Abstract
Background
Prostate cancer treatment is a significant source of morbidity and spending. Some men with prostate cancer, particularly those with significant health problems, are unlikely to benefit from treatment.
Objective
To assess relationships between financial incentives associated with urologist ownership of radiation facilities and treatment for prostate cancer.
Design, setting, and participants
A retrospective cohort of Medicare beneficiaries with prostate cancer diagnosed between 2010 and 2012. Patients were further classified by their risk of dying from noncancer causes in the 10 yr following their cancer diagnosis by using a mortality model derived from comparable patients known to be cancer-free.
Intervention
Urologists were categorized by their practice affiliation (single-specialty groups by size, multispecialty group) and ownership of a radiation facility.
Outcome measurements and analysis
Use of intensity-modulated radiation therapy (IMRT) and use of any treatment within 1 yr of diagnosis. Generalized estimating equations were used to adjust for patient differences.
Results
Among men with newly diagnosed prostate cancer, use of IMRT ranged from 24% in multispecialty groups to 37% in large urology groups (p < 0.001). Patients managed in groups with IMRT ownership (n = 5133) were more likely to receive IMRT than those managed by single-specialty groups without ownership (43% vs 30%, p < 0.001), regardless of group size. Among patients with a very high risk (> 75%) of noncancer mortality within 10 yr of diagnosis, both IMRT use (42% vs 26%, p < 0.001) and overall treatment (53% vs 44%, p < 0.001) were more likely in groups with ownership than in those without, respectively.
Conclusions
Urologists practicing in single-specialty groups with an ownership interest in radiation therapy are more likely to treat men with prostate cancer, including those with a high risk of noncancer mortality.
Patient summary
We assessed treatment for prostate cancer among urologists with varying levels of financial incentives favoring intervention. Those with stronger incentives, as determined by ownership interest in a radiation facility, were more likely to treat prostate cancer, even when treatment was unlikely to provide a survival benefit to the patient.
Keywords: Prostate, Cancer, Treatment
1. Introduction
Although many prostate cancers are slow-growing and do not affect survival even with conservative management, particularly in patients with multiple health problems and the elderly [1], the majority of diagnosed men in the USA are treated. Commensurate with broader trends in health care, spending for prostate cancer has increased more than 10% annually over the past decade and now approaches $12 billion in the USA alone [2,3]. Reasons for the spending growth are likely multifactorial, but almost certainly include the high rates of treatment and dissemination of new options for therapy [4], such as robotic surgery, intensity-modulated radiation therapy (IMRT), and proton beam radiation therapy, all of which are incrementally more expensive than the prior alternatives.
Recent trends in the organization of urological practice towards the formation of large single-specialty groups in the USA may also contribute to the increase in prostate cancer spending. Volume-based productivity bonus arrangements frequently implemented in larger practices may encourage the use of prostate cancer services. Further, some urology groups have purchased the equipment necessary to deliver IMRT and employed radiation oncologists to provide the treatment. These urology groups thus have the potential to collect both the professional and technical revenue from IMRT that otherwise would have been lost to other physicians, though not all groups are organized to take full advantage of these potential revenue streams.
For these reasons, we performed a national study to determine the association of urologist practice size and ownership of IMRT with patterns of treatment for prostate cancer. In particular, we were interested in assessing the effects of ownership on treatment among men with a high probability of noncancer-related death, or arguably those who stand the least to benefit from intervention.
2. Materials and methods
2.1. Data and study population
We performed a retrospective cohort study of fee-for-service Medicare beneficiaries with newly diagnosed prostate cancer between 2010 and 2012 using a 20% national sample. Incident cases of prostate cancer were identified using an algorithm validated with a cancer registry data (specificity and positive predictive value of 99.8% and 88.7%, respectively) [5]. Only patients eligible for both Parts A and B throughout this period were included in the study. Beneficiaries participating in Medicare managed care plans were excluded. Follow-up for all beneficiaries was available through December 31, 2013. Each patient was attributed to a urologist using well-established methods [6].
2.2. Characterizing urologist practice organization
We used explicit fields in the Healthcare Relational Spheres provider files (IMS Health) to characterize urologist practice organization (single-specialty vs multispecialty group). For urologists in single-specialty groups, we ascertained additional information from public records, including whether IMRT was provided onsite. Urologists were considered owners of IMRT when their practice website advertised providing IMRT onsite or when their group employed at least one radiation oncologist. Organizations technically owning an IMRT vault, but with weak incentives to profit from its use at the physician level (eg, some academic medical centers), were classified as nonowners. For practical reasons, ownership was ascertained once for each group in 2012, which was prior to the release of Medicare claims for 2012 and 2013. It was held constant for the entire study period. If a group containing urologists included one or more primary care physicians as well, the group was categorized as multispecialty. Using this approach, we identified 1546 urologists practicing in a multispecialty group and 4835 practicing in a single-specialty group. Single-specialty practices were further stratified according to the number of urologists in each group: one to two urologists (very small), three to five urologists (small), six to nine urologists (medium), and 10 or more urologists (large).
2.3. Outcomes
Our primary outcome was the use of IMRT within 12 mo of diagnosis and was measured at the beneficiary level. Additionally, we characterized other initial management strategies (none, other forms of external beam radiation, brachytherapy, cryotherapy, and surgery) using established methods [4]. Because our focus was on patterns of treatment with curative intent, patients managed with androgen deprivation only, watchful waiting, or surveillance were combined to form the “none” category. Patients treated with androgen deprivation and a curative treatment (eg, radiation) were categorized as having had that curative treatment. Patients having both surgery and radiation were classified as having had surgery.
Due to the protracted natural history of prostate cancer, including even for high-grade cancers in some cases, intervention is generally not recommended for those with a very high risk of death from other causes within 10 yr of their prostate cancer diagnosis [4,7]. We therefore developed a logistic regression model predicting noncancer mortality using a 5% sample of Medicare beneficiaries between 2004 and 2013 identified as cancer-free by the Surveillance, Epidemiology, and End Results Program (SEER). The model incorporated age, race, comorbidity [8], socioeconomic class measured at the 5-digit zip code level [9], census region, and time at risk (C-index 0.82) [4,10,11]. We applied the estimated coefficients from this model to our cohort of newly diagnosed prostate cancer patients, computing each patient’s predicted risk of death within 10 yr of diagnosis (the time at risk) absent their prostate cancer diagnosis. This predicted risk was then categorized as follows: risk of noncancer mortality less than or equal to 25% (“low”), greater than 25% to less than or equal to 50% (“intermediate”), greater than 50% to less or equal to 75% (“high”), and greather than 75% (“very high”).
