Abstract
The lymphatic vasculature is crucial for maintaining tissue-fluid homeostasis, providing immune surveillance and mediating lipid absorption. The lymphatic vasculature is tightly associated with the blood vasculature, although it exhibits distinct morphological and functional features. Endothelial cells (ECs) lineage fate specification is determined during embryonic development; however, accumulating evidence suggests that differentiated ECs exhibit remarkable heterogeneity and plasticity. In this review, we provide an overview of the molecular mechanisms promoting lymphatic cell fate specification in the mammalian embryo. We also summarize available data suggesting that lymphatic EC fate is reprogrammable in normal and pathological settings. We further discuss the possible advantages of cell fate manipulation to treat certain disorders associated with lymphatic dysfunction.
The mammalian vascular system consists of two closely associated and functionally specialized networks: the blood and the lymphatic vasculature. Both vasculatures are highly branched and lined by endothelial cells (ECs). The lymphatic vasculature consists of a network of thin-walled, blind-ended, highly permeable capillaries that lack pericyte coverage and a basement membrane. These capillaries converge to form larger-caliber collecting vessels surrounded by mural cells. Collecting lymphatic vessels have intraluminal valves to facilitate the transport of lymph back to the blood circulation through lymphovenous valve junctions located around the jugular region. Some of the main functions of the lymphatic vasculature are to drain fluid from the extracellular spaces and return it back to the blood circulation, lipid absorption from the intestinal tract, and transport of immune cells to lymphoid organs (76). Morphological or functional defects in the lymphatic vasculature have been associated among other ones, with congenital or acquired lymphedema, obesity, inflammation, atherosclerosis, and more recently glaucoma (18, 64).
Early during mammalian embryonic development, mesodermally derived ECs exhibit a primordial, nonspecialized endothelial phenotype, and they give rise to the primitive vasculature (vasculogenesis). Later on, this primary vascular plexus remodels, and, following endothelial sprouting and splitting (angiogenesis), these primitive ECs acquire specialized arterial or venous fates. Maintaining EC fate identity is crucial for normal specialized vasculature function. However, at least experimentally, EC identity can be easily manipulated by repressing a preexisting cell fate and/or by activating another one (48). In vivo, for example, establishment and maintenance of venous identity requires expression of the chicken ovalbumin upstream promoter transcription factor 2 (COUP-TFII), which, in turns, suppresses Notch signaling, thereby repressing the expression of arterial genes (78). Similarly, LEC fate can be reprogrammed back into its original blood endothelial cell (BEC) fate identity by downregulating the expression of the homeobox transcription factor Prox1 (28). In this review, we summarize our current knowledge of the molecular mechanisms regulating EC fate and discuss some of the possible advantages that EC plasticity and reprogramming can provide to gene-therapy approaches and tissue regeneration.
Blood Vascular Development and BEC Plasticity
Following the formation of ECs and a basic blood capillary network, arteriovenous EC fate specification and differentiation takes place. This process requires the activity of two master regulators, Notch and COUP-TFII. Notch signaling in ECs is required to promote arterial cell fate but concomitantly also suppresses venous fate (35). On the other hand, the orphan nuclear receptor COUP-TFII promotes venous identity mainly by inhibiting Notch signaling and other arterial-specific genes (78) (FIGURE 1). Mouse models of EC loss-of-function of COUP-TFII result in increased levels of Np-1 and Notch1 in veins, and the subsequent loss of venous fate (78). Alternatively, ectopic expression of COUP-TFII suppresses arterial fate by reducing Np-1 levels and inhibiting Notch signaling, leading to abnormal arterio/venous fusions (78). It is worth noting that the induction of arterial features upon conditional ablation of Coup-TFII in veins is not complete, suggesting that Coup-TFII was not fully removed and/or that venous ECs are not able to fully revert into an arterial fate once they become specified (78). Nevertheless, these data argue that arterial and venous fates are relatively plastic and reprogrammable. Interestingly, EC fate is not only genetically determined but also influenced by local environmental cues (46). For example, it was shown in quail-chick chimeras that grafted veins or arteries can colonize the endothelium of both arteries and veins during early stages and acquire the EC fate of the engrafted site (42). This plasticity is gradually lost at later stages of embryonic development such that, in the mouse and after embryonic day (E) 11.0, grafted aortic ECs mainly integrate into arteries and grafted venous ECs mainly into veins (42). This plasticity can be restored by keeping the ECs to be grafted separated from the vessel wall, a result suggesting that EC plasticity is influenced by external cues, provided in this particular case by signals derived from the vessel wall (42). In addition, flow manipulation in the yolk sac vitelline artery of chicken embryos transforms arteries into veins, and reperfusion of ligated vessels allows it to regain its arterial identity (45). Moreover, in mice, the coronary artery arises from differentiated mature venous ECs of the sinus venosus (55). These cells dedifferentiate as they invade the myocardium, becoming arteries in the myocardium and veins on the surface, suggesting that some mature venous cells retain developmental plasticity and are able to switch lineages in response to local cues (55). Certain extent of arterial-venous fate plasticity also appears to be retained in the adult organism, particularly during adaptation to arterial circulation, since veins from aged mice or adult humans grafted into arteries lose their venous markers, although they fail to induce aortic markers (34). Furthermore, ECs are able to go through endothelial-to-hematopoietic cell transition (EHT) to give rise to multi-lineage hematopoietic progenitors (12), as well as endothelial-to-mesenchymal transition (EndMT) to generate mesenchymal cells necessary for cardiac development, disease progression, and tissue regeneration (36). Interestingly, a recent study also identified the transient presence of venous progenitors in the mammalian dorsal aorta, a result suggesting at least a partial aortic origin for the cardinal vein (37). The authors showed that a subpopulation of ECs in the early DA express either venous markers (Coup-TFII and EphB4) or arterial markers (ephrin B2, Notch, and connexin 40), but not both at the same time (37). Later on, the DA expresses only arterial markers. Furthermore, the authors were able to image venous-fated ECs migrating from the heterogeneous DA to the CV (37). They concluded that the DA contains venous progenitors that eventually move and incorporate into the developing CV (37).
