Abstract
It has been 30 years since the intestinal sodium glucose cotransporter SGLT1 was cloned, and, in the intervening years, there have been many advances that have influenced physiology and medicine. Among the first was that SGLT1 is the founding member of the human gene family SLC5, containing 11 diverse transporters and a glucose sensor. Equally surprising was that SGLTs are members of a structural family of cotransporters and exchangers in different gene families. This led to the conclusion that these proteins operate by a mechanism where transport involves the opening and closing of external and internal gates. The mechanism is shared by a wide variety of transporters in different structural families, e.g., the human facilitated glucose transporters (SLC2) in the huge major facilitator superfamily (MFS). Not surprising is the finding that mutations in Sglt genes cause the rare diseases glucose-galactose-malabsorption (GGM) and familial renal glucosuria (FRG). However, it was not envisaged that SGLT inhibitors would be used to treat diabetes mellitus, and these drugs may be able to treat cancer. Finally, in 2017, we have just learned that SGLT1 may be required to resist infection and to avoid recurrent pregnancy loss.
SGLT Structure and Function
In the beginning, Bob Crane proposed that Na+/glucose cotransport explained active glucose transport across the brush border of the small intestine, and it took 27 years to identify and clone the transporter, SGLT1 (20, 48). This was followed by cloning of human SGLT1 and SGLT2, and the other members of the SLC5 family found in kidney, thyroid gland, the spinal cord, heart, muscle, and lung (46, 48). The SLC5 genes code for cotransporters for glucose and/or fructose (SGLT1, SGLT2, SGLT4, and SGLT5), inositol (SMIT1 and SMIT2), short-chain fatty acids (SMCT1 and SMCT2), iodide (NIS), choline (CHT1), and biotin (SMVT). SGLTs have no sequence homology with other transporters such as the facilitated glucose transporters (SLC2, GLUTs) or the neurotransmitter transporters (SLC6, e.g., GAT and DAT).
The SLC5A1 gene for SGLT1 codes for a 72-kDa protein with 14 transmembrane helices (TM1–TM13). The structure of the closely related bacterial sodium galactose cotransporter vSGLT has 14 TMs and a core structure of two inverted repeats (TM1–TM5 and TM6–TM10) (12). A surprise was that this core structure (FIGURE 1) was previously reported for a bacterial sodium-leucine cotransporter (LeuT; Ref. 49) and was later extended to include a total of nine cotransporters and exchangers in unrelated gene families (1). For all members of the LeuT structural family, the substrate-binding site is located in the middle of the protein adjacent to the unwound regions of TM1 and TM6. The sodium cotransporters share a common sodium-binding site, Na2, and in some, like SGLT1, there is a second Na1 site close to the substrate-binding site. Mutational analysis has confirmed the predicted side chains involved in glucose and sodium binding to SGLT1 (26, 35).
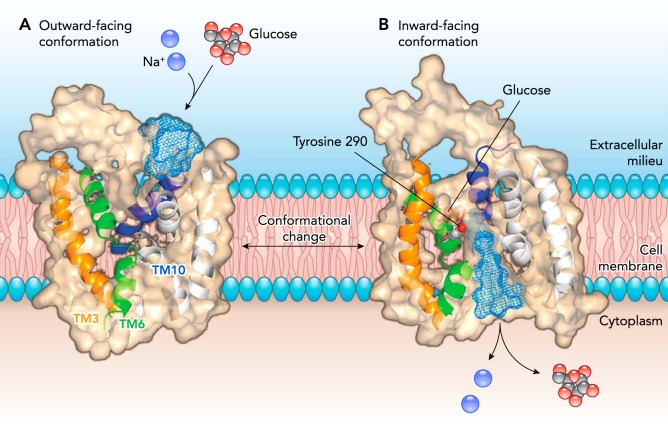
FIGURE 1.
Model of SGLT1 in the outward and inward facing conformations
In the open outward-facing conformation (left), a slice through the protein shows the opening to the external aqueous vestibule. External sodium binding to two Na+ binding sites (Na1 and Na2) causes the outer gate to open to allow external glucose to bind in the middle of the protein. A slice through the inward-facing protein (right) shows the bound glucose and the inward vestibule, through which Na+ and glucose escape into the cytoplasm. Helices showing structural rearrangements between the outward and inward conformations are shown in orange (TM3), green (TM6), and blue (TM10). Figure is adapted from Ref. 12, with permission, where the outward conformation is modeled on LeuT and the inward conformation is modeled on vSGLT.
