Abstract
Background
We previously reported that young men with chronic spinal cord injury (SCI) have a higher prevalence of testosterone deficiency when compared to an age-matched healthy control population. Young men with SCI are also at increased risk for developing cardiometabolic dysfunction after injury. It is unclear whether or not testosterone deficiency is associated with heightened cardiometabolic risk in men with SCI.
Objective
To investigate associations among levels of testosterone in young men with chronic SCI and surrogate markers of cardiometabolic risk.
Design
Secondary cross-sectional analysis.
Setting
Rehabilitation research centers in Washington, DC, and Miami, Florida, USA.
Participants
Men (n=58) aged 18–45 with chronic (≥1 year), motor complete SCI without comorbidities or use of testosterone therapy.
Methods
Plasma concentrations of testosterone, lipids, inflammatory markers (CRP and IL-6), HbA1C%, glucose, and insulin were measured in a fasting state using standard assays. A two-hour oral glucose tolerance test (OGTT) and Framingham Risk Score (FRS) were assessed for each subject. Body composition was assessed by DXA scan.
Main Outcome Measurements
Surrogate markers of cardiometabolic risk among men based on the level of total testosterone (TT)(≤300, 301–500, or >500 ng/dL) and free testosterone (fT) (≤9 or >9 ng/dL). Comparisons were made between men with normal and low TT or fT.
Results
FRS was significantly higher in men with low fT (P<.05). Percent body fat (P<.05) and waist-to-hip ratio (P<.05), but not body mass index (BMI) (P>.08), were higher in men with low TT or low fT. Men with low TT or low fT had lower high density lipoprotein cholesterol (HDL) levels (P<.05) without differences in fasting triglycerides (P>.1) or low density lipoprotein cholesterol (LDL) (P>.07). Men with low TT had higher levels of inflammatory markers CRP (P<.05) and IL-6 (P<.05). Men with low TT or low fT had higher fasting glucose (P<.05) and greater insulin resistance (P<.04), without differences in HbA1C% (P>.8).
Conclusions
In young men with chronic SCI, who undergo an accelerated aging process post-injury, hypogonadism is associated with an unfavorable cardiometabolic risk profile. Further research is needed to determine if a causal relationship exists between hypogonadism and heightened cardiometabolic risk in men with SCI, and whether routine screening for testosterone deficiency is warranted in this population.
INTRODUCTION
In the U.S., spinal cord injury (SCI) most commonly affects men under 50 years of age [1,2,3]. Following injury, individuals with SCI experience an accelerated risk of all-cause cardiovascular and metabolic diseases [4,5,6]. These disease processes are characterized by development of sarcopenic obesity, hyperlipidemia, insulin resistance, and a heightened inflammatory state. All of these conditions contribute to an overall increase in cardiometabolic risk at younger ages than in the non-disabled population [4,5,6].
The most common risk components for metabolic syndrome in individuals with SCI are obesity and dyslipidemia [7,8]. Among individuals with SCI, obesity is characterized primarily by sarcopenia, which further contributes to increased cardiometabolic disease risk by amplifying an already heightened pro-inflammatory state. Approximately 50–60% of SCI patients are overweight, and another 20–30% are obese when assessed by body mass index (BMI) criteria [9,10], striking statistics especially when one considers that BMI underestimates adiposity in this population due to the predominance of sarcopenia [11,12,13]. In fact, based on adjusted BMI categories proposed for adults with SCI [14], rates of overweight and obesity are even higher. In addition, individuals with SCI have high circulating levels of the inflammatory biomarker C-reactive protein (CRP), which is associated with elevated cardiovascular disease risk and which, in the SCI population, has been associated with greater degree of impairment (i.e., tetraplegia vs paraplegia), higher percent body fat, lower serum high-density lipoprotein cholesterol (HDL), and insulin resistance [15,16].
We and others have reported that men with chronic SCI also have an increased prevalence of testosterone deficiency after injury when compared to non-injured men [17,18,19,20,21,22,23,24]. Specifically, we recently reported that in a cohort of 58 otherwise healthy men between the ages of 18 and 45 with chronic (≥1 year) SCI, the prevalence of testosterone deficiency was 25%, compared to 7% in an age-matched historical control population (P<.001)[18]. Interestingly, we found that risk of hypogonadism in men with SCI was higher in those with higher percent body fat, suggesting a possible association between gonadal and metabolic dysfunction after SCI. We thus hypothesized that lower testosterone levels are associated with more unfavorable cardiometabolic risk profiles in young men with chronic SCI. To investigate the relationship between testosterone and cardiometabolic risk in men with SCI, we investigated associations among multiple surrogate markers of cardiometabolic disease risk and circulating levels of total and free testosterone in the same cohort of 58 otherwise healthy men with chronic SCI.
METHODS
The majority of study methods have been described previously [18]. Otherwise healthy men with chronic (≥1 year) SCI between C4 and T12 aged 18 to 45 years were recruited from an ongoing cross-sectional study evaluating cardiometabolic risk in SCI individuals. Men with motor complete SCI classified as American Spinal Injury Association (ASIA) Impairment Scale grade A or B and men with motor incomplete SCI who were primary wheelchair users were included (ambulatory men with motor incomplete SCI were excluded). Only men with traumatic SCI were included. Participants were recruited from the Medstar National Rehabilitation Hospital (MNRH), Washington, DC, and from the University of Miami Miller School of Medicine, Miami, FL, between 2008 and 2013.