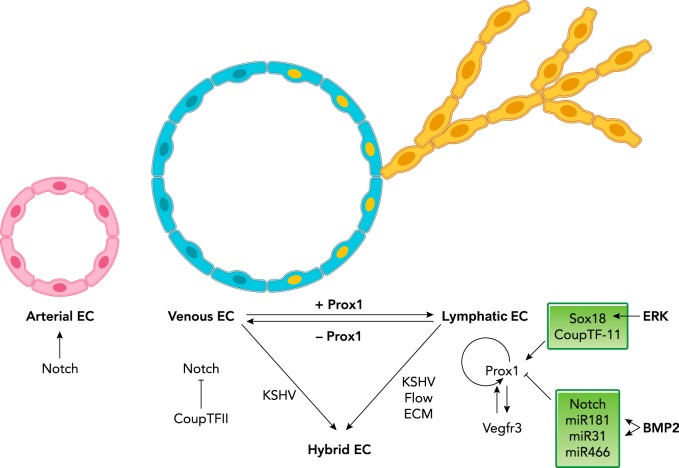
FIGURE 1.
Molecular players and signaling pathways involved in mammalian LEC specification
Notch signaling is required for arterial EC (in pink) identity, and COUP-TFII promotes venous EC (in light blue) fate by suppressing Notch signaling. The transcription factor Sox18 cooperates with COUP-TFII to activate Prox1 expression in a subpopulation of venous ECs. These cells become LEC progenitors (in light blue with yellow nuclei). These progenitors later on bud off from the cardinal vein (budding LECs in yellow). Prox1 activity is necessary and sufficient for LEC fate, and it regulates its own expression. Vegfr3 signaling is important for the maintenance of LEC fate specification, and it is directly regulated by Prox1. Notch signaling, miR181, miR31, and miR466 negatively regulate Prox1 in LECs. Biofactors such as blood flow and/or extracellular matrix (ECM) can cause LECs to lose lymphatic characteristics and/or acquire BEC features, resulting in a hybrid EC phenotype. Kaposi sarcoma-associated herpes virus (KSHV) infects both LECs and BECs, and transcriptionally reprograms these cells into hybrid ECs.
Origin and Source of LECs
In the mouse, lymphatic vasculature development starts at around E9.5, when a subpopulation of venous ECs in the anterior cardinal vein turn on Prox1 expression, becoming LEC progenitors committed into the lymphatic lineage (70, 71). Later on, these LEC progenitors bud off from the veins and migrate as associated strings that subsequently coalesce to form primitive lymph sacs (75). Most of the peripheral lymphatic vasculature develops from the lymph sacs through active lymphangiogenesis (the formation of a lymphatic network from preexisting lymphatic vessels).
Work performed at the beginning of the 20th century by the remarkable anatomist Florence Sabin using dye injections into pig embryos provided some of the first evidence to support the model that lymphatics were venous-derived (56). Instead, in contrast to Sabin’s centrifugal model, Huntington and McClure suggested a “centripetal” model in which lymphatic vessels originated from the fusion of perivascular mesenchyme-derived cells that later on connected to the venous network (26). Support for the venous model was provided later through the systematic anatomical characterization of mouse and human embryos (53, 54). These investigators concluded that, at least in mammals, all main lymphatics were derived from the endothelium lining the venous walls (54).
