Abstract
Cholinesterase inhibitors (ChEI) are the primary pharmacological treatment for symptom management of Alzheimer’s disease (AD), but they carry known risks during long-term use, and do not guarantee clinical effects over time. The balance of risks and benefits may warrant discontinuation at different points during the disease course. Indeed, while there is limited scientific study of deprescribing ChEI, clinicians routinely face practical decisions about whether to continue or stop medications. This review examined published practice recommendations for discontinuation of ChEI in AD. To characterize the scientific basis for recommendations, we first summarized randomized controlled trials of ChEI discontinuation. We then identified practice guidelines by professional societies and in textbooks and classified them according to (1) whether they made a recommendation about discontinuation, (2) what the recommendation was, and (3) the proposed grounds for discontinuation. There was no consensus in guidelines and textbooks about discontinuation. Most recommended individualized discontinuation decisions, but there was essentially no agreement about what findings or situations would warrant discontinuation, or even about what domains to consider in this process. The only relevant domain identified by most guidelines and textbooks was a lack of response or a loss of effectiveness, both of which can be difficult to ascertain in the course of a progressive condition. Well-designed, long-term studies of discontinuation have not been conducted; such evidence is needed to provide a scientific basis for practice guidelines. It seems reasonable to apply an individualized approach to discontinuation while engaging patients and families in treatment decisions.
Keywords: Alzheimer’s disease, cholinesterase inhibitor, treatment, discontinuation, deprescribing
The increasing national and international prevalence of Alzheimer’s disease (AD) and associated burden imparts a high priority on delivering safe and effective treatment options. While there is increased attention to early detection of neurocognitive disorders and initiation of treatment,(1) there is no cure. Cholinesterase inhibitors (ChEI) are widely used and typically employed as first-line pharmacotherapy for symptomatic treatment of major neurocognitive disorder caused by AD; however, treatment often continues through advanced disease stages.
Published findings and professional guidelines generally recommend initiating ChEI treatment for indicated conditions,(1) but some analyses have questioned the benefit of these medications. For instance, a focused review(2, p. 321) found that, “Because of flawed methods and small clinical benefits, the scientific basis for recommendations of cholinesterase inhibitors for the treatment of Alzheimer’s disease is questionable.” A recent Cochrane Review(3) found that ChEI treatment effects are small and of uncertain clinical importance. The trials reviewed did not elucidate the relationship between duration of treatment and response. Because cognitive and behavioral impairments change during the progressive disease course, the effects of medications may be unpredictable, especially over long durations of treatment.
Providers make meaningful decisions about continuation based not merely on measured effects of drug compared to placebo but rather on a risk-benefit balance, in the context of patients’ and families’ values and preferences. Common side effects such as diarrhea are typically considered to be a nuisance rather than a serious hazard but may significantly affect frail patients.(4) Serious adverse reactions associated with ChEI treatment have been reported in the Food and Drug Administration Adverse Event Reporting System database and include rhabdomyolysis, convulsion, falls, loss of consciousness, syncope, pneumonia, and death.(5) Additional epidemiological findings have highlighted that long-term ChEI treatment may be associated with greater risks than nontreatment, including weight loss,(6) urinary retention and addition of anticholinergic medications,(7) bradycardia,(8, 9) drug-drug interactions,(10) and depression.(11) Because many of these adverse events are unpredictable, it can be challenging to weigh predicted benefits against predicted harms of continued treatment.
Apart from harms and benefits measured across groups of patients, there may be personal factors that would influence continuation of medications for dementia. ChEI medications are now mainly generic but still confer economic costs. Persons with advanced dementia may receive overly aggressive care that does not align with person-centered care goals,(12, 13) and polypharmacy from medications that are minimally beneficial may place patients at unnecessary risk.(14) On the other hand, the act of giving a medication, regardless of its material effects, may signify that loved ones are not giving up hope and that they care for and love the patient. Thus, families may choose to continue ChEIs, even if they have not yielded positive effects. Furthermore, ChEI treatment may contribute to meaningful improvements in noncognitive domains, such as reduction in neuropsychiatric symptoms and behavioral disturbance.(4) These outcomes may be increasingly important in later stages of AD and deserve consideration alongside cognitive benefits.
To help patients, caregivers and providers make reasoned decisions about continuation of ChEI medications, it seems essential to ascertain the measured effects of their discontinuation. Observational studies are inherently unreliable, because the decision to cease treatment is often predicated on some other negative event. For instance, ChEI may be stopped because of an adverse health outcome or during a hospitalization, which may generate bias through confounding by indication.(15) Similarly, an open discontinuation trial may misrepresent the effects of discontinuation, because patients and caregivers may attribute normal worsening (part of the disease course) to the effects of stopping a medication. For these reasons, randomized controlled trials (RCTs) of discontinuation are required to clarify the potential benefits of ongoing use.
Prior RCT Evidence
A recent meta-analysis summarized the results of five RCTs of ChEI discontinuation in patients with possible or probable AD.(16) All involved outpatients, with varying degrees of severity. Another RCT,(17) published after this meta-analysis, examined discontinuation among institutionalized patients with probable AD. The primary outcomes across RCTs related to cognition, with little consistency in other outcomes. Some of the studies did not assess any behavioral or functional endpoints; those that did evaluate secondary outcomes included neuropsychiatric symptoms, quality of life, safety, and functioning (e.g., independence in daily living) among other variables; but null findings and lack of standardization across endpoints make it difficult to draw robust conclusions about noncognitive benefits of sustained ChEI treatment. The five outpatient studies reported poorer cognitive outcomes among those who discontinued ChEIs, although the clinical significance of such decline was difficult to ascertain. The inpatient study did not identify a significant difference between continuation/discontinuation groups. Among studies that examined functional or behavioral outcomes, there was no consistent difference between those who continued and those who stopped ChEI treatment. Indeed, there was some evidence that participants who had initially responded to ChEIs were those who showed the greatest declines after discontinuing.
While all these studies are considered high-quality RCTs, based on the Jadad scale,(18) they lacked consistency in some key parameters, especially the duration of treatment prior to discontinuation. Although each of the six RCTs made recommendations about discontinuation, the multiplicity of designs and outcomes and the absence of a consistent finding call into question the general conclusions about whether medications should or should not be continued in various cases.