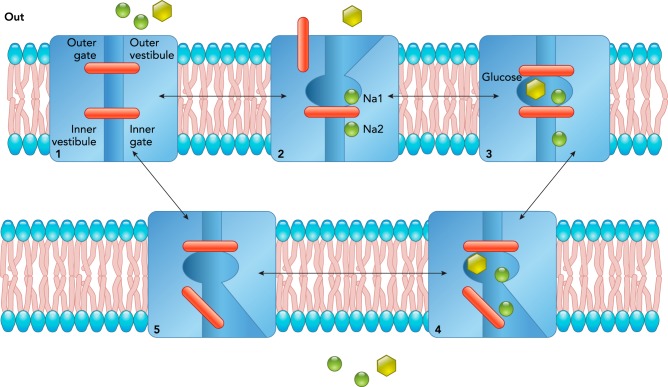
Intensive biophysical and biochemical experiments on SGLT1 expressed in oocytes and cultured cells (see Refs. 26, 48), and molecular dynamic studies on vSGLT (2), have resulted in a kinetic model of sodium and glucose transport by SGLTs (FIGURE 2). The starting point in the cycle is the outward-facing close conformation (state 1), where the transporter is poised to bind external ligands. External sodium binds first to the Na2 (and then Na1 in hSGLT1) sites to open the external gate (state 2) to permit external glucose to bind and close the external gate (state 3). After ligand binding, the protein isomerizes to face the cytoplasm where the internal gate opens to allow sodium and glucose to escape (state 4). Finally, the inner gate closes, and the protein isomerizes to the initial starting state. One complete cycle of hSGLT1 takes 20 ms and results in the transport of two Na+ ions and one glucose molecule across the membrane. The transport cycle is completely reversible. The rate and direction of transport simply depends on the driving forces across the membrane, the external and internal sodium and glucose concentrations, and the membrane potential. The binding order of the reverse cycle is unknown. A similar gated, alternating access transport mechanism is accepted for other Na+- and H+-coupled cotransporters and glucose uniporters (see Refs. 22, 29, 50, 51).
FIGURE 2.
A kinetic model of Na and glucose transport by SGLT1
The SGLT1 protein in the plasma membrane is represented as a double-gated structure. External Na binds first to the Na2 and Na1 sites to open the external gate and allow glucose to bind in a central pocket. This outer barrier closes to occlude the sugar from the external aqueous solution. Upon opening of the internal barrier, Na and glucose pass through an aqueous vestibule to reach the cytoplasm. The cycle is completed by the return of the protein to the starting position. The rate of transport is governed by the opening and closing of the outer and inner barriers.
The structures of [vSGLT in the sugar-bound, occluded, inward-facing conformation, the inward-facing, ligand-free conformations (12, 44)], and molecular dynamics of ligand release (2) provide direct support for the critical element of the ligand release steps in the model. Further biochemical and biophysical experiments provide evidence that external sodium binding opens a large vestibule to the glucose-binding site (FIGURES 1 AND 2). Structural analysis of the outward and inward facing homology models of SGLT1 suggests that the outer gates are formed by hydrophobic side-chains on the outer edge of TM1 (L87), TM2 (F101), and TM10 (F453), and that opening and closing is due in part to bending of the outer end of TM10 (yellow helix; FIGURE 1). Initially, we proposed that Y290 forms the inner gate and that opening and closing is due to rotation of the side chain, but this is not borne out in subsequent molecular dynamic studies of sugar release, i.e., the inner barrier to sugar release is more complex.
Phlorizin is a specific high-affinity competitive inhibitor of glucose transport by SGLTs with a Ki of 200 nM for SGLT1 and 10 nM for SGLT2 (21). This inhibitor only binds from the external surface in the Na+-bound state (FIGURE 2; state 2). It is proposed that the glucose moiety binds to the glucose-binding site and that the aglycone binds to the hydrophilic face of the outer vestibule (FIGURES 1 AND 2). Phloretin, the aglycone, inhibits transport, but with low affinity (300 µM) due to the absence of a sugar group to anchor the inhibitor in the glucose-binding site (5).
It is anticipated that the structure and transport mechanisms of sodium sugar cotransport by SGLT2, SGLT4, and SGLT5 will be similar to those for SGLT1. So far, homology models have not revealed the structural basis for differences in sugar selectivity, e.g., glucose vs. fructose, sodium to sugar coupling, e.g., 2/1 for SGLT1 and 1/1 for SGLT2, or the selectivity of inhibitors, e.g., the Ki for phlorizin is an order of magnitude lower for SGLT2 than for SGLT1.
A disconcerting issue with SGLT2 since it was first cloned was the very low level of activity in oocytes and cultured cells, e.g., in oocytes, the glucose transport activity was several orders of magnitude lower than for SGLT1, and this severely limited progress. However, expression of SGLT2 in HEK293 and COS cells gave an activity that was sufficient to enable testing of SGLT2 inhibitors and characterization of transport activity (21). This firmly established the restricted sugar selectivity, very low affinity for galactose, and 1:1 Na to glucose stoichiometry. Further electrophysiological experiments demonstrated that insulin rapidly increased SGLT2 activity mediated through protein kinases (16) and that SGLT2 inhibitors only act from the external surface of the cell (15). Michael Coady solved the mystery of low expression in oocytes with the discovery of the requirement for MAP17 (8, 9). Coexpression of MAP17 in HEK293 cells also increased SGLT2 activity, but the effect was not as dramatic as in oocytes. The properties of SGLT2 in MAP17 oocytes were identical to those in native HEK293 cells. The requirement for MAP17 expression in oocytes has yet to be explained.
SGLT1 is in the brush-border membrane of enterocytes where, along with GLUT2 in the basolateral membrane, it is responsible for glucose and galactose intestinal absorption. This gene is also expressed in the late proximal tubule in the kidney where it is responsible for reabsorption of glucose that escapes from the early proximal tubule. The gene and protein are also expressed in brain, heat, lung, liver, muscle, testes, and other organs and tissues (25, 52, 53), but the functional significance of SGLT1 in these tissues is unclear at this time (see below).