More recently, the discovery of LEC markers, such as Prox1, lymphatic vessel endothelial hyaluronan receptor 1 (Lyve1), and podoplanin, together with the use of lineage-tracing approaches provided additional tools that facilitated the characterization of the mechanisms participating in the formation of the lymphatic vasculature (76). Support for Sabin’s venous model was initially provided by the generation of novel mouse mutant models and the use of detailed Cre/loxP-based lineage-tracing approaches (60, 71). In particular, the analysis of Tie2-Cre;COUP-TFIIf/f mutant mouse embryos showed that loss of venous fate arrested LEC formation almost completely (60). Later on, detailed characterization of the early morphological events involved in lymphatic vasculature formation further illustrated that, in mouse embryos, lymphatic progenitors begin to form in the endothelium of the cardinal vein, intersomitic vessels, and superficial venous plexus at approximately E9.5 (21, 60, 75). The proposed venous origin of LECs was shown to be evolutionary conserved, at least in zebrafish embryos (77). However, the debate about LEC origin is not over yet, since it was reported that, in avian embryos and amphibians, a proportion of LECs originate in the mesenchyme (47, 72). The existence of mesenchymal lymphatic endothelial precursor cells was also reported to happen in mice based on the presence of few scattered cells with leukocyte and LEC characteristics (7). Although most evidence supports the argument that most murine embryonic LECs are venous derived (see Ref. 18 for a detailed review), recent results suggest that additional LEC sources might contribute to the lymphatic vasculature of some specific organs, although in a reduced percentage. The use of chick-quail chimeras and transgenic mice suggested a dual venous and mesenchymal origin for the gut lymphatics (38). In this context, Pitx2-driven Cxcl12-dependent asymmetric arteriogenesis in the left dorsal mensentery is required for the formation and assembly of a nonvenous-derived ventral LEC population (38). Other results also argued that part of the mouse mesenteric lymphatic vasculature originates from cKit-derived hemogenic endothelium (62). A heterogeneous cellular origin of LEC progenitors was also recently proposed for cardiac lymphatic vessels in mice, in which approximately one-fifth of the cardiac lymphatic network was proposed to be formed independently of venous sprouting and originate from yolk sac hemogenic endothelium (33). Finally, in the mouse dermis, recent work also argued that, although the anterior region of dermal LECs is venous derived, a significant proportion in the midline and lumbar region is not labeled when Tie2Cre is used to distinguish the blood endothelium lineage, or VavCre for the hemogenic endothelium (39). Instead, these authors propose that they form by the assembly of nonvenous cells into clusters and vessels (39). In conclusion, although most data seem to support Sabin’s original proposal that most LECs are venous derived, some results also argue that, at least in certain organs, a small percentage of LEC precursors might originate from other source(s) that have not yet been fully confirmed and identified. However, even in that case, we do not yet know whether those LECs originating from other sources are functionally significant.
During the past few decades, several key regulatory molecules and specific LEC markers have been identified. LEC progenitor fate is initially acquired by the expression of the transcription factors Sox18 and Prox1 in a subpopulation of COUP-TFII-expressing venous ECs (18). Sox18 appears to activate Prox1 expression by binding to two Sox18-binding sites on the Prox1 promoter (19). ERK activation was also shown to be important during LEC fate specification and might be responsible for activating Sox18 expression in the CV (16). COUP-TFII and Prox1 physically interact and cooperatively regulate the expression of other key players such as vascular endothelial growth factor receptor-3 (Vegfr3) (74). Recently, Vegfr3, the receptor for the lymphangiogenic growth factor Vegfc, was identified as a direct target of Prox1 in vivo (61). In addition, Vegfr3 also regulates Prox1 by establishing a feedback loop to maintain the identity of LEC progenitors (61). Notch1 signaling is also important during LEC progenitor specification since loss of Notch1, or reduced Notch signaling, leads to an increase in the number of LEC progenitors and larger lymph sacs (43).
A series of studies suggested that LEC identity is also regulated by miRNAs through posttranscriptional regulation of Prox1. MiR-31 was suggested to inhibit expression of LEC-specific transcripts in vitro, and its overexpression in LECs was shown to induce the preferential degradation of LEC signature genes such as Prox1 and FOXC2 (50). In addition, miR-181a was reported to bind the 3′ untranslated region of Prox1, resulting in its translational inhibition and transcript degradation (30). MiR-181a expression is significantly higher in embryonic BECs compared with LECs. Increased miR-181a in primary embryonic LECs resulted in reduced levels of Prox1 mRNA and protein, and reprogramming of LECs toward a BEC phenotype (30). Conversely, miR-181a antagomir treatment resulted in increased Prox1 mRNA levels in BECs, results suggesting that miR-181 might be required to repress Prox1 expression in BECs during development (30). In line with this notion, bone morphogenetic protein (BMP) 2 inhibits Prox1 expression and lymphatic differentiation during zebrafish and murine development via upregulation of miRNAs, including miR-31 and miR-181a (17). In addition, miR-466 was shown to suppress Prox1 expression and tube formation in human dermal LECs (59) (FIGURE 1).
Lymphatic Fate is Plastic
Recent in vitro and in vivo studies using gain- or loss-of-function models revealed that LEC fate is remarkably plastic and reprogrammable. Acquiring a LEC progenitor fate only requires Prox1 expression in venous BECs. Upon Prox1 expression, those venous ECs become LEC progenitors and start budding off from the CV, a process mediated by Vegfr3/Vegfc signaling (29, 61, 70, 71). Prox1-deficient mice are devoid of a lymphatic vasculature consequence of the lack of LEC progenitors, since in these embryos venous ECs fail to acquire a lymphatic phenotype and instead remain as BECs (70). Interestingly, genetic ablation of Prox1 during embryonic, postnatal, or adult stages is sufficient to reverse lymphatic cell fate toward the underlying blood vascular phenotype (28) (FIGURE 1). In vitro, ectopic expression of Prox1 is sufficient to induce lymphatic reprogramming of BECs via the upregulation of some key LEC markers, such as podoplanin and Vegfr3, and the suppression of typical BEC-specific genes (24, 51). These results argue that the mature LEC state is constantly and actively maintained by Prox1 activity.