Exclusion criteria included a history of traumatic brain injury, clinically significant cardiovascular disease, or gonadal dysfunction prior to SCI; medications to treat diabetes mellitus, hyperlipidemia, hypogonadism, or cardiovascular disease (with the exception of antihypertensive medications), any medication that alters serum testosterone levels, and glucocorticoids; >2 alcoholic drinks per day; daily use of opioid analgesics (as required use was considered acceptable); and self-reported type 2 or type 1 diabetes mellitus, liver disease, renal disease, heart failure (NYHA Class III or IV), obstructive sleep apnea, prostate cancer, testicular cancer, breast cancer, or benign prostatic hypertrophy (BPH). Men with polycythemia were excluded based on serum hemoglobin and hematocrit concentrations checked at study entry.
The Institutional Review Boards at both MNRH and the University of Miami approved this study, and all applicable institutional and governmental regulations concerning the ethical treatment of human volunteers were followed during this research. Informed consent was obtained from all study subjects before testing was initiated.
Subjects were instructed to fast for 12 hours and to avoid smoking, strenuous exercise, alcohol, and caffeine for 24 hours prior to laboratory testing. Fasting antecubital venous blood samples were collected under antiseptic conditions between 8 AM and 10 AM.
Total testosterone (TT) was measured by electrochemiluminescence immunoassay (ECLIA)[normal adult male reference range (RR): 300–1100 ng/dL, assay sensitivity 2.5 ng/mL, intra- and inter-assay coefficients of variation (CV): 2.4 and 2.9%, respectively]. Free testosterone (fT), an index of bio-available testosterone, was calculated using TT and sex hormone binding globulin (SHBG) concentration [25]. Serum concentrations of SHBG (RR: 16–54 nmol/L, sensitivity 0.80 nmol/L, intra-/inter-assay CV: 1.3%, 2.4%) were measured by ECLIA. Hormone measurements were tested only once for each participant. Morning serum concentrations of TT ≤300 ng/dL and calculated fT ≤9 ng/dL were considered to be in the hypogonadal range. We categorized study subjects by the level of morning TT (≤300, 301–500, or >500 ng/dL) and by the level of morning fT (≤9 or >9 ng/dL).
Standard assays were used to measure serum glucose, insulin, HbA1C%, inflammatory markers [high sensitivity-CRP (hs-CRP) and interleukin-6 (IL-6)], and lipid profiles. Glucose was assayed using the glucose oxidase method. Total cholesterol (TC), triglycerides, and HDL cholesterol were assayed on an automated analyzer (Roche Cobas-Mira; Roche Diagnostics, Indianapolis, IN) using commercially available kits according to manufacturer instructions and run procedures. LDL cholesterol was calculated by using the method of Friedewald: LDL = TC – [(fasting TG ÷ 5) – HDL] [27]. Glucose tolerance and insulin resistance were assessed by fasting and 2-hour glucose and insulin levels on standard oral glucose tolerance testing (OGTT). For the OGTT, subjects ingested 75 g of a commercial glucose solution (Trutol; NERL Clinical Diagnostics, East Providence, RI) within 5 minutes and underwent a blood draw 2 hours later for insulin and glucose levels. The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated to estimate insulin resistance using the following equation: HOMA-IR = [(fasting glucose (mg/dL) × fasting insulin (μU/mL)) ÷ 405] [28,29]. Quantitative Insulin sensitivity Check Index (QUICKI), a validated estimate of insulin sensitivity, was calculated using the formula: 1/[log (fasting insulin)+ (log fasting glucose)] [30].
Resting blood pressure was determined in the seated position by using the standard auscultation method previously described by the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [31]. Body composition was assessed by dual X-ray absorptiometry (DXA), body mass index (BMI), and waist-to-hip ratio (WHR). Height was determined by measurement in the supine position. Participants were weighed on a calibrated portable wheelchair scale. BMI was calculated as the quotient of body mass and the squared height (kg/m2). DXA was performed using a Hologic QDR series DXA scanner (Hologic Inc., Marlborough, MA) with individuals in the supine position, as described in adults with paraplegia [32], to determine whole body and regional fat and lean body mass.
Framingham Risk Score (FRS), an estimate of the 10-year risk of hard coronary events, was calculated for each subject. FRS is calculated using cardiovascular disease (CVD) risk factors—age, fasting TC, current smoking status (defined as any cigarette smoking within the previous month), fasting HDL, and resting systolic blood pressure—assigning each a point score, with higher point values corresponding to higher risk. FRS point totals, expressed as percentages, were calculated for each subject to estimate 10-year risk for developing CVD [33].
Statistical analyses
Data from study participants were compared by level of testosterone sufficiency. Participants were stratified according to serum concentrations of total T (≤300, 301–500, or >500 ng/dL) and calculated fT (≤9 or >9 ng/dL). Normality assumption was satisfied for total T; however, fT levels were not normally distributed. Accordingly, the non-parametric Wilcoxon rank sum test and Kruskal–Wallis test were used for comparisons between the two fT and three total T groups, respectively.
