Abstract
The burden of cardiovascular and metabolic diseases worldwide is staggering. The emergence of systems approaches in biology promises new therapies, faster and cheaper diagnostics, and personalized medicine. However, a profound understanding of pathogenic mechanisms at the cellular and molecular levels remains a fundamental requirement for discovery and therapeutics. Animal models of human disease are cornerstones of drug discovery as they allow identification of novel pharmacological targets by linking gene function with pathogenesis. The zebrafish model has been used for decades to study development and pathophysiology. More than ever, the specific strengths of the zebrafish model make it a prime partner in an age of discovery transformed by big-data approaches to genomics and disease. Zebrafish share a largely conserved physiology and anatomy with mammals. They allow a wide range of genetic manipulations, including the latest genome engineering approaches. They can be bred and studied with remarkable speed, enabling a range of large-scale phenotypic screens. Finally, zebrafish demonstrate an impressive regenerative capacity scientists hope to unlock in humans. Here, we provide a comprehensive guide on applications of zebrafish to investigate cardiovascular and metabolic diseases. We delineate advantages and limitations of zebrafish models of human disease and summarize their most significant contributions to understanding disease progression to date.
I. INTRODUCTION
“What is truly revolutionary about molecular biology in the post Watson-Crick era is that it has become digital.”
—Evolutionary biologist Richard Dawkins, 1941-
Over the past century, storage of electronic data as binary code and discovery of the genetic code have revolutionized biology. Biology can now be quantified: over 700 animal genomes sequenced, ~20 thousand genes in a human genome and ~1.25 gigabytes to store it; over a quarter of a million personal genomes sequenced (282). Just a few decades ago, biologic studies were largely characterized by relatively small sample sizes and single-gene approaches painstakingly studied in animal models, with broader studies taking advantage of cell culture, in vitro, or in silico data. Animal models remain indispensable for elucidating mechanism and pathophysiology, with different models best suited for different biological questions. Today, at the intersection of bench science and digital biology, the zebrafish animal model is poised to be ever more important as a high-throughput vertebrate model suitable for many questions in fundamental research, toxicology, or translational medicine.
Indeed, the zebrafish model system rose to prominence due to its utility in systematic, large-scale approaches to dissect genetic pathways during development (86, 109, 129). In the early 1990s, after large-scale screens had been established in Drosophila for a decade, the same researchers who revolutionized our understanding of the genetic control of the body plan of the fly learned of the work of George Streisinger, who pioneered the use of zebrafish, or Danio rerio, as a laboratory animal (120, 379). Streisinger showed that adult zebrafish, measuring just 2–3 cm in length, could be housed quite efficiently: up to 30 individuals in a tank the size of a standard mouse cage. He noted that zebrafish’s fecundity, external fertilization, and optical transparency of their embryos made them ideal for studying early development. Laboratories in Tübingen and Boston soon used zebrafish to carry out the first ever large-scale forward genetic screens in a vertebrate, identifying genes critically involved in the development of several organ systems (34, 86, 129, 130, 136, 176, 270, 292, 312, 338, 365, 370, 418).
During this pioneering effort, the roles of over 1,500 mutations in 400 genes were characterized mainly by visually examining developmental defects, taking advantage of the zebrafish’s rapid development, optical clarity, and anatomical resemblance to higher vertebrates. Soon, it became evident that mutations could be found in zebrafish orthologs of genes disrupted in human diseases, and, most importantly, that phenotypic characteristics of these mutant zebrafish resembled clinical hallmarks (48, 85, 419). Therefore, although studying the genetics of embryonic development and organogenesis have remained the principal research use of zebrafish, the same traits that originally led to its selection as a model are also making zebrafish an increasingly important animal model for studying human physiology and disease (19, 81, 212).
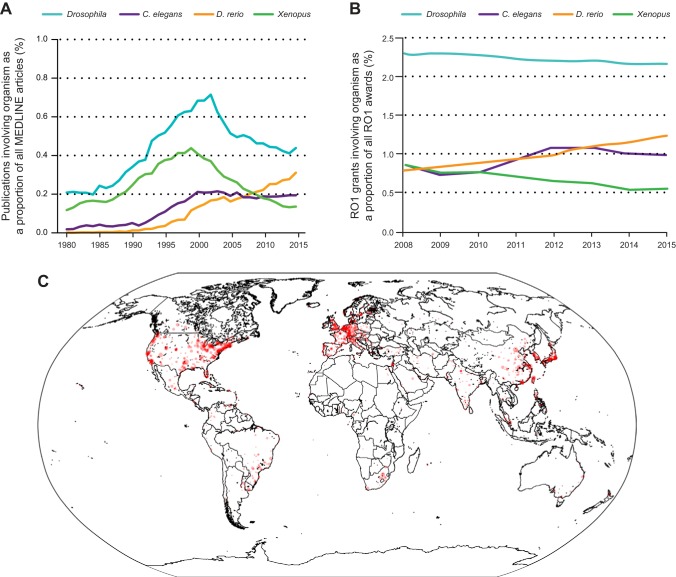
Advancements in genome engineering technologies and the emergence of zebrafish core facilities at many universities around the world make zebrafish a widely accessible model with easy-to-use genetics, lower cost, and fewer ethical restrictions than for mammalian models. Zebrafish research is increasing both as a percentage of publications in MEDLINE and as a percentage of National Institutes of Health (NIH)-funded R01 grants (199) (Figure 1). In addition to universities, many pharmaceutical companies have established zebrafish research to assist in drug discovery, including small molecule and target discovery, toxicology studies, as well as preclinical testing (226, 443). Several small molecules initially discovered in zebrafish screens have entered clinical trials, and more are expected to follow (67, 211, 226, 442). Here, we review state-of-the art approaches in zebrafish research as well as its translatability for human disease with a special emphasis on cardiovascular and metabolic disorders. As part of this introduction, we summarize the key features of zebrafish as an animal model, and we draw a laboratory perspective delineating the advantages and limitations of working with zebrafish.
FIGURE 1.
Size and shape of zebrafish research. A: percentage of publications involving the four most frequently used “lower” organisms Drosophila, C. elegans, Danio rerio, and Xenopus as a proportion of all articles published in MEDLINE. After the publication of the initial large-scale forward genetic screens in 1996, the zebrafish became increasingly popular as a model organism. B: percentage of NIH R01s involving zebrafish research is on the rise. [Graph modified from Lauer (199). Reprinted by permission from Macmillan Publishers Ltd. Data from Nature/The week in science: 5–11, August 2016; reproduced with permission from the NIH Office of Extramural Research/Open Mike blog.] C: geotagging authors’ affiliations from zebrafish papers in a MEDLINE search shows where in the world zebrafish research is taking place.
A. Early Development
Strengths of zebrafish for research in development, drug discovery, and disease must be viewed within the context of other useful models and of the zebrafish’s place in the phylogenetic tree. Zebrafish are part of the minnow family of teleost fish, an infraclass of ray-finned fishes that is thought to have arisen ~340 million years ago (156). Basic organ patterning is conserved among vertebrates, and therefore, zebrafish are useful for study of brain, eye, blood, gut, vessels, endocrine, and heart among other organ systems. Unlike other vertebrate models, zebrafish embryos develop a complete body plan with major organ systems, including a beating heart, major vessels with circulating blood, and a rudimentary gut, within 48 hours post fertilization (hpf) (182). Importantly, in addition to this rapid anatomical development, neuronal, hormonal, and paracrine feedback loops are established and become critical for homeostasis at early stages during development (171, 283, 342).
While cardiac and metabolic organ systems form early, the embryo initially relies on passive diffusion from water for oxygen, and on their yolks for nutrients; therefore, even experimental manipulations that severely perturb cardiometabolic organ systems can be studied (370). Furthermore, their short 2- to 3-mo generation time makes zebrafish faster to work with than most other vertebrates.
In addition to developing rapidly, zebrafish also develop externally, a great advantage over mammalian systems for imaging during development. Indeed, a myriad of reporter lines exist to image not only zebrafish morphology but also physiology such as electrical conduction, myocardial contraction, and more (9, 244, 317). Translucent embryos and larvae are amenable to fluorescent, confocal, time-lapse, and also three-dimensional tomographic imaging (160, 244, 317). External development also facilitates transplantation experiments, an often laborious but very powerful technique to investigate questions of cell autonomy, a technique that has been expanded recently to transparent adult zebrafish (54, 218, 432).
B. Scale and Size
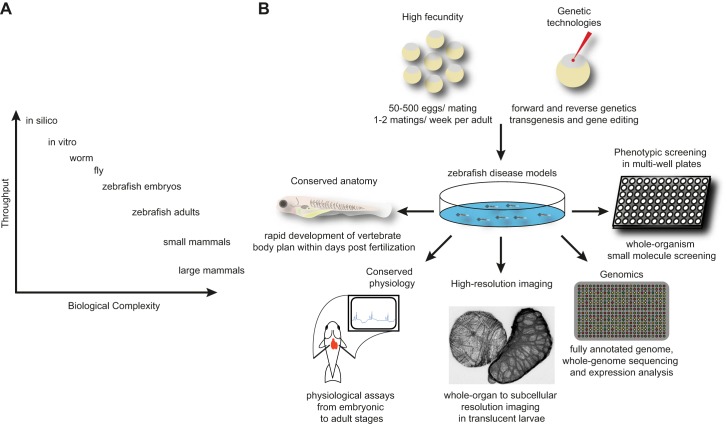
Zebrafish are small, but many, making them suitable for a range of assays. Manipulation of the zebrafish genome is fairly straightforward, as will be covered later. One pair of zebrafish can lay ~200 eggs in a single mating. Therefore, hundreds or even thousands of embryos from several mating pairs can quickly be collected and analyzed in a single experiment (Figure 2). This scale of production lies in contrast to the single-digit litters of murine models, for example, and allows investigation of rare genetic events and parallel hypothesis testing. The scale of zebrafish embryo production and ease of imaging have spawned technologies for automated embryo and larval phenotyping via high-throughput fluorescence biosorting and other approaches (10, 293, 309, 412).
FIGURE 2.
Advantages of zebrafish for biomedical research. A: zebrafish occupy a unique position in biomedical research as a vertebrate organism accessible to large-scale genetic and chemical screening. B: zebrafish are amenable to a broad spectrum of genomic, physiological, imaging, and small-molecule screening approaches.
The small size zebrafish embryos and adults can also be an advantage in the laboratory setting. Drug screens and other experiments can be set up without using a large amount of potentially precious reagents. Small size also facilitates whole-tissue (41), whole-organ, and whole-organism transcriptomic (170, 204, 318), proteomic (274), and other ’omics analyses (105), as well as whole organ clonal analysis (102, 122), cell-cell connectivity mapping (353, 366), and mosaicism analysis (54). Instead of analyzing only a biopsy of a larger heart for a given experimental condition, for example, an entire zebrafish heart chamber—or even the entire zebrafish heart—can be submitted for expression profiling. Combining whole-organism as well as whole-organ RNA expression with imaging, Junker et al. (170) created a RNA expression map of the entire zebrafish embryo by performing RNA-Seq on cryosectioned embryo samples along three dimensions. In another elegant study, McKenna et al. (240) recently used CRISPR genome editing to introduce progressive, combinatorial mutations in the zebrafish genome to create DNA “barcodes” used to perform organism-wide lineage tracing, sampling ~200,000 individual cells to reconstruct a fate map.
C. Limitations
Of course, some of the same potential strengths of the zebrafish model can also be drawbacks. Certain organ systems, such as the respiratory and reproductive ones, differ morphologically from those of humans. The small size of embryos and larvae that can be a benefit for some experiments can make it difficult to collect adequate amounts of tissue for others. Although growing, the number of zebrafish-specific antibodies for immunohistochemistry and the utility of zebrafish cell culture are limited compared with those for other animal models. While facilitating experiments involving water-soluble drug administration or biosorting, for example, the fish’s aquatic habitat can complicate certain other assays, such as EKG measurements. Finally, while zebrafish breeding is faster than for some other models, the timeline for generating mutants and transgenics is a critical consideration. To generate a stable transgenic adult or homozygous mutant embryos, ~4–6 mo are needed, although several strategies exist to accelerate this process for preliminary phenotyping (39, 404). Careful consideration of genetic strategies for creating an ideal model, and use of the many existing zebrafish resources and repositories (Table 1), can facilitate this process, as will be discussed in further detail in section II. Bearing in mind that every model system has some disadvantages, the numerous strengths of the zebrafish model from the laboratory perspective discussed above lay the groundwork for the design of disease and drug discovery models for which zebrafish is best suited.
Table 1.