The SGLT2 gene is virtually only expressed in the renal cortex, where it is largely responsible for the reabsorption of 160 g of glucose per day from the glomerular filtrate in the early and middle segments of the proximal tubule (48). The gene for the fructose and mannose cotransporters, SGLT5, is also solely limited to the renal cortex, but SGLT4 expression is widely distributed throughout the body. The physiological role of these fructose, mannose, and glucose transporters have yet to be elucidated (46).
Water Transport by SGLTs
Glucose stimulates intestinal salt and water absorption, and this has led to the very successful development of oral rehydration therapy (ORT) to treat secretory diarrheas such as cholera (48). We have found that SGLT1 behaves as a water (and urea) channel (27, 48). Osmotic water flow through SGLT1 is blocked by phlorizin, but water is independent of the presence of Na+ or glucose. Unlike Na+/glucose cotransport, the temperature dependence of passive water flow is low (4 kcal/mole), similar to that for conventional water channels (AQPs). The pathway for water permeation was identified by molecular dynamic studies on vSGLT (3, 7), which indicates that water follows the same pathway for Na+ and glucose. This was confirmed experimentally for SGLT1 by mutation of residues in the pathway (55). The physiological importance of these findings is that 60–70% of the passive water flow across the mouse small intestine occurs through SGLT1.
We proposed that SGLT1 is a water pump where water was cotransported with Na+ and sugar (28). This hypothesis was highly controversial, and the alternative explanation by Jean-Yves La Pointe is that coupled water flow is due to the accumulation of salt and sugar at the cytoplasmic surface of the transporter (see Refs. 27, 48, 54). Although it has proved difficult to distinguish between these two hypotheses, there is common agreement that there is a direct link between SGLT1 activity and water transport. This provides the rationale for ORT to treat secretory diarrhea. We estimated from the amount of intestinal glucose absorption (180 g/day) that SGLT1-mediated water transport could account for 5 liters of intestinal fluid absorption per day. In the kidney, the absorption of 180 g of glucose could also account for 5 liters of fluid absorption, but this is only a small fraction of the 170 liters of fluid reabsorbed each day.
SGLTs as Glucose Sensors
Glucose is known to depolarize cells expressing SGLTs, and this is best exemplified by the 70-mV depolarization of oocytes expressing SGLT1 (48). Clearly, the depolarization in native cells depends on the density of SGLTs and the contribution of other channels and transporters to the total conductance of the membrane. We are not aware of convincing experiments to examine the importance of SGLTs in determining cell membrane potentials in vivo.
Although SGLTs have been traditionally thought of as glucose transporters in the intestine and kidney, we have shown that they are functionally expressed in regions of rat brain (52, 53). Specifically, SGLT1 is found in glutamatergic pyramidal cells in the CA1 region of the hippocampus, Purkinje cells of the cerebellum, and SGLT2 in the thalamus. Given that GLUTs are expressed widely in the brain, we have offered two explanations for SGLT expression in these very metabolically active cells: 1) they may protect cells by providing glucose during anoxia, ischemia, or stroke; and 2) SGLTs may act as glucose sensors to regulate the membrane potentials following changes in extracellular glucose concentration.
The first definitive report that SGLTs may behave as glucose sensors was that human SGLT3 behaves as a glucose sensor (10). When expressed in oocytes, this protein does not transport glucose, but instead glucose caused a specific, phlorizin-sensitive depolarization of the membrane potential. The affinity of SGLT3 for sugar was low (20 mM); thus small changes in glucose concentration from normal would produce a linear change in the membrane potential. SGLT3 protein is located in enteric neurons and skeletal neuromuscular junctions, so we speculated that the glucose sensor was involved in regulating intestinal motility. Interestingly, imino sugars such as migitol and miglusta, drugs that used slow glucose absorption in diabetic patients, are potent agonist of SGLT3, with 40,000-fold higher apparent affinities than glucose (43).
Diez-Sampedro and others made considerable efforts to extend and test SGLT3 as a glucose sensor in rodent models, succinctly reviewed in Ref. 42. It has been a difficult road to follow, owing to the fact that mouse and rat genomes code for two proteins, SGLT3a and SGLT3b, and their properties do not precisely mimic those for human SGLT3. For example, SGLT3a only generates current at pH 5 in the presence and absence of glucose or the imino sugar 1-deoxynojirimycin, and SGLT3b only generates sugar-induced currents but not imino sugar-induced currents at neutral pHs. This has made it difficult to draw definitive conclusions about the physiology of rodent SGLT3. Nevertheless, there has been speculation that SGLT3 senses glucose in neurons, the hepatic portal vein, and the small intestine, and thereby influences food intake, gastric emptying, and hormone (GLP-1) secretion.
