Abstract
Farnesol, a sesquiterpene alcohol, potentiates the activity of β-lactam antibiotics against antibiotic-resistant bacteria. We document that farnesol and two synthetic derivatives (compounds 2 and 6) have poor antibacterial activities of their own, but they potentiate the activities of ampicillin and oxacillin against Staphylococcus aureus strains (including methicillin-resistant S. aureus). These compounds attenuate the rate of growth of bacteria, which has to be taken into account in assessment of the potentiation effect.
Keywords: Farnesol, β-Lactam antibiotics, Methicillin-resistant Staphylococcus aureus (MRSA), Antimicrobial activity, Potentiation
Graphical Abstract
Farnesol (1), a sesquiterpene, has been shown to exhibit a measurable antimicrobial activity against certain bacteria1–6 and it inhibits biofilm formation of Staphylococcus spp.7, 8 Furthermore, it has been reported to potentiate the activity of β-lactam antibiotics.3, 6 The mechanisms of action of these activities are not well understood. These activities are not unanticipated as farnesol is a chemical constituent of farnesyl pyrophosphate, which is ultimately converted to undecaprenyl pyrophosphate, the key bacterial lipid carrier. Lipid II (Scheme 1A), the precursor of the peptidoglycan, the major constituent of the cell wall, contains undecaprenyl pyrophosphate. All prenyl-containing metabolites, including undecaprenol, are present in limited quantity in bacteria, hence the biosynthetic pathways that utilize them are potential targets of antibiotics.
Scheme 1.
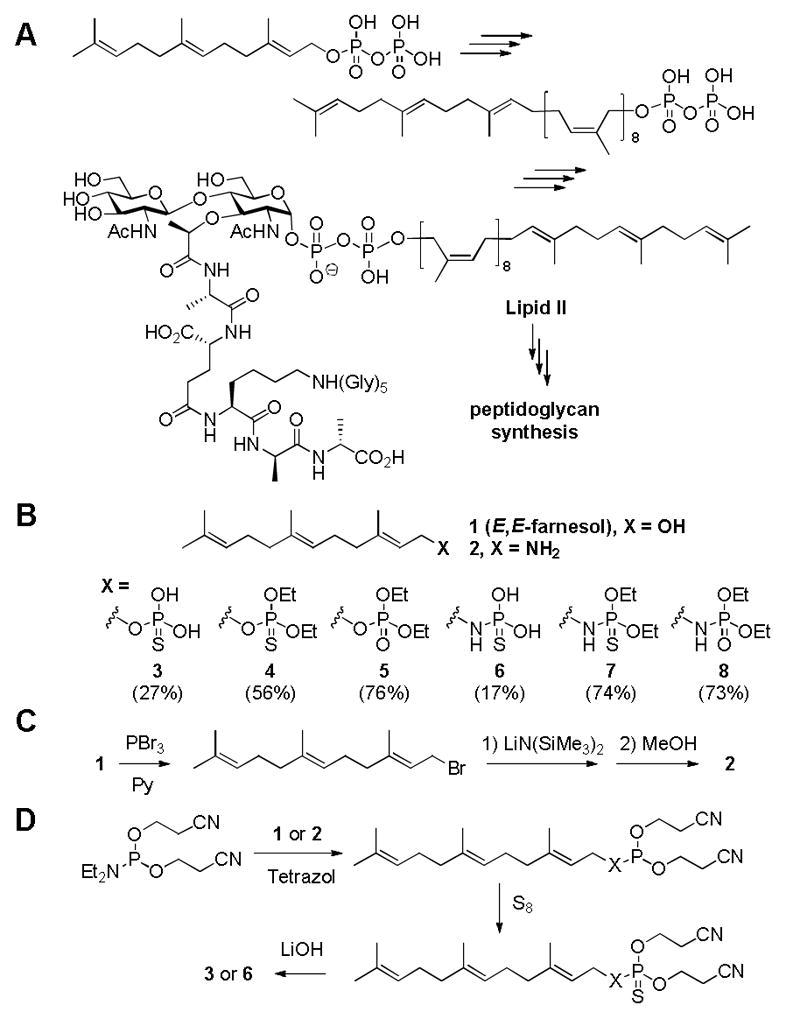
(A) Lipid II, anchored to the membrane by the undecaprenyl moiety, is the precursor of peptidoglycan. (B) Chemical structures of farnesyl derivatives used in this study. Overall synthetic yields are in parentheses. (C, D) Syntheses of farnesyl amine (2) and thiophosphoryl derivative.