Useful resources for zebrafish research
| Resource | Description | Website |
|---|---|---|
| Ensembl | Genome database for vertebrates and other eukaryotic species | www.ensembl.org |
| UCSC Genome Browser | Reference sequences and working draft assemblies of a large collection of genomes | http://Genome.ucsc.edu |
| NCBI Genome Browser | Genome database | https://www.ncbi.nlm.nih.gov/genome/ |
| UniProt | Database for protein sequences and functional domains | www.uniprot.org |
| Zfin|Zebrafish Model Organism Database | In situ hybridization atlas, genetic tools and genomics databases, developmental stages, publications; community website | http://zfin.org |
| Zebrafish International Resource Center | Stock center for zebrafish lines and plasmids in North America; fish pathology and health services | www.zirc.org |
| China Zebrafish Resource Center | Stock center for zebrafish lines in China | http://En.zfish.cn |
| Japanese Zebrafish Resource Center | Stock center for zebrafish lines in Japan | shigen.nig.ac.jp/zebra/index_en.html |
| European Zebrafish Resource Center | Stock center for zebrafish lines and plasmids in Europe; sequencing, genetic and chemical screening services | www.ezrc.kit.edu |
| Zebrafish Gene Trap and Enhancer Trap Database | Repository of gene trap and enhancer trap lines, including representative images of each line | Kawakami.laboratory.nig.ac.jp/ztrap/ |
| zfishbook|International Zebrafish Protein Trap Consortium | Repository of expression-tagged, revertible mutations in zebrafish genes using gene-breaking transposons | zfishbook.org/ |
| Digital Fish | Repository of flip trap zebrafish lines | Fliptrap.ucsc.edu/static/fliptraptech.html |
| CreZoo | Database for zebrafish Cre driver lines | crezoo.crt-dresden.de/crezoo/ |
| The Zebrafish Brain Browser | 3D Anatomy tool to visualize transgene and gene expression patterns in zebrafish larval brains | science.nichd.nih.gov/confluence/display/burgess/Brain+Browser |
| ZInC | Zebrafish insertional mutant collection. Joint efforts of UCLA and NIH to generate a genome-wide knockout resource for zebrafish | https://research.nhgri.nih.gov/ZInC/ |
| Zebrafish Neurophenome Project | Resource on neurobehavior and physiological data of adult zebrafish models | kaluefflab.com/znpindex.html |
| Bio-Atlas|Zebrafish Anatomy Atlas | Hematoxylin & eosin histological slides of zebrafish from embryonic to adult stages at anatomical resolution | Bio-atlas.psu.edu/zf/ |
| Sanger Zebrafish Genome Project | Zebrafish genome sequencing project | www.sanger.ac.uk/science/data/zebrafish-genome-project |
| DANIOCODE | Annotation of functional elements across the zebrafish genome | www.birmingham.ac.uk/generic/danio-code/index.aspx |
| CRISPR Design | Crispr design tool; scoring tool and calculation of off-target probabilities | crispr.mit.edu |
| CRISPRz | Database of validated CRISPR targets in zebrafish; database for methods and protocols related to the generation of zebrafish mutants | https://research.nhgri.nih.gov/CRISPRz/ |
| crisprScan | CRISPR design tool; scoring tool and calculation of off-target probabilities | www.crisprscan.org |
| BROAD Institute Genetic Perturbation Platform | Crispr design tool; gRNA efficiency scoring tool | www.broadinstitute.org/rnai/public/analysis-tools/sgrna-design |
| TALEN Design | TALEN and CRISPR design tool, including design of CRISPR Cas9 Nickase strategies | http://talendesign.org |
| Addgene | Repository for plasmids; distribution to academic laboratories only | www.addgene.org |
| BACPAC Resource Center | Repository for bacterial artificial chromosomes; not-for-profit distribution | http://Bacpac.chori.org |
| JOVE | Video-based protocols with a large number of protocols related to standard and specialized zebrafish procedures | www.jove.com |
II. THE GENETIC TOOLBOX FOR ZEBRAFISH RESEARCH
“What I cannot create, I do not understand.”
—Physicist Richard Feynman, 1918-1988
Biomedical researcher and entrepreneur Craig Venter coded this message into the DNA of a synthetic bacterium in a statement on the power of genome engineering (116). Biologists have developed a wide array of strategies for perturbing, and engineering, the genome; these tools are now a foundation to generate zebrafish disease models.
Easy breeding and injection of nucleic acids into the large, externally fertilized zygote make genetics in zebrafish relatively straightforward (Figure 2). A vast array of mutants, morphants, and transgenic animals in the literature attest to zebrafish’s genetic tractability (12, 22, 267, 349, 352, 392). To understand genomic engineering in zebrafish, it is important to understand the landscape of the zebrafish genome. First, zebrafish have a diploid genome. Second, zebrafish do not usually thrive when they are inbred, though isogenic lines have been generated. Third, teleosts underwent a whole-genome duplication event ~270 million years ago (150, 161). As a result, ~47% of human genes have a single zebrafish ortholog, while ~24% of human genes have more than one zebrafish ortholog (156). The remaining human genes have no recognized zebrafish ortholog to date, although improving sequence homology algorithms or focusing on functional homology over sequence homology should lead to recognition of more orthologs (Table 2).
Table 2.
Comparing technologies in zebrafish, mouse, and human
| Feature | Zebrafish | Mouse | Human |
|---|---|---|---|
| Genome size* | 1.4x109 bp | 2.7 × 109 bp | 3.0 × 109 bp |
| Genome assembly | High quality (GRCz10) | Very high quality (GRCm38) | Very high quality (GRCh38) |
| Number of genes* | 41,154; 26,373 protein coding | 46,062; 22,493 protein coding | 54,220; 20,433 protein coding |
| Genes shared with humans | 73% | 88% | 100% |
| Forward genetics | Established | Limited by cost and logistics | N/A |
| Reverse genetics | Established; TALEN, CRISPR/Cas9 | Established; homologous recombination, TALEN, CRISPR/Cas9 | N/A |
| Knockdown technologies | Established; easy-to-use, cost-efficient with morpholinos | Established; mostly viral delivery of RNAi constructs with variable efficiency depending on the tissue | Viral and nonviral methods feasible for some tissues as part of gene therapy strategies |
| Conditional alleles | Limited; difficult to insert Lox sites into genetic loci | Established; available for many cell types | N/A |
| Genome engineering | Established | Established | Proof-of-concept for correction of mutations in hematopoietic cells for human blood disorders; established for mutations in iPSC cells for in vitro studies |
| Cell culture | Limited: few cell lines available; possibility to culture different cell types | Cell lines; induced pluripotent stem cells; embryonic stem cells; primary cells well established | Cell lines; induced pluripotent stem cells; embryonic stem cells; primary cells well established |
| Antibodies | Limited | Broadly available | Broadly available |
| Imaging | Established; high-resolution in vivo imaging with standard confocal microscopy | Limited; in vivo imaging feasible at some stages and in some tissues, but requires highly sophisticated set-ups | Limited to clinical imaging and pathology |
| Physiology | Limited due to small organ size | Established | Established |
| Phenotypic small molecule screening | Established; large-scale screening in vivo | Limited to in vitro screening | Limited to in vitro screening |
Numbers were obtained from annotation releases z105, m106, and h108 of the NCBI Eukaryotic Genome Annotation Pipeline: https://www.ncbi.nlm.nih.gov/genome/annotation_euk/all/. The total number of genes includes coding, noncoding, and pseudogenes.
In some cases, multiple zebrafish orthologs confer a redundancy in gene function that can confound knockout models of human disease; a reported example is the redundant roles of zebrafish gata5 and gata6 in cardiomyocyte specification (151). In other cases, having multiple zebrafish orthologs for a single human gene has proven advantageous as they have evolved to have different tissue specificities. For example, knockout of the mouse sodium-calcium exchanger gene Ncx1 leads to embryonic lethality (49); in contrast, zebrafish have two ncx1 orthologs, one of which is heart specific, and so an ncx1 mutant was identified in a forward genetic screen for cardiac defects. Zebrafish was therefore a useful model to study the cardiac role of ncx1, showing its effect on cardiac calcium homeostasis, heart rhythm, and contraction (88, 197). Also, a whole-organism approach is necessary when the cause of cardiomyopathy is extracardiac, such as with high-output heart failure caused by anemia (114, 295, 407). The genetic tools most commonly used to manipulate the zebrafish genome are described in this section (Figure 3).
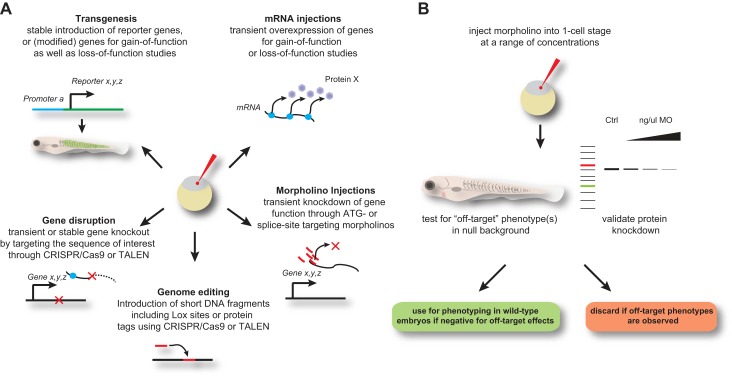
FIGURE 3.
Genome manipulations. A: overview of technologies to manipulate the zebrafish genome. B: guidelines for the correct use of morpholino (MO)-mediated gene knockdown technology. The current recommendation to identify a MO with minimal off-target effects is to test it in a null mutant background, as it should not induce any additional phenotypes.
A. Forward Genetics
Insights from forward genetic screens were one of the first demonstrations of the advantages of applying large-scale gene interrogation techniques to biological problems. One of the first researchers to use forward genetics was Thomas Hunt Morgan (1866–1945), who followed the segregation of spontaneous phenotypes in a population of inbred fruit flies and organized them on a genetic map (256). In the late 1970s and early 1980s, Christiane Nüsslein-Volhard and Eric Wieschaus revolutionized forward genetics in fruit flies by using chemical mutagens that induce random mutations within an organism to identify most genes involved in a specific developmental process, a method known as saturation screening (281). Saturation screens are based on two simple ingredients: an animal with a diploid genome which can be mutagenized in high numbers by mutagenic chemicals like N-ethyl-N-nitrosourea (ENU) or insertional mutagenesis with transposable elements or retroviruses, combined with a simple phenotypic readout. This powerful combination led to the discovery of virtually every gene regulating body plan segmentation in the fly, earning Nüsslein-Volhard and Wieschaus a Nobel Prize in 1995 (4, 280). Many successful screens were also carried out in models like yeast and worms (139, 278). Vertebrate forward genetics remained elusive, however, due to unsuitable breeding schemes (chicken), unsuitable genomic architecture (the allotetraploid Xenopus laevis), unsuitability for large-scale husbandry (mouse, chicken), or difficulty in scoring early developmental phenotypes (mouse).
1. Genetic screens in zebrafish
This situation changed fundamentally with the establishment of the zebrafish as a genetic model organism (379). Zebrafish embryos fulfill all genetic, logistical, and phenotypic criteria essential for a successful genetic screen, as mentioned above. Consequently, a wealth of zebrafish screens have identified mutants with highly interesting phenotypes (2, 44, 86, 297, 370). Intriguingly, even screens scoring fish-specific phenotypes have led to the discovery of human disease-associated genes, as fish and human organ systems rely on the function of similar cell types (271). Protocols for efficient zebrafish mutagenesis have been optimized over the years (324), reaching yields of one to two loss-of-function alleles per 1,000 screened genomes for average-sized genes, and show the feasibility of saturation screens if the phenotypic readout is chosen carefully. In other words, to find a potential loss-of-function allele, an average of 1,000 F1 germlines have to be screened. While the first genetic screens in zebrafish relied on relatively simple readouts like body axis formation, skin integrity or the establishment of circulation, the availability of transgenic lines which can serve as markers of specific cell types like microglia, pancreatic cells, blood or liver cells have greatly enhanced the resolution of structures accessible to forward genetic screens (297).
Phenotype-based forward genetic screens have specific advantages and disadvantages over reverse genetic approaches. A clear advantage is that any identified mutant in a phenotypic screen is by definition relevant to the developmental process under study. While there may be some bias in the genomic location of mutations especially in the case of insertional mutagenesis, forward genetic screens are by definition random and thus independent of hypothesis-driven decisions that can be based on wrong assumptions. Furthermore, forward genetic screens allow the identification of a range of alleles including hypomorphs, when null alleles can be lethal before the desired biological process can be studied (80). A challenge of forward genetic screens has been the need for efficient protocols to isolate the mutations causing the phenotypes. While in most cases these procedures have been relatively straightforward and as of late have become much more efficient, depending on the chromosomal location, cloning can pose a significant bottleneck (318, 372).
2. Positional cloning
Traditionally, cloning was performed using a large set of known simple sequence length polymorphism (SSLP) markers, or z markers, which were identified and mapped to the zebrafish genome. This work was further extended by the use of radiation hybrid mapping (112), leading to a countless number of precisely mapped SSLP markers. Briefly, mutant zebrafish are sorted by phenotype and subsequently genotyped with various SSLPs. Linkage of an SSLP to the mutation indicates physical proximity. Based on these results, a genetic map is constructed resulting in an interval that is progressively narrowed down to the location of the causal mutation. After genotyping between 1,500 and 2,000 animals and fine mapping, candidate genes within the small remaining interval can be sequenced to look for mutations. Fortunately, the z marker collection is extensive and can be accessed via ZFIN, the zebrafish model organism database. Many SSLP markers have also been mapped to the current assembly of the zebrafish genome (GRCz10) available at http://www.ensembl.org/. ZFIN, ENSEMBL, and other important databases and resources for zebrafish research are summarized in Table 1.
3. Advances in screening and cloning
Incorporating newer genomic techniques into forward genetic approaches has expanded their utility. For example, in contrast to chemical- or radiation-induced mutagenesis, insertional mutagenesis can generate loss-of-function alleles by insertion of a transgene that disrupts gene function (transgenesis will be discussed in detail below). The disrupted gene can usually be easily recovered by sequencing around the inserted DNA fragment, and insertional mutagenesis has been successfully established through the use of retroviral vectors (2, 109, 215) as well as the use of transposable elements (362). Insertional mutagenesis has been further developed with the use of a reporter transgene, which has facilitated the analysis not just of coding but also of noncoding regions such as enhancer elements (55, 76, 290, 310, 395). Insertional mutagenesis offers the significant advantage of rather straightforward identification of the insertion site, but insertional bias limits the number of targetable genes. Several protocols to identify the disrupted genes including inverse PCR and adapter ligation-based PCR have been established for zebrafish and other organisms (399).