SGLTs and Diabetes
Concurrent with the cloning of SGLT1 in 1987, DeFronzo’s team found that phlorizin reversed the symptoms of diabetes in a rat model (33). It was argued that the reduction in fasting and post-prandial glucose levels and the increase in insulin sensitivity were due to the inhibition of glucose reabsorption by the kidney. The subsequent cloning of SGLT2 (23, 45) led to the race to develop specific SGLT2 inhibitors to treat Type 2 diabetes mellitus. The finding that a benign disorder, familial glucosuria, was due to mutations in SGLT2 bolstered this quest (37). The pharmaceutical industry has developed oral, specific SGLT2 inhibitors that reduce blood glucose levels (and HbA1c levels) in T2DM patients without producing hypoglycemia or any other remarkable adverse effect (see Refs. 13, 25). However, these drugs produce a sustained loss of weight. Since 2013, the FDA has approved three SGLT2 inhibitors for the treatment of T2DM, Invokana, Forgixa, and Jardiance, and three others are in clinical use in Japan. Jardiance and Invokana protect against life-threatening cardiovascular events in diabetic patients, but the underlying mechanism is not yet known.
Surprisingly, these inhibitors only inhibit 50% of glucose reabsorption in the kidney. This is because SGLT1 in the late proximal tubule is able to reabsorb a substantial fraction of the glucose that was not reabsorbed in the early proximal tubule. This has been established in studies of Sglt1, Sglt2, and double Sglt1/Sglt2-null mice (32), and the use of the potent dual SGLT1/SGLT2 inhibitor LP-925219 (31).
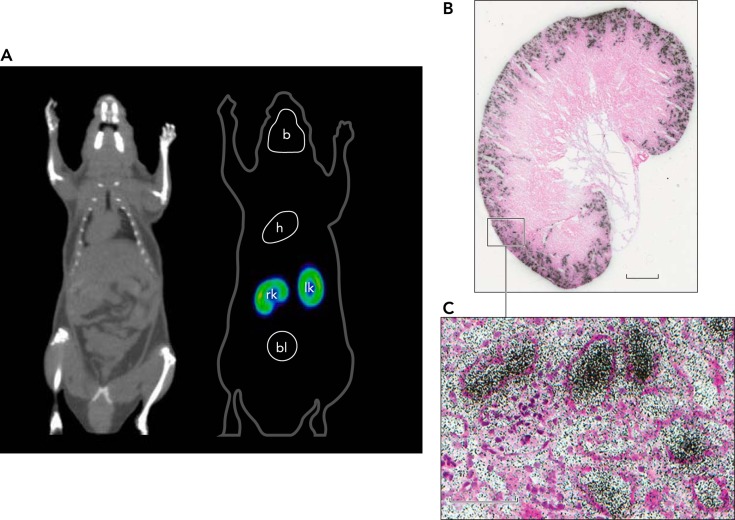
Our studies have clarified the mode of action of SGLT2 inhibitor (15, 18). In whole-cell patch-clamp experiments, we showed that phlorizin and a specific SGLT2 inhibitor blocks transport only from the external surface of the cell. Then, using [18F]-dapagliflozin (Forgixa), we showed that the drug is filtered by the glomerulus and binds to SGLT2 in the early proximal tubule to inhibit SGLT2-mediated glucose reabsorption (FIGURE 3). When displaced from SGLT2 by excess drug or phlorizin, [18F]-dapagliflozin is reabsorbed back into blood and excreted by the liver into bile. We found that there was no measurable [18F]-dapagliflozin binding to any other organ in the mouse, consistent with the lack of extra-renal Sglt2 expression and SGLT2 immunohistochemistry (25).
FIGURE 3.
Location of SGLT2 in a mouse using [18F]-dapagliflozin micro-PET and ex vivo autoradiography
A: dapagliflozin, a selective high-affinity SGLT2 inhibitor (Kd 2 nM) labeled with [18F], was injected intravenously into a mouse, and the distribution was recorded using micro-PET (16). Binding was only observed in the outer cortex of the kidney (bright yellow band), and this was rapidly displaced with intravenous injection of dapagliflozin or phlorizin (not shown). No binding was observed in Sglt2-null mice. B: after [18F]-dapagliflozin binding to the kidney reached a steady state (15 min), a kidney was rapidly removed and prepared for ex vivo autoradiography. A slice of the whole kidney shows [18F]-dapagliflozin binding to the outer cortex, and a higher magnification view shows the inhibitor within some tubules adjacent to the glomerulus. Redrawn from Ref. 18.
In clinical practice, SGLT2 inhibitor monotherapy only produces about a one-point drop in HbA1c levels. It is now common to use these drugs in combination with others to achieve the desired clinical end points. This has led to a consideration of SGLT1 inhibitors to reduce glucose absorption in diabetic patients (see also Ref. 41). So far, there have been preclinical advances with two classes of inhibitors: 1) specific SGLT1 inhibitors, e.g., KGA-2727 (40); and 2) non-absorbable SGLT inhibitors, e.g., LX 2761 (19, 30). In animal models of diabetes and one small clinical trial (11), oral delivery of the drugs lowered fasting blood glucose and HbA1c levels, and, at least in mice and rats, the occasional diarrhea due to malabsorption decreased in frequency and severity over time.