Flow as Modulator of Lymphatic Fate
Although vessel identity is established by genetic programs early during development, it is well known that environmental cues such as blood flow and cell-cell interactions also contribute to lineage commitment. Hemodynamic forces regulate blood vessel identity, vasculature development, and remodeling. Although lymphatic flow is slower and more irregular than blood flow, LECs still sense flow (11, 13, 44). In fact, LECs and BECs respond differently when placed under interstitial fluid flow and shear flow in vitro (44). Under interstitial flow, LECs form large vacuoles and long extensions; instead, BECs exhibit enhanced networking and multicellular tubulogenesis (44). Under planar shear flow, LECs and BECs align along the axis of the flow, but only LECs downregulate cell-cell adhesions, and BECs do not (44). In addition, LECs are able to sense changes in shear stress and adjust their barrier function by a mechanism dependent on Rac1-mediated actin dynamics (6). In developing mouse embryos, LECs become elongated and show increased proliferation and enhanced VEGFR3 tyrosine phosphorylation in response to increased interstitial fluid, whereas a reduction in the amount of interstitial fluid causes the opposite effect (52).
Recent studies demonstrated that lymphatic fluid flow is required for lymphatic fate maintenance, lymphatic vessel patterning, development of lymphatic valves, maturation of collecting lymphatics, lymphatic barrier function, and lymphatic vessel stabilization (9, 11, 13, 57, 58, 63, 68). In the lymphatic vasculature, the unidirectional transport of the lymphatic fluid depends on the formation of lymphatic intraluminal valves and the proper separation of the blood and lymphatic vascular systems. Lymphovenous valves are important gate-keepers to prevent blood from entering the lymphatic vessel; however, these valves are not sufficient to prevent blood from back flow into the lymphatic system (11, 23) (FIGURE 2A). Mouse embryos with genetic deletion of Clec-2 and Pdpn fail to form platelet aggregates at the LV valves, so they display blood/lymphatic miss-separation (11, 40, 66) (FIGURE 2C). Interestingly, a recent report indicates that not only the formation of the thrombi clot is required for lymphovenous hemostasis but that regulation of LV thrombus degradation is also important (15). These authors showed that loss of the chromodomain helicase DNA-binding 4 (CHD4) in Lyve1+ LECs increases plasmin activation, which degrades fibrin-rich thrombi at the LV valves, subsequently allowing blood to enter into the lymphatics (15) (FIGURE 2C). Therefore, deficiency in either lymphovenous valve formation or lymphovenous hemostasis results in severe lymphedema, and defective blood/lymphatic separation results in blood backflow into the lymphatic vessels, which impedes forward lymph flow. The blood-filled lymphatic vessel phenotype reported in T-syn or C1galt1 (20), Slp-76 (1, 11), Syk (1), and PLC- γ2 (27) mutant mice appears to promote formation of hybrid vessels with mosaic expression of blood and lymphatic EC markers (FIGURE 2B). Exposure of lymphatic vessels to blood without flow did not alter vessel identity in vivo; however, LECs exposed to blood vessel levels of fluid shear stress ex vivo rapidly reduced expression of Prox1, suggesting that flow can reprogram lymphatic vessels to blood vessels through transcriptional pathways that establish and maintain endothelial identity (11). In addition, lymph flow is also required for maturation of the lymphatic vasculature. Loss of normal lymph flow failed to induce the shear stress response transcription factors GATA2 and FOXC2, and failed to coordinate a flow-induced gene expression program along with Prox1, leading to a reduction in lymphatic valve development (63). Recently, another study indicated that oscillatory shear stress triggers Wnt/β-catenin signaling and that lymphatic β-catenin regulates lymphatic vessel patterning and is required for the formation of lymphatic and lymphovenous valves (9). Despite the role of fluid flow in lymphatic vasculature development, the molecular mechanisms involved in mechanotransduction in the lymphatic endothelium remain elusive. In addition, whether the effect of hemodynamic forces on LEC identity and lymphatic valve is reversible in vivo is still unknown.
FIGURE 2.
Flow is important for lymphatic fate maintenance
A: lymphovenous valves (LVV) form at the junction of the internal jugular vein (IJV) and subclavian veins (SCV). Platelet thrombi block retrograde blood flow into lymphatic vessels (LV). These structures ensure the drainage of lymph into venous circulation. B: usually, extracellular fluid drains into lymphatic vessels (left). When lymphovenous homeostasis is defective, blood enters into lymphatic vessels and disrupts forward lymph flow (right). LECs will lose their lymphatic fate and adopt BEC characteristics, such as acquiring mural coverage (arrow indicates directional lymph flow and retrograde flow). C: flow-rate laminar flow causes calcium influx in LECs, which upregulates Notch E3 ligase heterodimer encoded by Dtx1and Dtx3l, suppressing Notch1 activity. Notch1 is known as a negative regulator of Prox1. Loss of the chromatin-remodeling enzyme CHD4 results in upregulation of uPAR and subsequently elevated plasmin activity to degrade fibrin-rich thrombi that help seal the LVVs. Pdpn and T-synthase, the enzyme that is required for its O-glycosylation, activate platelets via the CLEC-2 receptor. CLEC-2 activates the SYK-Slp76-PLC-γ2 cascade to promote platelet aggregation, a process important for lymphovenous homeostasis. Loss of the platelet thrombi results in blood backflow in lymphatic vessels, leading to reprogramming of LECs into BECs.