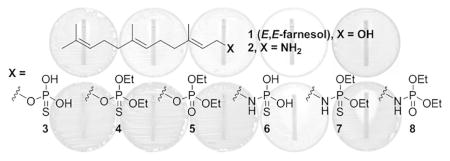
The report of potentiation of β-lactam activity by farnesol intrigued us.3, 6 We wondered since farnesol, a substrate for several enzymes, exhibited some antibacterial activity, whether structural variants of farnesol could in principle be even better, as they would potentially inhibit enzymes that would bind them as mimetics of farnesol and its phosphorylated derivatives. As an effort to explore this possibility, seven derivatives of E,E-farnesol were synthetically prepared (Scheme 1B). First, farnesyl amine (2) was prepared by a literature method,9 as shown in Scheme 1C. Briefly, the hydroxyl group was converted to the corresponding bromo derivative, which was substituted with an amine by the use of lithium bis(trimethylsilyl)amide. Subsequently, both the alcohol in farnesol and amine in compound 2 were further derivatized with various phosphoryl or thiophosphoryl chlorides to yield compounds 4, 5, 7, and 8 using a general literature procedure.10 The synthesis of compounds 3 and 6 are given as representative examples in Scheme 1D.11 The overall synthesis of thiophosphate derivatives was similar to that of farnesylphosphate using a base-labile cyanoethyl group as the protective group. The insertion of sulfur was accomplished by a literature method using sulfur powder.12, 13 Among them, compounds 2 and 6 (discussed below) showed improved MIC values compared to the parental farnesol. These two were selected for the study of potentiation with ampicillin (AMP) and oxacillin (OXA), both penicillins.
Three methicillin-resistant Staphylococcus aureus (MRSA) strains (NRS100, NRS123 and NRS70) and one methicillin-susceptible S. aureus (MSSA) strain (ATCC29213) were used to determine susceptibility to farnesol and its derivatives. The MIC values were examined by the micro-dilution method using cation-adjusted Mueller-Hinton II medium (Difco, USA) supplemented with 1% Tween-80 (CAMHB-T), as described by Kuroda and colleagues.3 The concentration range was from 2 to 2048 μg/mL for all compounds. The MIC value of the parental farnesol was decidedly poor at 2048 μg/mL, consistent with the data that had been reported.3 Compounds 2 and 6 (among the synthetic derivatives) exhibited improved yet modest MICs. The MIC values of Compound 2 were 256 μg/mL for strains NRS100 and NRS70, and 512 μg/mL for strains ATCC29213 and NRS123, whereas compound 6 exhibited MIC of 512 μg/mL to all strains. The MIC values of farnesol compounds could not be determined without Tween-80 since they are immiscible with the media. Tween-80 at 1% of concentration did not affect the MICs of β-lactam antibiotics.
In order to investigate potentiation of β-lactam antibiotics—we used ampicillin (AMP) and oxacillin (OXA)—by farnesol and its derivative, the MIC determination was performed using the Etest (bioMereux, USA) on CAMHB-T agar plates containing farnesol derivatives at half-MIC concentrations: farnesol, 1000 μg/mL for all strains; compound 6, 256 μg/mL for all strains; compound 2, 128 μg/mL for NRS100 and NRS70 and 256 μg/mL for ATCC29213 and NRS123. The results are given in Table 1.
Table 1.
| S. aureus strain | SCCmec type | MIC (μg/mL) of ampicillin | MIC (μg/mL) of oxacillin | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| None | Farnesol | 2d | 6 | None | Farnesol | 2d | 6 | ||
| ATCC29213 | 0.5 | 0.5 (1) | 0.2 (2.5) | 0.5 (1) | 0.4 | 0.5 (0.8) | 0.5 (0.8) | 0.4 (1) | |
| NRS100 (COL) | I | 16 | 4 (4)e | 8 (2) | 3 (5.3)e | >256 | 8 (>32)e | >256 (−) | 2 (>128)e |
| NRS70 (N315) | II | 16 | 4 (4)e | 16 (1) | 6 (2.7)e | 32 | 12 (2.7)e | 32 (1) | 1 (32)e |
| NRS123 (MW2) | IV | 12 | 3 (4)e | 1 (12)e | 8 (1.5) | 24 | 2 (12)e | 0.75 (32)e | 16 (1.5) |
MIC was determined by Etest on CAMHB-T agar without/with half-MIC of farnesol or its derivatives: 1000 μg/mL of farnesol; 256 μg/mL of 2 and 6.