While traditional mapping using polymorphic markers remains an important tool, next-generation sequencing technology now significantly facilitates the isolation of the phenotype causing mutations (32, 207, 249, 329). Mapping and identification of mutations affecting particular physiological or morphological processes using whole-genome sequencing (WGS) relies on the same basic principles as traditional protocols, namely, linkage of SNPs to a phenotype. The considerable size of the zebrafish genome in combination with a lack of isogenic lines initially delayed the establishment of WGS as a reliable tool to map mutations cost effectively. Progress in sequencing technology, multiplexing, and cost reduction eventually made WGS an efficient method to map mutations, which is now widely used in zebrafish research. Recent studies show that even multiplexing and low coverage WGS of DNA pools of phenotyped mutants allows direct identification or precise mapping of previously unknown causative mutations (32, 140, 207).
B. Tools for Reverse Genetics
1. Morpholino antisense technology
Zebrafish first gained prominence as a vertebrate model because of the successful forward genetic screens, but in recent years they have become an attractive model for reverse genetics as well (Figure 3). The global or tissue-specific expression of proteins with dominant negative function was the principal method of functional knockdown in the early 90s and remains an excellent tool for functional studies. By the late-1990s, however, antisense oligonucleotides joined the toolbox. These small, synthetic single-stranded DNA fragments hybridize to specific regions of an endogenous mRNA and are easy to use in zebrafish because they can be injected into embryos in a high-throughput fashion and at variable concentrations (Figure 3). The development of morpholino-modified oligonucleotides (simply called “morpholinos”) dramatically improved their stability against nucleases and degradation, so the use of morpholinos became the standard approach for gene knockdown in zebrafish (267).
Morpholinos work by hybridizing to a defined target sequence. However, they become diluted as cell divisions in the growing zebrafish embryo continue, mostly limiting their effectiveness to the first 2–3 days of development. Morpholinos are most effective when targeting the translational start site, thereby inhibiting protein synthesis. Other morpholinos are designed to target a splice acceptor or donor site, leading to exon skipping or intron retention which in turn can cause frameshifts, insertions, missense, or nonsense mutations within the target gene coding sequence. Translation-inhibiting and splice-targeting morpholinos each have their own advantages and disadvantages. The use of splice site morpholinos are by definition limited to targets that contain introns, and they have no effect on mature mRNAs (from maternal contribution for example). Therefore, morpholinos targeting the translational start site usually work more efficiently. On the other hand, evaluation of the effectiveness of translation-inhibiting morpholinos is significantly more difficult. The only valid test for translational inhibitors is to quantify the gene product, which is often problematic due to the lack of specific antibodies against zebrafish proteins. For splice-targeting morpholinos, quantification of mature versus mis-spliced mRNA by RT-PCR is easily done (Figure 3).
The specificity of morpholinos is a concern recently revived following direct comparison of phenotypes in morpholino-injected embryos/larvae versus genome-edited mutants generated via transcription activator-like effector nucleases (TALENs) or clustered regularly interspaced short palindromic repeats (CRISPRs) (188, 200, 277, 325). In most cases reported so far, there is a clear discrepancy between these phenotypes with the mutants exhibiting less severe phenotypes, leading to vigorous debate within the fish community, as well as others, as to whether morpholino knockdown of gene function should still be an accepted tool for gene perturbation (28, 201, 346, 371). The current recommendation to identify a morpholino with minimal off-target effects is to test it in a null mutant background as it should not induce any additional phenotypes (154, 325). Such an approach of course relies on the ability to generate and validate null alleles, for example, by whole-gene deletion (214), without disrupting other genes in the process. Once validated, morpholinos could be used to generate large numbers of embryos with reduced gene function facilitating downstream analyses.
C. Genome Editing
1. Zinc finger nucleases
As concerns about morpholinos’ off-target effects were becoming more acute, sequential advances in reverse genetics—genome editing with zinc finger nucleases (ZFNs), TALENs, and then CRISPRs—revolutionized the zebrafish genetic toolbox (Table 3). The age of genome editing in zebrafish started with a report that an artificial hybrid assembly of DNA-binding zinc finger modules, each recognizing a specific nucleotide triplet, could be coupled with the sequence-nonspecific nuclease domain of the restriction enzyme FokI to induce double-stranded breaks (DSBs) at specific loci of the zebrafish genome in vivo (84). The cellular repair machinery then recognizes the DSB as a substrate for the nonhomologous end joining (NHEJ) DNA repair pathway (400). As NHEJ is an efficient but error-prone system, DSBs often lead to faulty repair and the generation of small local insertions, deletions, or combination indels (84, 242).
Table 3.
Genetic toolbox for the generation of genetically modified zebrafish
| Tool | Application | Advantages | Disadvantages | Reference Nos. |
|---|---|---|---|---|
| Transgenesis tools | ||||
| I-SceI transgenesis | Genomic insertion of transgenic constructs | Low bias towards genomic position. Usually single insertion site. Stable expression. | Relatively low efficiency. Possible generation of concatemers. | 118, 368 |
| Tol2 transgenesis | Genomic insertion of transgenic constructs | Bias towards open chromatin (see gene trap). High efficiency. No concatemers. | Commonly insertion into several loci. | 193, 387 |
| BAC transgenesis | Generation of transgenic lines | Independence from identification of regulatory elements. High chance of endogenous expression pattern. | Very low efficiency in the transgenesis step due to size of the transgene (usually ~150 kb). | 40, 108 |
| Gene trap | Generation of transgenic reporter lines; forward genetic screening | Use of endogenous regulatory elements. Fusion proteins. Generation of mutants | Lack of site specificity. | 12, 56, 395 |
| Gene knockout tools | ||||
| TILLING | Reverse genetics | Can be combined with forward genetic screening. Generates single nucleotide polymorphisms. | Cost intensive and laborious. Possibly outdated with the availability of TALENs and CRISPRs. | 177, 254, 375, 424 |
| Zinc finger nucleases | Reverse genetics | Very high sequence specificity due to two proximal recognition sequences. | Time-consuming assembly and testing. Became outdated with the availability of TALENs and CRISPRs. | 104 |
| TALEN | Reverse genetics | Very high sequence specificity due to two proximal recognition sequences. Highly efficient. Design tools available. | Somewhat time-consuming assembly (4–5 days). Maintenance of modular assembly library. Risk of library cross-contamination. | 22, 43, 83 |
| CRISPR | Reverse genetics | High sequence specificity. Highly efficient. Very simple and rapid assembly (2 days). | Possibly higher risk for off-target lesions compared with TALEN’s or Zinc Finger Nucleases. | 157, 167, 390 |
| Gene knockdown | ||||
| Morpholinos | Transient gene knockdown | Highly established, easy to use, inhibition of target translation or splicing. | Need to validate sequence and amount of morpholino by injecting mutant embryos. | 188, 267, 325 |
| CRISPRi | Transient gene knockdown | Easy to use. | Not widely used. Not highly efficient. Possibility of off-target effects. | 220, 325 |
| Forward genetic screening | ||||
| ENU mutagenesis | Forward genetics/tilling | Mutagenizes during S-phase with no positional bias. Allows the induction of hypomorphic mutations. | Demands mapping efforts to identify the mutation causative of the observed phenotype. | 297, 324 |
| Insertional mutagenesis | Forward genetics/gene trap | Insertion site relatively easy to identify. Generates reporter lines. | Bias towards euchromatic regions does not allow saturation screens. | 265 |
| Genome editing tools | ||||
| TALEN | Site-directed genome editing (gene tagging, reporter insertion, generation of large deletions) | Very high sequence specificity. | Somewhat time consuming assembly (4–5 days). Maintenance of modular assembly library. Risk of library cross-contamination. | 22, 152 |
| CRISPR | Site-directed genome editing (gene tagging, reporter insertion, generation of large deletions) | High sequence specificity. | Site specificity not always a given due to potential lack of a guide RNA target sequence. | 14, 145, 164 |
| Wild-type lines | ||||
| AB | Wild-type strain | Highly inbred, cleared from background mutations, widely used. | Low rate of polymorphisms but not isogenic. | |
| TÜ | Wild-type strain | Highly inbred, widely used. Original reference genome strain. | Medium amount of polymorphisms. | |
| WIK | Wild-type strain | Highly polymorphic wild strain used for mapping purposes. | Wild strain, normally not used for experimental work other than recombination mapping. |
A significant drawback in the use of ZFNs is that the zinc finger modules are bulky and interfere with each other, creating difficulty in predicting their binding affinities to a given DNA sequence. Consequently, ZFN assembly and selection demand screening assays to identify working assemblies, which then need to be tested for specificity. While these methods significantly improved over time (229, 332) and mutant alleles for several genes were successfully created using ZFNs in zebrafish, the complicated and time-consuming process of making ZFNs prevented their widespread adoption. Nevertheless, two important lessons were learned: first, modular DNA-binding peptides could be combined with FokI to create sequence-specific DNA scissors. Second, it was possible to induce site-specific DNA mutations in zebrafish through error-prone nonhomologous NHEJ repair of DSBs. Two new technologies incorporating these lessons soon appeared on the horizon.
2. TALENs
Only shortly after the first reports of ZFN activity in zebrafish, a group working on the bacterial plant pathogen Xanthomonas was able to break the code for the modular DNA-binding domain of the transcription activator-like effectors (TALEs), a group of type III secreted virulence factors that Xanthomonas uses to modulate its hosts’ gene expression program (29). Unlike zinc fingers, each individual TALE module, called a repeat variable diresidue (RVD), recognizes a single nucleotide and does not influence other RVDs’ affinity, allowing highly predictable sequence-specific assemblies of RVDs to target a DNA sequence of interest. The knowledge obtained from ZFNs now paved the way for the first highly efficient, yet easy to use genome-editing tool for zebrafish: TALE-nucleases, or TALENs. A TALEN consists of two sequences of RVDs each coupled to a FokI nuclease; each of these RVD “arms” binds to the same target DNA sequence to provide specificity, while FokI induces DSBs.
Major efforts have led to clear and robust design guidelines for TALENs which are available online and do not demand expert knowledge (43, 83, 250, 263) (Table 3). Clever and cost efficient molecular cloning and assembly approaches have made it possible to assemble the two DNA-binding arms each consisting of 17–21 nucleotide-specific RVDs and the FokI nuclease rapidly and in large quantities (43, 321). Additionally, several good methods have been established to test TALENs for their efficiency and to genotype embryos with indel mutations (69, 386, 441).
3. CRISPR/Cas9
The discovery of the CRISPR/Cas9 system changed the game once again as it made genome editing and reverse genetics in zebrafish easier than ever before. Clustered regularly interspaced short palindromic repeats (CRISPRs), together with their associated proteins (Cas), help bacteria recognize and cleave foreign DNA and were therefore adapted to create DNA scissors for any sequence of interest. The general principle of the CRISPR/Cas9 system has already been reviewed many times (82, 158, 231, 405, 423), and thus will not be discussed here in detail. In short, CRISPR/Cas9 is a two-component system that recognizes specific DNA targets by hybridization of a guide RNA (gRNA) and induces DSBs through the nuclease activity of the Cas9 protein.
Unlike ZFNs and TALENs for which creating the DNA-binding part of the scissors is a laborious assembly of zinc fingers or RVDs, target specificity for CRISPR/Cas relies on a single gRNA of ~20 nucleotides in length. Therefore, for each desired DNA target, only a sequence of the length of a PCR primer needs to be cloned while the rest of the system remains identical. Due to its remarkable simplicity and adaptability, the CRISPR/Cas9 system rapidly gained popularity and can now be considered the genome-editing tool of choice for most zebrafish researchers. Like TALENs, CRISPRs can be modified not just to cut DNA, but also to inhibit or activate gene transcription (333). CRISPRs have enabled single laboratories to carry out larger-scale knockouts of several genes at a time, for example, to dissect a genetic pathway (352), to generate mutants from genomic regions of interest (318), or to investigate candidate mutations identified through next-generation sequencing of human disease samples (110, 300). CRISPRs have accelerated existing collaborative efforts to systematically knock out every zebrafish gene (177). Of all the genome-editing technologies, it is the speed and ease of CRISPR that is allowing genome editing at a “high-throughput” pace, a pace that the zebrafish model is well-suited to match.
4. Challenges with CRISPR and other genome editing technologies
Despite CRISPRs’ ease of use, there are still some drawbacks, partially shared by the other genome editing technologies. Cas9 nuclease activity depends on the protospacer adjacent motif (PAM) immediately following the sequence targeted by the guide RNA, thereby often limiting potential target recognition sites. TALENs offer an alternative way to cleave DNA sequences not targetable by CRISPRs, and for this reason, may not become entirely obsolete. This consideration becomes particularly relevant when specific alleles (e.g., phosphorylation sites, DNA binding domains,…) or AT-rich regions need to be targeted for mutagenesis. Statistically, CRISPRs’ short DNA target sequences are present in multiple places in the zebrafish genome, raising concern for off-target mutations (428), whereas TALENs with their two-component recognition offer higher stringency. Finally, NHEJ creates an unpredictable array of alleles that subsequently need to be outbred and screened in what can be a time-consuming process. While NHEJ will likely produce a useful missense or nonsense allele, it is hard to control the kind of mutation generated (see below for the use of oligonucleotides to this end).
Each of these challenges is being addressed. CRISPR design and strategies to mitigate off-target effects are improving (128, 354), and the accepted standards for proving the effect of a mutation now include careful evaluation including RNA and protein expression analyses, as well as functional readouts (Figure 4). Generation of specific mutations has also improved and is most frequently achieved by co-injecting a CRISPR with a template oligonucleotide that cells can use to repair the DNA by homologous recombination rather than NHEJ. This template contains a specific mutation of interest, for example, a specific point mutation, or an epitope tag (8). The ability to generate specific mutations is very important for disease modeling, when disease can hinge on a single point mutation, and different mutations even within the same gene can cause different phenotypes.