2.4. Analysis
We first compared patient and regional characteristics across urologist practice types using chi-square statistics. To examine the independent effect of urologist practice type, or IMRT ownership, on rates of treatment for prostate cancer, we fit multivariable logistic regression models using patient-level treatment as the outcome. To account for the clustered nature of the data (patient within a Hospital Referral Region, defined by the Dartmouth Atlas of Health Care) [12], we utilized generalized estimating equations. All models were adjusted for patient age, race, comorbidity [8], socioeconomic class [9], urban versus nonurban residence and regional variables, including supply of urologists, radiation oncologists, hospital beds, and Medicare managed care penetration. For analyses focusing on treatment rates by risk of noncancer mortality, models were stratified by urologist ownership of IMRT. In all cases, we derived the adjusted percentages of patients treated across urologist practice type or IMRT ownership status by back-transforming the predicted use from the models. This was achieved by taking the predicted population marginal means at each level of the variable of interest derived from the multivariable logistic regression model. The means were then converted from the log-odds scale to percentages via an inverse logit transformation.
2.5. Sensitivity analysis
To mitigate concerns that differences in treatment patterns across urology practice affiliations may relate to the misclassification of incident prostate cancer using Medicare claims alone or to differences in disease severity, we repeated all analyses using SEER cancer registry data linked to Medicare claims and adjusted for cancer grade and stage. The SEER-Medicare cohort included incident cases of prostate cancer diagnosed between 2010 and 2011.
All analyses were carried out using computerized software (SAS 9.4, Cary, NC, USA). All tests were two-sided and the probability of type 1 error was set at 0.05. The study protocol was judged to be exempt by the institutional review board of the University of Michigan.
3. Results
There were 31 274 patients with newly diagnosed prostate cancer between 2010 and 2012, the years of the study. Of these, 28 164 (or 90%) had complete information and at least 1 yr of follow-up. Demographics and clinical characteristics of these 28 164 newly diagnosed prostate cancer patients varied according to urologist practice affiliation (Table 1), although many of these differences were small in magnitude. Of note, IMRT ownership increased with group size, and was present in 64% of the 110 large single-specialty urology practices.
Table 1.
Clinical and regional characteristics of newly diagnosed prostate cancer patients by urologist practice affiliation
| Characteristics | Urologist practice affiliation | p value* | ||||
|---|---|---|---|---|---|---|
| Single specialty group | Multispecialty group | |||||
| 1–2 urologists |
Small (3–5) |
Medium (6–9) |
Large (10 or more) |
|||
| Urology group characteristics: | ||||||
| No. of urology groups | — | 332 | 113 | 110 | — | — |
| No. of urologists | 1711 | 1006 | 630 | 1488 | 1546 | — |
| No. of groups with IMRT ownership | 0 | 5 (2) | 13 (12) | 70 (64) | 0 | — |
| Patient characteristics: | ||||||
| No. of patients | 6905 | 4859 | 2981 | 7043 | 6376 | — |
| Age (yr) | <0.001 | |||||
| 66–69 | 1889 (27) | 1426 (29) | 939 (31) | 2133 (30) | 2143 (34) | |
| 70–74 | 2284 (33) | 1586 (33) | 1003 (34) | 2407 (34) | 2177 (34) | |
| 75–79 | 1576 (23) | 1128 (23) | 623 (21) | 1555 (22) | 1313 (21) | |
| 80–84 | 822 (12) | 493 (10) | 292 (10) | 673 (10) | 545 (9) | |
| 85+ | 334 (5) | 226 (5) | 124 (4) | 275 (4) | 198 (3) | |
| Race/ethnicity | <0.001 | |||||
| White | 6074 (88) | 4351 (90) | 2688 (90) | 6121 (87) | 5717 (90) | |
| Black | 591 (9) | 396 (8) | 222 (7) | 759 (11) | 465 (7) | |
| Other/unknown | 240 (3) | 112 (2) | 71 (2) | 163 (2) | 194 (3) | |
| Comorbidity | <0.001 | |||||
| 0 | 3976 (58) | 2819 (58) | 1852 (62) | 4173 (59) | 4022 (63) | |
| 1 | 1732 (25) | 1208 (25) | 697 (23) | 1756 (25) | 1448 (23) | |
| 2 | 669 (10) | 468 (10) | 249 (8) | 665 (9) | 511 (8) | |
| 3+ | 528 (8) | 364 (7) | 183 (6) | 449 (6) | 395 (6) | |
| Risk of noncancer mortality | <0.001 | |||||
| Low | 2056 (30) | 1569 (32) | 1089 (37) | 2482 (35) | 2529 (40) | |
| Intermediate | 2422 (35) | 1646 (34) | 1024 (34) | 2461 (35) | 2143 (34) | |
| High | 1581 (23) | 1063 (22) | 581 (19) | 1413 (20) | 1130 (18) | |
| Very high | 846 (12) | 581 (12) | 287 (10) | 687 (10) | 574 (9) | |
| Socioeconomic status (zip code level) | <0.001 | |||||
| Low | 2722 (39) | 1598 (33) | 930 (31) | 1879 (27) | 1833 (29) | |
| Medium | 2285 (33) | 1862 (38) | 1036 (35) | 2254 (32) | 2305 (36) | |
| High | 1898 (27) | 1399 (29) | 1015 (34) | 2910 (41) | 2238 (35) | |
| Residing in urban area | <0.001 | |||||
| Nonurban | 1989 (29) | 1018 (21) | 606 (20) | 585 (8) | 1587 (25) | |
| Urban | 4916 (71) | 3841 (79) | 2375 (80) | 6458 (92) | 4789 (75) | |
| Urologists per 100 000 | <0.001 | |||||
| Low (≤ 34) | 2803 (41) | 1675 (34) | 1121 (38) | 1663 (24) | 2119 (33) | |
| Intermediate | 2359 (34) | 1807 (37) | 980 (33) | 2194 (31) | 2086 (33) | |
| High (≥ 71) | 1743 (25) | 1377 (28) | 880 (30) | 3186 (45) | 2171 (34) | |
| Radiation oncologists per 100 000 | <0.001 | |||||
| Low (≤ 13) | 2714 (39) | 1767 (36) | 943 (32) | 1756 (25) | 2180 (34) | |
| Intermediate | 2420 (35) | 1760 (36) | 1055 (35) | 2211 (31) | 1995 (31) | |
| High (≥ 29) | 1771 (26) | 1332 (27) | 983 (33) | 3076 (44) | 2201 (35) | |
| Hospital beds per 100 000 | <0.001 | |||||
| Low (≤ 3340) | 2667 (39) | 1702 (35) | 955 (32) | 2086 (30) | 1989 (31) | |
| Intermediate | 2442 (35) | 1696 (35) | 1011 (34) | 2110 (30) | 2205 (35) | |
| High (≥ 6342) | 1796 (26) | 1461 (30) | 1015 (34) | 2847 (40) | 2182 (34) | |
| Medicare managed care penetration | <0.001 | |||||
| Low (≤ 13.5%) | 2421 (35) | 1854 (38) | 1154 (39) | 1762 (25) | 2138 (34) | |
| Intermediate | 2107 (31) | 1514 (31) | 1031 (35) | 2674 (38) | 2046 (32) | |
| High (≥ 25.0%) | 2377 (34) | 1491 (31) | 796 (27) | 2607 (37) | 2192 (34) | |
IMRT = intensity-modulated radiation therapy.
