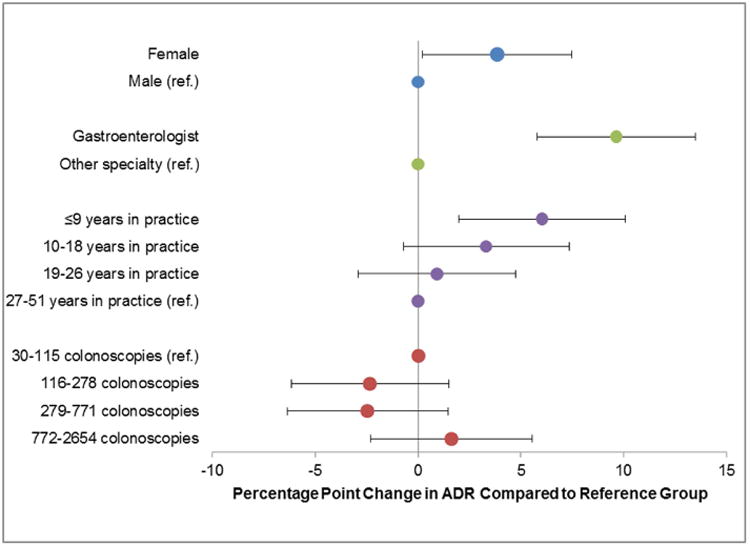
Association between physician characteristics and performance on adenoma detection rate
Notes: (1) Reference groups are: gender: male, specialty: non-gastroenterologist, years in practice: 27-51, colonoscopy volume: 30-115
(2) Percentage point differences and standard errors come from a multivariable linear regression in which the outcome was the physician's adenoma detection rate and predictor variables were physician gender, specialty, years in practice and colonoscopy volume. The physician's adenoma detection rate was risk-adjusted for differences in patient population.
We conducted a series of sensitivity analyses: (1) limited sample to screening colonoscopies, (2) excluded colonoscopies with inadequate preparation or where the procedure was incomplete, (3) excluded adults over 80, (4) and limited to only the first colonoscopy (when patients who had more than one colonoscopy). The direction and the magnitude of the association between ADR and physician characteristics were similar (Supplementary Table 3). The associations between advanced ADR and physician gender, specialty, and years of practice were attenuated relative to the association between ADR and these characteristics (Appendix 5).
Discussion
We found considerable variation in physician ADR in a sample of over 200 physicians across four health systems. Female physicians, gastroenterologists, and those with fewer years in practice had higher performance on ADR whereas volume of procedures was unrelated to ADR. Our results on the prevalence of adenomas by patient characteristics are consistent with prior work (35, 36).
In our sample, female physicians detected roughly 10% more adenomas than male physicians; their average risk-adjusted ADR was 3.8 percentage points higher than the average risk-adjusted ADR among male physicians (36.3% vs 32.5%, after adjusting for differences in patient characteristics and other physician characteristics). Although the differences are only marginally statistically significant, they are consistent with recent work in which patients treated by female hospitalists had 0.42 percentage point lower 30-day mortality rates than patients treated by male physicians.(37) A deliberate and meticulous approach to colonoscopy may facilitate achievement of a high ADR,(38) and this method may be more common among female physicians. This is supported by research showing that female physicians are more likely to adhere to clinical guidelines(39, 40) and to provide preventive care.(41-43) In addition, research in other fields has shown men to be more risk-seeking,(44, 45) which may undermine the deliberate approach needed for adenoma detection. Sex differences in color perception may make it easier for female physicians to identify adenomas.(46, 47)
The improved detection of adenomas by physicians with fewer years in practice is echoed in research outside gastroenterology. A systematic review found that physician years in practice is often negatively associated with quality of care.(48) These differences in colonoscopy quality could be driven by improved fellowship training, higher likelihood of using newer equipment, or simply decay of performance with age. Similar to others(13, 14, 16, 49, 50) we find that non-gastroenterologists have lower ADR. Future work that explores what drives the associations between physician characteristics and performance might provide useful insights on how to improve the care provided by all physicians.
Our study has several strengths. This is one of the first studies to use NLP to measure quality across multiple, geographically dispersed healthcare systems with varying electronic repositories and systems for documentation. Consistent with prior research in the Veterans Administration(51) and the Kaiser Permanente system,(8) our study demonstrates that the automated evaluation of colonoscopy and pathology reports through NLP could be used to regularly measure physician ADR. Instead of excluding non-screening colonoscopies, we used risk adjustment, a technique commonly used in comparing physician and hospital performance,(8, 33, 34) to address differences in patient populations. Avoiding exclusion criteria increases the number of colonoscopies used to assess physician performance and reduces the potential for physicians to game their documentation (eg, stating bowel preparation is inadequate) in order to improve their performance.(52) (53) The risk adjustment methods we describe could be used by the gastroenterology community when profiling physicians on ADR or other quality metrics.