Means and standard deviations for continuous variables and frequencies and percentages for categorical variables were calculated for all groups. For continuous variables, the differences in means between groups were tested by ANOVA when normality assumption was satisfied, and by the non-parametric Kruskal–Wallis test when normality assumption was not satisfied. Chi-square and Fisher exact tests were used to investigate the differences between categorical variables. P<.05 was considered significant.
RESULTS
Framingham Risk Score
Men with low fT had higher FRS than men with normal fT; there was no difference in FRS based on TT levels (TT≤300, 3.5±4.1%; TT 301–500, 2.4±2.9%; TT >500, 1.5±0.8%, P=.41) (fT≤9, 3.3±3.4%; fT>9, 1.7±2.0%, P=.04)(Figure 1). That said, all men with SCI in our cohort had FRS<10% (defined as low risk).
Figure 1.
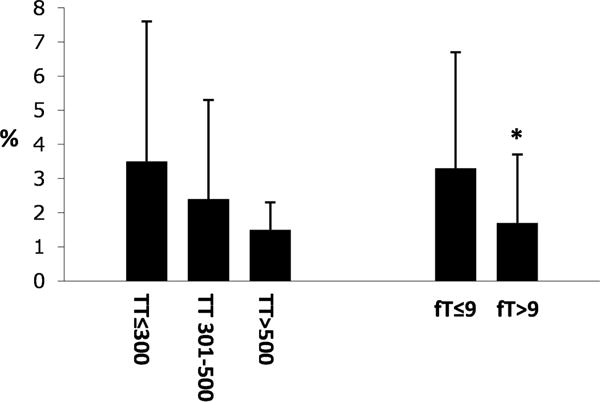
Framingham Risk Scores (%) based on serum TT (left, ng/dL) or fT (right, ng/dL) in men with SCI. *P<.05 versus TT≤300 or fT≤9 ng/dL.
Body Composition
Men with both low TT and low fT had significantly higher body fat percentages compared to men with normal TT and fT levels (TT≤300, 40±12%; TT 301–500, 35±9%; TT >500, 25±8%, P<.001) (fT≤9, 37±12%; fT>9, 30±9%, P=.04), without differences in BMI (TT≤300, 27.3±7.9; TT 301–500, 26.4±4.2; TT >500, 24.8±3.4 mg/kg2, P=.75) (fT≤9, 29.2±7.4; fT>9, 24.2±3.9 mg/kg2, P=.09) (Figure 2). Waist-to-hip ratio (WHR) was significantly higher in men with low total T compared to men with the highest level of TT (>500 ng/dL) (TT≤300, 0.97±0.1; TT 301–500, 1.01±0.02; TT >500, 0.9±0.1, P<.05); however, WHR did not differ based on level of fT (fT≤9, 0.97±0.05; fT>9, 0.92±0.1, P=.2).
Figure 2.
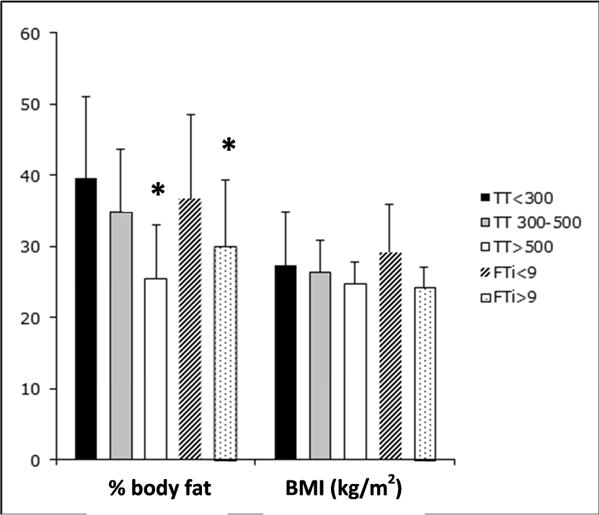
Mean percent body fat and BMI (kg/m2) based on serum TT (left, ng/dL) or fT (right, ng/dL) in men with SCI. *P<.05 versus TT≤300 or fT≤9 ng/dL.
Fasting Lipids
Men with low TT and low fT had significantly lower HDL levels compared to men with normal T levels (TT≤300, 37±7; TT 301–500, 40±10; TT >500, 46±9 mg/dL, P<.01)(fT≤9, 39±9; fT>9, 44±10 mg/dL, P<.05). There were no statistically significant differences in levels of fasting triglycerides (TT≤300, 121±69; TT 301–500, 102±39; TT >500, 95±50 mg/dL, P=.28)(fT≤9, 115±61; fT>9, 97±44 mg/dL, P=.19) or LDL (TT≤300, 88±27; TT 301–500, 108±32; TT >500, 112±28 mg/dL, P=.08)(fT≤9, 95±27; fT>9, 111±30 mg/dL, P=.12) based on TT or fT levels, although there was a trend toward lower TG levels with higher TT or fT levels (Figure 3).
Figure 3.