SGLTs and Cancer
In tumor cells, glycolysis is a major source of ATP required for growth, and glucose uptake has long been a potential target for cancer therapy, e.g. Ref. 14. In most tumors, GLUT1 is upregulated to supply the increasing demand for glucose. It is not practical to use GLUT1 inhibitors for therapy, since blood-brain-barrier GLUT1 is essential in supplying glucose to the brain. The expression of SGLTs in some tumors has been reported, but their importance has been difficult to judge, owing to the use of suspect antibodies, a problem that has plagued the SGLT field (see Ref. 48).
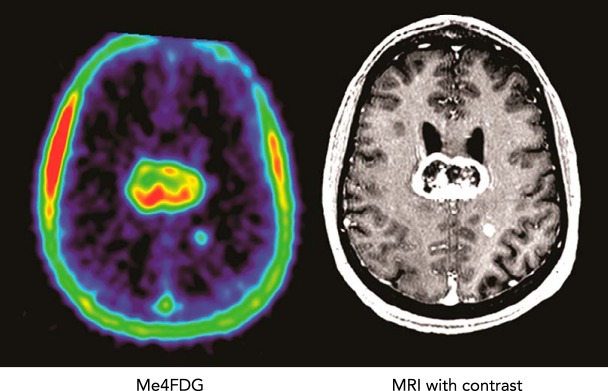
Through serendipity, we have established that SGLT2 protein is expressed in brain, pancreas, and prostate tumors. This finding has raised the possibility of using SGLT2 inhibitors to treat these cancers. These observations occurred through our invention of a SGLT-specific tracer for positron emission tomography (PET) in animal and human subjects, Me-4FDG (34, 47, 52, 53). In human subjects, PET imaging shows that, unlike the classical glucose PET tracer 2-FDG, Me-4FDG does not enter the brain across the blood brain barrier and is only excreted into the urine in patients with familial renal glycosuria or in Sgl1- and/or Sglt2-null mice (35). This is explained by the fact Me-4FDG is a substrate for renal SGLTs, but not for blood-brain-barrier GLUT1, and 2-FDG is not a substrate for SGLTs. We did find that Me-4FDG was accumulated in high-grade astrocytomas (FIGURE 4) (24). Immunocytochemistry with specific SGLT antibodies on tumors resected from WHO grade IV astrocytoma patients revealed that SGLT2 was located in the capillaries of the tumor micro-vessels, in microglia/macrophages surrounding these capillaries, and in cancer cells (FIGURE 5).
FIGURE 4.
SGLT activity in brain cancer patients using Me-4FDG PET
Me-4FDG PET scans and a contrast MRI were conducted on a patient with a grade IV astrocytoma in the corpus callosum. The co-registered images of the brain show uptake of Me-4FDG and the contrast agent into a large 46-mm tumor with a non-enhancing necrotic core in the corpus callosum and a small 6-mm tumor in the left parietal gray matter. The PET signal was approximately twice as high as that in the blood in the sagittal sinus, and the signal-to-noise ratio for the tumor relative to the rest of the brain was 12. See Ref. 24.
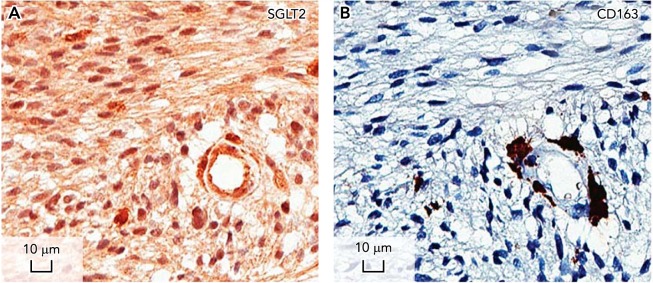
FIGURE 5.
Location of SGLT2 in a brain tumor using immunocytochemistry
IHC was carried out to examine the distribution of SGLT2 in a grade IV astrocytoma. A: the specific SGLT2 immunoreaction found in a tumor microvessel, the surrounding glia, and macrophages and cancer cells. The antigen used to generate the SGLT2 polyclonal antibody blocked the reactivity (not shown). B: an adjacent section stained with the glial marker CD163. The results suggest that Me-4FDG gains access to the brain through SGLT2 expressed in the microvasculature of the tumor where it is accumulated by SGLT2 in the plasma membrane of tumor cells. See Ref. 24.
Functional and IHC studies of fresh tumors harvested from patients further demonstrated that SGLT2 was expressed in pancreatic and prostate adenocarcinomas (38). SGLT2 was also expressed in these cancer cells grown in mice, as judged by Me-4FDG micro-PET, ex vivo autoradiography, and IHC. Uptake of Me-4FDG was inhibited by dapagliflozin. In preliminary trials, canagliflozin reduced the rate of tumor growth and increased necrosis. We are currently extending the human Me-4FDG PET studies to include pancreatic and other cancers, and to test the effect of SGLT2 inhibitors on Me-4FDG uptakes. At a minimum, we hope to determine whether Me-4FDG PET can be used for the early detection and staging of tumors.
SGLTs and Infection
Recent studies have suggested that SGLT1 is expressed in activated murine cytotoxic T-lymphocytes (4). In a very convincing follow-up study to their hypothesis that SGLT1 contributes to the immune response, the Lang team infected wild-type and Sglt1-null mice with Listeria and found that all the Sglt1-null mice died within 5 days (39). The authors conclude that SGLT1 in lymphocytes plays a decisive role in the defense against the Listeria pathogen. It will be interesting to know whether SGLT1 is also important in the defense of other infections in mice and humans.