Our study also has several important limitations. The physicians in our sample may not be representative of the larger community of physicians who perform colonoscopies in and outside the United States. Our sample of gastroenterologists is comparable in gender but has fewer years in practice than the overall U.S. population of gastroenterologists (Appendix 3). Also, although this is one of the largest studies of colonoscopy quality in terms of number of physicians conducted in the United States, our sample only includes 201 physicians. Although we accounted for differences in patient characteristics such as age, gender, and procedure indication, it is possible that there are unmeasured differences in patients that might explain some of the differences in ADR observed across the physicians. We also could not measure other physician factors that might explain some of the variation we observed, such as type of endoscopes used.
In conclusion, across a large sample of physicians in multiple health systems we find non-gastroenterologists, male physicians, and physicians with more years of practice had significantly worse ADR than their counterparts. Efforts to target physicians with lower quality performance are needed.
Supplementary Material
Supplementary Table 1: Adenoma detection rate, excluding serrated lesions and carcinomas, by selected patient characteristics (unadjusted)
Supplementary Table 2. Sensitivity analyses for relationship between physician characteristics and adenoma detection rate with varying exclusion criteria as well as model specifications
Supplementary Table 3: Variation in physician quality among all colonoscopies (top panel, main results from manuscript) and screening colonoscopies (bottom panel, sensitivity analysis)
Supplementary Table 4. Details of risk adjustment for 5 highest-volume physicians
Supplementary Table 5. Risk adjustment models for patient characteristics associated with detection of pre-cancerous lesions (n=101,761)
Acknowledgments
This research was supported by the National Cancer Institute (5R01CA168959).
Glossary
- ADR
Adenoma Detection Rate
- NLP
Natural Language Processing
- KPW
Kaiser Permanente Washington
- CIE
Central Illinois Endoscopy
- UNC
University of North Carolina
- UPMC
University of Pittsburgh Medical Center
- OR
Odds Ratio
- 95% CI
95% Confidence Interval
- COV
Coefficient of Variation
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 2.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. The New England Journal of Medicine. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holme Ø, Løberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: A randomized clinical trial. JAMA. 2014;312:606–615. doi: 10.1001/jama.2014.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segnan N, Armaroli P, Bonelli L, et al. Once-Only Sigmoidoscopy in Colorectal Cancer Screening: Follow-up Findings of the Italian Randomized Controlled Trial— SCORE. JNCI: Journal of the National Cancer Institute. 2011;103:1310–1322. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 5.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. The Lancet. 348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 6.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC's survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 8.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. The New England Journal of Medicine. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boroff ES, Gurudu SR, Hentz JG, et al. Polyp and Adenoma Detection Rates in the Proximal and Distal Colon. American Journal of Gastroenterology. 2013;108:993–999. doi: 10.1038/ajg.2013.68. [DOI] [PubMed] [Google Scholar]
- 10.Shaukat A, Oancea C, Bond JH, et al. Variation in Detection of Adenomas and Polyps by Colonoscopy and Change Over Time With a Performance Improvement Program. Clinical Gastroenterology and Hepatology. 2009;7:1335–1340. doi: 10.1016/j.cgh.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Mehrotra A, Dellon ES, Schoen RE, et al. Applying a natural language processing tool to electronic health records to assess performance on colonoscopy quality measures. Gastrointestinal Endoscopy. 2012;75:1233–1239 e14. doi: 10.1016/j.gie.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 13.Rabeneck L, Paszat LF, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8:275–9. doi: 10.1016/j.cgh.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 14.Bressler B, Paszat LF, Chen Z, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Lamiraud K, Holly A, Burnand B, et al. The effect of nonmedical factors on variations in the performance of colonoscopy among different health care settings. Med Care. 2010;48:101–9. doi: 10.1097/MLR.0b013e3181c160ee. [DOI] [PubMed] [Google Scholar]
- 16.Baxter NN, Sutradhar R, Forbes SS, et al. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson DA, Gurney MS, Volpe RJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. American Journal of Gastroenterology. 1990;85:969–74. [PubMed] [Google Scholar]