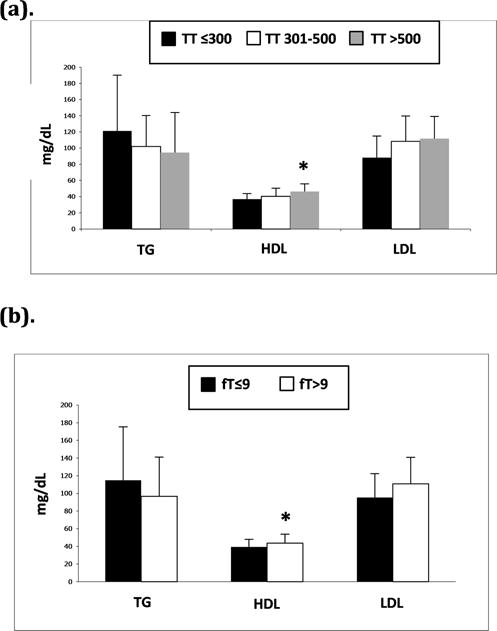
a and b. Mean fasting triglycerides (TG), HDL, and LDL in (a) men with TT≤300, 301–500, and >500 ng/dL and (b) men with fT≤9 and >9 ng/dL. *P<.05 versus TT≤300 or fT≤9 ng/dL.
Inflammatory Markers
Men with low TT had higher levels of the circulating inflammatory markers hsCRP and IL-6. There were no differences in levels of IL-6 or hsCRP based on fT concentrations (Table 1).
Table 1.
Mean±SD serum levels of inflammatory markers, hsCRP and IL-6, in men with SCI based on level of TT or fT (ng/dL).
| TT≤300 | TT 301-500 | TT>500 | P | fT≤9 | fT>9 | P | |
|---|---|---|---|---|---|---|---|
| hsCRP (mg/dL) | 5.3±4.6 | 5.9±8.6 | 3.4±5.4* | .03* | 6.1±8.6 | 4.0±5.0 | .24 |
| IL-6 (mg/dL) | 2.8±1.8 | 2.2±1.2 | 1.7±2.1* | .02* | 2.2±1.5 | 2.0±1.8 | .66 |
P<.05 versus TT≤300 or fT≤9 ng/dL.
Glycemic Control
Men with low TT and low fT had higher fasting glucose levels compared to men with higher TT and fT levels (TT≤300, 99±33; TT 301–500, 87±10; TT >500, 82±8 mg/dL, P=.04)(fT≤9, 95±27; fT>9, 84±10 mg/dL, P<.01). On a standard 2-hour oral glucose tolerance test, men with low fT had higher two-hour glucose levels than men with normal fT (fT≤9, 131±73; fT>9, 101±25 mg/dL, P<.01). Two-hour glucose levels were not significantly different based on the level of TT (TT≤300, 140±92; TT 301–500, 105±23; TT >500, 104±28 mg/dL, P=.09), although there was a trend toward lower two-hour glucose with higher levels of TT (Figure 4). HbA1C% did not differ among men with SCI based on level of TT or fT (TT≤300, 5.6±1.5; TT 301–500, 5.1±0.3; TT >500, 5.1±0.3, P=.86)(fT≤9, 5.4±1.2; fT≥9, 5.1±0.3 mg/dL, P=.93). HOMA-IR and QUICKI calculations estimated that men with lower TT or fT were more insulin resistant (Figure 5a), and accordingly, that men with higher TT or fT were more insulin sensitive (Figure 5b). Men who were deficient in TT (≤300 ng/dL) or fT (≤9 ng/dL) had the highest estimated insulin resistance by HOMA-IR (TT≤300, 5.5±6.9; TT 301–500, 3.4±4.2; TT >500, 1.9±1.6, P<.01)(fT≤9, 4.8±6.1; fT>9, 2.5±3.3, P=.03) and the lowest estimated insulin sensitivity by QUICKI (TT≤300, 3.23±0.44; TT 301–500, 3.43±0.45; TT >500, 3.66±0.43, P<.01)(fT≤9, 3.27±0.46; fT>9, 3.59±0.44, P<.01). Men with intermediate levels of TT (301–500 ng/dL) had HOMA-IR and QUICKI scores that were intermediate between men with low TT and men with TT>500 ng/dL.
Figure 4.
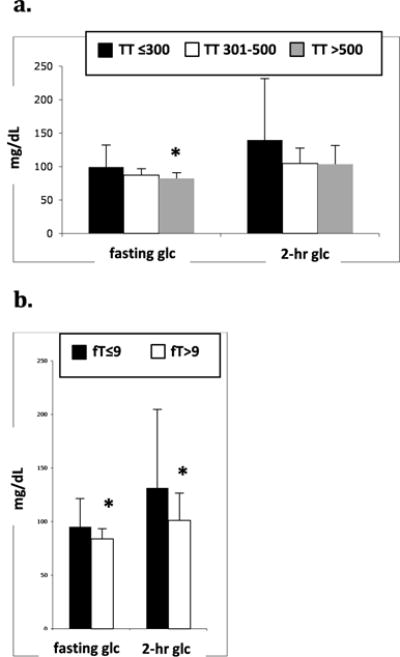
a and b. Mean fasting and 2hr glucose (glc) levels during 2hr OGTT based on serum TT (a) or fT (b)(ng/dL) in men with SCI. *P<.05 versus TT≤300 ng/dL or fT≤9 ng/dL.