Fold ratios of increased susceptibility are in parentheses.
Entry with fold increases of 4 or higher are in bold.
Compound 2 was used at 128 μg/mL for NRS70 (N315) and NRS100 (COL).
The bacterial growth got slower at the half-MICs of farnesol and its derivatives; highlighted. The MIC determination was performed in triplicate; each experiment exhibited similar results.
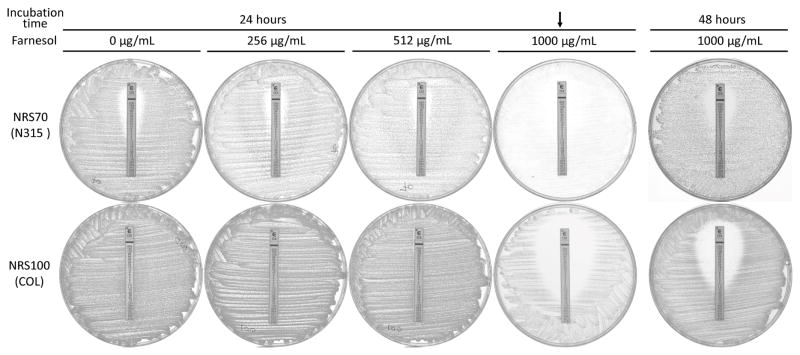
The potentiation of AMP and OXA by farnesol was different from what Kuroda et al. described.3 They reported dramatic improvement in susceptibilities to AMP and OXA by farnesol. MIC values of AMP and OXA were reported decreased by 24- and >64-fold for NRS100 and 256- and 96-fold for NRS70, respectively. In our hands, the improvement in susceptibility by farnesol was modest, with MICs for AMP and OXA attenuated by 4- and >32-fold for NRS100, and a mere 4- and 2.7-fold for NRS70, respectively. Farnesol reduced MICs of AMP and OXA as much as 4- and 12-fold for NRS123 as well. We attributed the difference between our determinations and those of Kuroda et al. by our observation of slow growth of all strains, especially NRS70, on CAMHB-T agar plates containing 1000 μg/mL of farnesol. The reported large effect of farnesol on susceptibility to β-lactams might be the result of not taking into account the slow growth of bacteria in the presence of farnesol. That is to say that the MIC values determined after the 24-hour incubation in the presence of farnesol were similar to those reported by Kuroda and colleagues (Figure 1). However, the MIC values were significantly larger against the MRSA strains when read after a 48-hour incubation period (Figure 1). Chemical stability of compounds 2 and 6 was checked by LC-MS.14 A total of 88(±1)% of compound 2 remains after 48-hour incubation in the growth medium. However, compound 6 was not detected beyond 24-hour of incubation. Compound 6 is a prodrug of compound 2.
Figure 1.
Effect of farnesol on the growth of NRS70 and NRS100 at half-MIC (1000 μg/mL). Etest for oxacillin was performed on CAMHB-T agar plates containing from zero to 1000 μg/mL of farnesol. The plates were incubated for 24 and 48 hours. NRS70 and NRS100 were grown slowly at the half-MIC of farnesol (indicated by the arrow), leading false reading of MIC values. The MIC values were substantially increased after 48 hour-incubation (the rightmost column) compared to 24-hour incubation.
Compound 2 produced larger improvement in susceptibility of strain NRS123 to both β-lactams—12-fold for AMP and 32-fold for OXA—compared to the case of farnesol. As for compound 6, it exhibited the largest effect with OXA against strains NRS100 (>128-fold) and NRS70 (32-fold); compared to that of farnesol (>32-fold for NRS100 and 2.7-fold for NRS70). When concentration of farnesol was reduced further (256 and 512 μg/mL) and then strains NRS100 and NRS70 were challenged by AMP and OXA, we saw no effect on bacterial growth. The MIC values for the β-lactams under these conditions were the same as those in the absence of farnesol. Compounds 2 and 6 exhibited similar effects on β-lactam susceptibility as the parental farnesol, except they were larger. The MIC drop or potentiation by farnesol and the synthetic derivatives on AMP and OXA against the strains NRS100 and NRS70 was due to attenuation of the rate of the growth.