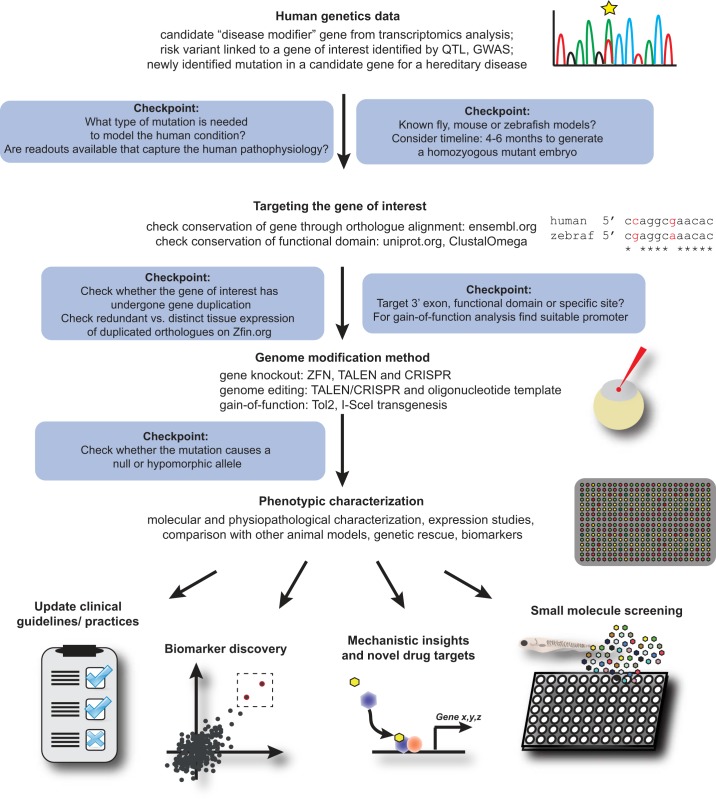
FIGURE 4.
Overview of decision roadmap to model genetic disease in zebrafish. Complex and monogenic human diseases can be modeled in zebrafish using different strategies, including genome editing, morpholinos, or transgenic technologies. Several considerations have to be made such as the conservation of genes between zebrafish and human, the timeline of generating genetically modified zebrafish, existence of more suitable animal models, and the availability of meaningful assays to match phenotypic findings to clinical symptoms.
For CRISPRs as for the other genome-editing technologies, when the sequence of interest allows a choice of where to target the DSB, it is important to target the exon that gives the best likelihood of causing a null allele. Targeting exon 1 carries the danger that an alternative start codon is used, thereby leading to a functional protein, whereas out-of-frame mutations in later exons can lead to exon-skipping, sometimes preserving protein function. The general recommendation therefore is to consider functional domains and alternative translational start sites, as well as the potential for nonsense-mediated decay of the mutant mRNA (Figure 4). Deleting large parts of a gene’s coding sequence is also an option, although one has to consider potential regulatory elements in introns as well as protein(s) and RNA(s) encoded by the reverse strand.
C. Tools for Transgenesis
Key for many zebrafish studies is the ability to generate stable transgenic lines that express genes (e.g., fluorescent marker genes) under specific regulatory elements to perform insertional mutagenesis-based screens, to drive the conditional expression of specific genes, and to design complex transgenic strategies for drug discovery or toxicology. Protocols to stably introduce foreign DNA into the zebrafish genome were pioneered by the Westerfield laboratory in the late 1980s, generating the first transgenic zebrafish that transmitted a bacterial plasmid DNA to their progeny after injecting linearized DNA into the cytoplasm of a fertilized egg (380). Southern Blot analysis revealed that the injection of linear DNA alone was sufficient to achieve high amounts of mosaic somatic transgenesis, and germline transmission was observed in up to 15% of the injected animals (380). The expression of genes from transgenic cassettes, however, turned out not to always occur, probably as a result of rearrangements of the foreign DNA into various types of tandem repeats that potentially induced transcriptional silencing and antisense expression (65). These problems were overcome by the establishment of a protocol that included the flanking of the transgenic cassette with the 18-bp recognition site for the i-Sce meganuclease enzyme. Co-injecting this rare-cutting nuclease with the cassette-carrying plasmid linearizes the circular DNA within the cell and effectively reduces the chance of concatemerization before genome integration. This trick also significantly increased transgenesis efficiency by increasing the integration of low-molecular-weight DNA (392). The use of i-Sce meganuclease has since become widely adopted to generate transgenic zebrafish lines and was further optimized into a standardized protocol (368).
A second method for efficient transgenesis in zebrafish came from the identification and characterization of a hAT transposable element in the Japanese rice fish Medaka (Oryzias latipes). Medaka and zebrafish, distant cousins within the teleost branch, do not share the hAT element which made it ideal as a vector to allow transposon-based transgenesis in zebrafish without inducing undesired mobilization of endogenous transposable elements (356). The basic principle of this system is the separation of the cis transposable elements called long terminal repeats (LTRs) from the transposase enzyme. The transposase is transcribed in vitro into mRNA, and the cis regulatory elements are used to flank the transgenesis cassette in a circular plasmid. Co-injection leads to very high transgenesis rates, which frequently translate into high germline transmission (175). With the use of this Tol2 system, up to 40% of the injected fish end up having one or more insertions in their germline. Due to the mechanisms of transposon-mediated DNA insertion, plasmid backbones are only rarely integrated using this method. Additionally, Tol2-mediated transgenesis typically results in single insertions per locus, thereby avoiding the formation of concatemers. On the downside, single insertions usually express somewhat lower amounts of the transgene, and transposon-mediated transgenesis has an intrinsic bias for uncondensed chromatin, raising the chances to integrate into gene-harboring loci (191, 210).
With the establishment of meganuclease- and transposon-based protocols, the high transgenesis efficiency has made this technology accessible even to small zebrafish facilities that can quickly develop transgenic lines suited for their research. The limiting factor today is the availability of cloned regulatory elements, enhancers or promoters, to drive time- and tissue-specific expression of genes of interest. The standard way of identifying regulatory elements has been to use PCR to amplify regions located 5′ to the transcriptional start site of the gene of interest, but even since the emergence of promoter prediction algorithms, as well as more powerful techniques to identify open chromatin regions such as ATAC-seq, choosing and testing fragments of different sizes can be time-consuming, laborious, and risky. Another strategy to drive gene expression in specific patterns arose from the availability of bacterial artificial chromosome (BAC) libraries. BACs, each containing up to 300 kb of genomic DNA, were originally developed to aid in genome mapping, sequencing, and assembly. In a landmark paper published in 1997, it was shown that the large genomic fragments carried in BACs could be manipulated in bacteria through homologous recombination (431). This finding was adapted to insert a gene of interest at the ATG start codon of an endogenous gene within a BAC, preserving the regulatory sequences without having to know their location, and then integrate the modified BAC into the zebrafish genome. Optimization of BAC transgenesis protocols has led to highly accurate, simple, and efficient methods (40).
III. DISEASE MODELS
“For a large number of problems there will be some animal of choice or a few such animals on which it can be [most] conveniently studied.”
—Physiologist August Krogh, 1874-1949
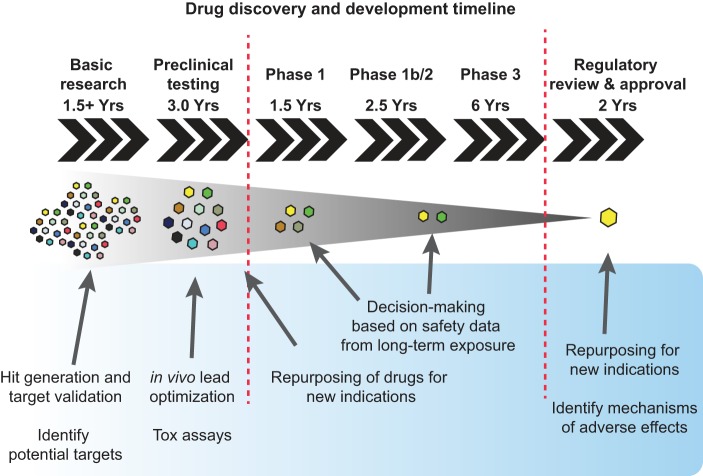
This Nobel Prize winner knew that a good disease model accurately predicts how perturbing a signaling pathway by genetic, nutritional, or pharmacological means affects human disease outcome. To date, mice and rats have been most commonly used to translate scientific knowledge into clinical predictions, which may then be proven or disproven in human clinical trials. Nevertheless, high-impact rodent studies have had translational success only ~30% of the time (from a median citation count of 889), and only 10% of these successful cases eventually led to a marketed drug (402). The reasons for this low translational efficiency are certainly manyfold and not all solved by using alternative animal models. However, using other model organisms like Caenorhabditis elegans, Drosophila melanogaster, and zebrafish has helped overcome several methodological shortcomings common with rodent models (342), such as underpowered statistics due to high costs and laborious manipulation (402), inbred strains that do not reflect natural genetic diversity (6), as well as frequent lack of randomization and blinding in research design (23, 106, 236, 402). Zebrafish can fill some of the gaps in current animal experimentation. In this section, we review paradigmatic examples of disease models that have been described in zebrafish and how these models have contributed to our understanding of disease mechanisms (Figure 5 AND Tables 4–7).
FIGURE 5.
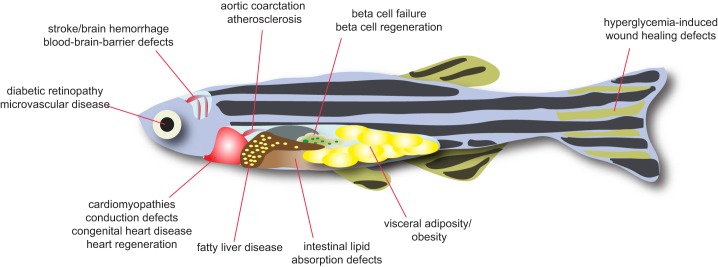
Cardiovascular and metabolic diseases studied in zebrafish. Zebrafish have a similar anatomy as that of higher vertebrates. Tissue-intrinsic pathological processes can be studied for a broad range of human cardiovascular and metabolic diseases.
Table 4.
Zebrafish models of cardiovascular disease
| Model | Phenotype | Key Findings Reported | Reference Nos. |
|---|---|---|---|
| Cardiomyopathy | |||
| tnnt2a mutant; tnnt2a knockdown | Sarcomere assembly defects | Tnnt2 is essential for myocardial sarcomere assembly. Blood flow is essential for ventricular trabeculation. Hemodynamics regulate Notch activity in the endocardium. | 73, 301, 350 |
| Inducible myocardial expression PKGB 2057del2 | Arrhythmogenic cardiomyopathy (ACM) | Model was used in small compound screen leading to the identification of a drug that reduced ACM in mouse models and human patients iPSC-derived cardiomyocytes. | 13 |
| nexilin morphant/overexpression of a pathogenic human NXN allele | Z-disk abnormalities | Nexilin is a component of Z-disks and is essential for Z-disk stability and function. Nexilin mutations associated with dilated cardiomyopathy in humans. | 133 |
| erbb2 mutant, expression of dnErbb2 | Contractility, trabeculation | Erbb2 is a regulator of myofibril localization and bundling. Erbb2 regulates trabeculation through cell shape changes. Erbb2 is a regulator of cardiomyocyte proliferation. | 218, 317, 374 |
| Cardiac expression of an atrial fibrillation associated MYL4 p.Glu11Lys allele | Disruption of sarcomeric structure, atrial enlargement, and electrical abnormalities | Pathogenic mutations in structural components of the contractile machinery can cause subtypes of atrial fibrillation. | 289 |
| myl3 mutant | Cardiac insufficiency, haploinsuffiency, and stress sensitivity | Essential light chain S195 phosphorylation is essential to adapt cardiac contractility under cardiac stress. | 337 |
| desma mutant | Desminopathy | Loss of desmin and desmin aggregates cause excitation-contraction coupling defects and reduction of cardiac function. Inhibition of desmin aggregation can restore cardiac function. | 311 |
| plcg1 mutant | Progressive loss of cardiac contractility | VEGF signaling through PLCgamma1 modulates cardiac contractility. | 326 |
| Injection of human amyloid light-chain proteins into circulation | Amyloidosis | Circulating human amyloid light-chain proteins induce cardiomyopathy in zebrafish through cardiomyocyte death in a p38 MAPK dependent pathway. Autophagic flux is critical for the induction of mitochondrial dysfunction and development of amyloid cardiomyopathy. | 121, 253 |
| ttna mutants | Low contractility | Analysis of different loss-of-function alleles led to identification of a conserved COOH-terminal internal promoter that is able to restore expression of a truncated, but functional Titin protein. This finding may explain the stronger phenotype of human mutations affecting the COOH-terminal vs the NH2-terminal regions. | 444 |
| slc4a1a mutant | Severe anemia, chronic cardiac overload | Chronic cardiac overload induces cardiomegaly through hypertrophy and hyperplasia. Haploinsufficiency of TOR is cardioprotective and attenuates cardiomyopathy in zebrafish. Wnt/β-catenin signaling modulates cardiomyocyte proliferation and hypertrophy. | 78, 148, 384 |
| Bolus of doxorubicin and various zebrafish lines derived from an insertional mutagenesis screen affecting heart function | Endoplasmic reticulum stress and reduced survival | dnajb6b is a susceptibility gene for doxorubicin-induced cardiac myopathy. Ectopic expression of the gene protects against cardiac damage in zebrafish and mouse. | 77 |
Table 7.