Figure 5.
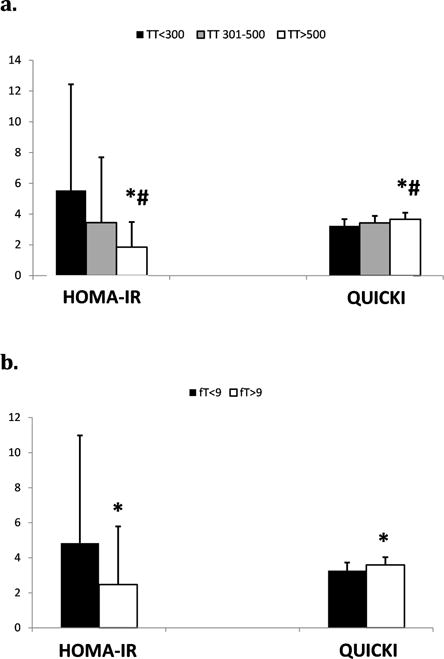
a and b. HOMA-IR and QUICKI indices for men with SCI based on level of TT (a) or fT (b) (ng/dL). *P<.05 versus TT≤300 or fT≤9 ng/dL. # P<.05 versus TT 301–500.
DISCUSSION
We found that multiple surrogate markers of cardiometabolic risk in young men with chronic SCI were associated with low TT or low fT levels. Overall, our data suggest that men with SCI with low testosterone may have significantly higher cardiometabolic risk compared to men with SCI with normal testosterone levels. Although we cannot assess causality between hypogonadism and increased cardiometabolic risk in men with SCI given the cross-sectional design of our study, our data demonstrate a clear association between testosterone deficiency and heightened cardiometabolic risk in this population. Our finding that increasing cardiometabolic risk exists on a continuum with decreasing testosterone levels, such that men with TT in the low normal range (i.e., 301–500 ng/dL) have more unfavorable risk profiles compared to men with TT levels in the upper range of normal (>500 ng/dL), supports this association. That said, we cannot determine from our study whether testosterone deficiency preceeded any exacerbations in cardiometabolic risk, or vice versa. Indeed, prospective studies evaluating cardiometabolic risk profiles and serum testosterone levels over time after injury are needed to establish whether or not such a causal relationship exists.
We calculated Framingham Risk Scores for men with SCI and found that men with low fT levels had significantly higher scores when compared to men with normal fT. FRS did not differ among men based on levels of TT, although there was a trend toward higher FRS as TT levels declined. That said, all men in our cohort had FRS that were less than 10%, indicating overall low ten-year cardiovascular disease risk, and supporting previous data suggesting that FRS may underestimate actual CVD risk in the SCI population [34,35].
We previously reported that percent body fat increased with decreasing levels of TT or fT among the young SCI men in our cohort [18]. Specifically, men who were deficient in TT or fT had significantly higher body fat percentages compared to men with sufficient levels of testosterone. These differences in body fat percentage were present despite similar BMI among the TT and fT groups. The lack of differences in BMI was not surprising given that BMI has been shown to be a poor measure of body composition in the SCI population [11,12,13]. In the present study, we also found small but statistically significant differences in WHR based on levels of TT, with men who were deficient in TT having higher WHR compared to men with TT>500 ng/dL. In this regard, WHR, similarly to waist circumference, has been shown to be a reliable marker of visceral fat in men with SCI, and is a major contributor to metabolic syndrome in this population [32].
When assessing fasting lipid levels, we found no differences in serum triglycerides or LDL concentrations among the TT or fT groups, although there were non-significant trends toward men with lower TT or fT having higher serum triglycerides and LDL levels. Interestingly, HDL, a marker of protection against CVD, was significantly higher in men with TT>500 ng/dL and fT>9 ng/dL compared to men who were deficient in TT or fT, providing evidence for an association between hypogonadism and increased atherogenic risk in young men with SCI. Interestingly, the majority of men with SCI have low circulating HDL cholesterol levels [36,37]. Our data suggest that testosterone deficiency may be one factor associated with HDL ‘deficiency’ in this population.
We assessed several measures of glycemic control to determine if hypogonadism is associated with changes in glucose tolerance or insulin sensitivity in young men with SCI. HbA1C% did not differ among men with SCI based on the level of TT or fT. On standard two-hour OGTTs, fasting glucose was significantly lower in men with TT>500 and fT>9 ng/dL compared to men who were deficient in TT or fT. Two-hour glucose levels were lower in men with normal fT compared to men with low fT; however, there was only a trend toward lower two-hour glucose levels based on the level of TT. The two-hour glucose level on OGTT is a more robust indicator of cardiovascular disease risk compared to either fasting glucose or HbA1C% [38]. Our finding of a significant association between low free T, but not low total T, and higher two-hour glucose levels perhaps suggests that free T, rather than total T, may be a more sensitive measure of gonadal function and may be more strongly associated with metabolic risk in men with SCI. Taken together, our findings of higher fasting and two-hour glucose levels in men with low testosterone levels suggest that hypogonadism may be associated with insulin resistance and impaired glucose tolerance even in young men with SCI.