Objective
Although clinical trials are meant to inform evidence-based practice, providers generally lack the time to consult the primary literature for clinical decision making. Practice guidelines and textbook recommendations synthesize research findings and clinical experience and account for contextual factors, such as the prevalence, significance and course of an illness. Such recommendations, because they define a standard and are usually written in straightforward terms, are likely to influence care across a variety of settings. To characterize the guidance that providers might obtain through guidelines and recommendations, we conducted a systematic review of those recently published. Given the ambiguity in the primary literature synthesized above, we anticipated that our review findings could guide and improve future recommendations about dementia treatment. For the sake of parsimony, AD is used throughout this article to refer to dementia, or major neurocognitive disorder, caused by AD.
Methods
This systematic review was conducted in accordance with the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.(19)
Practice Guidelines
English-language professional/practice/clinical guidelines were sourced from the National Guideline Clearinghouse, Guidelines International Network, Guideline Central, PubMed, Alzheimer’s Association website, Google search engine, seven websites of relevant specialty societies (American Medical Association, American Academy of Neurology, American Geriatrics Society, Gerontological Society of America, American Psychiatric Association, American Association for Geriatric Psychiatry and American Academy of Home Care Medicine), and email correspondence with a representative of the Alzheimer’s Association. A snowball search strategy supplemented these sources, in which reference lists of guidelines were consulted for other guidelines. PubMed was searched using the MeSH term Alzheimer's disease treatment guidelines. Search terms considered for other databases, search engines, and websites included combinations of the terms acetylcholinesterase inhibitor, cholinesterase inhibitor, treatment, dementia, Alzheimer, Alzheimer’s, and variants of the terms deprescribe and discontinuation.
Guidelines were selected for review if they addressed patient care or treatment recommendations for dementia broadly or AD specifically. They were excluded if they exclusively pertained to a disorder other than AD, specific pharmacological treatments other than cholinesterase inhibitors, specific aspects of patient care (e.g., wandering, depression, non-pharmacological interventions), or to a nonprescribing discipline (e.g., occupational therapy, nursing protocols). Exclusion at screening was conservative; any content that did not clearly fit the exclusion criteria was reviewed by the senior author (MEK) and, when necessary, a full-text copy was obtained for review. English translations of international guidelines were included if available. Guidelines were crosschecked to ensure the most recent version was included in the present review. Expert consensus was sought from local clinicians and researchers in a large metropolitan academic medical center until saturation of sources was achieved. Given the growth in research about diagnosis, treatment and management of AD over the last decade, guidelines published prior to 2005 were not included in the present review.
Textbooks
A search of top selling and top reviewed English-language medical texts was conducted on Amazon.com, using the “medical books” sort function across five pertinent disciplines (neurology, geriatrics, psychiatry, medicine, pharmacology) and one specialty topic (dementia). Psychiatry was subsequently expanded to include (1) general psychiatry and (2) geriatric and neuropsychiatry, and the search terms geriatric psychiatry and neuropsychiatry were used to search within the medical books category. The most recently published and top selling texts in each category were screened for inclusion. These texts were supplemented with searches, using relevant search engines (American Psychiatric Association Publishing, Clinical Key, Google search engine). Expert advisors, comprising five physicians and doctoral-level practitioners and faculty in the respective disciplines at a large academic medical center (including authors AAAA, AC, SRM, and BGM), reviewed the textbook list and provided recommendations for other widely accepted and referenced texts. For initial screening, materials were inclusively selected for review if they were a medical textbook reviewing diagnosis, treatment and practice in the relevant subspecialty; texts were excluded only if they were specific to an unrelated aspect of medicine or practice (e.g., nursing guides, pediatric psychiatry). Full-text hardcopy or electronic books meeting initial inclusion criteria were reviewed independently by two reviewers (BNR and MEK). Texts were subsequently excluded at this stage if they were not appropriate to the category (e.g., a text in the neurology category that was largely focused on internal medicine) or if they did not address dementia (e.g., a psychiatry text with no attention to neurodegenerative conditions). Disagreements were resolved by consensus. This iterative search and review process continued until there were at least five texts in each of the seven categories. Both authors independently applied selection criteria and extracted data into tables, which the first author (BNR) collated. If the content under review met exclusion guidelines, the two reviewers noted and discussed the reason for exclusion.
Results
Search Results
Identification of practice guidelines
Guidelines were searched across multiple sources. The PubMed database search yielded 221 citations; we identified an additional 310 citations through guidelines databases (National Guideline Clearinghouse = 177, Guidelines International Network = 108, Guideline Central = 25). Searching the websites of seven relevant professional societies yielded three relevant guidelines since 2005.(20–22) One additional guideline was revealed using Google search engine.(23) The primary reasons for exclusion were that the article was (1) not a practice guideline, (2) not about AD, (3) specific to one symptom (e.g., agitation, wandering) and (4) not about treatment (e.g., specific to diagnosis). One US guideline(24) was excluded, as it was not in the public domain. See Figure 1 for a flowchart of search results of practice guidelines. Sixteen practice guidelines, both domestic and international, were included in this review. Six were from the United States, five were from Western Europe, two were from Canada, one was from Singapore, one was from Australia, and one was multinational.
Figure 1.