The p values are from chi-square tests of independence comparing the distribution of each variable across all different categories of group size.
Examining by group size and affiliation, we found that the use of IMRT ranged from 24% in multispecialty groups to 37% in large urology groups (Fig. 1A; p < 0.001). Overall rates of curative treatment varied less, ranging from 67% for those seen by urologists practicing in small groups to 70% for those managed by large groups (p = 0.004). However, IMRT use was more common among urology groups with ownership compared with those without (43% vs 30%, p < 0.01), or to multispecialty groups (24%, p < 0.001). Stratifying by both IMRT ownership and group size (Fig. 1B), the gap in use of IMRT between owners and nonowners was consistent across the size of the urology group.
Fig. 1.
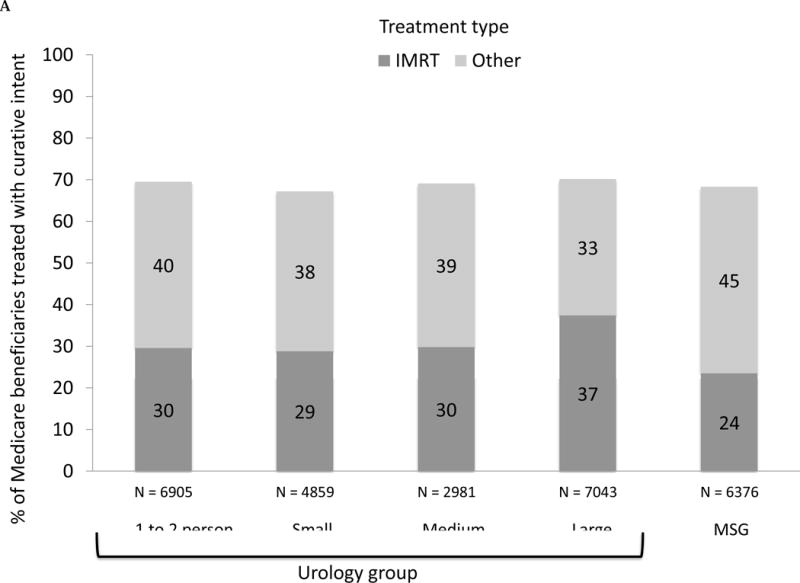
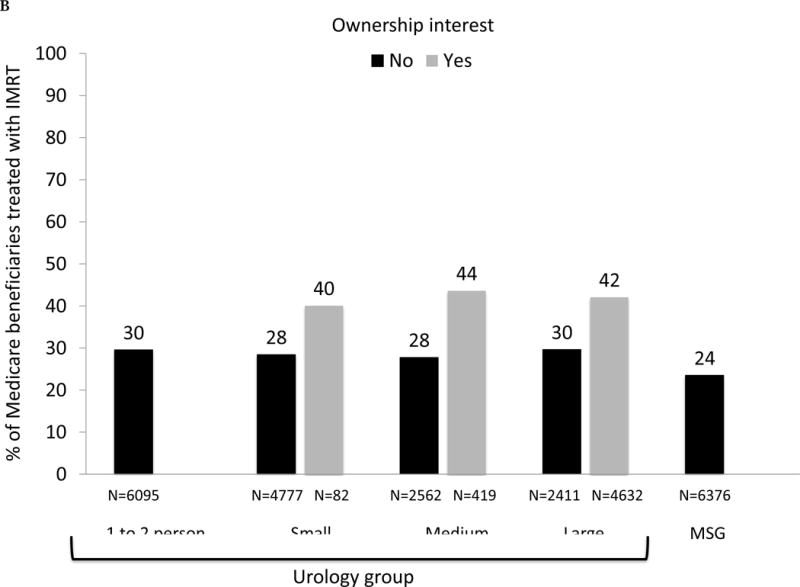
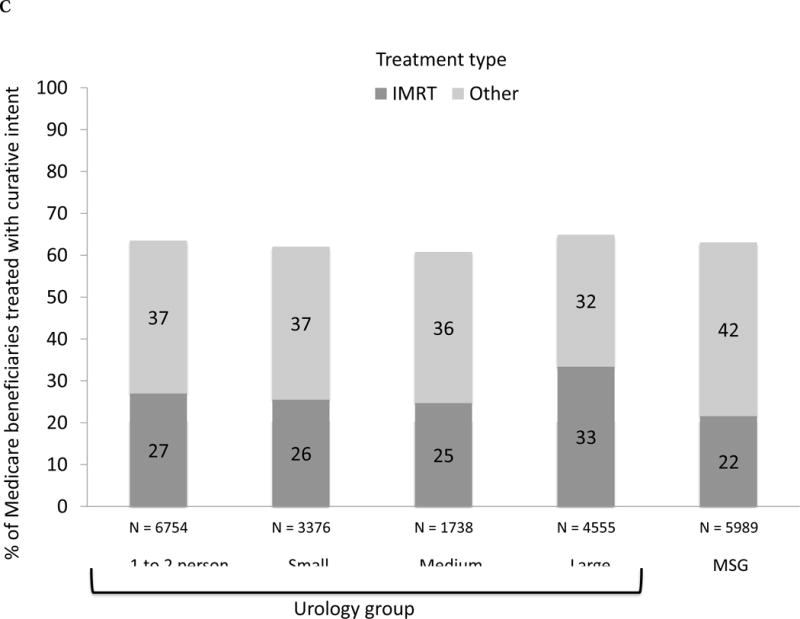
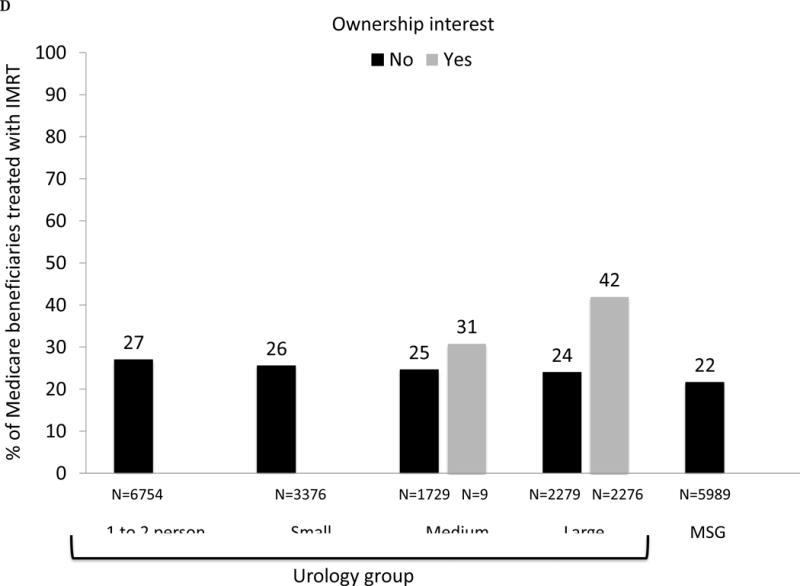
(A) Adjusted percent of Medicare beneficiaries with newly diagnosed prostate cancer in 2010–2012 (n = 28 164) treated with curative intent within 1 yr of diagnosis, presented as stacked bars. Dark gray bars represent treatment with intensity-modulated radiation therapy (IMRT). Light gray bars represent other treatments, including external beam radiation therapy other than IMRT, brachytherapy, cryotherapy, and surgery. Results presented are stratified by type of urology practice: urology group practice (further divided by size of group) or multispecialty group (MSG). Comparing IMRT use across the practice types, p <0.001. Comparing overall curative treatment (IMRT plus other) across the practice types, p =0.004. (B) Adjusted percent of Medicare beneficiaries with newly diagnosed prostate cancer in 2010–2012 (n = 28 164) treated with IMRT within 1 yr of diagnosis, stratified by IMRT ownership status of urology practice (gray bars represent owners, black bars represent nonowners) and practice type: urology group practice (further divided by size of group) or MSG group. There