To further explore a potential relationship between hypogonadism and dysglycemia, we used OGTT data to derive scores on two validated measures of glycemic control for each subject: HOMA-IR, a validated measure of insulin resistance, and QUICKI, a validated measure of insulin sensitivity. Interestingly, we found that as total or free T levels increased, insulin resistance as estimated by HOMA-IR decreased, and insulin sensitivity as estimated by QUICKI increased. In fact, men who were deficient in TT (≤300 ng/dL) and fT (≤9 ng/dL) had significantly greater insulin resistance and significantly lower insulin sensitivity compared to men with testosterone sufficiency, providing support for a potential association between hypogonadism and increased risk of dysglycemia, insulin resistance, pre-diabetes, and ultimately, type 2 diabetes mellitus in men with SCI. Interestingly in this regard, we previously reported that men with low TT had significantly lower serum SHBG levels compared to men with normal TT [18]. It is well established that low SHBG levels are correlated with increased insulin resistance and predict the development of metabolic syndrome [39,40].
Finally, we found that circulating levels of the inflammatory markers CRP and IL-6 were significantly lower in men with total T >500 ng/dL compared to men with TT deficiency. We did not observe differences in either inflammatory marker in men based on their level of fT, although we observed a trend toward higher CRP and IL-6 concentrations in men with fT≤9 ng/dL. Overall, differences in levels of inflammatory markers based on testosterone sufficiency provide further support for an association between hypogonadism and cardiovascular disease risk in men with SCI, albeit less robustly than the other surrogate markers.
We recently reported a significantly higher prevalence of low testosterone among young men with chronic SCI compared to similarly aged, non-injured men [18], supporting previous data suggesting that men with SCI are at high risk for T deficiency post-injury [17,19,20,21,22,23,24]. Interestingly, in our previous study, we also found that the men with SCI who met criteria for testosterone deficiency based on serum TT≤300 ng/dL had significantly higher body fat percentages compared to men with normal TT, albeit without differences in BMI [18]. This is in accordance with previous data demonstrating that BMI is a poor indicator of body adiposity in the SCI population [11,12,13], and importantly, lead us to hypothesize that hypogonadism may also be associated with heightened cardiometabolic risk in men with SCI.
Several previous studies have provided evidence that cardiometabolic risk significantly increases after spinal cord injury [4,5,7,8]. Our data extend these findings by suggesting that cardiometabolic risk in young men with SCI may be associated with concomitant hypogonadism. Specifically, we provide evidence suggesting that hypogonadism may be associated with increased adiposity, dyslipidemia, insulin resistance, and increased systemic inflammation in young men with SCI. Certainly, determining whether or not these modifiable cardiometabolic risk factors are exacerbated by testosterone deficiency, or whether testosterone deficiency exacerbates cardiometabolic risk in men with SCI, will be important in improving overall morbidity and mortality in this population.
When identified, testosterone deficiency is easily treated with marketed testosterone replacement therapies and monitored with simple blood tests. To date, few studies have investigated effects of testosterone replacement therapy (TRT) in men with SCI [19,41,42]. These studies suggest that TRT may have numerous benefits, including improvement in energy expenditure and lean tissue mass [41,42] and decreased cardiac arrhythmic potential [19]. However, other studies in non-SCI populations inform the potential risks of TRT, particularly increased risk of cardiovascular-related adverse events such as myocardial infarction [43, 44]. Cardiac adverse events associated with TRT use are generally seen in older men ≥65 years of age, rather than in younger, otherwise healthy men similar in age to our cohort. Certainly, both the potential risks and benefits of TRT must be considered when determining whether or not to treat hypogonadal SCI men with TRT, particularly given the paucity of clinical data regarding the safety of TRT in the SCI population.
Because testosterone levels are expected to decline more quickly after the insult of a spinal cord injury compared to changes in cardiometabolic risk factors (e.g., lipids, body adiposity, insulin sensitivity), testosterone deficiency could potentially be an early ‘marker’ of heightened cardiometabolic risk after SCI. It remains to be determined, however, whether routine screening for testosterone deficiency after SCI is warranted. When testosterone deficiency is diagnosed, clinicians may recommend not only TRT, but also preventative strategies to reduce cardiometabolic risk, such as healthy lifestyle changes and insulin-sensitizing or lipid-lowering medications. Indeed, determining whether testosterone deficiency is an early risk marker predicting increased cardiometabolic risk in men with SCI will be an important advancement in our ability to care for these individuals.
There are several limitations to our study. First, due to the cross-sectional, retrospective design, we were unable to determine whether or not a causal relationship exists between hypogonadism and cardiometabolic risk in young men with SCI. That said, our demonstration of an association between lower testosterone levels and worsening cardiometabolic risk profiles may inform future prospective studies evaluating cardiometabolic risk and testosterone levels over time after injury. Second, our study enrolled a relatively small number of subjects (n=58), limiting our study power. Also due to the retrospective design of our study, we were able to assess only a single morning testosterone level for each subject, and we were unable to assess potential physical manifestations or symptoms of low testosterone. Strengths of this study include our ability to asses multiple cardiometabolic risk markers in each study subject, including markers of glycemic control, fasting lipids, and inflammatory markers; measurement of both total and free testosterone levels; a well-characterized, overall healthy, young population of men with chronic SCI; and ability to accurately assess body composition, particularly percent body fat, using DEXA measurements.