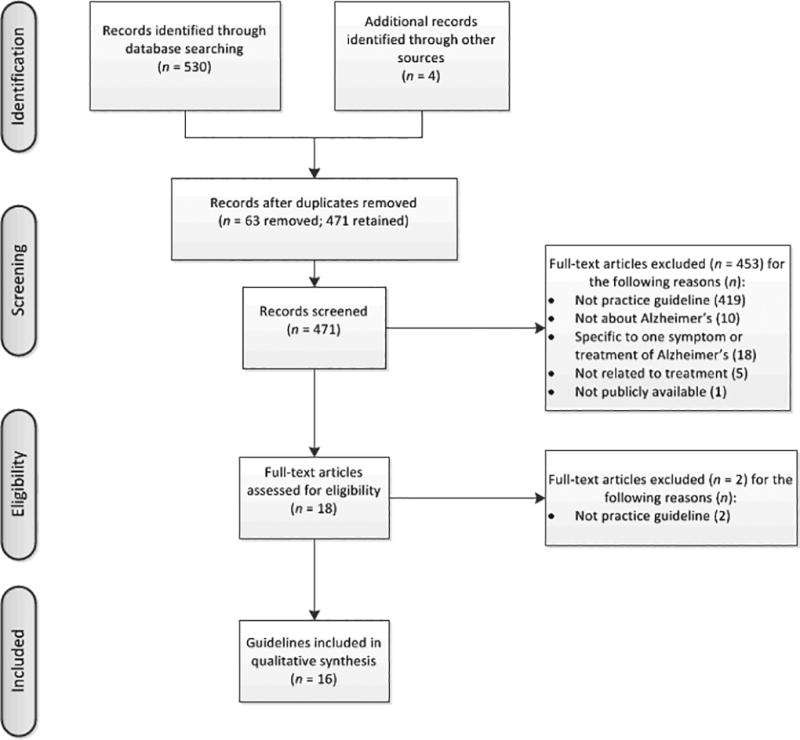
Flowchart of professional guideline selection strategy.
Identification of textbooks
The initial textbook search on Amazon.com yielded 41 texts for review across seven disciplines/categories (dementia = 3, neurology = 6, general psychiatry = 6, geriatric psychiatry/neuropsychiatry = 7, family medicine = 6, geriatrics = 7, pharmacology = 6). Search engines and expert consultation yielded an additional 26 texts. The primary reasons for exclusion were that texts were (1) specific to an unrelated topic (e.g., anatomy texts) or disease/procedure (e.g., EKG interpretation), (2) not for prescribers (e.g., nursing guides/texts), (3) pocket guidebooks or abbreviated editions, (4) nonmedical (e.g., self-help, popular culture), (5) study guides, or (6) referred specifically to a single population unrelated to this review (e.g., gynecology, pediatrics). Although our aim was to include five texts in each relevant category, one category had six eligible texts result after our iterative review. A total of 36 texts (dementia = 5, neurology = 6, psychiatry = 5, geriatric psychiatry/neuropsychiatry = 5, family medicine = 5, geriatrics = 5, pharmacology = 5) was included in the present review.
Search Results
Recommendations of professional guidelines
Of the 16 guidelines, three (18.8%) offered no recommendation regarding discontinuation.(21, 25, 26) Another two (12.5%) recommended against discontinuing ChEI treatment.(27, 28) However, one of these guidelines(28) was rather equivocal in its recommendation. The authors recommended continuation of ChEI therapy, even in patients with advanced AD, based on RCT evidence for worsening upon discontinuation among those with mild-to-moderate AD. However, these same guidelines also observed that, in some cases, discontinuation could be considered with caution when the efficacy of treatment is doubtful. Similarly, a second guideline(27) suggested that, although continuation of ChEI was unlikely to have benefit in severe AD, it was unclear when to discontinue treatment.
The remaining 11 guidelines (68.8% of those included) offered recommendations to discontinue ChEI treatment under specific circumstances. These recommendations across professional guidelines were issued based on a variety of resources and references. Of 13 guidelines issuing a recommendation for or against discontinuation, all but one formulated recommendations at least in part on expert consensus; of these, only six included references to RCT evidence. Two practice recommendations referenced the National Institute for Health and Care Excellence guidelines in drawing up their own recommendations. See Table 1 for a synthesis of findings and details of each guideline’s source of recommendation.
Table 1.
Practice Guidelines for ChEI Discontinuation
| Reference (Year) |
Country | No Recom menda tion |
Recom- mend to Not Discontinue |
Discontinue Based On: | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Lack of Respons e/Loss of Effective ness |
Side Effects / Adverse Events |
Severity of Cognitive Impairme nt |
Functional Level / Stage |
Institutio nail- zation |
Behavior | Medical Status |
Family/ CG/ Patient Preferenc e |
||||
| AAFP (2011)(32) | USA | X ‡ⱶ | |||||||||
| ACP & AAFP (2008)(25) | USA | X | |||||||||
| AGS (2014, 2015)(20, 22) | USA | X †§ | X § | ||||||||
| APA (2014)(21) | USA | X | |||||||||
| BAP (2017)(45) | UK | X †§ | |||||||||
| BPS & RCPsych (2007)(33) | UK | X §ⱶ | X1 ⱶ | X §ⱶ | X §ⱶ | ||||||
| CCCDTD (2014)(29) | Canada | X § | X § | X2§ | X § | X § | |||||
| CWGAD (2011)(35) | USA | X §‡ | |||||||||
| EFNS (2010)(27) | Multinational (Europe) | X †§ | |||||||||
| IAP (2005)(28) | Italy | X †§ | |||||||||
| MoH (2013)(30) | Singapore | X § | X § | ||||||||
| NICE (2016)(34) | UK | X †‡§ | X3 †‡§ | ||||||||
| PHN (2016)(23) | Australia | X †‡§ | X †‡§ | X †‡§ | |||||||
| RNAO (2016)(26) | Canada | X | |||||||||
| WFSBP (2011)(31) | Multinational | X § | X § | ||||||||
AAFP = American Academy of Family Physicians; ACP = American College of Physicians; APA = American Psychiatric Association; BAP = British Association for Psychopharmacology; BPS = British Psychological Society; CCCDTD = Canadian Consensus Conference on the Diagnosis and Treatment of Dementia; CWGAD = California Workgroup on Guidelines for Alzheimer’s Disease management; EFNS = European Federation of Neurological Societies; IAP = Italian Association of Psychogeriatrics; MoH = Singapore Ministry of Health; NICE = National Institute for Health and Care Excellence; PHN = Primary Health Tasmania; RCP = Royal College of Physicians; RCPsych = Royal College of Psychiatrists; RNAO = Registered Nurses’ Association of Ontario; WFSBP = World Federation of Societies of Biological Psychiatry
ChEI discontinuation indicated when Mini Mental State Examination (MMSE score < 10)
ChEI discontinuation indicated when dementia progresses to a stage (e.g., Global Deterioration Scale stage 7) where no benefit is expected from continued pharmacotherapy.
ChEI discontinuation indicated using clinical judgement of AD severity rather than rigid adherence to MMSE alone
Evidence from randomized, controlled trial.