Extracellular Environment Affects Lymphatic Fate
The extracellular environment has also been reported to affect the expression of lymphatic genes. It also contributes to vascular bed-specific gene expression and EC heterogeneity (2). Transcriptional characteristics that define BECs and LECs in vivo are lost rapidly upon culture, suggesting that the in vitro culture conditions do not fully recapitulate the in vivo environment, and can influence LEC and BEC fate (3, 69). It was reported that, when BECs are cultured within or on top of matrices such as type I collagen, fibrin, and Matrigel, they show altered gene expression toward a lymphatic phenotype (14). Induction of the lymphatic phenotype is reversible once cells are removed from the culture and is partially inhibited by coculture of HUVECs with perivascular cells (14). In addition, different from venous ECs, arterial ECs do not reprogram toward a LEC fate upon ectopic Prox1 expression in vivo (31). Interestingly, abnormal recruitment of mural cell to initial lymphatics is observed in several models of lymphatic malfunction characterized by the partial reprogramming of LECs toward BECs, although it is still unclear whether mural cell recruitment is the cause or consequence of the fate change. These data further indicate that the LEC phenotype is plastic, and is at least partially regulated by the extracellular matrix environment and/or local cues from surrounding cells. Furthermore, it is known that BEC transcriptional profile changes among different organs and parts of the same organ to adapt to the various functional and physiological requirements and to the environment (46). How and when these cells change their molecular signatures is not yet known.
Pathological Conditions Change Lymphatic Fate
ECs may lose their original identity and exhibit new mixed cell phenotypes in pathological conditions. Kaposi's sarcoma (KS) is the most common cancer in untreated HIV patients and is caused by infection with the Kaposi sarcoma-associated herpes virus (KSHV) (10). Breiteneder-Geleff et al. showed that angiosarcomas and early stage KS co-express the LEC markers Vegfr3 and podoplanin, as well as markers of blood vessel endothelium, such as CD31 and podocalyxin (5). KSHV infects LECs and BECs in vitro and transcriptionally reprograms both cell types (67). Infection of differentiated BECs with KSHV results in lymphatic reprogramming, inducing expression of lymphatic lineage-specific genes and downregulating blood vascular genes (Ho44; Wa44; see Refs. 8, 25, 67). KSHV infection of LECs increases BEC gene expression (22). However, the role of lymphatic reprogramming in KS, its origin, and the mechanism causing this mixed phenotype are still not fully understood. JAK2/STAT3 and PI3K/Akt cell signaling pathways are activated in KSHV infection through the gp130 receptor and are necessary for KSHV-induced lymphatic reprogramming of ECs, at least partially via Prox1 (41). In addition, KSHV influences EC motility to promote KS progression by downregulating miR-221/miR-222 and upregulating miR-31 (73). As mentioned above, miR-31 appears to act as a negative regulator of lymphatic development via direct repression of Prox1 (50). KSHV-encoded miRNAs also contribute to EC reprogramming by downregulating the cellular transcription factor c-MAF, which represses BEC marker genes in LECs (22).
BEC/LEC Hybrid Vessels
Interestingly, recent studies revealed that some vessels exhibit mixed BEC and LEC molecular features. Among those is the Schlemm’s Canal (SC), an endothelium-lined vascular channel in the eye required to maintain fluid homeostasis by draining aqueous humor from the intraocular chamber into the systemic circulation (32). Dysfunction of the SC appears to elevate ocular pressure, leading to glaucoma, a degenerative, age-related eye disease that is the second leading cause of blindness worldwide (4, 32, 65). At the molecular level, the SC is a hybrid vessel expressing typical LEC markers (Prox1, integrin-a9, Vegfr3, and CCL21) as well as BEC markers (Vegfr2, endomucin, vWF); however, the expression of other typical LEC markers such as Lyve1 or Pdpn is marginal or not detected (4, 32, 49). Lineage-tracing studies indicated that the SC forms by postnatal migration of choroidal vein-derived progenitors that fuse to form the primitive Prox1-expressing SC (4, 32, 49). Notably, although the morphogenesis of the SC appears somehow similar to that of primitive lymph sacs and is also dependent on Vegfc activity, Prox1 is not expressed on the choroidal veins and Sox18 is not detected in the SC-forming cells (49). Interestingly, aqueous humor outflow positively regulates SC formation and influences Prox1 expression (49). The SC in aged mice exhibits a senescent endothelium and reduced AHO; meanwhile, they have reduced expression of lymphatic vessel makers but increased expression of that of blood vessels and mesenchymal features (49).