Computational survey of cardiometabolic diseases modeled in zebrafish
| Disease Class | Disease Examples | Number of Citations | Number of Genes | First Citation Year | Representative Genes |
|---|---|---|---|---|---|
| Cardiomyopathy/heart Failure | Dilated, hypertrophic, restrictive, amyloid, noncompaction, arrhythmogenic, toxic, muscular dystrophy-associated | 121 | 105 | 1999 | ERBB2, EYA4, HAND1, JUP, MYH7, TCAP, TNNT2, TTN |
| Arrhythmia | Long QT syndrome, short QT syndrome, Brugada syndrome, sick sinus syndrome, atrial fibrillation, other conduction disease | 138 | 91 | 2000 | GREM2, KCNH2, KCNK1, KCNQ1, MYL4, PITX2, SCN5A, TBX5 |
| Myocardial injury and regeneration | Myocardial injury, cardiac regeneration | 144 | 62 | 2002 | ALDH1, CAV1, ERBB2, GATA4, IGF1R, NFKB, NRG1, WT1 |
| Structural and congenital heart disease | Ciliopathies, heterotaxy, RASopathies, cohesinopathies, mitral valve prolapse, endocardial cushion defects, septal defects, diGeorge syndrome, tetralogy of Fallot | 74 | 97 | 1999 | A2ML1, BBS, CEP290, DCHS1, DHAND, MEF2C, NIPBL, NPHP4, PTPN11, RAD21, RAF1, RIT1, TBX1 |
| Rare congenital syndromes | CHARGE syndrome, congenital diaphragmatic hernia, Holt-Oram syndrome, hypoplastic left heart syndrome, orphan congenital syndromes | 114 | 157 | 1999 | BMP4, CHD7, FOXH1, GATA4, SAL4, SOX10, TBX1, TBX5, TBX20 |
| Vascular disease | Cerebrovascular disease, atherosclerosis, fibromuscular dysplasia, aortic and branchial arch abnormalities, pulmonary hypertension, hereditary hemorrhagic telangectasia, other vascular disease | 76 | 118 | 1996 | BARX1/2, CXCR4, DLL4, EDNRA, EPHB4, FGF8, FLT4, GATA1, GATA2, HAND2, NOTCH, RNF213, SOX18, VEGF |
| Diabetes, obesity, insulin resistance | Diabetes, obesity, insulin resistance | 247 | 300 | 1996 | AHCY, BBS, FABP, FTO, GCG, INS, LEP, MTOR, PCK1, PDX1, POMC, PPARG, PTF1A, SLC16A6 |
| Lipid metabolism | Hepatic steatosis, hyperlipidemia, hypertriglyceridemia | 167 | 239 | 1974 | APOB, APOE, ATF6, CETP, FASN, HMGCR, INS, LDLR, SCAP |
| Inborn errors of metabolism | Enzyme deficiencies, mitochondrial deficiencies, hemochromatosis, Barth syndrome, hereditary spastic paraplegia | 121 | 176 | 2000 | ALAS2, ETFA/B, FKRP, GLRX5, HFE, KIF, SPAST, TAZ |
A custom Python script was written to search MEDLINE and return number of citations, PMIDs, and genes listed in the titles or abstracts as search results. Searches were specifically designed for zebrafish models of human disease, PMIDs returned by the Python script were entered into https://www.ncbi.nlm.nih.gov/pubmed/ and the corresponding abstracts were validated for relevance.
A. Cardiovascular Disease Models
1. Advantages and disadvantages of the zebrafish cardiovascular system for disease modeling
Zebrafish hold specific anatomic and physiological advantages and disadvantages that are important for cardiovascular disease modeling. Like the human heart, the zebrafish heart develops from a linear heart tube formed by fusion of mesodermal progenitors from a primary heart field (192, 433). The heart tube begins beating via peristaltic motion and loops to form separate atrial and ventricular chambers (317, 373). Cells from the secondary heart field, responsible for the right ventricle and right ventricular outflow tract in mammals, further contribute to ventricular growth (203) (Figure 6). The atrium and ventricle initially have thin myocardial walls comprising a single cell layer, but soon the ventricle undergoes a process of trabeculation in which clusters of cardiomyocytes of the wall delaminate towards the cardiac lumen to form sheetlike projections, a process which is conserved among higher vertebrates (218). Notably, zebrafish hearts do not septate and do not undergo significant myocardial compaction after trabeculation.
FIGURE 6.
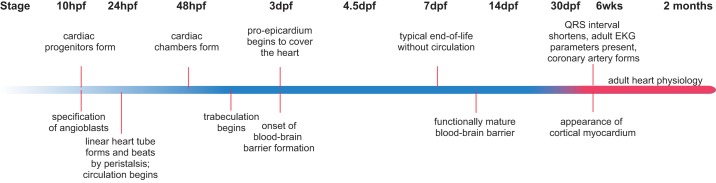
Development of cardiovascular physiology. Cardiac peristalsis and blood circulation can be detected by 24 hpf, making the zebrafish a prime model to study early events of cardiovascular development and physiology. The full complement of anatomical and physiological characteristics of an adult heart develops gradually and is considered to be complete by around 6 wk post fertilization. Analogous to cardiac development, the different cell types of the vascular system develop progressively; for example, a fully mature blood-brain barrier is not established before 14 dpf.
At a border specified by chamber-specific gene expression patterns, atrioventricular and aortic valves form from endocardial cushions by endothelial-to-mesenchymal transition, in a process driven by hemodynamic as well as genetic factors (24, 162, 301, 411). Finally, in juvenile stages ~6 wk post fertilization (wpf), trabecular cardiomyocytes give rise to a new layer of cortical myocardium that surrounds the heart (122). The zebrafish heart is innervated by sympathetic and parasympathetic nerve fibers (377), with cardiac conduction beginning in the sinoatrial node, pausing at the atrioventricular junction, and then speeding through the ventricle resulting in apex-to-base contraction that ejects blood from the heart (47, 246). Unlike murine models, heart rate and EKG parameters representing atrial, atrioventricular, and ventricular depolarization as well as ventricular repolarization are similar to those of humans; for example, heart rate in zebrafish is similar to the 60–100 beats/min in humans, while murine heart rate is 600 beats/min. However, zebrafish lack a specialized His-Purkinje system (9, 246, 289). Zebrafish hearts can also regenerate (179, 230, 307, 413). Physiological assays have been developed for zebrafish hearts including exercise, EKG, telemetry, cardiac output and ejection fraction measurements, and ultrasound, as well as drug screening and drug-inducible gene expression (284, 382, 438). Together, these characteristics make zebrafish an excellent model to study early heart development, arrhythmia, and myocardial function both organ-wide and at high resolution (36, 344, 373).
The zebrafish vascular system also lends itself to disease modeling offering superb visualization of vascular development (Figure 6). In a process termed vasculogenesis, endothelial progenitors first migrate to form a cord by 20 hpf (18, 137, 142, 202, 372); by 24 hpf, the single vascular cord has given way to a dorsal aorta and cardinal vein, and circulation begins. Angiogenesis then initiates, first sprouting new intersegmental vessels from the dorsal aorta and then the cardinal vein (137, 141, 202, 360). During vasculogenesis and angiogenesis, arterial and venous fates are specified and aortic arch vessels are defined (138, 141, 202).
Organ-specific vasculature develops at different stages. For example, the blood-brain barrier develops during larval stages, and the zebrafish has been used to study blood-brain barrier permeability to various drugs and infectious agents (92, 168, 403). Coronary vessels develop much later, several weeks post fertilization, as the zebrafish ventricle has grown significantly since hatching. These vessels arise from angiogenic sprouting and migration of endothelial cells (132, 141). Finally, zebrafish have been used as a highly accessible organism to model vessel injury and inflammation (57), and zebrafish fed a high-fat diet develop lipid depositions in their arteries, creating an in vivo model of atherosclerosis important for studying coronary artery disease as will be covered later (97, 98, 376).
2. Cardiomyopathy
One might expect the most highly conserved cardiac functions across vertebrates to be at the cell level. Indeed, some of the first zebrafish heart disease models focused on cell-autonomous cardiomyocyte defects. In 2002, mutations in genes encoding two sarcomeric proteins, Titin and Troponin, were reported to cause embryonic lethal cardiomyopathies (350, 370, 429). Linkage analyses in human families implicated these genes in dilated and hypertrophic cardiomyopathy around the same time (184, 361, 393, 415), followed by several years of dual discovery of various sarcomeric cardiomyopathy mutations in human families and zebrafish forward genetics screens (17, 21, 149, 364, 430). In fact, modern transcriptome analyses show that ~96% of genes associated with human cardiomyopathy are expressed in the zebrafish heart (355).
Reduced cardiac contractility and resultant pericardial edema are clearly scorable in zebrafish larvae, so zebrafish became an easy way to test the pathogenicity of novel variants found in human cardiomyopathy patients, mostly using morpholino gene knockdown. Genes associated with cardiomyopathy soon expanded from those encoding sarcomeric proteins to transcription factors and mitochondrial, transmembrane, and other regulatory proteins (17, 316, 345, 436) (Table 4). Several genes governing cell-cell connections, causing arrhythmogenic and noncompaction cardiomyopathies when defective, were also investigated in zebrafish (133, 186, 235, 257). Morpholinos were used as a quick-and-dirty validation of in silico predictions of mutations causing dilated cardiomyopathy (74). While one might argue that cell-autonomous causes of cardiomyopathy may be just as readily studied in cell culture, zebrafish models provided an organism-wide readout of the various phenotypes caused; for example, tax1bp3 causes both cardiomyopathy and cranial abnormalities (345). Also, a whole-organism approach is necessary when the cause of cardiomyopathy is extracardiac, such as with high-output heart failure caused by anemia (384).
More recent studies are moving past pericardial edema phenotyping and morpholino gene knockdown to provide a more sophisticated analysis of mechanisms behind several cardiomyopathies. For example, Zou et al. (444) took advantage of the ease of CRISPR technology in zebrafish to create several different titin mutations, showing that an internal titin promoter may provide partial rescue of some mutant proteins but not others, perhaps explaining some of the range of clinical severity observed in different titin-associated cardiomyopathies. Several assays for larval and adult zebrafish have emerged to parse phenotypes more complex than embryonic lethal pericardial edema, and these studies now define a new standard in zebrafish heart failure phenotyping (9, 36, 47, 135, 147, 219, 244, 382). Examples of cardiomyopathies in adult zebrafish include genetically, anemia-, and chemically induced models (13, 76, 78, 384). In a study that exemplifies well the advantage of combining high-resolution imaging with genetic models, Reischauer et al. (317) created a novel transgenic line affinity tagging cardiac actin to show myofibril assembly and architecture during early heart development. Using a dominant-negative erbb2 transgene, the authors created a model of adult cardiomyopathy in zebrafish. They then used genetic and chemical manipulations of the Erbb2 pathway, associated with chemotherapy-induced cardiomyopathy and of major interest as a therapeutic target, to show that Erbb2 inhibition modulates myofibril organization on a cellular level (317). A recent study by Ding et al. (77) further shows the powerful translational potential towards mammalian cardiomyopathy models: From a transposon-based insertional mutagenesis screen, the authors identified 44 zebrafish lines with cardiac expression of a fluorescent protein indicating genetic lesions at loci that may regulate heart function. Using these lines the authors performed a modifier screen of doxorubicin-induced cardiomyopathy, which pointed to a long isoform of dnajb6b that, when disrupted, dramatically increases the susceptibility of adult zebrafish to cardiac damage. In contrast, overexpression of the long isoforms of dnajb6/DNAJB6 in zebrafish and mice showed to be protective against doxorubicin-induced cardiac myopathy and extended the survival of the animals. Furthermore, ectopic expression of rare DNAJB6 variants from human cardiomyopathy patients reduced survival in doxorubicin-treated zebrafish (77).
3. Cardiac conduction
Zebrafish models also added new dimensions to the study of cardiac electrophysiology (9, 47, 88, 414) (see Figure 7). One of the most powerful examples of this contribution involves the human delayed rectifier potassium channel HERG, defects in which are responsible for Long QT Syndrome Type 2 (LQT2). A range of mutations as well as drugs can prolong the QT interval, creating risk for lethal arrhythmias, and every drug approved by the United States Food and Drug Administration (FDA) requires testing for its effects on the QT interval. Therefore, HERG was already the subject of intense study in in vitro and animal models. Mutations in the mouse ortholog, however, had failed to recapitulate human disease phenotypes. Hence, researchers studied LQT2 in larger animal models that were more difficult to maintain and less genetically tractable.
FIGURE 7.