SGLTs and Pregnancy
In early pregnancy before the onset of placental perfusion, the embryo is dependent on anaerobic glycolysis for ATP production, and an important source of glucose is glycogen stored in the endometrium (6). Glycogen synthesis in the endometrium requires glucose transporters, either GLUTs or SGLTs. In both humans and mice, Sglt1 transcripts were detected in endometrial cells, especially in humans during the early to mid-luteal phase (36). The Sglt1 gene and protein was found in mouse endometrial glandular and surface epithelial cells, but not in Sglt1-null mice. SGLT1 was functional in mouse uterine tissue (glucose-inducted short-circuit currents) but not for Sglt1-null mice. Endometrial glycogen, litter size, and pup weight were all lower in Sglt1-null mice than in wild-type mice; finally, in patients with recurrent pregnancy loss during the implantation window, Sglt1 gene and protein expression were significantly lower than in control patients. It is concluded that SGLT1 deficiency in the human endometrium at implantation may predispose one to early pregnancy failure, obstetrical compilations, and low fetal growth. This suggests that it may not be advisable to prescribe SGLT inhibitors to women of childbearing years.
Summary
It is gratifying for us as physiologists to see that SGLTs were the founding members of a gene family (SLC5) and that their structure and transport mechanism has had such an impact on transporters in diverse gene families, such as those containing the amino acid and neurotransmitter cotransporters. Advances in the SGLT field have already resulted in application to diseases like GGM and T2DM. We are hopeful that recent progress will also have practical applications to the early detection, and possibly treatment, of pancreatic cancer. We also look forward to the development of recent findings on the role of SGLT1 in the immune response and in the nurturing of early embryos. There are still many unresolved problems in the field of SGLT biology ranging from the mechanism of Na+, glucose, and water transport to their physiological role in the brain, lung, and muscle. There is room for physiologists to help answer these and other emerging problems, and to contribute to new therapies to disease.
Acknowledgments
We are grateful for all the contributions made to SGLT biology by members of the Wright Laboratory and our collaborators in the Abramson, Barrio, Grabe, Zampighi, and Zeuthen Laboratories.
Our work was supported by grants from the National Institutes of Health.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: E.M.W., C.G., and D.D.L. conceived and designed research; E.M.W., C.G., and D.D.L. analyzed data; E.M.W. prepared figures; E.M.W. drafted manuscript; E.M.W., C.G., and D.D.L. edited and revised manuscript; E.M.W., C.G., and D.D.L. approved final version of manuscript; C.G. and D.D.L. performed experiments; C.G. and D.D.L. interpreted results of experiments.
References
- 1.Abramson J, Wright EM. Structure and function of Na(+)-symporters with inverted repeats. Curr Opin Struct Biol 19: 425–432, 2009. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adelman JL, Ghezzi C, Bisignano P, Loo DD, Choe S, Abramson J, Rosenberg JM, Wright EM, Grabe M. Stochastic steps in secondary active sugar transport. Proc Natl Acad Sci USA 113: E3960–E3966, 2016. doi: 10.1073/pnas.1525378113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adelman JL, Sheng Y, Choe S, Abramson J, Wright EM, Rosenberg JM, Grabe M. Structural determinants of water permeation through the sodium-galactose transporter vSGLT. Biophys J 106: 1280–1289, 2014. doi: 10.1016/j.bpj.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhavsar SK, Singh Y, Sharma P, Khairnar V, Hosseinzadeh Z, Zhang S, Palmada M, Sabolic I, Koepsell H, Lang KS, Lang PA, Lang F. Expression of JAK3 sensitive Na+ coupled glucose carrier SGLT1 in activated cytotoxic T lymphocytes. Cell Physiol Biochem 39: 1209–1228, 2016. doi: 10.1159/000447827. [DOI] [PubMed] [Google Scholar]
- 5.Bisignano P, Kalyanaraman C, Ghezzi C, Wright EM, Abramson J, Paz A, Jacobson MP, Friemann R, Grabe M. Structural insights into sodium-dependent sugar transporters and their inhibition mechanism. Biophys J 112, Suppl 1: 128a, 2017. doi: 10.1016/j.bpj.2016.11.714. [DOI] [Google Scholar]
- 6.Burton GJ, Scioscia M, Rademacher TW. Endometrial secretions: creating a stimulatory microenvironment within the human early placenta and implications for the aetiopathogenesis of preeclampsia. J Reprod Immunol 89: 118–125, 2011. doi: 10.1016/j.jri.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Choe S, Rosenberg JM, Abramson J, Wright EM, Grabe M. Water permeation through the sodium-dependent galactose cotransporter vSGLT. Biophys J 99: L56–L58, 2010. doi: 10.1016/j.bpj.2010.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, Dumayne C, Bichet DG, Lapointe JY. MAP17 is a necessary activator of renal Na+/glucose cotransporter SGLT2. J Am Soc Nephrol 28: 85–93, 2017. doi: 10.1681/ASN.2015111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coady MJ, Wallendorff B, Lapointe JY. Characterization of the transport activity of SGLT2/MAP17, the renal low-affinity Na(+)-glucose cotransporter. Am J Physiol Renal Physiol 313: F467–F474, 2017. doi: 10.1152/ajprenal.00628.2016. [DOI] [PubMed] [Google Scholar]