Conclusions and Perspectives
It has been proposed that EC lineage specification is determined during embryonic development, after which ECs are irreversibly committed to their fate. However, recent studies using gain- or loss-of-function approaches demonstrated that ECs remain plastic through life, and their fate could be altered at least in certain pathological settings, under some functional and environmental requirements, and by changes in hemodynamic forces. For example, pathological conditions such as inflammation, tumorigenesis, and wound healing are associated with lymphangiogenesis. In these conditions, the vessel permeability as well as the amounts and types of miRNAs, local chemokines, and growth factors, among others, are subjected to dramatic changes, possibly triggering fate change of BECs into LECs to meet the increasing demand for fluid drainage and immune cell transportation. On the other hand, dedifferentiation of LECs to BECs might also promote the accessibility of oxygen and nutrients to the fast-growing tumor tissue. However, the extent and precise role of LEC plasticity remains to be defined. These findings raise some interesting questions. Are all the ECs in different vascular beds equally competent to changes in their cell fate? What is the molecular mechanism triggering and coordinating this fate switch? Answering these questions should be instrumental for the understanding of lymphatic vessel physiology and should open new opportunities for cell-based therapies for vascular diseases.
Acknowledgments
Research discussed in this manuscript was supported by National Heart, Lung, and Blood Institute Grant R01 HL-073402.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: W.M. prepared figures; W.M. and G.O. drafted manuscript; W.M. and G.O. edited and revised manuscript; W.M. and G.O. approved final version of manuscript.
References
- 1.Abtahian F, Guerriero A, Sebzda E, Lu M-M, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299: 247–251, 2003. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2: a006429, 2012. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amatschek S, Kriehuber E, Bauer W, Reininger B, Meraner P, Wolpl A, Schweifer N, Haslinger C, Stingl G, Maurer D. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood 109: 4777–4785, 2007. doi: 10.1182/blood-2006-10-053280. [DOI] [PubMed] [Google Scholar]
- 4.Aspelund A, Tammela T, Antila S, Nurmi H, Leppänen V-M, Zarkada G, Stanczuk L, Francois M, Mäkinen T, Saharinen P, Immonen I, Alitalo K. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest 124: 3975–3986, 2014. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154: 385–394, 1999. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslin JW, Kurtz KM. Lymphatic endothelial cells adapt their barrier function in response to changes in shear stress. Lymphat Res Biol 7: 229–237, 2009. doi: 10.1089/lrb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buttler K, Kreysing A, von Kaisenberg CS, Schweigerer L, Gale N, Papoutsi M, Wilting J. Mesenchymal cells with leukocyte and lymphendothelial characteristics in murine embryos. Dev Dyn 235: 1554–1562, 2006. doi: 10.1002/dvdy.20737. [DOI] [PubMed] [Google Scholar]
- 8.Carroll PA, Brazeau E, Lagunoff M. Kaposi’s sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 328: 7–18, 2004. doi: 10.1016/j.virol.2004.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha B, Geng X, Mahamud MR, Fu J, Mukherjee A, Kim Y, Jho E-H, Kim TH, Kahn ML, Xia L, Dixon JB, Chen H, Srinivasan RS. Mechanotransduction activates canonical Wnt/β-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev 30: 1454–1469, 2016. doi: 10.1101/gad.282400.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266: 1865–1869, 1994. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-Y, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vicente A, Mericko P, Levy RJ, Makinen T, Oliver G, Kahn ML. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest 122: 2006–2017, 2012. doi: 10.1172/JCI57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457: 887–891, 2009. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi D, Park E, Jung E, Seong YJ, Yoo J, Lee E, Hong M, Lee S, Ishida H, Burford J, Peti-Peterdi J, Adams RH, Srikanth S, Gwack Y, Chen CS, Vogel HJ, Koh CJ, Wong AK, Hong Y-K. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J Clin Invest 127: 1225–1240, 2017. doi: 10.1172/JCI87442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley LS, Handsley MM, Zhou Z, Lafleur MA, Pennington CJ, Thompson EW, Pöschl E, Edwards DR. Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro. J Cell Sci 123: 3808–3816, 2010. doi: 10.1242/jcs.064279. [DOI] [PubMed] [Google Scholar]
- 15.Crosswhite PL, Podsiadlowska JJ, Curtis CD, Gao S, Xia L, Srinivasan RS, Griffin CT. CHD4-regulated plasmin activation impacts lymphovenous hemostasis and hepatic vascular integrity. J Clin Invest 126: 2254–2266, 2016. doi: 10.1172/JCI84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Y, Atri D, Eichmann A, Simons M. Endothelial ERK signaling controls lymphatic fate specification. J Clin Invest 123: 1202–1215, 2013. doi: 10.1172/JCI63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunworth WP, Cardona-Costa J, Bozkulak EC, Kim J-D, Meadows S, Fischer JC, Wang Y, Cleaver O, Qyang Y, Ober EA, Jin S-W. Bone morphogenetic protein 2 signaling negatively modulates lymphatic development in vertebrate embryos. Circ Res 114: 56–66, 2014. doi: 10.1161/CIRCRESAHA.114.302452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escobedo N, Oliver G. Lymphangiogenesis: origin, specification, and cell fate determination. Annu Rev Cell Dev Biol 32: 677–691, 2016. doi: 10.1146/annurev-cellbio-111315-124944. [DOI] [PubMed] [Google Scholar]