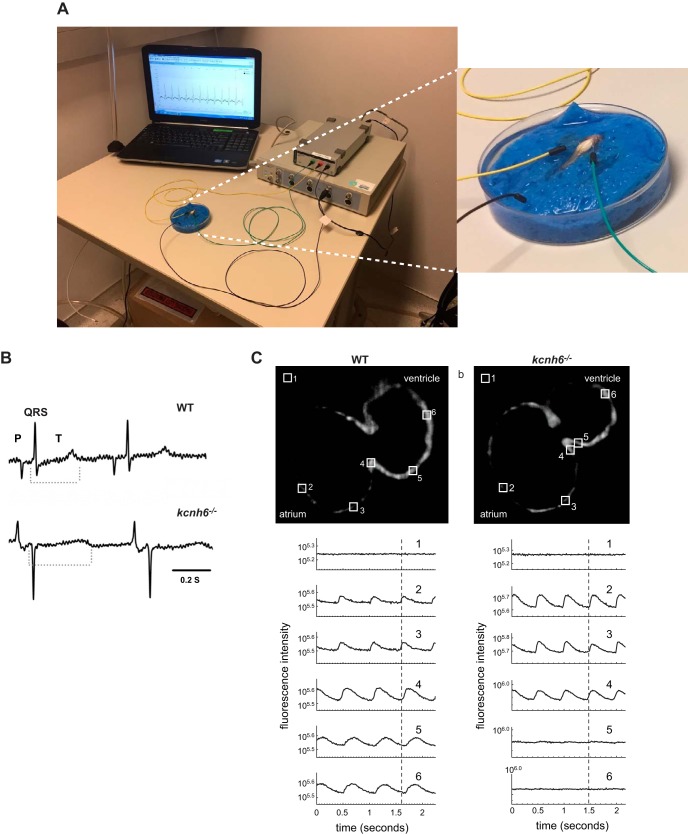
Modeling and phenotyping of cardiac disease. A: schematic of adult zebrafish EKG measurement. An anesthetized zebrafish is positioned ventral side up on a damp sponge; a gill perfusion pump may be used. Needle electrodes are positioned under the skin, and the EKG signal is amplified, filtered, and acquired using standard equipment (246). B: the zebrafish model for Long QT Syndrome Type 2 (LQT2) has a clinically relevant phenotype. Human LQT2 mutations are autosomal dominant and cause a long QT interval on EKG. Analogously, heterozygous kcnh6 adults also demonstrate prolonged QT interval compared with wild-type siblings. C: homozygous kcnh6−/− embryos are amenable to detailed phenotyping, showing absence of ventricular conduction by optical mapping. Wild-type and mutant embryos were crossed into the Tg(myl7:gCaMP) fluorescent calcium sensor transgenic background, and imaged using selective plane illumination microscopy. Hearts were stopped to facilitate imaging of conduction. Fluorescence intensity over time was plotted from several regions of interest from the embryos’ atrium and ventricle, showing that cyclic fluorescence, and therefore conduction, was absent in the mutant ventricle. This observation was confirmed by patch-clamping the embryonic hearts. [B and C from Arnaout et al. (9).]
Zebrafish forward genetic screens identified mutants with 2:1 heart block and a silent ventricle; it was soon realized that these different phenotypes all mapped to the HERG zebrafish ortholog kcnh6 (9, 198). The zebrafish LQT2 model proved to hold several advantages in the study of QT prolongation. First, while homozygous mutants exhibited the severe phenotypes mentioned, more careful phenotyping showed that heterozygotes, or wild-type fish treated with QT-prolonging drugs, manifested the same clinical phenotype as humans: a long QT interval on EKG (9, 246). While zebrafish can demonstrate a clinical long QT phenotype, the model can also be designed to show an easily scorable, mechanism-based, binary phenotype of 2:1 heart block—a “high-throughput” phenotype for a clinically relevant disease (196). With the use of zebrafish embryos and this rapid phenotyping, several drug screens were performed validating the zebrafish model’s response to many known QT-prolonging drugs, finding new genes involved in repolarization, and identifying possible drug therapies for long-QT syndrome (9, 247, 248, 298, 420). The genetic tractability of the zebrafish LQT model, in contrast to the dog and rabbit models, also allowed a more systematic search for genetic modifiers to the LQT phenotype to help explain the variability in penetrance observed clinically (26, 247).
The kcnh6 example also illustrates an important lesson about designing phenotypic readouts in zebrafish disease models. An ideal zebrafish model has a documented, clinically relevant phenotype: in this case, long QT on EKG. An alternate, high-throughput phenotype for the same model is also highly desirable, but must be based on the specific disease mechanism at hand, in the way that 2:1 heart block is a mechanistically based high-throughput phenotype for long QT syndrome, while pericardial edema is a not a specific phenotype and should not be relied upon.
Second, studying Kcnh6 and other ion channels in zebrafish has demonstrated a new role for cardiac conduction in structural heart development, at embryonic times when mammalian studies would have simply shown in utero death (9, 45, 46). Similarly, zebrafish were used to study the role of electrical conduction and repolarization during cardiac regeneration, a phenomenon not widely possible in mammalian models (383, 435). As with the cardiomyopathy work, study of conduction in zebrafish first focused on cell-autonomous functions, then cell-cell connections such as gap junctions, while having the advantage of an organ-wide phenotypic readout (353). In studying zebrafish cardiac conduction, researchers have learned how zebrafish hearts are innervated (377), how sinoatrial pacing is set, and how atrioventricular conduction is managed (237, 245, 378). In a remarkable partnership of genetics and imaging, Arrenberg et al. (11) gained optogenetic control of the zebrafish heart (244). Finally, zebrafish has been a useful model for investigating GWAS loci associated with arrhythmia and other novel candidate genes for common arrhythmias found using next generation sequencing (225, 322, 396). In a recent example, whole-exome sequencing identified a cardiac myosin light-chain mutation as a novel cause of heritable atrial fibrillation, and a transgenic zebrafish modeling the same mutation was used to demonstrate that atrial fibrillation in this family was due to atrial myopathy (289). In addition to underscoring the complex relationship between myopathy and arrhythmia, this study highlighted a fact often observed in electrophysiology, where different mutations in the same gene can cause very different phenotypes. As another example, different HERG mutations can cause either Long or Short QT Syndrome (9, 37, 134). Therefore, the recent emergence of reverse genetic techniques to efficiently engineer specific mutations, combined with the powerful ability to image and clinically phenotype arrhythmia, will continue to make the zebrafish a powerful model to investigate conduction disease.
4. Complex congenital cardiovascular disease
Caution must be taken when modeling complex congenital heart disease in fish. Specific phenotypes of human congenital heart diseases, such as septal defects or transposition of great vessels, will be quite different in zebrafish, even when studying orthologous genes. Structural cardiovascular disease is also sometimes part of complex congenital syndromes, as with heterotaxy, diGeorge syndrome, or tetralogy of Fallot.
Even in the case of structural cardiovascular disease, however, the zebrafish model can contribute. Whether genetic or environmental, congenital heart disease has developmental origins. Also, many congenital heart diseases are quite rare (defined by the NIH as affecting fewer than 200,000 people in the United States), greatly limiting clinical research. As a vertebrate model extremely well suited to studying development, the zebrafish is an ideal animal model partner for rare congenital heart disease research. According to the NIH Genetic and Rare Diseases Information Center, roughly one-third of rare diseases affecting the heart have zebrafish models.
Consider Noonan syndrome as an example. Noonan syndrome is an autosomal dominant disease consisting of a range of craniofacial, musculoskeletal, hematologic, and cardiac phenotypes, including stenosis of the pulmonic valve and/or pulmonary arteries. Noonan syndrome is caused by mutations in several different genes, including PTPN11, SOS1, RAF1, and RIT1 (7, 30, 187, 291, 315). The zebrafish Noonan cardiac phenotype was a heart laterality and looping defect, much more simple and nonspecific than pulmonary stenosis, namely because zebrafish lack a pulmonary valve (30). However, the looping phenotype was scorable and extracardiac phenotypes in zebrafish also echoed Noonan syndrome, adding increased phenotypic specificity to what otherwise is not a highly specific cardiac phenotype on its own. Through work in zebrafish and other models, causative genes were found to all take part in the RAS signaling pathway, redefining Noonan and other related syndromes as RASopathies. Using zebrafish genetics and scorable, if simple, phenotypes to redefine congenital heart diseases by their molecular mechanisms will improve our understanding of structural heart diseases. Similar principles have been applied to ciliopathies, cohesinopathies, mitral valve prolapse, and vascular diseases including aortic coarctation, arteriovenous malformations, and hereditary hemorrhagic telangiectasia (15, 75, 264, 394, 427). With careful interpretation of analogous phenotypes, and/or use of a supplementary animal model, zebrafish models of complex structural heart disease can improve mechanistic understanding of congenital heart disease and potentially lead to high-throughput screens for therapies for these rare diseases.
5. Cardiovascular regeneration
Throughout this section, the focus has been on whether zebrafish can measure up as a high-throughput, mechanistically informative, and phenotypically sophisticated model for human cardiovascular disease at the cell, tissue, and organ levels. There is one human cardiovascular disease, however, for which researchers are not trying to model zebrafish after human disease, but rather to get human hearts to mimic those of zebrafish. That disease is cardiac injury, which is all too common as a result of myocardial infarction and a leading cause of heart failure. Adult zebrafish hearts regenerate, while adult mammalian hearts do not (307).
Cardiac regeneration is a very active area of zebrafish research and has been discussed in detail in several excellent reviews (103, 328, 389). Notably, researchers have learned that adult zebrafish can regenerate their ventricles without noticeable scar when subjected to surgical amputation or cryoinjury (307). They can also regenerate in response to genetic cardiomyocyte ablation of both atrium and ventricle, even when that ablation is widespread enough to initially cause clinical heart failure (413). Several studies attest to the role of the epicardium as providing mitogenic signals, such as retinoic acid (178); these signals direct subsequent dedifferentiation, proliferation, and redifferentiation of zebrafish cardiomyocytes in response to injury (169). Signaling pathways invoked during development are again seen during cardiac regeneration, such as the Erbb/Neuregulin pathway (113, 174, 179).
Given that even the two-chambered, mononucleate-celled, adult zebrafish myocardium is considered evolutionarily immature in many ways compared with the mammalian heart (306), questions arose as to whether mammals could ever demonstrate cardiac regeneration. In 2011, Porrello et al. (305) reported that neonatal mouse hearts were indeed capable of limited regeneration, finally crossing the divide from fish to mammal. In-depth study of the various pathways allowing cardiac regeneration in response to injury will undoubtedly continue making use of both zebrafish and mammalian systems.
B. Metabolic Disease Models
As with cardiovascular disease, zebrafish have emerged as a popular model for metabolic disease. While the study of zebrafish metabolism is quite valuable, it was slower to develop due to several obstacles that must be recognized and respected to properly model human disease.
1. Advantages and disadvantages of the zebrafish metabolic system for disease modeling
Metabolic disease involves lifestyle, socioeconomic, and behavioral factors unique to humans. Studying energy metabolism in zebrafish also adds technical challenges. For example, assays routine in mammals, like glucose- and insulin-tolerance tests and hormone testing, are cumbersome in zebrafish (87, 185, 234). Zebrafish dietary interventions are difficult to translate to human dietary recommendations due to fundamental differences in macro- and micronutrient requirements (359, 363). Within a tank of zebrafish, it is difficult to track individual food intake; however, food intake is closely linked to growth rate, which in turn influences adipocyte formation, as zebrafish favor somatic growth over body fat deposition through young adulthood (163, 205, 252). This problem becomes evident when rearing a family of wild-type siblings; competition for food, differences in the genetic background, and other factors can lead to large differences in growth rates and adiposity. Temperature also affects growth, metabolic rate, and body fat composition in this poikilothermic animal (216, 408, 409).
The microbiome may also affect metabolism, a factor not controlled for in zebrafish facilities. Compared with conventional or even specific germ-free (SPF) housing of mice, the water circulation carries a large number of potential pathogens. A core microbiome has been reported in zebrafish, and several groups have now begun to explore how naturally or infection-induced differences in the microbiota of the zebrafish intestine alter lipid and glucose homeostasis (20, 94, 95, 144, 303, 313, 314, 426), a concept that is gaining great attention in mammals.
Another angle that remains largely undescribed in zebrafish is the control of systemic metabolism through innervation of glands like the thyroid, chromaffin cells, and the pancreas or that of metabolic organs like enterocytes, the liver, and adipose tissue by sympathetic and parasympathetic inputs. Similarities in the development of the peripheral nervous system as well as the modulation of glucose metabolism by drugs targeting the serotonergic or adrenergic systems suggest the existence of such control mechanisms in zebrafish, but this notion needs to be further explored (91, 124, 224, 258). For these and other reasons, metabolism research in zebrafish has been slow compared with the rapid progress in the cardiovascular field.
Despite these challenges, the fundamental principles of metabolic control appear to be strikingly similar between zebrafish and mammals. Common medicines like the anti-diabetic metformin or the cholesterol-lowering simvastatin have “therapeutic” effects in zebrafish (16, 93, 124), suggesting that new drugs discovered in zebrafish can also benefit humans (226, 443). In this section, we highlight examples that illustrate the utility and value of studying metabolic disease in zebrafish (Tables 5 AND 6). While zebrafish have been used to study many metabolic diseases including inborn errors of metabolism (238, 391), hyper- and hypothyroidism (153, 437), disorders of the hypothalamus-pituitary-adrenal (HPA) axis (119, 416), dysregulation of the circadian clock (72, 417), and cancer metabolism (62), we will focus on diabetes, obesity, and dyslipidemia as the three most common metabolic disorders (60, 412a).
Table 5.