were no multispecialty groups or urology groups with one to two urologists that had IMRT ownership. For differences in IMRT use comparing owners with nonowners, p =0.18 for small urology groups, p =0.004 for medium urology groups, and p <0.001 for large urology groups. (C) Adjusted percent of beneficiaries in Surveillance, Epidemiology, and End Results-Medicare data with newly diagnosed prostate cancer in 2010–2011 (n = 22 412) treated with curative intent within 1 yr of diagnosis, presented as stacked bars. Dark gray bars represent treatment with IMRT. Light gray bars represent other treatments, including external beam radiation therapy other than IMRT, brachytherapy, cryotherapy, and surgery. Results presented are stratified by type of urology practice: urology group practice (further divided by size of group) or MSG. Comparing IMRT use across the practice types, p =0.033. Comparing overall curative treatment (IMRT plus other) across the practice types, p =0.08. (D) Adjusted percent of beneficiaries in Surveillance, Epidemiology, and End Results-Medicare data with newly diagnosed prostate cancer in 2010–2011 (n = 22 412) treated with IMRT within 1 yr of diagnosis, stratified by IMRT ownership status of urology practice (gray bars represent owners, black bars represent nonowners) and practice type: urology group practice (further divided by size of group) or MSG group. There were no multispecialty groups, urology groups with one to two urologists, or small urology groups that had IMRT ownership. For differences in IMRT use comparing owners to nonowners, p =0.66 for medium urology groups, and p <0.001 for large urology groups.
The initial management strategy varied with respect to physician ownership interest in IMRT (Fig. 2A). Owners were more likely to use radiation therapy (44% vs 31%) and less likely to use brachytherapy (8.7% vs 15%) or no curative treatment (24% vs 28%) after adjustment for the characteristics noted in Table 1 (each p < 0.001). The use of surgery among newly diagnosed prostate cancer patients was similar (p = 0.11).
Fig. 2.