- 19.François M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KSE, Stacker SA, Muscat GEO, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature 456: 643–647, 2008. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 20.Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, Nye E, Ju T, Ramirez MI, Carmeliet P, Cummings RD, Lupu F, Xia L. Endothelial cell O-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest 118: 3725–3737, 2008. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hägerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 32: 629–644, 2013. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen A, Henderson S, Lagos D, Nikitenko L, Coulter E, Roberts S, Gratrix F, Plaisance K, Renne R, Bower M, Kellam P, Boshoff C. KSHV-encoded miRNAs target MAF to induce endothelial cell reprogramming. Genes Dev 24: 195–205, 2010. doi: 10.1101/gad.553410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G, Makinen T, Xia L, Kahn ML. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest 124: 273–284, 2014. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong Y-K, Harvey N, Noh Y-H, Schacht V, Hirakawa S, Detmar M, Oliver G. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225: 351–357, 2002. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- 25.Hong Y-KK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet 36: 683–685, 2004. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- 26.Huntington GS, McClure CFW. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica). Am J Anat 10: 177–312, 1910. doi: 10.1002/aja.1000100108. [DOI] [Google Scholar]
- 27.Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development 136: 191–195, 2009. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- 28.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22: 3282–3291, 2008. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80, 2004. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 30.Kazenwadel J, Michael MZ, Harvey NL. Prox1 expression is negatively regulated by miR-181 in endothelial cells. Blood 116: 2395–2401, 2010. doi: 10.1182/blood-2009-12-256297. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Cruz M, Bourdeau A, Dumont DJ. Cell-cell interactions influence vascular reprogramming by Prox1 during embryonic development. PLoS One 8: e52197, 2013. doi: 10.1371/journal.pone.0052197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kizhatil K, Ryan M, Marchant JK, Henrich S, John SWM. Schlemm’s canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol 12: e1001912, 2014. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522: 62–67, 2015. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol 27: 1562–1571, 2007. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 35.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Lin F, Wang N, Zhang T-C. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life 64: 717–723, 2012. doi: 10.1002/iub.1059. [DOI] [PubMed] [Google Scholar]
- 37.Lindskog H, Kim YH, Jelin EB, Kong Y, Guevara-Gallardo S, Kim TN, Wang RA. Molecular identification of venous progenitors in the dorsal aorta reveals an aortic origin for the cardinal vein in mammals. Development 141: 1120–1128, 2014. doi: 10.1242/dev.101808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahadevan A, Welsh IC, Sivakumar A, Gludish DW, Shilvock AR, Noden DM, Huss D, Lansford R, Kurpios NA. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev Cell 31: 690–706, 2014. doi: 10.1016/j.devcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, John SWM, Alitalo K, Ortega S, Makinen T. Nonvenous origin of dermal lymphatic vasculature. Circ Res 116: 1649–1654, 2015. doi: 10.1161/CIRCRESAHA.116.306170. [DOI] [PubMed] [Google Scholar]
- 40.May F, Hagedorn I, Pleines I, Bender M, Vögtle T, Eble J, Elvers M, Nieswandt B. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood 114: 3464–3472, 2009. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 41.Morris VA, Punjabi AS, Lagunoff M. Activation of Akt through gp130 receptor signaling is required for Kaposi’s sarcoma-associated herpesvirus-induced lymphatic reprogramming of endothelial cells. J Virol 82: 8771–8779, 2008. doi: 10.1128/JVI.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyon D, Pardanaud L, Yuan L, Bréant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development 128: 3359–3370, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Murtomaki A, Uh MK, Choi YK, Kitajewski C, Borisenko V, Kitajewski J, Shawber CJ. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development 140: 2365–2376, 2013. doi: 10.1242/dev.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng CP, Helm C-LE, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc Res 68: 258–264, 2004. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 45.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Bréant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development 131: 361–375, 2004. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 46.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding B-S, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219, 2013. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ny A, Koch M, Schneider M, Neven E, Tong RT, Maity S, Fischer C, Plaisance S, Lambrechts D, Héligon C, Terclavers S, Ciesiolka M, Kälin R, Man WY, Senn I, Wyns S, Lupu F, Brändli A, Vleminckx K, Collen D, Dewerchin M, Conway EM, Moons L, Jain RK, Carmeliet P. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med 11: 998–1004, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Oliver G, Srinivasan RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development 137: 363–372, 2010. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park DY, Lee J, Park I, Choi D, Lee S, Song S, Hwang Y, Hong KY, Nakaoka Y, Makinen T, Kim P, Alitalo K, Hong YK, Koh GY. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest 124: 3960–3974, 2014. doi: 10.1172/JCI75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedrioli DML, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, Marino D, Kälin RE, Leidel S, Cinelli P, Schulte-Merker S, Brändli AW, Detmar M. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol 30: 3620–3634, 2010. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrova TV, Mäkinen T, Mäkelä TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Ylä-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21: 4593–4599, 2002. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Planas-Paz L, Strilić B, Goedecke A, Breier G, Fässler R, Lammert E. Mechanoinduction of lymph vessel expansion. EMBO J 31: 788–804, 2012. doi: 10.1038/emboj.2011.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Putte SC. The early development of the lymphatic system in mouse embryos. Acta Morphol Neerl Scand 13: 245–286, 1975. [PubMed] [Google Scholar]