CONCLUSION
Altogether, our data suggest that testosterone deficiency, which is significantly more prevalent in men with SCI compared to non-injured men, may be associated with cardiometabolic disease development after spinal cord injury. From these data, one may hypothesize that screening for testosterone deficiency after SCI and treatment of hypogonadal men with testosterone replacement therapy may help to ameliorate at least some of the cardiometabolic health risks sustained following injury. Certainly, further research is needed to determine the nature of the relationship between hypogonadism and cardiometabolic risk after spinal cord injury, as well as the potential risks and benefits of TRT in hypogonadal men with SCI.
Acknowledgments
We thank the staff of the Immunoassay and Biomarker Core Laboratory at the Diabetes Research Institute, University of Miami Miller School of Medicine, and particularly Armando J. Mendez, Ph.D., Director, for coordinating and performing all serum hormone assays.
We thank Medstar Health Research Institute for statistical support.
We sincerely thank all the individual study participants for their generous provision of time and willingness to help advance clinical research in patients with SCI.
Grant support:
This research was supported by Grant Number H133B090002 from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR), Administration for Community Living (ACL), Department of Health and Human Services (HHS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDILRR, ACL, or HHS. Funding was also provided by a Sam Schmidt Young Investigator’s research grant to SDS from the American Spinal Injury Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no financial conflicts of interest.
References
- 1.National Spinal Cord Injury Statistical Center. Annual Report, Complete Public Version, University of Alabama. :2014. Available at https://wwwnsciscuabedu/reports. Published Nov, 2014. Accessed February 1, 2015.
- 2.Chen Y, Tang Y, Vogel LC, Devivo MJ. Causes of spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19(1):1–8. doi: 10.1310/sci1901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chistropher and Dana Reeve Foundation website. Available at http://wwwchristopherreeveorg/site/cmtKZKgMWKwG/b5184189/k5587/Paralysis_Facts_Figures.htm. Published 2009. Accessed May 1, 2014.
- 4.Groah SL, Nash MS, Ward EA, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31(2):73–80. doi: 10.1097/HCR.0b013e3181f68aba. [DOI] [PubMed] [Google Scholar]
- 5.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466–476. doi: 10.1038/sj.sc.3102161. [DOI] [PubMed] [Google Scholar]
- 6.Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37(9):601–616. doi: 10.1038/sj.sc.3100911. [DOI] [PubMed] [Google Scholar]
- 7.Libin A, Tinsley EA, Nash MS, et al. Cardiometabolic risk clustering in spinal cord injury: results of exploratory factor analysis. Top Spinal Cord Inj Rehabil. 2013;19(3):183–194. doi: 10.1310/sci1903-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash MS, Tractenberg RE, Mendez AJ, et al. Cardiometabolic Syndrome in People With Spinal Cord Injury/Disease: Guideline-Derived and Nonguideline Risk Components in a Pooled Sample. Arch Phys Med Rehabil. 2016;97(10):1696–1705. doi: 10.1016/j.apmr.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, White KT, Sandford PR. Body mass index in spinal cord injury – a retrospective study. Spinal Cord. 2006;44(2):92–94. doi: 10.1038/sj.sc.3101790. [DOI] [PubMed] [Google Scholar]
- 10.Weaver FM, Collins EG, Kurichi J, et al. Prevalence of obesity and high blood pressure in veterans with spinal cord injuries and disorders: a retrospective review. Am J Phys Med Rehabil. 2007;86(1):22–29. doi: 10.1097/phm.0b013e31802b8937. [DOI] [PubMed] [Google Scholar]
- 11.Jones LM, Goulding A, Gerrard DF. DEXA: a practical and accurate tool to demonstrate total and regional bone loss, lean tissue loss and fat mass gain in paraplegia. Spinal Cord. 1998;36(9):637–640. doi: 10.1038/sj.sc.3100664. [DOI] [PubMed] [Google Scholar]
- 12.Spungen AM, Bauman WA, Wang J, Pierson RN., Jr Measurement of body fat in individuals with tetraplegia: a comparison of eight clinical methods. Paraplegia. 1995;33(7):402–408. doi: 10.1038/sc.1995.90. [DOI] [PubMed] [Google Scholar]
- 13.Ravensbergen HR, Lear SA, Claydon VE. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma. 2014;31(3):292–300. doi: 10.1089/neu.2013.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47(10):757–762. doi: 10.1038/sc.2009.33. [DOI] [PubMed] [Google Scholar]
- 15.Gibson AE, Buchholz AC, Martin Ginis KA. C-Reactive protein in adults with chronic spinal cord injury: increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord. 2008;46(9):616–621. doi: 10.1038/sc.2008.32. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Mojtahedi MC, Chen D, Braunschweig CL. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil. 2008;89(1):36–41. doi: 10.1016/j.apmr.2007.08.121. [DOI] [PubMed] [Google Scholar]