Zebrafish diabetes models
| Model | Phenotype | Key Findings Reported | Reference Nos. |
|---|---|---|---|
| Diabetes | |||
| Overexpression of a gene associated with a genetic risk variant for fasting glucose levels: Tg(fabp10a:foxn3,EGFP) | Increased hepatic gluconeogenesis and fasting glucose levels | A SNP in the first exon of FOXN3 is associated with human fasting glucose levels. Transgenic experiments in zebrafish confirm this risk variant by showing that Foxn3 overexpression increases hepatic gluconeogenesis and fasting glucose levels. | 173 |
| A gene knockout model for monogenic diabetes: pdx1−/− | Reduced beta cell numbers, decreased insulin and elevated glucose levels; growth retardation | pdx1−/− show characteristic features of human MODY, type IV. Primary islet forms, but secondary islets do not. | 183 |
| Streptozotocin-induced ablation of beta cells | Increased blood glucose levels, followed by beta cell regeneration and recovery to normoglycemia | First study showing that streptozotocin efficiently decreases beta cell mass in adult zebrafish, which is followed by a full recovery within two weeks. | 260 |
| Overfeeding a zebrafish line where beta cells are constitutively ablated: Tg(ins:Cre) x Tg(ins:loxp:BFPloxp:DTA) | Rapid proliferation of existing beta cells and increased differentiation from a progenitor pool within the intrapancreatic duct | High nutrient conditions suppress Notch signaling in progenitor cells within the intrapancreatic duct and trigger their differentiation to beta cells in an mtor dependent mechanism. | 273 |
| A zebrafish line with insulin resistance in skeletal muscle: Tg(actc1b:dnigf1ra-EGFP) | Skeletal muscle insulin resistance and blunted glucose uptake. Compensatory increase of beta cell numbers | Plasticity of beta cells leading to an increase in beta cell numbers in young animals. Beta cell failure and increased fasting glucose after overnutrition. | 228 |
| A model of nonproliferative retinopathy induced by repeated incubation in glucose-enriched water | Prevalent cone photoreceptor dysfunction and impaired vision. Thickening of the retinal vessels in conjunction with the breakdown of interendothelial cell-cell junctions. Absence of neovascularization. | First description of a zebrafish model of diabetic retinophaty (DR). Impairment of cone photoreceptor function independently of neovascularization, the clinical hallmark of DR and primary therapeutic target. | 1 |
| Streptozotocin-induced ablation of beta cells | Hyperglycemia followed by return to normoglycemia within 2 wk while wound healing is persistently impaired | Persistent DNA hypomethylation and differences in gene regulation during the wound-healing response in this transient hyperglycemia model. First proof of a “diabetic memory” concept in zebrafish. | 286 |
| Streptozotocin-induced ablation of beta cells | Impaired angiogenesis during wound healing in zebrafish that experienced hyperglycemia earlier in life | Transient hyperglycemia impairs angiogenesis during regeneration after return to normoglycemia. Inhibition of poly-ADP ribose polymerase prevents impairment of angiogenesis. | 335 |
| A nitroreductase-mediated beta cell ablation system using the prodrug metronidazole: Tg(insa:NTR-CFP) | Ablation of beta cells followed by beta cell regeneration and return to normoglycemia within days after ablation | The first model showing efficient regeneration of pancreatic beta cells in zebrafish larvae. | 5, 66, 304 |
Table 6.
Zebrafish models of obesity, NAFLD, and atherosclerosis
| Model | Phenotype | Key Findings Reported | Reference Nos. |
|---|---|---|---|
| NAFLD | |||
| A genetic model of hepatic steatosis: slc16a6−/− | Fasting-induced fatty liver disease that is reversed by feeding | slc16a6 encodes a ketone body exporter expressed in hepatocytes | 159 |
| Gain- and loss-of-function models of the human LXRα and LXRβ orthologs: Tg(actb2:EGFP-nr1h3); Tg(fabp2:EGFP-nrl1h3; lxr−/−) | Various effects on lipid metabolism | Enterocyte-specific overexpression of lxr promotes storage of fatty acids in the intestine and protects from hypercholesterolemia and hepatic steatosis | 64 |
| Diet-induced obesity | Hepatic steatosis and fibrosis | Ovarian senescence increases the risk for progression to fibrosis in patients with hepatic steatosis | 398 |
| ahcy−/− | Hepatic steatosis and liver degeneration | Mutations in ahcy cause hepatic steatosis in a TNFa-dependent manner | 238 |
| Fructose-induced hepatic steatosis | Hepatic lipid accumulation, lipogenesis, inflammation, and ER defects in response to fructose treatment of zebrafish larvae | Rescue of hepatic steatosis by rapamycin treatment | 334 |
| Obesity | |||
| Low- and high-density husbandry in combination with caloric restriction or ad libitum feeding | Positive effect of high caloric intake on postembryonic development, metamorphosis, somatic growth, body weight, fat storage, and reproduction | This paper highlights a number of important issues when studying obesity in zebrafish, i.e., juvenile zebrafish are largely resistant against DIO and transform elevated energy intake into an accelerated growth rate. In contrast, middle-aged zebrafish develop severe DIO. | 205 |
| Feeding-induced formation of the first adipocytes and visceral fat depots | The first fat-storing cells after the onset of feeding are visceral adipocytes in proximity to the pancreas. Formation of visceral fat depots occurs at late larval stages. | This study is the first to visualize adipocyte formation by making use of nile red staining. | 101 |
| plxnd1−/− | Plxnd1 selectively regulates expansion of visceral adipose tissue. | This paper describes a new role for Plxnd1 in adipose tissue plasticity as well as a number of important assays to monitor adipose tissue formation and metabolic health. | 251 |
| lepr−/− | Absence of obesity, hyperphagia, or infertility. Alterations in glucose homeostasis and beta cell plasticity. | Evolutionary diversification of obesogenic and glucose regulatory functions of leptin between fish and mammals. | 243 |
| Overexpression of agrp | Increased obesity and somatic growth | This study reports the first teleost obesity model in response to modulation of central mechanisms of fat storage. | 367 |
| Atherosclerosis/dyslipidemia | |||
| High-cholesterol diet | Vascular lipid accumulation; accumulation of myeloid cells in the vascular wall. Vascular leakage in response to chronic high-cholesterol diet | First model of high-cholesterol diet-induced atherosclerosis in zebrafish; regulation of lipid uptake into macrophages by Toll-like receptor 4. | 376 |
| High-cholesterol diet | Vascular lipid accumulation | Visualization of an immunogenic atherosclerotic plaque in vivo using an EGFP-labeled single-chain human monoclonal antibody. | 96 |
| apoc2 mutant | Hypertriglyceridemia, chylomicronemia | apoc2 deficiency leads to accumulation of lipid-laded macrophages in blood vessels | 217 |
2. Development of metabolic control in zebrafish
The control of energy homeostasis through feedback loops across cells and tissues forms the basis of an organism’s survival and function. When this fine-tuned homeostasis is lost, a toxic build-up of carbohydrate, protein, and lipids forms. In diabetes, elevated blood glucose causes both micro- and macrovascular disease (223), which in turn leads to stroke, heart attack, blindness, kidney failure, and neuropathy (60, 223, 262, 410). Due to its multi-organ effects, metabolic control must be understood at a system-wide, organismal level to develop new therapies.
Just like body plan formation, metabolic homeostasis is an important intrinsic feature of development. Evolving dynamics of metabolic feedback circuitry can be observed alongside the zebrafish’s anatomical development: organs such as the thyroid and the endocrine pancreas have developed within 48 hpf and actively secrete hormones that are critical for energy homeostasis (153, 171, 234, 283, 287). Development of metabolic homeostasis begins with establishment of glucose regulation. Glucose levels follow well-defined patterns, demonstrating two distinct peaks at 24 hpf and 5 dpf (Figure 8). Interestingly, both peaks are followed by increased beta cell proliferation, suggesting a delay of homeostatic feedback signals or even a developmental role for glucose in triggering beta cell proliferation (124, 171, 397). Between 48 hpf and 5 dpf, the whole-organism glucose concentration drops, followed by a second decline when the yolk has been consumed and the zebrafish transitions to a fasting state. Like mammals, zebrafish can adapt to low energy supply by shutting down energy consumption in most tissues, while activating a gluconeogenic program in the liver (124, 171). Early glucose dynamics are counterregulated by insulin; impaired beta cell development or ablation of beta cells as early as 2 dpf leads to a steep rise in glucose levels that recovers when beta cells are allowed to regenerate (5, 183).
FIGURE 8.
Development of metabolic regulation in zebrafish. A: timeline of metabolic characteristics throughout development. Glucose levels are highly dynamic during development and are regulated at early stages by endogenous glucose production, glucose utilization, as well as hormones, as in mammalian glucoregulation. Lipid homeostasis is determined at early stages by release of stored lipids within the yolk sac. The first adipose cells appear after feeding commences, and larger fat depots are not formed before ~30 dpf. B: energy substrates in the yolk sustain growth and metabolism, but are depleted around 4.5 dpf. If larvae are not fed by this time, they switch to a fasted state, and metabolic adaptations set in that are reminiscent of mammalian energy-sensing mechanisms. This defined yolk-feeding to fasting transition can be leveraged to monitor energy-sensing mechanisms. In addition, feeding of zebrafish larvae can be used to monitor adipocyte formation or intestinal lipid processing [Modified from Schlegel and Gut (341).]
Recently, Karanth et al. (173) showed that monitoring gluconeogenesis in zebrafish can be used to probe genetic variants. A SNP located within the first intron of FOXN3 had been identified in genome-wide association studies as a risk variant for elevated fasting blood glucose levels. Overexpression and loss-of-function studies in zebrafish revealed that FOXN3/foxn3 stimulates hepatic gluconeogenesis in larval and adult zebrafish consistent with the higher mRNA expression of FOXN3 in human carriers of the risk allele.
3. Yolk-feeding to fasting transition as an experimental advantage
The yolk-feeding to fasting transition provides an easy experimental system to study metabolic adaptations to energy scarcity in thousands of animals and without external feeding bias. A study that exemplifies well the power of this approach screened hundreds of zebrafish families from an ENU mutagenesis screen for animals that develop fatty liver. The group identified a mutant, red moon, which is characterized by hepatic steatosis during late larval stages. Mechanistic analyses found that the mutated gene encodes Slc16a6a, a monocarboxylate transporter which helps export ketone bodies from the liver to supply energy to tissues during fasting (159). Chronically fasted slc16a6a−/− mutants die from lack of sufficient ketone body supply to peripheral tissues. Importantly, hepatic lipid accumulation in slc16a6a−/− mutants is reversible with feeding, when endogenous ketone body production is suppressed.
Cellular energy sensing during the transition to fasting is a key feature of cells to adapt metabolism to nutrient deprivation. Of the four major energy-sensing complexes, insulin/insulin-like growth factor 1 (IGF1), mTOR, and AMPK have been found to have well-conserved functions. Disruption of the major energy sensor complex AMPK in zebrafish that lack the upstream kinase Lkb1 die prematurely at 7 dpf, 2 days after the yolk has been consumed (401). A similar phenotype is observed in tsc2−/− animals, which are characterized by a constitutive activation of mTOR signaling. These animals show cellular hyperplasia and, similar to lkb1−/− mutants, premature death at 7 dpf. Consistent with conserved mechanisms of mTOR signaling, the tsc2 mutant phenotype can be reversed by treating with rapamycin, an inhibitor of the mTOR complex downstream of Tsc2 (180). The fourth major class of energy-sensing complexes, sirtuins, which are NAD+-dependent deacylases, are to date not well studied in zebrafish (125, 155). Morpholino knockdown of the zebrafish sirt1 ortholog impairs endothelial sprouting and vascular morphogenesis (308). However, stable loss-of-function models and proof for a role of sirtuins in zebrafish metabolic control are still lacking, despite evolutionary conservation with 1-to-1 orthologs for each of the seven sirtuin genes.
The yolk-feeding to fasting transition can also be leveraged for drug screening. A small-molecule screen for endogenous induction of pyruvate carboxykinase 1 (pck1) expression during the transition identified compounds that modulate organismal fasting metabolism. The screen of ~3,000 compounds revealed that several ligands of Tspo, a protein located to the outer mitochondrial membrane, are activators of hepatic gluconeogenesis while potently suppressing whole-body glucose levels. These ligands activate a Ppara-mediated gene signature resembling a canonical mammalian fasting response. These compounds were also able to protect mice with diet-induced obesity against hepatic steatosis and improve glucose handling (123, 124).
4. Adipose tissue plasticity and obesity
Obesity results from incrementally storing large amounts of dietary triglycerides in adipose tissue. Although obesity is now catalogued as a disease in itself, an individual’s propensity to develop diabetes, non-alcoholic fatty liver disease (NAFLD), atherosclerosis, and their fatal complications varies dramatically based on genetic background, gender, lifestyle, and environmental factors. The existence of “healthy obese” individuals is debated, but reflects the idea that a subset of individuals are considered healthy with respect to cardiovascular parameters, insulin resistance, glucose handling, and blood lipid profiles despite profound adiposity. A high capacity to store neutral lipids and avoid systemic inflammation rather than exposing peripheral tissues to triglycerides, cholesterol, and fatty acids is believed to have a major influence towards preventing obesity-associated complications (166, 194, 381, 421). As such, identifying factors regulating adipose tissue development and plasticity is key to understanding why some individuals escape the detrimental consequences of obesity. Genetic and nutritional factors that regulate the plasticity and expansion of adipose tissues, mechanisms of lipid absorption, packaging and transport, as well as inflammatory responses to aggressive lipid moieties are starting to be investigated in zebrafish (3, 339, 341, 351).
Adipose development in zebrafish follows the same basic principles as in mammals. Larvae soaked in the neutral lipid dye nile red first show adipocytes shortly after they commence feeding around 7 dpf, whereas no adipocytes develop in food-deprived animals (101). The yolk sac stores and synthesizes lipids during the first 4 days of development (105), but its anatomical structure and packaging of energy substrates has little resemblance to that of human adipose tissue. Subcutaneous and visceral fat pads that anatomically match human adipose tissue first appear around 30 dpf when the energy is no longer completely converted into somatic growth (101, 163, 205, 252) (Figure 8). This late appearance of adipocyte tissue weakens many of the advantages of the zebrafish model such as easy in vivo imaging or rapid drug testing in multi-well format. Along these lines, attempts to identify a promoter that selectively labels zebrafish adipocytes for lineage tracing or spatio-temporal control of gene expression have been unsuccessful thus far. Several studies have shown that lipid uptake, metabolism, and storage are tightly regulated in zebrafish and that chronic overfeeding with lipid-rich diets ultimately leads to insulin resistance (101, 209, 228, 252), NAFLD (64), obesity (205, 228, 252, 285), and atherosclerosis (97, 98, 376) (Tables 5 AND 6).