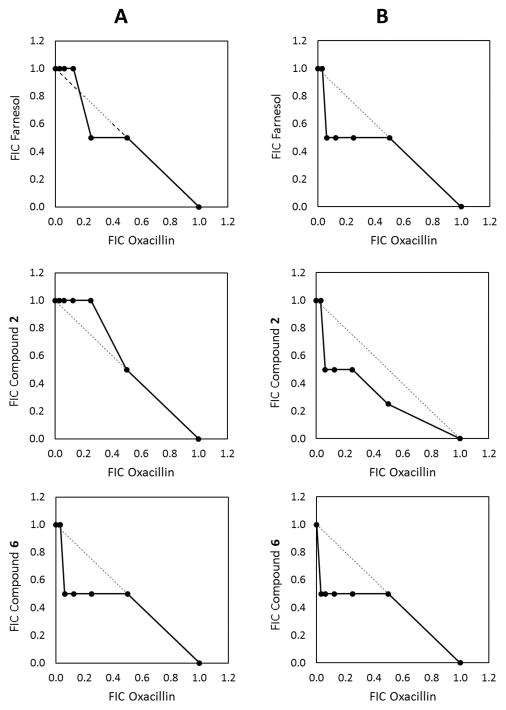
The potential for synergism between OXA and farnesol (or its derivatives) was checked in the checkerboard assay with measurements of the fractional inhibitory concentration index (FICI).15–17 We could not observe any synergy between the β-lactams and farnesol or its derivatives with 0.53 ≤ FICI ≤ 1.13 (Figure 2). Farnesol may best be characterized as a potentiator of β-lactam antibiotics, rather than a synergistic effector.
Figure 2.
Isobolograms showing no interactions between oxacillin and farnesol or its derivatives for NRS70 (A) and NRS100 (B). Data are the fractional inhibitory concentrations (FICs) of two drugs in combination. A FIC value of 1 corresponds to the MIC value of the particular antimicrobial agent. The dotted lines represent the theoretical additive interaction between two agents. The filled circles indicate FICI of each combination of two drugs. Results are from a single experiment performed in triplicate. Experiments were repeated 3 times, each with similar results. FICI ≤ 0.5, synergy; 0.5 < FICI ≤ 4, no interaction; FICI > 4, antagonism.
In conclusion, it would appear that there exists a biochemical consequence to S. aureus on exposure to high concentrations of farnesol and its derivatives. The mechanism for this effect of compound 2 and 6 has not been studied, although two possible mechanisms have been reported for farnesol; inhibition of lipase activity18 and disruption of cytoplasmic membrane through the leakage of potassium ions by farnesol.1, 19 No doubt that variants of the structure of farnesol could be engineered for more potent effect in potentiating the activities of β-lactams than what we have documented with the synthetic compounds 2 and 6. Nonetheless, these findings are worthy of further discovery of novel molecules as mechanistic tools and as potential clinical recourse in countering ubiquitous β-lactam resistance in bacteria.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (AI104987).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Shimada J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol Lett. 2004;237(2):325–331. doi: 10.1016/j.femsle.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Jabra-Rizk MA, Shirtliff M, James C, Meiller T. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 2006;6(7):1063–1073. doi: 10.1111/j.1567-1364.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuroda M, Nagasaki S, Ohta T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to β-lactams in methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2007;59(3):425–432. doi: 10.1093/jac/dkl519. [DOI] [PubMed] [Google Scholar]
- 4.Derengowski LS, De-Souza-Silva C, Braz SV, et al. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann Clin Microbiol Antimicrob. 2009;8:13. doi: 10.1186/1476-0711-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaneko M, Togashi N, Hamashima H, Hirohara M, Inoue Y. Effect of farnesol on mevalonate pathway of Staphylococcus aureus. J Antibiot. 2011;64(8):547–549. doi: 10.1038/ja.2011.49. [DOI] [PubMed] [Google Scholar]