- 10.Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci USA 100: 11753–11758, 2003. doi: 10.1073/pnas.1733027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbins RL, Greenway FL, Chen L, Liu Y, Breed SL, Andrews SM, Wald JA, Walker A, Smith CD. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol 308: G946–G954, 2015. doi: 10.1152/ajpgi.00286.2014. [DOI] [PubMed] [Google Scholar]
- 12.Faham S, Watanabe A, Besserer GM, Cascio D, Specht A, Hirayama BA, Wright EM, Abramson J. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science 321: 810–814, 2008. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 12: 78–89, 2015. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther 121: 29–40, 2009. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Ghezzi C, Hirayama BA, Gorraitz E, Loo DD, Liang Y, Wright EM. SGLT2 inhibitors act from the extracellular surface of the cell membrane. Physiol Rep 2: e12058, 2014. doi: 10.14814/phy2.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghezzi C, Wright EM. Regulation of the human Na+-dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol 303: C348–C354, 2012. doi: 10.1152/ajpcell.00115.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghezzi C, Yu AS, Hirayama BA, Kepe V, Liu J, Scafoglio C, Powell DR, Huang SC, Satyamurthy N, Barrio JR, Wright EM. Dapagliflozin binds specifically to sodium-glucose cotransporter 2 in the proximal renal tubule. J Am Soc Nephrol 28: 802–810, 2017. doi: 10.1681/ASN.2016050510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodwin NC, Ding ZM, Harrison BA, Strobel ED, Harris AL, Smith M, Thompson AY, Xiong W, Mseeh F, Bruce DJ, Diaz D, Gopinathan S, Li L, O’Neill E, Thiel M, Wilson AG, Carson KG, Powell DR, Rawlins DB. Discovery of LX2761, a sodium-dependent glucose cotransporter 1 (SGLT1) inhibitor restricted to the intestinal lumen, for the treatment of diabetes. J Med Chem 60: 710–721, 2017. doi: 10.1021/acs.jmedchem.6b01541. [DOI] [PubMed] [Google Scholar]
- 20.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature 330: 379–381, 1987. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 21.Hummel CS, Lu C, Loo DD, Hirayama BA, Voss AA, Wright EM. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300: C14–C21, 2011. doi: 10.1152/ajpcell.00388.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaback HR. A chemiosmotic mechanism of symport. Proc Natl Acad Sci USA 112: 1259–1264, 2015. doi: 10.1073/pnas.1419325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest 93: 397–404, 1994. doi: 10.1172/JCI116972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kepe V, Scafoglio C, Liu J, Yong WH, Bergsneider M, Huang SC, Barrio JR. A positron emission tomography tracer targeting a sodium glucose cotransporter in high grate astrocytomas. In Preparation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koepsell H. The Na(+)-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol Ther 170: 148–165, 2017. doi: 10.1016/j.pharmthera.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Loo DD, Jiang X, Gorraitz E, Hirayama BA, Wright EM. Functional identification and characterization of sodium binding sites in Na symporters. Proc Natl Acad Sci USA 110: E4557–E4566, 2013. doi: 10.1073/pnas.1319218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loo DD, Wright EM, Zeuthen T. Water pumps. J Physiol 542: 53–60, 2002. doi: 10.1113/jphysiol.2002.018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo DD, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA 93: 13367–13370, 1996. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nomura N, Verdon G, Kang HJ, Shimamura T, Nomura Y, Sonoda Y, Hussien SA, Qureshi AA, Coincon M, Sato Y, Abe H, Nakada-Nakura Y, Hino T, Arakawa T, Kusano-Arai O, Iwanari H, Murata T, Kobayashi T, Hamakubo T, Kasahara M, Iwata S, Drew D. Structure and mechanism of the mammalian fructose transporter GLUT5. Nature 526: 397–401, 2015. doi: 10.1038/nature14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell DR, Smith MG, Doree DD, Harris AL, Greer J, DaCosta CM, Thompson A, Jeter-Jones S, Xiong W, Carson KG, Goodwin NC, Harrison BA, Rawlins DB, Strobel ED, Gopinathan S, Wilson A, Mseeh F, Zambrowicz B, Ding ZM. LX2761, a sodium/glucose cotransporter 1 inhibitor restricted to the intestine, improves glycemic control in mice. J Pharmacol Exp Ther 362: 85–97, 2017. doi: 10.1124/jpet.117.240820. [DOI] [PubMed] [Google Scholar]