Evidence from general review of scientific literature and industry reports.
Evidence from expert consensus/clinical expertise.
Evidence from another guideline.
The guidelines recommending discontinuation emphasized employing clinical judgment and balancing perceived cost and benefit instead of following any rigid algorithm. The most common reason for considering discontinuation was lack of response or loss of treatment effectiveness (n = 7). Other reasons were severity of cognitive impairment (n = 3), family/caregiver preference (n = 4), side effects/adverse events (n = 3), medical status (n = 2), functional level (n = 1), institutionalization (n = 1), and behavior (n =1). These guidelines recommended that clinicians monitor cognitive, behavioral, functional and affective status to assess response and gauge the appropriateness of ongoing treatment. Some supplemented this clinical judgment with consensus from the caregivers and family members, and encouraged providers to collaboratively discuss cognitive, functional, and behavioral goals of treatment with families for both initiation and discontinuation decisions.(20, 29–31) Several offered more defined guidance about early discontinuation, such as stopping if there was no improvement after 6–8 weeks despite maximal therapy,(32) or if there was no response after 12 weeks.(22) Guidelines from Tasmania(23) advised a reassessment after six months if the patient has not responded, or had unclear response. One guideline(29) recommended stopping if there was accelerated decline, defined as a decrease of 3 points or more on the Mini-Mental State Examination (MMSE) over six months.
The articles generally provided little discussion of the scientific basis for their recommendations. Three pointed out that RCT evidence of the long-term benefits of ChEI therapy, particularly among those with severe disease, was limited.(22, 30, 32) Another(25) suggested that the evidence was insufficient to determine the optimal duration of therapy, but that initial benefits (either improvement or stabilization) should be expected within three months. Two(23, 33) warned against exclusively relying on MMSE score and recommended considering functional and behavioral assessment, in conjunction with the family/caregiver assessment of the patient’s condition. Along those same lines, another guideline(34) suggested not using test scores but instead considering the level of overall disease severity in the determination about when to stop medication treatment. Finally, one guideline(35) mentioned discontinuation of ChEI treatment as indicated only prior to surgery.
Recommendations of textbooks
Table 2 presents a summary of recommendations from the 36 textbooks reviewed. Of these, 24 (66.7%) did not address discontinuation. Only two texts (n = 1 each in neurology and psychiatry; 5.5% of all texts) recommended against discontinuing ChEI treatment. The neurology textbook(36) noted that ChEI, particularly in combination with memantine, had clinically meaningful benefit for patients across AD severity. However, the authors cautioned that this conclusion was based on a few brief trials and urged further research to examine the benefit of long-term use, considering adverse events and other cost-benefit analyses. The psychiatry text(37) gave a tentative recommendation to continue ChEI treatment indefinitely, as discontinuation had been associated with cognitive deterioration. However, authors noted that the empirical basis for the duration of treatment was unclear, as clinical trials demonstrating efficacy typically ranged from six months to two years.
Table 2.
ChEI Discontinuation Guidelines in Textbooks
| No Recommendation |
Recommend to Not Discontinue |
Discontinue Based On: | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lack of Response/ Loss of Effective ness |
Side Effects / Adverse Events |
Severity of Cognitive Impairment |
Functional Level / Stage |
Institutionali -zation |
Behavio r |
Medica l Status |
Family/ CG/ Patient Preference |
|||
| Neurology | ||||||||||
| (46) | X | |||||||||
| (47) | X | |||||||||
| (48) | X | |||||||||
| (49) | X | |||||||||
| (36) | X | |||||||||
| (50, 51) | X | |||||||||
| Geriatrics | ||||||||||
| (52) | X | |||||||||
| (53) | X | |||||||||
| (54) | X | |||||||||
| (55) | X | X | X | |||||||
| (56) | X | |||||||||
| Psychiatry | ||||||||||
| (57) | X | |||||||||
| (37) | X | |||||||||
| (58) | X | X | ||||||||
| (59) | X | |||||||||
| (60) | X | |||||||||
| Geriatric Psychiatry/Neuropsychiatry | ||||||||||
| (61) | X | |||||||||
| (62) | X | |||||||||
| (63) | X | |||||||||
| (41) | X | |||||||||
| (64) | X | |||||||||
| Clinical Pharmacology | ||||||||||
| (65) | X | |||||||||
| (66) | X | |||||||||
| (42) | X | X | ||||||||
| (67) | X | |||||||||
| (68) | X | |||||||||
| Family Medicine/Internal Medicine | ||||||||||
| (69) | X | |||||||||
| (70) | X | |||||||||
| (71) | X | |||||||||
| (40) | X | |||||||||
| (72) | X | X | ||||||||
| Dementia | ||||||||||
| (38) | X | |||||||||
| (39) | X | X | X | |||||||
| (73) | X | |||||||||
| (74) | X | |||||||||
| (75) | X | |||||||||
CG = caregiver
Ten of the texts (27.8%) offered guidance on ChEI discontinuation in particular circumstances: lack of response or loss of treatment effectiveness (n = 6), significantly impaired functional level (n = 4), severe cognitive impairment (n = 3), side effects/adverse events (n = 3), and family/caregiver preference (n = 1). However, none of these texts offered specific guidance or standardized recommendations for how to determine when a patient’s functional level or cognitive status was severely impaired enough to warrant discontinuation.
Discipline-specific textbook recommendations
Dementia-specific texts offered the most guidance on discontinuation guidelines. Three of the five texts (60%) advised discontinuation based on declining functional level, severity of cognitive impairment, untoward side effects, and/or lack of response to treatment. One dementia text(38) suggested that when the patient could no longer interact meaningfully with others, the severity of impairment was likely beyond a level that would benefit from ongoing ChEI treatment. The most comprehensive recommendation for discontinuation came from a dementia text,(39) which advised discontinuation when a patient’s care goals transitioned away from active treatment toward palliative care. The authors suggested that this transition of care was typically signaled by such severely impaired functioning that the individual was institutionalized, was no longer able to find comfort in visits with family members, or was no longer able to perform basic daily functions such as feeding him/herself.