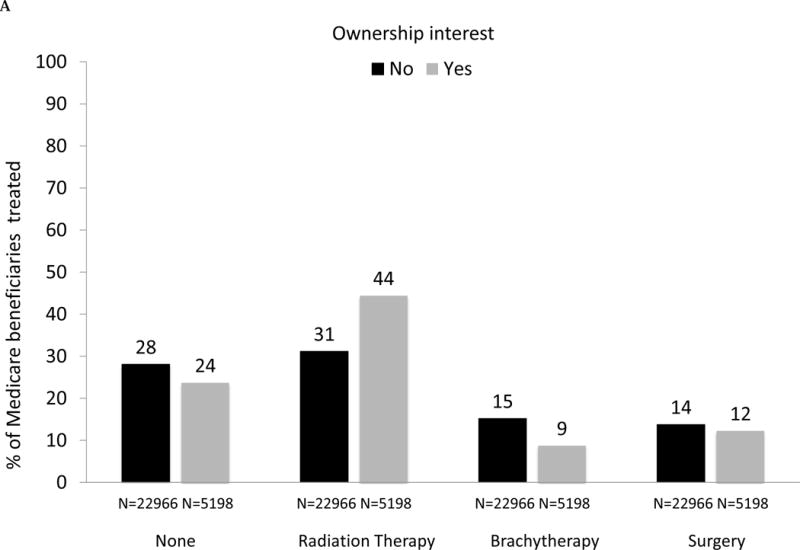
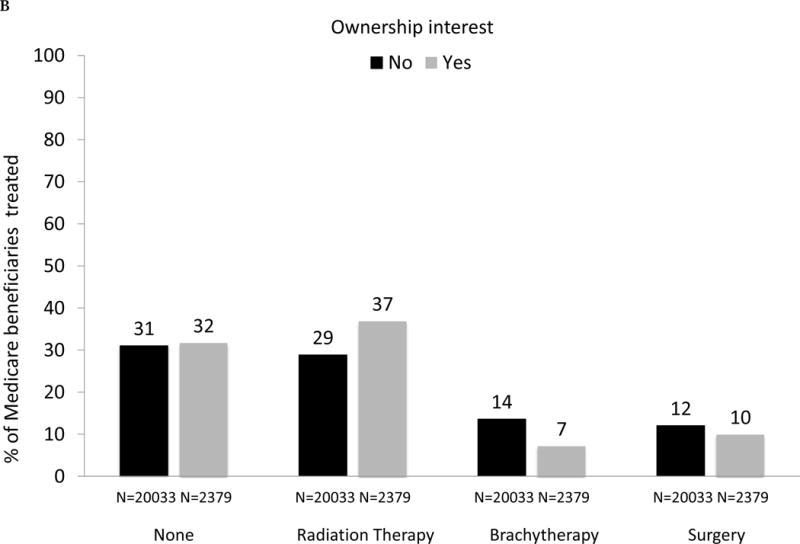
(A) Adjusted percent of Medicare beneficiaries with newly diagnosed prostate cancer (n = 28 164), by management strategy within 1 yr of diagnosis (none, radiation therapy, brachytherapy, surgery), stratified by intensity-modulated radiation therapy (IMRT) ownership status of urology practice (gray bars represent owners, black bars represent nonowners). Comparing owners with nonowners, p <0.001 for the use of no treatment, radiation therapy, and brachytherapy; p =0.11 for surgery. Cryotherapy was extremely uncommon, with only 783 cases (or, 3% of all study participants) and is not shown. (B) Adjusted percent of beneficiaries in Surveillance, Epidemiology, and End Results-Medicare data with newly diagnosed prostate cancer (n = 22 412), by management strategy within 1 yr of diagnosis (none, radiation therapy, brachytherapy, surgery), stratified by IMRT ownership status of urology practice (gray bars represent owners, black bars represent nonowners). Comparing owners with nonowners, p =0.76 for the use of no treatment, p =0.003 for radiation therapy, p <0.001 for brachytherapy, and p =0.25 for surgery. Cryotherapy was extremely uncommon, with only 502 cases (or, 2% of all study participants) and is not shown.
As demonstrated in Fig. 3A, large and significant differences in the use of IMRT for men with prostate cancer between owners and nonowners were evident across all levels of risk of noncancer mortality (p values for all comparisons between owners and nonowners < 0.001). For patients with a greater than 75% risk (“very high” category) of mortality within 10 yr and absent a cancer diagnosis, 42% were treated by owners compared with only 26% by nonowners (p < 0.001). As shown in Figure 3B, there was a consistent decline in the use of treatment with curative intent with increasing risk of noncancer mortality for both owners and nonowners (both p values for trend < 0.001). Rates of treatment with curative intent were higher in groups with IMRT ownership versus nonownership across all categories of risk of noncancer mortality (though not statistically significant for the high-risk category), with the largest difference in the “very high” risk category (53% vs 44%, p = 0.002).
Fig. 3.