- 31.Powell DR, Smith MG, Doree DD, Harris AL, Xiong WW, Mseeh F, Wilson A, Gopinathan S, Diaz D, Goodwin NC, Harrison B, Strobel E, Rawlins DB, Carson K, Zambrowicz B, Ding ZM. LP-925219 maximizes urinary glucose excretion in mice by inhibiting both renal SGLT1 and SGLT2. Pharmacol Res Perspect 3: e00129, 2015. doi: 10.1002/prp2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 306: F188–F193, 2014. doi: 10.1152/ajprenal.00518.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515, 1987. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sala-Rabanal M, Hirayama BA, Ghezzi C, Liu J, Huang SC, Kepe V, Koepsell H, Yu A, Powell DR, Thorens B, Wright EM, Barrio JR. Revisiting the physiological roles of SGLTs and GLUTs using positron emission tomography in mice. J Physiol 594: 4425–4438, 2016. doi: 10.1113/JP271904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sala-Rabanal M, Hirayama BA, Loo DD, Chaptal V, Abramson J, Wright EM. Bridging the gap between structure and kinetics of human SGLT1. Am J Physiol Cell Physiol 302: C1293–C1305, 2012. doi: 10.1152/ajpcell.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salker MS, Singh Y, Zeng N, Chen H, Zhang S, Umbach AT, Fakhri H, Kohlhofer U, Quintanilla-Martinez L, Durairaj RRP, Barrios FSV, Vrljicak P, Ott S, Brucker S, Wallwiener D, Madunic IV, Brelijak D, Sabolic I, Koepsell H, Brosens JJ, Lang F. Endometrial sodium glucose cotransporter SGLT1 is essential for embryo survival and fetal growth in pregnancy. Sci Rep. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14: 2873–2882, 2003. doi: 10.1097/01.ASN.0000092790.89332.D2. [DOI] [PubMed] [Google Scholar]
- 38.Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio JR, Wright EM. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci USA 112: E4111–E4119, 2015. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma P, Khairnar V, Madunić IV, Singh Y, Pandyra A, Salker MS, Koepsell H, Sabolić I, Lang F, Lang PA, Lang KS. SGLT1 deficiency turns listeria infection into a lethal disease in mice. Cell Physiol Biochem 42: 1358–1365, 2017. doi: 10.1159/000479197. [DOI] [PubMed] [Google Scholar]
- 40.Shibazaki T, Tomae M, Ishikawa-Takemura Y, Fushimi N, Itoh F, Yamada M, Isaji M. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther 342: 288–296, 2012. doi: 10.1124/jpet.112.193045. [DOI] [PubMed] [Google Scholar]
- 41.Song P, Onishi A, Koepsell H, Vallon V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin Ther Targets 20: 1109–1125, 2016. doi: 10.1517/14728222.2016.1168808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soták M, Marks J, Unwin RJ. Putative tissue location and function of the SLC5 family member SGLT3. Exp Physiol 102: 5–13, 2017. doi: 10.1113/EP086042. [DOI] [PubMed] [Google Scholar]
- 43.Voss AA, Díez-Sampedro A, Hirayama BA, Loo DD, Wright EM. Imino sugars are potent agonists of the human glucose sensor SGLT3. Mol Pharmacol 71: 628–634, 2007. doi: 10.1124/mol.106.030288. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe A, Choe S, Chaptal V, Rosenberg JM, Wright EM, Grabe M, Abramson J. The mechanism of sodium and substrate release from the binding pocket of vSGLT. Nature 468: 988–991, 2010. doi: 10.1038/nature09580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wells RG, Pajor AM, Kanai Y, Turk E, Wright EM, Hediger MA. Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am J Physiol Renal Physiol 263: F459–F465, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Wright EM. Glucose transport families SLC5 and SLC50. Mol Aspects Med 34: 183–196, 2013. doi: 10.1016/j.mam.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Wright EM, Barrio JR, Hirayama BA, Kepe V. Tracers for Monitoring the Activity of Sodium/Glucose Cotransporters in Health and Disease. U.S. Patent US884599 B2. September 30, 2014.
- 48.Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl–dependent neurotransmitter transporters. Nature 437: 215–223, 2005. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 50.Yan N. A century’s investigation of glucose uptake-structural biology of the major facilitators superfamily glucose transporters GLUTS. J Mol Biol. In press. [Google Scholar]
- 51.Yan N. Structural biology of the major facilitator superfamily transporters. Annu Rev Biophys 44: 257–283, 2015. doi: 10.1146/annurev-biophys-060414-033901. [DOI] [PubMed] [Google Scholar]
- 52.Yu AS, Hirayama BA, Timbol G, Liu J, Diez-Sampedro A, Kepe V, Satyamurthy N, Huang SC, Wright EM, Barrio JR. Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol 304: C240–C247, 2013. doi: 10.1152/ajpcell.00317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu AS, Hirayama BA, Timbol G, Liu J, Basarah E, Kepe V, Satyamurthy N, Huang SC, Wright EM, Barrio JR. Functional expression of SGLTs in rat brain. Am J Physiol Cell Physiol 299: C1277–C1284, 2010. doi: 10.1152/ajpcell.00296.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeuthen T. Water-transporting proteins. J Membr Biol 234: 57–73, 2010. doi: 10.1007/s00232-009-9216-y. [DOI] [PubMed] [Google Scholar]
- 55.Zeuthen T, Gorraitz E, Her K, Wright EM, Loo DD. Structural and functional significance of water permeation through cotransporters. Proc Natl Acad Sci USA 113: E6887–E6894, 2016. doi: 10.1073/pnas.1613744113. [DOI] [PMC free article] [PubMed] [Google Scholar]