In both geriatrics and family/internal medicine specialties, 40% (two of five books in each discipline) provided guidance on discontinuation. The geriatrics texts advised discontinuation when patients evidenced a lack of treatment response, or when the benefits of treatment were no longer commensurate with the individual’s functional level or burden of side-effects. Similarly, the family/internal medicine texts both advised discontinuation in cases of cognitive or functional worsening such that treatment benefit was no longer realized. One family/internal medicine text specifically recommended discontinuation if there was no improvement within three months of treatment initiation.(40)
Within geriatric psychiatry and neuropsychiatry, one of the five books (20%) reviewed provided practical guidance on discontinuation in case of a lack of response (i.e., no improvement or stabilization apparent after six months of treatment).(41) The authors recommended that providers closely monitor for marked decline after discontinuation and consider reinitiation of treatment accordingly. Likewise, one of the five clinical pharmacology (20%) texts provided a practice recommendation to discontinue ChEI if there was a lack of treatment response and/or when the patient’s cognitive functioning declines into the severe impairment range (as assessed by an MMSE score < 10).(42) None (0%) of the six neurology texts provided recommendations about when discontinuation might be appropriate.
Overall, we observed that dementia-specific texts were the most likely to offer some consideration for discontinuation, followed by texts in geriatrics and family medicine. None of the texts reviewed from these disciplines asserted that ChEI treatment should be indefinite. General psychiatry textbook recommendations were mixed, with one text recommending against discontinuation and the other providing guidance on deprescribing. Texts from neurology, pharmacology and geriatric psychiatry generally did not comment either for or against discontinuing medication. Despite this variability, the most consistent finding in our review of the textbooks was that most texts did not mention discontinuation of ChEI.
Conclusions
Our review of published guidelines and recommendations about ChEI discontinuation revealed a striking lack of consistency. There is a paucity of informative clinical trial data to provide an evidence base for practice, and some of the results are inconsistent. While all of the publications presumably had access to the same body of scientific research, they reached notably different conclusions about the general advisability of discontinuation and the circumstances in which discontinuation should be considered. Furthermore, these recommendations were often vague, lacking any comprehensive guidance for discontinuation and, thus, impeding implementation of such guidelines. Some guidelines were tentative and recognized the limited evidence; others presented clear and strong recommendations, sometimes without empirical support. The recommendations from guidelines and textbooks did not uniformly agree with the best available scientific evidence. Indeed, the previously published meta-analysis(16) of the discontinuation literature concluded that ChEI discontinuation may deleteriously affect cognition and neuropsychiatric symptoms and generally argued against discontinuation. Textbooks and professional guidelines generally offered more consideration (often based on clinical consensus) for circumstances under which to consider discontinuation. However, the best available scientific evidence base is limited. The small number of discontinuation RCTs to date involved relatively few participants and used heterogeneous designs and outcomes. None were powered to differentiate the effects of discontinuation in different groups of patients; for instance, among those at different stages of the disease. Indeed, the most recent RCT(17) did not find compelling differences among inpatients who continued or discontinued ChEI treatment. Notably, this recent study is the only double-blind RCT to date to examine ChEI discontinuation in institutionalized patients with moderate-to-severe AD. However, because of its recent publication, it was not included in any of the guidelines or textbooks reviewed here. This heterogeneity across a small number of trials is reflected in the practice guidelines and texts, few of which offer comprehensive guidance on using clinical outcomes or assessments to guide practice decisions.
These inconsistencies underline the lack of any compelling scientific findings about the benefits or risks of long-term ChEI use. Fundamentally, there is no clear evidence that continuing treatment yields consistent or predictable benefits of a substantive nature to patients or families. The observation by Qaseem and colleagues(25, p. 375) about pharmacological treatment for dementia applies equally well to discontinuation: “Many of the improvements demonstrated in the trials, although statistically significant, were not clinically important or their relative importance cannot be determined at this time.” This serves to remind providers that any treatment decisions in dementia should be, as a recent review suggested,(43, p. 615) based on “judicious individualization.” Given the inherent heterogeneity of illness, patient factors and family preferences, there is no single recommendation to universally address this dilemma. Rather, an individual approach requires trial and error, with meticulous monitoring of risks relative to benefits.
Few guidelines explicitly considered a fundamental dilemma in dementia care: given the progressive course of AD, it can be difficult to ascertain a meaningful response to treatment, particularly in any one individual patient. The expectation of treatments is, therefore, not absolute improvement but rather an improvement compared to what the condition would have been like in the absence of treatment. Patients, families, or providers can always reason that, although the condition has worsened, it might be even worse without medication. For this reason, it may be difficult to follow the predominant suggestion that treatment cease for lack of response or loss of treatment effectiveness. The guidelines and recommendations did not offer any meaningful way to reason about such counterfactual thinking (i.e., “What might have been…”) in making determinations about treatment effects. Further work is needed to understand treatment response in a progressive condition and to provide clinically meaningful benchmarks according to disease severity. In particular, it is important to consider the limitations of prior studies and design rigorous trials to evaluate the risks and benefits of long-term ChEI treatment. Moreover, such trials need to investigate treatment among those very patients most susceptible to long-term use—those with advanced AD.
Despite the absence of well-supported or consistent recommendations that would direct providers to continue or discontinue ChEI medications as a matter of course or in all patients, we argue that discontinuation decisions should be made on an individual basis, evaluated in reference to patients’ values and preferences. In the interest of patient-centered care, it would be productive to identify realistic outcomes that mattered to patients and families prior to treatment initiation (e.g., minimizing behavioral disturbances, slowing cognitive worsening to maximize quality of life and functioning, etc.).(42) Then these outcomes could be reevaluated at the end of a predetermined period. If these goals have not been reached, providers might consider a trial period of dose reduction and discontinuation, with reassessment of these outcomes both on and off medication. Our review supports this type of “n-of-1” trial to help prescribers reach a reasoned decision about whether to continue treatment. Clinicians must further consider adverse events in discontinuation decisions. Although adverse side effects associated with ChEI have been clearly delineated in placebo-controlled RCTs, determining whether a side effect in an individual is attributable to a ChEI rather than some other factor (progression of disease, medical comorbidities, etc.) remains challenging. Discontinuation in the presence of such side effects with ongoing monitoring of adverse events following medication withdrawal may be justified in light of a careful balance of risks and benefits of treatment.