- 17.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med. 2014;37(1):32–39. doi: 10.1179/2045772313Y.0000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan SD, Nash MS, Tefera E, Tinsley E, Blackman MR, Groah S. Prevalence and Etiology of Hypogonadism in Young Men with Chronic Spinal Cord Injury: A Cross-Sectional Analysis from Two University-Based Rehabilitation Centers. PM R. 2016 doi: 10.1016/j.pmrj.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Fountaine MF, Wecht JM, Cirnigliaro CM, Kirshblum SC, Spungen AM, Bauman WA. Testosterone replacement therapy improves QTaVI in hypogonadal men with spinal cord injury. Neuroendocrinology. 2013;97(4):341–346. doi: 10.1159/000347070. [DOI] [PubMed] [Google Scholar]
- 20.Clark MJ, Schopp LH, Mazurek MO, et al. Testosterone levels among men with spinal cord injury: relationship between time since injury and laboratory values. Am J Phys Med Rehabil. 2008;87(9):758–767. doi: 10.1097/PHM.0b013e3181837f4f. [DOI] [PubMed] [Google Scholar]
- 21.Schopp LH, Clark M, Mazurek MO, et al. Testosterone levels among men with spinal cord injury admitted to inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(8):678–684. doi: 10.1097/01.phm.0000228617.94079.4a. quiz 685–677. [DOI] [PubMed] [Google Scholar]
- 22.Celik B, Sahin A, Caglar N, et al. Sex hormone levels and functional outcomes: a controlled study of patients with spinal cord injury compared with healthy subjects. Am J Phys Med Rehabil. 2007;86(10):784–790. doi: 10.1097/PHM.0b013e318151fa70. [DOI] [PubMed] [Google Scholar]
- 23.Safarinejad MR. Level of injury and hormone profiles in spinal cord-injured men. Urology. 2001;58(5):671–676. doi: 10.1016/s0090-4295(01)01353-x. [DOI] [PubMed] [Google Scholar]
- 24.Durga A, Sepahpanah F, Regozzi M, Hastings J, Crane DA. Prevalence of testosterone deficiency after spinal cord injury. PM R. 2011;3(10):929–932. doi: 10.1016/j.pmrj.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 27.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K. Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocr J. 2001;48(1):81–86. doi: 10.1507/endocrj.48.81. [DOI] [PubMed] [Google Scholar]
- 30.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 31.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 32.Emmons RR, Garber CE, Cirnigliaro CM, Kirshblum SC, Spungen AM, Bauman WA. Assessment of measures for abdominal adiposity in persons with spinal cord injury. Ultrasound Med Biol. 2011;37(5):734–741. doi: 10.1016/j.ultrasmedbio.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–187. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 34.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: the Stockholm spinal cord injury study. J Rehabil Med. 2011;43(3):237–242. doi: 10.2340/16501977-0658. [DOI] [PubMed] [Google Scholar]
- 35.Finnie AK, Buchholz AC, Martin Ginis KA. Current coronary heart disease risk assessment tools may underestimate risk in community-dwelling persons with chronic spinal cord injury. Spinal Cord. 2008;46(9):608–615. doi: 10.1038/sc.2008.21. [DOI] [PubMed] [Google Scholar]
- 36.Laclaustra M, Van Den Berg EL, Hurtado-Roca Y, Castellote JM. Serum lipid profile in subjects with traumatic spinal cord injury. PLoS One. 2015;10(2):e0115522. doi: 10.1371/journal.pone.0115522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koyuncu E, Nakipoglu Yuzer GF, Yenigun D, Ozgirgin N. The analysis of serum lipid levels in patients with spinal cord injury. J Spinal Cord Med. 2016:1–6. doi: 10.1080/10790268.2016.1228286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lind M, Tuomilehto J, Uusitupa M, et al. The association between HbA1c, fasting glucose, 1-hour glucose and 2-hour glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS One. 2014;9(10):e109506. doi: 10.1371/journal.pone.0109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joyce KE, Biggs ML, Djousse L, et al. Testosterone, Dihydrotestosterone, Sex Hormone Binding Globulin and Incident Diabetes among Older Men: the Cardiovascular Health Study. J Clin Endocrinol Metab. 2016:jc20162623. doi: 10.1210/jc.2016-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang YH, Zhao MJ, Zhou SJ, et al. Is serum sex hormone-binding globulin a dominant risk factor for metabolic syndrome? Asian J Androl. 2015;17(6):991–995. doi: 10.4103/1008-682X.150845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bauman WA, Cirnigliaro CM, La Fountaine MF, et al. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. 2011;43(8):574–579. doi: 10.1055/s-0031-1280797. [DOI] [PubMed] [Google Scholar]
- 42.Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, Spungen AM. Lean tissue mass and energy expenditure are retained in hypogonadal men with spinal cord injury after discontinuation of testosterone replacement therapy. J Spinal Cord Med. 2015;38(1):38–47. doi: 10.1179/2045772314Y.0000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. NEJM. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finkle WD, Greenland S, Ridgeway GK, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;19:e85805. doi: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]