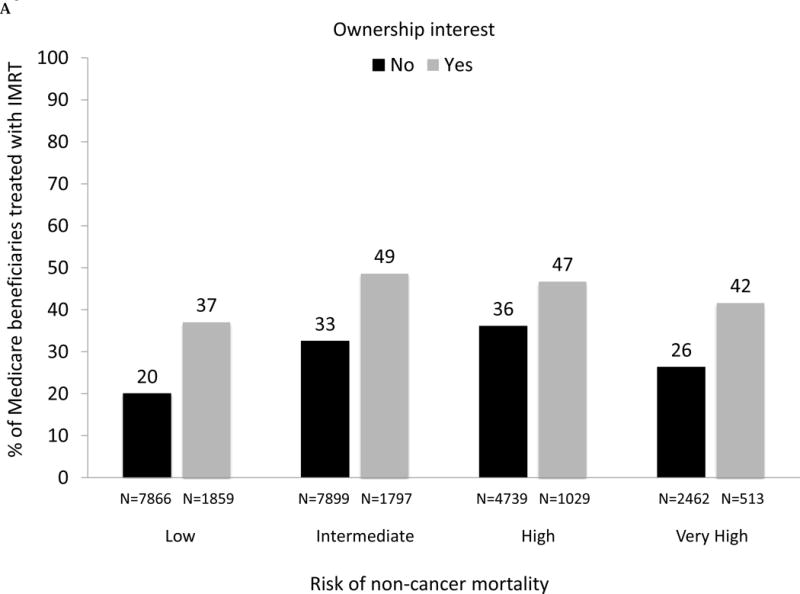
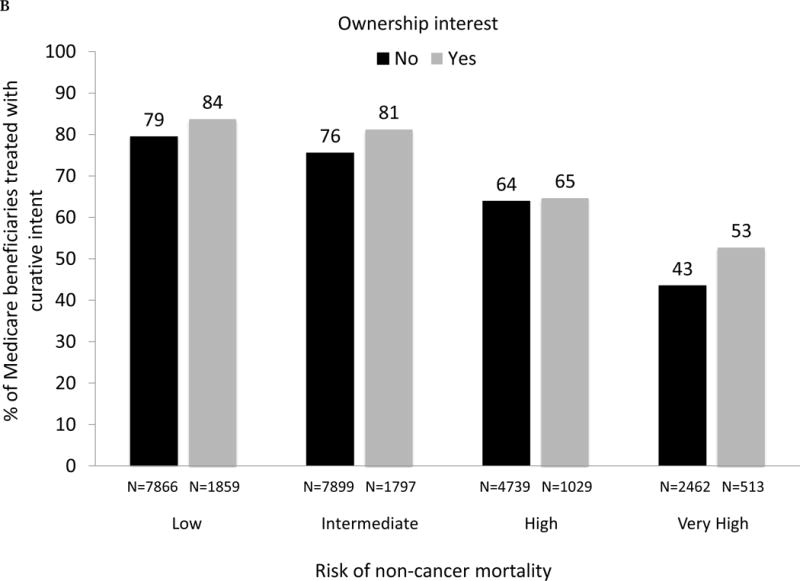
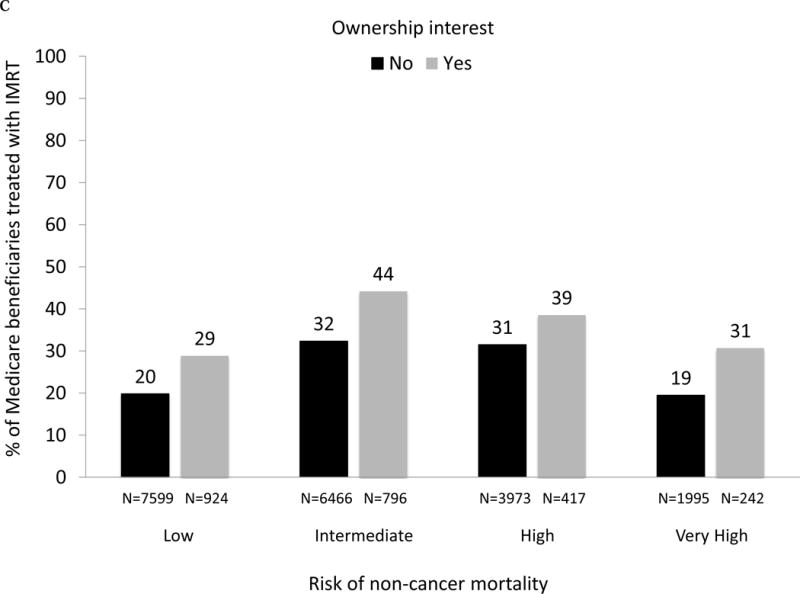
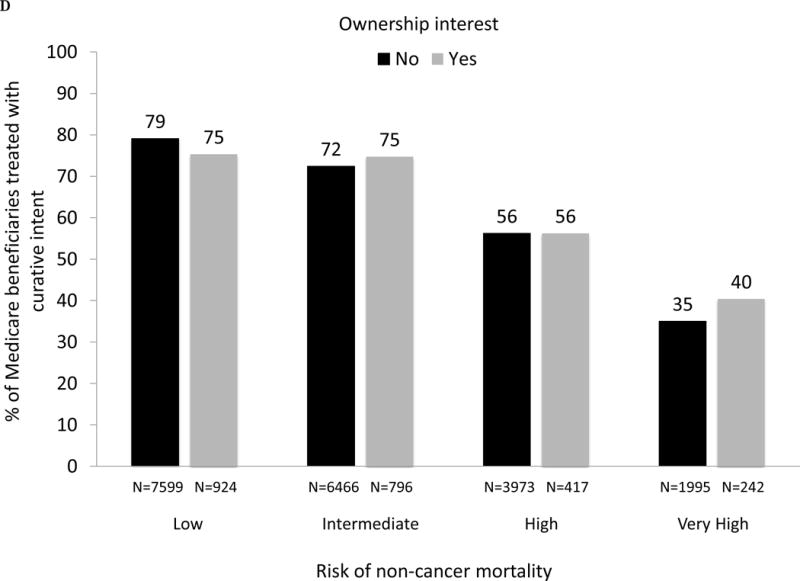
(A) Adjusted percent of Medicare beneficiaries with newly diagnosed prostate cancer in 2010–2012 (n = 28 164) treated with intensity-modulated radiation therapy (IMRT) within 1 yr of diagnosis, according to risk of noncancer mortality (“low” representing 0–25% risk of death within 10 yr absent a prostate cancer diagnosis; “intermediate” representing 25–50% risk; “high” representing > 50–75% risk; “very high” representing > 75–100% risk) and stratified by IMRT ownership status (black bars represent nonowners, gray bars represent owners). For the overall cohort, comparing use of IMRT between owners versus nonowners, p <0.001. Comparing IMRT use between owners versus nonowners within each mortality risk category, p <0.001 for every category. (B) Adjusted percent of Medicare beneficiaries with newly diagnosed prostate cancer in 2010–2012 (n = 28 164) treated with curative intent (any external beam radiation, brachytherapy, cryotherapy, or surgery) within 1 yr of diagnosis, according to risk of noncancer mortality and stratified by IMRT ownership (black bars represent nonowners, gray bars represent owners). Comparing use of curative treatment between owners versus nonowners for the overall cohort, p <0.001. Comparing use of curative treatment between owners versus nonowners within each noncancer mortality risk category, p <0.001 for the low and intermediate noncancer mortality risk categories, p =0.72 for the high noncancer mortality risk category and p =0.002 for the very high noncancer mortality risk category. (C) Adjusted percent of men with newly diagnosed prostate cancer in Surveillance, Epidemiology, and End Results-Medicare in 2010 and 2011 (n = 22 412) treated with IMRT within 1 yr of diagnosis, according to risk of noncancer mortality, further adjusted for cancer grade and stage, stratified by IMRT ownership (black bars represent nonowners, gray bars represent owners). Comparing IMRT use between owners versus nonowners for the overall cohort, p =0.005. Comparing IMRT use between owners versus nonowners within each noncancer mortality risk category, p =0.002 for the low noncancer mortality risk category, p =0.001 for the intermediate noncancer mortality risk category, p =0.039 for the high noncancer mortality risk category and p <0.001 for the very high noncancer mortality risk category. (D) Adjusted percent of men with newly diagnosed prostate cancer in Surveillance, Epidemiology, and End Results-Medicare in 2010 and 2011 (n = 22 412) treated with curative intent (any external beam radiation, brachytherapy, cryotherapy, or surgery) within 1 yr of diagnosis, according to risk of noncancer mortality, further adjusted for cancer grade and stage, stratified by IMRT ownership (black bars represent nonowners, gray bars represent owners). Comparing use of curative treatment between owners versus nonowners for the overall cohort, p =0.76. Comparing use of curative treatment between owners versus nonowners within each noncancer mortality risk category, p =0.16 for the low noncancer mortality risk category, p =0.31 for the intermediate noncancer mortality risk category, p =0.99 for the high noncancer mortality risk category, and p =0.042 for the very high noncancer mortality risk category.