The use of an absolute threshold of functioning or cognition as a point to recommend discontinuation may be a plausible goal but difficult to establish. Research about the effect of ChEIs at different stages of dementia has not produced any definitive findings, and it is likely that individuals respond idiosyncratically to medications depending on disease stage and other factors. A broader recommendation for a threshold at which continuation has no clear benefit would require more controlled research of the sort conducted by Herrmann and colleagues.(17) Furthermore, researchers and clinicians may consider other important endpoints, other than frank cognitive functioning, in evaluating discontinuation. For instance, noncognitive endpoints such as medication effects on mood, apathy, energy, or other neuropsychiatric symptoms (e.g., agitation, delusions, and visual hallucinations) may have important meaning to patients and families, and RCTs should systematically evaluate them.
Limitations
This review aimed to extend the scientific literature beyond the limited RCT evidence and instead emphasized the professional guidelines and textbooks that often inform routine clinical practice. As such, we relied on the gray literature and secondary sources with varying degrees of evidence-based recommendations. Of note, textbook chapters are often written by a single author or limited number of contributors, whereas professional guidelines are typically compiled by a panel of experts that employs a systematic approach to reviewing the literature and establishing consensus. Thus, the texts reviewed here may represent the viewpoints of a singular few rather than an expert consensus. Furthermore, texts are typically based on published literature, although not necessarily based on an exhaustive or systematic review. These factors may explain in part the overwhelming lack of discontinuation guidelines presented in texts. For those interested in a formal review of the primary scientific literature base, we refer to the previously mentioned meta-analysis(16) and more recent RCT of institutionalized patients with AD.(17)
Implications
Our primary findings of limited empirical investigation of discontinuation, considerable variability across practice guidelines and recommendations, and the absence of any definitive guideline or recommendation all argue against the use of a formulaic approach to ChEI discontinuation. Instead, we propose individualized decisions about stopping dementia medication, based on the patients’ and families’ values and preferences, to include a balanced discussion of potential risks and potential benefits. Our suggestion for an individualized process is in line with recent opinion pieces,(43, 44) as well as many publications we considered. Given that primary care providers manage the majority of AD patients, translating and disseminating the primary evidence to these professionals is particularly important to assist them with weighing the risks and benefits of discontinuation. For example, decision aids may offer support when making such individualized decisions and could be one way of implementing evidence-based practice.
We have demonstrated in a systematic manner that there is a large amount of variability in the guidance given about ChEI discontinuation. Far from discouraging further efforts, this variability is the starting point for identifying the current gaps in knowledge, for serving as an impetus for discontinuation studies, and for developing more balanced and accurate clinical messages. Rigorous studies of the effects of discontinuation could inform clearer practice guidelines, which would in turn assist clinicians in making informed deprescribing decisions. None of this would be a substitute for eliciting patient and family values and preferences for care, or for having a thoughtful discussion about the risks of and realistic expectations for treatment. These are, we propose, the best ways to help patients and families make reasoned decisions to optimize their care by using or not using the treatments available.
Acknowledgments
This research was partially supported by the US Department of Veterans Affairs (VA) HSR&D Houston Center for Innovations in Quality, Effectiveness and Safety (CIN# 13-413), Michael E. DeBakey VA Medical Center, Houston, TX, and by a training grant from the National Institutes of Health (T32MH073553-11). The views expressed reflect those of the authors and not necessarily the policy or position of the Department of Veterans Affairs, the US government, Baylor College of Medicine, or the University of Washington. None of these bodies played a role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Portions of this work were presented at the Annual Meeting of the American Association for Geriatric Psychiatry, held March 24–27, 2017 in Dallas, TX.
The authors report no financial conflicts of interest.
References
- 1.Ngo J, Holroyd-Leduc JM. Systematic review of recent dementia practice guidelines. Age Ageing. 2015;44(1):25–33. doi: 10.1093/ageing/afu143. [DOI] [PubMed] [Google Scholar]
- 2.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt HP, et al. Cholinesterase inhibitors for patients with Alzheimer's disease: systematic review of randomised clinical trials. BMJ. 2005;331(7512):321–7. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birks JS, Chong LY, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev. 2015;9:CD001191. doi: 10.1002/14651858.CD001191.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birks JS. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;1:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali TB, Schleret TR, Reilly BM, et al. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the United-States and Canada. PloS One. 2015;10(12):e0144337. doi: 10.1371/journal.pone.0144337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheffrin M, Miao Y, Boscardin WJ, et al. Weight loss associated with cholinesterase inhibitors in individuals with dementia in a national healthcare system. J Am Geriatr Soc. 2015;63(8):1512–8. doi: 10.1111/jgs.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–13. doi: 10.1001/archinte.165.7.808. [DOI] [PubMed] [Google Scholar]
- 8.Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169(9):867–73. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Brown RT, Ding EL, et al. Dementia medications and risk of falls, syncope, and related adverse events: meta-analysis of randomized controlled trials. J Am Geriatr Soc. 2011;59(6):1019–31. doi: 10.1111/j.1532-5415.2011.03450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavassoli N, Sommet A, Lapeyre-Mestre M, et al. Drug interactions with cholinesterase inhibitors: an analysis of the French pharmacovigilance database and a comparison of two national drug formularies (Vidal, British National Formulary) Drug Saf. 2007;30(11):1063–71. doi: 10.2165/00002018-200730110-00005. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds CF, III, Butters MA, Lopez O, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68(1):51–60. doi: 10.1001/archgenpsychiatry.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell SL, Morris JN, Park PS, et al. Terminal care for persons with advanced dementia in the nursing home and home care settings. J Palliat Med. 2004;7(6):808–16. doi: 10.1089/jpm.2004.7.808. [DOI] [PubMed] [Google Scholar]
- 13.Parsons C, Hughes CM, Passmore AP, et al. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs Aging. 2010;27(6):435–49. doi: 10.2165/11536760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Vetrano DL, Tosato M, Colloca G, et al. Polypharmacy in nursing home residents with severe cognitive impairment: results from the SHELTER Study. Alzheimers Dement. 2013;9(5):587–93. doi: 10.1016/j.jalz.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiologic terminology. Am J Epidemiol. 1999;149(11):981–3. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 16.O'Regan J, Lanctot KL, Mazereeuw G, et al. Cholinesterase inhibitor discontinuation in patients with Alzheimer's disease: a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2015;76(11):e1424–31. doi: 10.4088/JCP.14r09237. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann N, O'Regan J, Ruthirakuhan M, et al. A randomized placebo-controlled discontinuation study of cholinesterase inhibitors in institutionalized patients with moderate to severe Alzheimer disease. J Am Med Dir Assoc. 2016;17(2):142–7. doi: 10.1016/j.jamda.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Geriatrics Society. [Accessed Nov 1, 2016];Choosing wisely: ten things clinicians and patients should question [American Geriatrics Society website] 2015 Available at: http://www.choosingwisely.org/societies/american-geriatrics-society/
- 21.Rabins PV, Rovner RB, Rummans T, et al. [Accessed Nov 1, 2016];Guideline watch: practice guideline for the treatment of patients with Alzheimer’s disease and other dementias [American Psychiatric Association website] 2014 doi: 10.1176/appi.focus.15106. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/alzheimerwatch.pdf. [DOI] [PMC free article] [PubMed]
- 22.American Geriatrics Society Workgroup. American Geriatrics Society identifies another five things that healthcare providers and patients should question. J Am Geriatr Soc. 2014;62(5):950–60. doi: 10.1111/jgs.12770. [DOI] [PubMed] [Google Scholar]
- 23.Tenni P, Dunbabin D. [Accessed Aug 19, 2017];A guide to deprescribing cholinesterase inhibitors [Primary Health Tasmania website] 2016 Available at: https://www.primaryhealthtas.com.au/resources/deprescribing-cholinesterase-inhibitors.