- 19.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. American Journal of Gastroenterology. 1991;86:946–51. [PubMed] [Google Scholar]
- 20.Rex DK, Lehman GA, Ulbright TM, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. American Journal of Gastroenterology. 1993;88:825–31. [PubMed] [Google Scholar]
- 21.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointestinal Endoscopy. 2011;74:656–65. doi: 10.1016/j.gie.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Aberdeen J, Bayer S, Yeniterzi R, et al. The MITRE Identification Scrubber Toolkit: Design, training, and assessment. International Journal of Medical Informatics. 2010;79:849–859. doi: 10.1016/j.ijmedinf.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein MJ, Lunn MR, Peng L. What Makes a Top Research Medical School? A Call for a New Model to Evaluate Academic Physicians and Medical School Performance. Academic Medicine. 2015;90:603–608. doi: 10.1097/ACM.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 24.Blumenthal DM, Olenski AR, Yeh RW, et al. Sex Differences in Faculty Rank Among Academic Cardiologists in the United States. Circulation. 2017;135:506–517. doi: 10.1161/CIRCULATIONAHA.116.023520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jena AB, Olenski AR, Blumenthal DM. Sex Differences in Physician Salary in US Public Medical Schools. JAMA Internal Medicine. 2016;176:1294–1304. doi: 10.1001/jamainternmed.2016.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harkema H, Chapman WW, Saul M, et al. Developing a natural language processing application for measuring the quality of colonoscopy procedures. Journal of the American Medical Informatics Association : JAMIA. 2011;18(1):i150–6. doi: 10.1136/amiajnl-2011-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carrell DS, Schoen RE, Leffler DA, et al. Challenges in adapting existing clinical natural language processing systems to multiple, diverse healthcare settings. Journal of the American Medical Informatics Association. 2017;ocx039 doi: 10.1093/jamia/ocx039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leggett B, Whitehall V. Role of the Serrated Pathway in Colorectal Cancer Pathogenesis. Gastroenterology. 2010;138:2088–2100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 29.Snover DC. Update on the serrated pathway to colorectal carcinoma. Human Pathology. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Marcondes FO, Dean KM, Schoen RE, et al. The impact of exclusion criteria on a physician's adenoma detection rate. Gastrointestinal Endoscopy. 2015;82:668–675. doi: 10.1016/j.gie.2014.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointestinal Endoscopy. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 33.Jensen CD, Doubeni CA, Quinn VP, et al. Adjusting for Patient Demographics Has Minimal Effects on Rates of Adenoma Detection in a Large, Community-based Setting. Clinical Gastroenterology and Hepatology. 2015;13:739–746. doi: 10.1016/j.cgh.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucholz EM, Butala NM, Ma S, et al. Life Expectancy after Myocardial Infarction, According to Hospital Performance. New England Journal of Medicine. 2016;375:1332–1342. doi: 10.1056/NEJMoa1513223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corley DA, Jensen CD, Marks AR, et al. Variation of Adenoma Prevalence by Age, Sex, Race, and Colon Location in a Large Population: Implications for Screening and Quality Programs. Clinical Gastroenterology and Hepatology. 2013;11:172–180. doi: 10.1016/j.cgh.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointestinal Endoscopy. 2011;74:135–140. doi: 10.1016/j.gie.2011.03.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsugawa Y, Jena AB, Figueroa JF, et al. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA internal medicine. 2016 doi: 10.1001/jamainternmed.2016.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rex DK. Who is the best colonoscopist? Gastrointest Endosc. 2007;65:145–50. doi: 10.1016/j.gie.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 39.Kim C, McEwen LN, Gerzoff RB, et al. Is Physician Gender Associated With the Quality of Diabetes Care? Diabetes Care. 2005;28:1594–1598. doi: 10.2337/diacare.28.7.1594. [DOI] [PubMed] [Google Scholar]
- 40.Berthold HK, Gouni-Berthold I, Bestehorn KP, et al. Physician gender is associated with the quality of type 2 diabetes care. Journal of Internal Medicine. 2008;264:340–350. doi: 10.1111/j.1365-2796.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 41.Andersen MR, Urban N. Physician Gender and Screening: Do Patient Differences Account for Differences in Mammography Use? Women and Health. 1997;26:29–39. doi: 10.1300/J013v26n01_03. [DOI] [PubMed] [Google Scholar]