We performed a sensitivity analysis among men with newly diagnosed prostate cancer in SEER-Medicare data in 2010 and 2011, allowing us to adjust for cancer grade and stage. As shown in Figures 1C, 1D, 2B, 3C, and 3D, patterns of treatment across practice size and IMRT ownership status was similar to those in the Medicare cohort. In particular, rates of treatment with curative intent were also higher in groups with IMRT ownership among patients with a “very high” risk of noncancer mortality (40% vs 35%, p = 0.04).
4. Discussion
Between 2010 and 2012, more than two-thirds of male Medicare beneficiaries received treatment, regardless of whether the urologist practiced in a single-specialty or multispecialty group. Larger urology groups were much more likely to use IMRT as the treatment modality, but this appeared to be primarily because they were more likely to have an ownership interest in IMRT. Urology practices with an ownership interest were 43% more likely to use IMRT for treatment, regardless of group size, and were less likely to use other modalities. Among those with the highest risk of death within 10 yr of diagnosis absent a cancer diagnosis, arguably the men least likely to benefit from intervention with curative therapy, male beneficiaries managed by owners were 58% more likely to receive IMRT compared with nonowners and 21% more likely to be treated with curative intent overall.
Prior work has demonstrated that the integration of IMRT into a urology group practice is associated with changes in treatment patterns for men with prostate cancer [13,14]. The first study described a 16% and 29% increase in the use of IMRT after ownership acquisition relative to nonself-referring private practice urologists and urologists working in National Comprehensive Cancer Network centers, respectively [13]. The study was criticized for its poor choice of control groups that did not illustrate established trends in treatment patterns gleaned from population-based data [15,16]. The second study demonstrated a similar change in IMRT use following its integration into a single urology practice [14]. Because of selection issues, proponents of self-referral have argued that findings from these studies lack generalizability to the broader population of urology practices and prostate cancer patients. Our national study represents the first population-based study to demonstrate a strong association between ownership and IMRT use.
More importantly, the association between IMRT ownership and utilization is evident across the full range of patient health status, including among men with the highest risk of noncancer mortality. Although it remains among the most common causes of cancer-related mortality among men [17], a significant proportion of prostate cancers are slow growing such that death from other causes is much more likely [1]. For this reason, professional guidelines recommend assessment of health status at the time of diagnosis and favor observation for those with significant comorbidity, as these men are unlikely to benefit from curative intervention [7]. Our sensitivity analyses incorporating cancer grade and stage suggest that higher disease severity in practices with IMRT ownership is an unlikely explanation for the observed treatment patterns. One possible motivation for the higher intensity practice patterns is the financial incentives associated with physician ownership. In the context of IMRT, urology groups additionally collect the technical payment component (ie, operating expenses), strengthening incentives to maximize throughput. Our findings are consistent with previous research suggesting that physicians are most responsive to financial incentives in clinical settings where treatment benefit is uncertain as opposed to circumstances where it is clear-cut [18]. In this context, the greatest gap in use of curative treatment among IMRT owners versus nonowners occurred in the patients with the highest risk of noncancer mortality, where treatment benefit is least certain.
Beyond relevance for patients seeking counseling about a new diagnosis, our study results also have implications for practicing clinicians. Referring physicians should carefully consider the practice structure of the urology group to which they send their patients, as this is likely to importantly influence how their patients are managed. Furthermore, urologists themselves should consider the setting in which they practice, and be more aware of the incentives which may influence how they manage the men under their care, particularly in clinical scenarios where the benefits of therapy are uncertain.
Our findings should be considered in the context of several limitations in the data. First, while urology groups with IMRT ownership were more likely to use it to treat patients, our study design prevents us from asserting a causal link between ownership and utilization. However, prior longitudinal studies on the topic [13,14] and strong relationships between ownership and utilization in other contexts [19–23] support that possibility. Second, to ensure complete medical information for the patients in the study, we excluded those with Medicare managed care plans. While this is a necessary exclusion to maintain internal validity, its practical effect has grown as Medicare Advantage has expanded. We addressed this secular trend by including a covariate adjusting for Medicare Advantage penetration at the county level in all analyses. Third, while there are some urology groups that overuse IMRT, there are others who use it appropriately. There is nothing fundamentally wrong with large integrated urology groups, just a concern that financial incentives—in certain circumstances—can lead to unnecessary treatment. Fourth, our national Medicare analyses could not adjust for grade and stage. In the SEER-Medicare analyses, the effect of ownership on the delivery of radiation therapy was attenuated but remained significant (Supplementary data). SEER catchment areas are not random samples of Medicare physicians or patients. While the registry has robust internal validity, it therefore has shortcomings in terms of external validity (generalizability). In fact, 70% of the practices with IMRT ownership that most often overtreated patients lie outside of SEER regions, explaining in part the attenuated effect.