- 24.American Medical Directors Association. [Accessed Nov 1, 2016];Dementia in the long term care setting [National Guideline Clearinghouse website] 2012 Available at: https://www.guideline.gov/summaries/summary/45525/dementia-ini-the-long-term-care-setting.
- 25.Qaseem A, Snow V, Cross JT, Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Annals Intern Med. 2008;148(5):370–8. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 26.Registered Nurses' Assocation of Ontario. [Accessed Nov 1, 2016];Delirium, dementia, and depression in older adults: assessment and care [Registered Nurses' Association of Ontario website] 2016 Available at: http://rnao.ca/sites/rnao-ca/files/3Ds_BPG_WEB_FINAL.pdf.
- 27.Hort J, O'Brien JT, Gainotti G, et al. EFNS guidelines for the diagnosis and management of Alzheimer's disease. Eur J Neurol. 2010;17(10):1236–48. doi: 10.1111/j.1468-1331.2010.03040.x. [DOI] [PubMed] [Google Scholar]
- 28.Caltagirone C, Bianchetti A, Di Luca M, et al. Guidelines for the treatment of Alzheimer's disease from the Italian Association of Psychogeriatrics. Drugs Aging. 2005;22(Suppl 1):1–26. doi: 10.2165/00002512-200522001-00002. [DOI] [PubMed] [Google Scholar]
- 29.Moore A, Patterson C, Lee L, et al. Fourth Canadian Consensus Conference on the Diagnosis and Treatment of Dementia: recommendations for family physicians. Can Fam Physician. 2014;60(5):433–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Nagaendran K, Chen LH, Chong MS, et al. Ministry of health clinical practice guidelines: dementia. Singapore Med J. 2013;54(5):293–8. doi: 10.11622/smedj.2013112. [DOI] [PubMed] [Google Scholar]
- 31.Ihl R, Frolich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer's disease and other dementias. World J Biol Psychiatry. 2011;12(1):2–32. doi: 10.3109/15622975.2010.538083. [DOI] [PubMed] [Google Scholar]
- 32.Winslow BT, Onysko MK, Stob CM, et al. Treatment of Alzheimer disease. Am Fam Physician. 2011;83(12):1403–12. [PubMed] [Google Scholar]
- 33.National Collaborating Centre for Mental Health. NICE Clinical Guidelines No. 42. Leicester, UK: British Psychological Society and the Royal College of Psychiatrists; 2007. [Accessed Nov 1, 2016]. A NICE–SCIE guideline on supporting people with dementia and their carers in health and social care. Available at: https://www.scie.org.uk/publications/misc/dementia/dementia-fullguideline.pdf?res=true. [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. [Accessed Nov 1, 2016];Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease: technology appraisal guideline [NICE website] 2016 May 31; Available at: https://www.nice.org.uk/guidance/ta217.