- 42.Franks P, Bertakis KD. Physician Gender, Patient Gender, and Primary Care. Journal of Women's Health. 2003;12:73–80. doi: 10.1089/154099903321154167. [DOI] [PubMed] [Google Scholar]
- 43.Smith AW, Borowski LA, Liu B, et al. U.S. Primary Care Physicians' Diet-, Physical Activity–, and Weight-Related Care of Adult Patients. American Journal of Preventive Medicine. 2011;41:33–42. doi: 10.1016/j.amepre.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell M, Ansic D. Gender differences in risk behaviour in financial decision-making: An experimental analysis. Journal of Economic Psychology. 1997;18:605–628. [Google Scholar]
- 45.Charness G, Gneezy U. Strong Evidence for Gender Differences in Risk Taking. Journal of Economic Behavior & Organization. 2012;83:50–58. [Google Scholar]
- 46.Shibasaki M, Masataka N. The color red distorts time perception for men, but not for women. Scientific Reports. 2014;4:5899. doi: 10.1038/srep05899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.RodrÍguez-Carmona M, Sharpe LT, Harlow JA, et al. Sex-related differences in chromatic sensitivity. Visual Neuroscience. 2008;25:433–440. doi: 10.1017/S095252380808019X. [DOI] [PubMed] [Google Scholar]
- 48.Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142:260–73. doi: 10.7326/0003-4819-142-4-200502150-00008. [DOI] [PubMed] [Google Scholar]
- 49.Baxter NN, Warren JL, Barrett MJ, et al. Association Between Colonoscopy and Colorectal Cancer Mortality in a US Cohort According to Site of Cancer and Colonoscopist Specialty. Journal of Clinical Oncology. 2012;30:2664–2669. doi: 10.1200/JCO.2011.40.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang M, Sewitch MJ, Barkun AN, et al. Endoscopist specialty is associated with colonoscopy quality. BMC Gastroenterology. 2013;13:78–78. doi: 10.1186/1471-230X-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imler TD, Morea J, Kahi C, et al. Multi-Center Colonoscopy Quality Measurement Utilizing Natural Language Processing. American Journal of Gastroenterology. 2015;110:543–552. doi: 10.1038/ajg.2015.51. [DOI] [PubMed] [Google Scholar]
- 52.Gerard DP, Foster DB, Raiser MW, et al. Validation of a New Bowel Preparation Scale for Measuring Colon Cleansing for Colonoscopy: The Chicago Bowel Preparation Scale. Clin Trans Gastroenterol. 2013;4:e43. doi: 10.1038/ctg.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Narins CR, Dozier AM, Ling FS, et al. The influence of public reporting of outcome data on medical decision making by physicians. Archives of Internal Medicine. 2005;165:83–87. doi: 10.1001/archinte.165.1.83. [DOI] [PubMed] [Google Scholar]
- 54.Hong S, Cai Q, Chen D, et al. Abdominal Obesity and the Risk of Colorectal Adenoma: a Metaanalysis of Observational Studies. European Journal of Cancer Prevention. 2012;21:523–531. doi: 10.1097/CEJ.0b013e328351c775. [DOI] [PubMed] [Google Scholar]
- 55.Ben Q, An W, Jiang Y, et al. Body Mass Index Increases Risk for Colorectal Adenomas Based on Meta-analysis. Gastroenterology. 2012;142:762–772. doi: 10.1053/j.gastro.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 56.Murphy CC, Martin CF, Sandler RS. Racial Differences in Obesity Measures and Risk of Colorectal Adenomas in a Large Screening Population. Nutrition and Cancer. 2015;67:98–104. doi: 10.1080/01635581.2015.976316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lebwohl B, Capiak K, Neugut AI, et al. Risk of colorectal adenomas and advanced neoplasia in Hispanic, black and white patients undergoing screening colonoscopy. Alimentary Pharmacology and Therapeutics. 2012;35:1467–1473. doi: 10.1111/j.1365-2036.2012.05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butterly L, Robinson CM, Anderson JC, et al. Serrated and Adenomatous Polyp Detection Increases With Longer Withdrawal Time: Results From the New Hampshire Colonoscopy Registry. American Journal of Gastroenterology. 2014;109:417–426. doi: 10.1038/ajg.2013.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark BT, Protiva P, Nagar A, et al. Quantification of Adequate Bowel Preparation for Screening or Surveillance Colonoscopy in Men. Gastroenterology. 2016;150:396–405. doi: 10.1053/j.gastro.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Adenoma detection rate, excluding serrated lesions and carcinomas, by selected patient characteristics (unadjusted)
Supplementary Table 2. Sensitivity analyses for relationship between physician characteristics and adenoma detection rate with varying exclusion criteria as well as model specifications
Supplementary Table 3: Variation in physician quality among all colonoscopies (top panel, main results from manuscript) and screening colonoscopies (bottom panel, sensitivity analysis)
Supplementary Table 4. Details of risk adjustment for 5 highest-volume physicians
Supplementary Table 5. Risk adjustment models for patient characteristics associated with detection of pre-cancerous lesions (n=101,761)