Finally, we agree that there is a potential for misclassification of IMRT ownership. However, this misclassification would be random and independent of the values of the other variables in the model because the data for the classification did not come from Medicare claims. We wanted to ensure that the exposure was assessed independently of all other variables. Indeed, when we assessed IMRT ownership in 2012, Medicare data for 2012 and 2013 were not yet available and thus we were effectively blinded to the Medicare part of the picture. While the general effect of such misclassification would be to bias results toward the null, we decided to investigate this in our study with a sensitivity analysis on the radiation therapy results in Figure 2A. We limited the cohort to patients diagnosed in 2012, the year we classified ownership, and found that the effect size of ownership increased when we improved the accuracy of the ownership classification by shrinking the period between diagnosis and classification. This is consistent with misclassification biasing results toward the null.
5. Conclusions
Notwithstanding these limitations, urologists practicing in single-specialty groups with an ownership interest in radiation therapy are more likely to treat, and even potentially overtreat, patients with IMRT than those affiliated with a multispecialty practice or a group without an ownership stake. Further work is needed to examine whether the impact of such self-referral arrangements on utilization is counterbalanced by improvements in efficiency or quality, as many proponents claim.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: This work was supported by research funding from the NCI (R01 CA168691) to BKH and VBS. BKH is further supported by funding from the NIA (R01 AG048071). FRS is supported by the Department of Veterans Affairs, Veterans Health Administration, VISN1 Career Development Award.
Funding/Support and role of the sponsor: The funding sources provided financial support but had no role in the design, conduct, interpretation, or dissemination of the study. Because this study uses SEER-Medicare data, the final version of the manuscript was approved by the NCI to ensure the absence of confidentiality issues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Brent K. Hollenbeck had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hollenbeck, Shahinian, Jacobs, Skolarus, Borza, Schroeck, Herrel.
Acquisition of data: Hollenbeck, Shahinian, Kaufman, Yan.
Analysis and interpretation of data: Kaufman, Yan.
Drafting of the manuscript: Hollenbeck, Shahinian.
Critical revision of the manuscript for important intellectual content: Hollenbeck, Kaufman, Yan, Herrel, Borza, Schroeck, Jacobs, Skolarus, Shahinian.
Statistical analysis: Kaufman, Yan.
Obtaining funding: Hollenbeck, Shahinian.
Administrative, technical, or material support: Hollenbeck.
Supervision: Hollenbeck, Shahinian.
Other: None.
Financial disclosures: Brent K. Hollenbeck certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript
We assessed treatment for prostate cancer among urologists with and without ownership of radiation facilities. Owners were more likely to treat prostate cancer, even when treatment was unlikely to provide a survival benefit to the patient.
References
- 1.Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996;124:577–84. doi: 10.7326/0003-4819-124-6-199603150-00007. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roehrig C, Miller G, Lake C, Bryant J. National health spending by medical condition, 1996–2005. Health Aff. 2009;28:w358–67. doi: 10.1377/hlthaff.28.2.w358. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309:2587–95. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollenbeck BK, Bierlein MJ, Kaufman SR, et al. Implications of evolving delivery system reforms for prostate cancer care. Am J Manag Care. 2016;22:569–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NCCN Guidelines for Patients. Prostate cancer. http://www.nccn.org/patients.
- 8.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 9.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 10.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145:646–53. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Cutler DM, Glaeser EL, Rosen AB. Is the US population behaving healthier? http://www.nber.org/aging/rrc/papers/orrc06-13.pdf.
- 12.The Dartmouth Atlas of Health Care: data by region. Lebanon (NH): http://www.dartmouthatlas.org/data/region. [Google Scholar]
- 13.Mitchell JM. Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629–37. doi: 10.1056/NEJMsa1201141. [DOI] [PubMed] [Google Scholar]
- 14.Bekelman JE, Suneja G, Guzzo T, Pollack CE, Armstrong K, Epstein AJ. Effect of practice integration between urologists and radiation oncologists on prostate cancer treatment patterns. J Urol. 2013;190:97–101. doi: 10.1016/j.juro.2013.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saylor BP, Kerr RR. Intensity-modulated radiation therapy study reignites urologist self-referral controversy. Urology Times. 2013 http://urologytimes.modernmedicine.com/urology-times/content/tags/aacu/intensity-modulated-radiation-therapy-study-reignites-urologist-self.
- 16.Jacobs BL, Schroeck FR, Hollenbeck BK. Intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;370:679. doi: 10.1056/NEJMc1314524. [DOI] [PubMed] [Google Scholar]
- 17.American Cancer Society. Cancer Facts & Figures. 2017 https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
- 18.Shen J, Andersen R, Brook R, Kominski G, Albert PS, Wenger N. The effects of payment method on clinical decision-making: physician responses to clinical scenarios. Med Care. 2004;42:297–302. doi: 10.1097/01.mlr.0000114918.50088.1c. [DOI] [PubMed] [Google Scholar]
- 19.Hillman BJ, Olson GT, Griffith PE, et al. Physicians’ utilization and charges for outpatient diagnostic imaging in a Medicare population. JAMA. 1992;268:2050–4. [PubMed] [Google Scholar]
- 20.Hughes DR, Bhargavan M, Sunshine JH. Imaging self-referral associated with higher costs and limited impact on duration of illness. Health Aff. 2010;29:2244–51. doi: 10.1377/hlthaff.2010.0413. [DOI] [PubMed] [Google Scholar]
- 21.Baker LC. Acquisition of MRI equipment by doctors drives up imaging use and spending. Health Aff. 2010;12:2252–9. doi: 10.1377/hlthaff.2009.1099. [DOI] [PubMed] [Google Scholar]
- 22.Hollenbeck BK, Dunn RL, Suskind AM, Zhang Y, Hollingsworth JM, Birkmeyer JD. Ambulatory surgery centers and outpatient procedure use among Medicare beneficiaries. Med Care. 2014;52:926–31. doi: 10.1097/MLR.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingsworth JM, Ye Z, Strope SA, Krein SL, Hollenbeck AT, Hollenbeck BK. Physician-ownership of ambulatory surgery centers linked to higher volume of surgeries. Health Aff. 2010;29:683–9. doi: 10.1377/hlthaff.2008.0567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