- 35.Segal-Gidan F, Cherry D, Jones R, et al. Alzheimer's disease management guideline: update 2008. Alzheimers Dement. 2011;7(3):e51–9. doi: 10.1016/j.jalz.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Snyder CH, Woodruff BK, Caselli RJ. Cognitive disorders: mild cognitive impairment and Alzehimer's disease. In: Demaerschalk BM, Wingerchuk D, editors. Evidence-Based Neurology: Management of Neurological Disorders. 2. West Sussex, UK: Wiley Blackwell; 2015. pp. 174–190. [Google Scholar]
- 37.Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock's Synopsis of Psychiatry. 11. Philadelphia, PA: Wolters Kluwer; 2015. [Google Scholar]
- 38.Weiner MF, Lipton AM. The American Psychiatric Publishing Textbook of Alzheimer Disease and Other Dementias. Washington, DC: American Psychiatric Publishing; 2009. [Google Scholar]
- 39.Budson AE, Solomon PR. Memory Loss, Alzheimer's Disease, and Dementia: A Practical Guide for Clinicians. 2. Edinburgh, UK: Elsevier; 2016. [Google Scholar]
- 40.Goroll AH, Mulley AG. Primary Care Medicine: Office Evaluation and Management of the Adult Patient. 7. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014. [Google Scholar]
- 41.Abou-Saleh MT, Katona CLE, Kumar A. Principles and Practice of Geriatric Psychiatry. 3. Chichester, West Sussex, UK: Wiley-Blackwell; 2011. [Google Scholar]
- 42.Bennett PN, Brown MJ, Sharma P. Clinical Pharmacology. 11. London, UK: Churchill Livingstone; 2012. [Google Scholar]
- 43.Rockwood K. For how long should we use symptomatic therapies to treat people with Alzheimer disease? Can J Psychiatry. 2014;59(12):615–7. doi: 10.1177/070674371405901201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons C. Withdrawal of antidementia drugs in older people: who, when and how? Drugs Aging. 2016;33(8):545–56. doi: 10.1007/s40266-016-0384-z. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien JT, Holmes C, Jones M, et al. Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31(2):147–168. doi: 10.1177/0269881116680924. [DOI] [PubMed] [Google Scholar]
- 46.Daroff RB, Jankovic J, Mazziotta JC, et al. Bradley's Neurology in Clinical Practice. 7. Philadelphia, PA: Elsevier/Saunders; 2016. [Google Scholar]
- 47.Ropper AH, Samuels MA, Klein J. Adams and Victor's Principles of Neurology. 10. New York, NY: McGraw-Hill Education Medical; 2014. [Google Scholar]
- 48.Louis ED, Mayer SA, Rowland LP. Merritt's Neurology. 13. Philadelphia, PA: Wolters Kluwer; 2016. [Google Scholar]
- 49.Aminoff MJ, Greenberg DA, Simon RP. Clinical Neurology. 9. New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 50.Grossman M. Dementia overview. In: Lisak RP, Truong DD, Carroll WM, Bhidayasiri R, editors. International Neurology. 2. Hoboken, NJ: John Wiley & Sons; 2016. p. 132. [Google Scholar]
- 51.Wolk D, Vaishnavi S. Mild cognitive impairment and Alzheimer's disease. In: Lisak RP, Truong DD, Carroll WM, Bhidayasiri R, editors. International Neurology. 2. Hoboken, NJ: John Wiley & Sons; 2016. pp. 133–9. [Google Scholar]
- 52.Kane RL, Ouslander JG, Abrass IB, et al. Essentials of Clinical Geriatrics. 7. New York, NY: McGraw-Hill Education; 2013. [Google Scholar]
- 53.Williams BA, Chang A, Ahalt C, et al. Current Diagnosis and Treatment: Geriatrics. 2. New York, NY: McGraw-Hill Education Medical; 2014. [Google Scholar]
- 54.Halter JB, Ouslander JG, Tinetti M, et al. Hazzard's Geriatric Medicine and Gerontology. 6. New York, NY: McGraw-Hill Professional Publishing; 2009. [Google Scholar]
- 55.Fillit HM, Rockwood K, Young JB. Brocklehurst's Textbook of Geriatric Medicine and Gerontology. 8. Philadelphia, PA: Elsevier; 2017. [Google Scholar]
- 56.Hutchison LC, Sleeper RB. Fundamentals of Geriatric Pharmacotherapy. 2. Bethesda, MD: American Society of Health-System Pharmacists; 2015. [Google Scholar]
- 57.Stern TA, Fava M, Wilens TE, et al. Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 58.Gabbard GO. Gabbard's Treatments of Psychiatric Disorders. 5. Washington, DC: American Psychiatric Publishing; 2014. [Google Scholar]
- 59.Hales RE, Yudofsky SC, Gabbard GO. Essentials of Psychiatry. 3. Washington, DC: American Psychiatric Publishing; 2011. [Google Scholar]
- 60.Taylor DM, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. 12. West Sussex, UK: Wiley Blackwell; 2015. [Google Scholar]
- 61.Steffens DC, Blazer DG, Thakur ME. The American Psychiatric Publishing Textbook of Geriatric Psychiatry. 5. Washington, DC: American Psychiatric Publishing; 2015. [Google Scholar]
- 62.Moore DP, Puri BK. Textbook of Clinical Neuropsychiatry and Behavioral Neuroscience. 3. London, UK: Hodder Education; 2012. [Google Scholar]
- 63.Coffey CE, Cummings JL, George MS, et al. The American Psychiatric Publishing Textbook of Geriatric Neuropsychiatry. 3. Washington, DC: American Psychiatric Publishing; 2011. [Google Scholar]
- 64.Yudofsky SC, Hales RE. The American Psychiatric Publishing Textbook of Neuropsychiatry and Behavioral Neurosciences. 5. Washington, DC: American Psychiatric Publishing; 2008. [DOI] [PubMed] [Google Scholar]
- 65.Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 12. New York, NY: McGraw-Hill Medical; 2011. [Google Scholar]
- 66.Wecker L, Crespo LM, Dunaway G. Brody's Human Pharmacology: Molecular to Clinical. 5. Philadelphia, PA: Mosby/Elsevier; 2010. [Google Scholar]
- 67.Katzung BG, Trevor AJ. Basic and Clinical Pharmacology. 13. New York, NY: McGraw-Hill Medical; 2015. [Google Scholar]
- 68.Stahl SM. Stahl's Essential Psychopharmacology: Prescriber's Guide. New York, NY: Cambridge University Press; 2014. [Google Scholar]
- 69.Papadakis MA, McPhee SJ, Rabow MW. Current Medical Diagnosis & Treatment. 55. New York, NY: McGraw-Hill Education; 2016. [Google Scholar]
- 70.Kasper DL, Fauci AS, Hauser SL, et al. Harrison's Principles of Internal Medicine. 19. New York, NY: McGraw-Hill Education; 2015. [Google Scholar]
- 71.Rakel RE, Rakel DP. Textbook of Family Medicine. 9. Philadelphia, PA: Elsevier / Saunders; 2016. [Google Scholar]
- 72.Press D, Alexander M. Cholinesterase inhibitors in the treatment of dementia, in UpToDate. Waltham, MA: UpToDate; [Accessed June 3, 2017]. 2017. Available at: www.uptodate.com. [Google Scholar]
- 73.Huang J. [Accessed March 12, 2017];Alzheimer disease [Merck Manual: Professional Version website] 2016 Available at: http://www.merckmanuals.com/professional/neurologic-disorders/delirium-and-dementia/alzheimer-disease.
- 74.Larner AJ. Dementia in Clinical Practice: A Neurological Perspective. 2. New York, NY: Springer; 2014. [Google Scholar]
- 75.Husain M, Schott JM. Oxford Textbook of Cognitive Neurology and Dementia. Oxford, UK: Oxford University Press; 2016. [Google Scholar]