- 6.Brilhante RSN, Valente LGA, Rocha MFG, et al. Sesquiterpene farnesol contributes to increased susceptibility to β-lactams in strains of Burkholderia pseudomallei. Antimicrob Agents Chemother. 2012;56(4):2198–2200. doi: 10.1128/AAC.05885-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabra-Rizk MA, Meiller TF, James CE, Shirtliff ME. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob Agents Chemother. 2006;50(4):1463–1469. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes FIA, Teixeira P, Azeredo J, Oliveira R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr Microbiol. 2009;59(2):118–122. doi: 10.1007/s00284-009-9408-9. [DOI] [PubMed] [Google Scholar]
- 9.Coppola GM, Prashad M. A convenient preparation of farnesylamine. Synth Commun. 1993;23(4):535–541. [Google Scholar]
- 10.Nowotny S, Tucker CE, Jubert C, Knochel P. Chromium(II)-mediated stereodivergent additions of allylic phosphates and halides to aldehydes. J Org Chem. 1995;60:2762–2772. [Google Scholar]
- 11.Compounds 3 and 6 were prepared by a literature procedure from reference 12. Spectral data of compound 3: 1H NMR (500 MHz, CD3CO2D) δ 1.56, 1.57, 1.63, 1.64 (4 X s, 12H), 1.91 – 2.11 (m, 8H), 3.40 (dd, J = 9.4, 8.6 Hz, 1H), 5.05 – 5.19 (m, 2H), 5.30 (t, J = 7.7 Hz, 1H); 31P NMR (202 MHz, D2O) δ 43.24 (s); HRMS (ESI-QTOF) m/z [M–H]− Calcd for C15H26O3PS 317.1335; found 317.1369. Spectral data of compound 6: 1H NMR (500 MHz, D2O) δ 1.57, 1.58, 1.64, 1.66 (4 X s, 12H), 1.98 – 2.11 (m, 8H), 2.80 – 2.88 (m, 2H), 5.07 – 5.17 (m, 2H), 5.38 – 5.43 (m, 1H); 31P NMR (202 MHz, D2O) δ 44.39 (s); HRMS (ESI-QTOF) m/z [M–H]− Calcd for C15H27NO2PS 316.1494; found 316.1473.
- 12.Maung J, Campbell TY, Shieh CC, Berkman CE. Synthesis of N-thiophosphoryl amino acids via phosphoramidite amine-exchange. Synth Commun. 2004;34(4):571–577. [Google Scholar]
- 13.Poulter CD, Mautz DS. Solvolysis of allylic isoprene phosphorothioate esters - a mechanistic study of the thiono-> thiolo rearrangement. J Am Chem Soc. 1991;113(13):4895–4903. [Google Scholar]
- 14.Compound 2 was first dissolved at 50 mg/ml in DMSO and diluted to 128, 256, 512 and 1024 μg/ml with CAMHB-T for standard curve. On the other hand, compound 6 was directly dissolved at 2048 μg/ml in CAMHB-T as it exhibited high solubility in aqueous solution, followed by the same dilution as compound 2. The diluted samples were immediately stored at −20 degree until use. Compounds 2 and 6 at the concentration of 512 μg/ml were used for the stability test. They were incubated for 2, 24 and 48 hours at 37 degree and stored at −20 degree. Each sample was applied to LC-MS for analysis.
- 15.Dougherty PF, Yotter DW, Matthews TR. Microdilution transfer plate technique for determining in vitro synergy of antimicrobial agents. Antimicrob Agents Chemother. 1977;11(2):225–228. doi: 10.1128/aac.11.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall MJ, Middleton RF, Westmacott D. The fractional inhibitory concentration (FIC) index as a measure of synergy. J Antimicrob Chemother. 1983;11(5):427–433. doi: 10.1093/jac/11.5.427. [DOI] [PubMed] [Google Scholar]
- 17.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda M, Nagasaki S, Ito R, Ohta T. Sesquiterpene farnesol as a competitive inhibitor of lipase activity of Staphylococcus aureus. FEMS Microbiol Lett. 2007;273(1):28–34. doi: 10.1111/j.1574-6968.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- 19.Inoue Y, Togashi N, Hamashima H. Farnesol-induced disruption of the Staphylococcus aureus cytoplasmic membrane. Biol Pharm Bull. 2016;39:653–656. doi: 10.1248/bpb.b15-00416. [DOI] [PubMed] [Google Scholar]