- 54.van der Putte SC, van Limborgh J. The embryonic development of the main lymphatics in man. Acta Morphol Neerl Scand 18: 323–335, 1980. [PubMed] [Google Scholar]
- 55.Red-Horse K, Ueno H, Weissman IL, Krasnow MA. Coronary arteries form by developmental reprogramming of venous cells. Nature 464: 549–553, 2010. doi: 10.1038/nature08873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabin FR. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat 1: 367–389, 1902. doi: 10.1002/aja.1000010310. [DOI] [Google Scholar]
- 57.Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, Kwak BR, Mäkinen T, Martinez-Corral I, Ortega S, Delorenzi M, Kiefer F, Davis MJ, Djonov V, Miura N, Petrova TV. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 125: 3861–3877, 2015. doi: 10.1172/JCI80454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hägerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, Calmes J-M, Adams RH, Mäkinen T, Kiefer F, Kwak BR, Petrova TV. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell 22: 430–445, 2012. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Seo M, Choi J-S, Rho CR, Joo C-K, Lee SK. MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. J Biomed Sci 22: 3, 2015. doi: 10.1186/s12929-014-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Srinivasan RS, Dillard ME, Lagutin OV, Lin F-J, Tsai S, Tsai M-J, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21: 2422–2432, 2007. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srinivasan RS, Escobedo N, Yang Y, Interiano A, Dillard ME, Finkelstein D, Mukatira S, Gil HJ, Nurmi H, Alitalo K, Oliver G. The Prox1-Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev 28: 2175–2187, 2014. doi: 10.1101/gad.216226.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Laviña B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, Alitalo K, Graupera M, Mäkinen T. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Reports 10: 1708–1721, 2015. doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 63.Sweet DT, Jiménez JM, Chang J, Hess PR, Mericko-Ishizuka P, Fu J, Xia L, Davies PF, Kahn ML. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J Clin Invest 125: 2995–3007, 2015. doi: 10.1172/JCI79386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140: 460–476, 2010. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 65.Thomson BR, Heinen S, Jeansson M, Ghosh AK, Fatima A, Sung HK, Onay T, Chen H, Yamaguchi S, Economides AN, Flenniken A, Gale NW, Hong YK, Fawzi A, Liu X, Kume T, Quaggin SE. A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest 124: 4320–4324, 2014. doi: 10.1172/JCI77162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fässler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood 115: 3997–4005, 2010. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 67.Wang H-W, Trotter MWB, Lagos D, Bourboulia D, Henderson S, Mäkinen T, Elliman S, Flanagan AM, Alitalo K, Boshoff C. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat Genet 36: 687–693, 2004. doi: 10.1038/ng1384. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Baeyens N, Corti F, Tanaka K, Fang JS, Zhang J, Jin Y, Coon B, Hirschi KK, Schwartz MA, Simons M. Syndecan 4 controls lymphatic vasculature remodeling during mouse embryonic development. Development 143: 4441–4451, 2016. doi: 10.1242/dev.140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wick N, Saharinen P, Saharinen J, Gurnhofer E, Steiner CW, Raab I, Stokic D, Giovanoli P, Buchsbaum S, Burchard A, Thurner S, Alitalo K, Kerjaschki D. Transcriptomal comparison of human dermal lymphatic endothelial cells ex vivo and in vitro. Physiol Genomics 28: 179–192, 2007. doi: 10.1152/physiolgenomics.00037.2006. [DOI] [PubMed] [Google Scholar]
- 70.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21: 1505–1513, 2002. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778, 1999. doi: 10.1016/S0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 72.Wilting J, Aref Y, Huang R, Tomarev SI, Schweigerer L, Christ B, Valasek P, Papoutsi M. Dual origin of avian lymphatics. Dev Biol 292: 165–173, 2006. doi: 10.1016/j.ydbio.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 73.Wu Y-H, Hu T-F, Chen Y-C, Tsai Y-N, Tsai Y-H, Cheng C-C, Wang H-W. The manipulation of miRNA-gene regulatory networks by KSHV induces endothelial cell motility. Blood 118: 2896–2905, 2011. doi: 10.1182/blood-2011-01-330589. [DOI] [PubMed] [Google Scholar]
- 74.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 14: 425–434, 2009. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 75.Yang Y, García-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120: 2340–2348, 2012. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y, Oliver G. Development of the mammalian lymphatic vasculature. J Clin Invest 124: 888–897, 2014. doi: 10.1172/JCI71609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaniv K, Isogai S, Castranova D, Dye L, Hitomi J, Weinstein BM. Live imaging of lymphatic development in the zebrafish. Nat Med 12: 711–716, 2006. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 78.You L-R, Lin F-J, Lee CT, DeMayo FJ, Tsai M-J, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104, 2005. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]