Genetic loss- and gain-of-function studies have also shown that many genetic factors underlying adiposity in zebrafish are similar to those in mammals (51, 241, 367). However, knockout of one of the most extensively studied signaling pathways that determines body fat mass, leptin signaling, fails to show a conserved role. Human patients with mutations affecting leptin signaling show excessive eating patterns and are morbidly obese and less fertile compared with healthy individuals. Mouse models show similar phenotypes; leptin is highly expressed in mammalian adipocytes, and its secretion is stimulated by insulin after food intake (107). In contrast, zebrafish that lack the leptin receptor lepr show normal growth, fat mass, feeding behavior, and fertility (243). Expression of the lep gene in zebrafish is nearly absent in fat, whereas mRNA levels are increased during fasting in the liver.
Instead, leptin signaling in zebrafish appears to affect glucose homeostasis. In depth phenotyping of zebrafish lacking lepr−/− or lepa−/− orthologs revealed increased insulin sensitivity and glucose clearance after a glucose tolerance test in adult zebrafish. Larval zebrafish showed a higher beta cell number while having a reduced compensatory increase in the number of beta cells in response to overfeeding. Lastly, wound healing after fin clipping was slower in lepr−/− animals compared with wild-type. This recent study points out important molecular differences between zebrafish and mammalian adipose tissues, highlighting the possibility of dissecting the divergent roles of leptin signaling in glucose homeostasis without the confounding effect of excessive fat mass (243).
Zebrafish work has already led to truly novel insights in obesity research. For example, recent work has revealed a mechanism for PLXND1, a gene associated in population genetics studies with differences in human hip-to-waist fat distribution as well as an increased risk for type 2 diabetes mellitus (T2DM) (358). The mouse knockout of Plexin D1 causes embryonic lethality before visceral fat development (251), but plxnd1−/− zebrafish are amenable to study. Interestingly, visceral fat depots in the zebrafish mutant are hyperplastic with a reduced capacity to store lipids (251). In contrast, subcutaneous fat appears unaffected, suggesting a role for plxnd1 in adipose tissue distribution. In high-fat diet (HFD) conditions, the visceral fat tissue was unable to expand thereby forcing the redistribution of excess lipids to subcutaneous depots. The underlying mechanism was shown to depend on the remodeling of the surrounding extracellular matrix. In addition to revealing novel plxnd1 functions, this work describes an interesting set of in vivo and ex vivo adipose tissue imaging techniques that should be of great use for similar investigations in the future (251, 252).
Despite the absence of adipose tissue during early embryonic and larval zebrafish development, other aspects of lipid metabolism can be studied at these early stages; for example, the gastrointestinal system of zebrafish is anatomically and functionally closely related to that of mammals (3, 99, 341). Feeding of fluorescently labeled lipid moieties to zebrafish larvae has been leveraged to visualize lipid absorption and processing in enterocytes. A forward genetic screen using this assay, for example, identified the fat-free mutant, which showed unaltered intestinal morphology despite reduced phospholipid and cholesterol processing (99). It was later shown that fat-free encodes Ang2, a conserved subunit of the Golgi-associated retrograde protein (GARP) complex, which regulates lysosomal enzyme sorting, endosomal cholesterol trafficking, and autophagy (146, 299).
5. Non-alcoholic fatty liver disease
NAFLD is the most frequent lipid storage disorder in the liver independent of alcohol consumption and is driven mainly by high-fat diets and carbohydrate-rich foods (213). It affects over 30% of the general population and an even higher percentage of obese individuals and is considered a complication of obesity. It is reversible through dieting, but if untreated predisposes to a sequence of severe liver pathologies: non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (221). Which genetic factors drive the progression from benign NALFD towards inflammation, fibrosis, and ultimately malignant liver cancer are poorly understood (59).
Studying NAFLD in zebrafish has the advantage that steatosis can be visualized in larvae by whole-mount Oil Red O staining for neutral lipids (340, 343). With the onset of feeding at 4 dpf, zebrafish can be fed lipid-rich diets such as an emulsion of egg yolk (209) or Artemia brine shrimp (285). Alternatively, ketogenic or fructose-based diets have been used to trigger steatosis (172, 334). The larval liver is big enough to be dissected with relative ease, and transcriptomic, histological, and biochemical analyses can be performed on these samples (124, 172). A broad range of transgenic lines exist to visualize different hepatic cell types, including hepatocytes, biliary cells, endothelial cells, and stellate cells (331, 336, 434). Adult zebrafish can be chronically exposed to defined diets and lipid content of the liver measured by conventional enzymatic assays or metabolomics (159, 172). Radiolabeled tracers can be used to follow carbon flux into different biosynthetic pathways (172).
The first zebrafish models of NAFLD were again found in large-scale forward genetics screens, suggesting that systematic screening for NAFLD-causing mutations and investigating their role in disease progression can reveal important insights into disease mechanisms (159, 238, 279, 330) (Table 6). A single recent ENU mutagenesis screen alone identified 19 new mutant lines that are characterized by hepatomegaly, with most of them displaying prominent neutral lipid staining. Many of these mutants exhibit unique histological pathological features ranging from mild micro- or macrovesicular steatosis to severe signs of NASH characterized by ballooning and necrosis of hepatocytes (181). Cloning the genes affected by these mutations will likely contribute important insights into the development of chronic liver disease.
6. Dyslipidemia and atherosclerosis
Studying atherosclerosis in zebrafish has similar technical advantages as studying NAFLD including the possibility to stain lipid deposits within blood vessels in vivo. A set of groundbreaking studies by the Miller laboratory showed that unlike in rodents, wild-type zebrafish that are chronically exposed to high-cholesterol diets have a propensity to develop histopathological changes similar to those seen in human atherosclerosis (96–98, 376). [Murine atherosclerosis studies therefore rely on a sensitized background such as ApoE−/− animals (35).] An evolutionary basis for this phenomenon is that the ortholog of the human cholesteryl ester transfer protein (CETP) is conserved in zebrafish, while it has disappeared from rodent genomes. Therefore, as in humans, cholesteryl esters in zebrafish are diverted from protective HDL cholesterol particles to “bad” LDL, thereby increasing the susceptibility for atherogenic events. To exploit the advantages of zebrafish for in vivo imaging, the Miller group (96) also showed that oxidization of LDL by malondialdehyde can be visualized using single-chain monoclonal antibodies linked to GFP. Furthermore, recruitment of macrophages to vascular lesions can be monitored and quantified to find new treatment strategies.
Liver X receptor (encoded by LXRA and LXRB in humans) is a master regulator of cholesterol homeostasis in mammals (33) which appears to have functionally conserved roles in zebrafish. In contrast to mice, however, only one lxr ortholog exists in zebrafish (encoded by lxra/nr1h3) allowing one to capture its physiological roles with relatively straightforward gain- and loss-of-function experiments. lxra−/− knockout zebrafish are viable, but mirror the cholesterol intolerance of Lxra−/−, Lxrb−/− double knockout mice which show excessive cholesterol levels in the liver and blood after a chronic HFD. Through overexpression experiments, the authors also showed that increased Lxr function specifically in enterocytes could cause the opposite effect, i.e., in response to HFD the accumulation of cholesterol in the blood and liver was strongly slowed down (64). Mechanistically, it was found that Lxr activates an acyl-CoA synthetase (encoded by acsl3a) that metabolizes absorbed lipids and targets them for storage in lipid droplets instead of a quick release into the blood. Clinically, this discovery is of importance as several studies indicate that postprandial increases of cholesterol drive atherosclerosis. Postprandial dynamics of cholesterol, however, is a process that is not particularly well controlled by statins (which inhibit the de novo synthesis of cholesterol in the liver). Targeting the Lxr-Acsl3a axis may therefore be an interesting strategy to slow the release of absorbed dietary lipids into the circulation, thereby acting synergistically with statin treatment (64, 341).
7. Regenerative approaches to diabetes
Zebrafish have a remarkable plasticity of beta cell mass, in response to both injury and metabolic challenges (5, 209, 228, 260). Dissection of the genetic cues that determine the development, maturation, and plasticity of functional beta cells has been a primary focus in zebrafish research and is starting to contribute important insights for beta cell regeneration and replacement therapies. Several distinct approaches are being probed in this context, ranging from the differentiation of mature beta cells from induced pluripotent stem cells in vitro to coaxing remaining beta cells, progenitor pools, or even non-beta cells into restoring adequate numbers of functional beta cells in vivo (5, 68, 208, 406, 439, 440).
Anatomically, zebrafish are well suited for this purpose because each progenitor and cell type within the islet can be visualized and monitored over time in vivo using fluorescent reporter proteins until the formation of a mature pancreas throughout embryonic, larval, juvenile, and adult stages (70, 80, 143, 261, 272, 273, 432). Pancreatic islets in zebrafish include a single primary islet in the head of the pancreas as well as secondary islets dispersed throughout the organ (100, 296).
The first studies suggesting that zebrafish could be used to dissect the genetic network governing endocrine pancreas development came once again from forward genetic screens (292, 385). An insertional mutagenesis screen identified a line with a mutation in the homeobox gene vhnf1, which fails to express normal levels of pdx1, a transcription factor gene necessary for the induction of the insulin gene (385). The disease relevance of these findings became clear as lesions in these genes are known to cause a subtype of monogenic diabetes: mature-onset diabetes of the young (MODY) type V for VHNF1 and type IV for PDX1. Indeed, it was later shown that deletion of pdx1 in zebrafish causes a reduction in beta cells, decreased insulin levels, and disrupted glucose homeostasis (183) (Table 5). To date, a large number of studies have shown that genes specifying hepatopancreatic cell lineages have highly conserved functions (52–54, 79, 100, 117, 143, 232, 233, 269, 283, 369).
Drug screens are now being carried out to find small molecules that induce or block different steps of endocrine lineage specification and maturation. These molecules may ultimately be used to improve the efficiency of the generation of mature beta cells from iPS cells in vitro or to activate progenitor pools that can generate new beta cells in vivo (68). A screen that exemplifies this approach used a double-transgenic line Tg(Tp1:hmgb1-mCherry), Tg(pax6b:GFP) to visualize pax6b-positive progenitor cells within the intrapancreatic duct (327). Tg(Tp1:hmgb1-mCherry) animals carry a Notch activity reporter that marks cells of the intrapancreatic duct, and upon suppression of Notch signaling, these progenitor cells become activated to become beta cells in a process similar to the differentiation of progenitor cells in the mammalian pancreatic duct (266). This line was subsequently screened for compounds that induce the occurrence of secondary islets as marked by pax6b:GFP expression at 5 dpf (296, 327), a stage when no secondary islets have formed during normal development.
Zebrafish have also been used to systematically interrogate pathways required to enhance the regeneration of beta cells after tissue injury using a chemical genetics approach. Beta cell ablation studies have shown that normal numbers of beta cells recover within days even after a near-total ablation of beta cells (Table 5) (66, 260, 304). With the use of this ablation paradigm, a screen of over 7000 small molecules revealed that the largest group of hit compounds restoring beta cell mass activates adenosine signaling and triggers compensatory proliferation of the regenerating beta cells. The strongest hit, the pan-adenosine receptor agonist NECA, was also able to increase proliferation of beta cells in mice subjected to streptozotocin-induced beta cell ablation, and to restore glucose homeostasis (5). In a follow-up study, it was shown that the effects on beta cell proliferation were mediated by the adenosine receptor A2a, which in fact is specifically implicated during compensatory beta cell proliferation, including post-injury as well as during pregnancy (347).
Lineage tracing and beta cell ablation tools have been used to study aspects of beta cell plasticity in response to metabolic challenges, a phenotype that has been observed in obese individuals, but is mechanistically poorly understood (27). Similar to what is observed in human obesity, zebrafish beta cells are transiently able to adapt to the increased demand for insulin by increasing overall beta cell mass. Overnutrition of zebrafish larvae and juveniles leads to a suppression of Notch signaling in endocrine progenitors which causes their differentiation into beta cells. This plasticity was further shown to depend on mTOR-induced suppression of Notch signaling (273). Beta cell plasticity has also been shown to occur in adult zebrafish in response to excess food intake. Lipid-rich diets as well as glucose trigger compensatory beta cell expansion based on distinct mechanisms, i.e., whereas the response to high glucose levels requires active mTOR signaling, the response to lipids depends on insulin/IGF1 signaling (209, 227). The newly formed beta cells could be further characterized by two subpopulations of postmitotic precursor cells positive for nkx2.2 or mnx1 expression.
In a recent report, zebrafish studies contributed to the identification of artemisinins as small molecules that in response to beta cell ablation repress alpha cell identity and lead to enhanced beta cell regeneration as well as improved glucose control (208). On the basis of this and many other examples, the vast collection of transgenic reporter lines and islet-cell ablation tools hold promise to systematically interrogate all steps of beta cell development and plasticity in healthy and stressed conditions. A close collaboration with groups specialized in stem cell biology and regenerative medicine will be necessary to support the best therapeutic strategies with knowledge collected from zebrafish studies.
IV. DRUG DISCOVERY USING ZEBRAFISH
“A very interesting set of compounds that were waiting for the right disease.”
—Doctor Jerome Horwitz, 1919-2012
That is how researcher Jerome Horowitz described the dideoxythymidine compounds he developed in the 1960s, hoping to cure cancer. Instead, these drugs—the most famous of them being zidovudine, or AZT—became the first to successfully fight HIV infections, decades after they had been forgotten as a failed cancer treatment. Drug discovery has powered some of the most revolutionary advances in modern medicine. Zebrafish are increasingly used both in the academic and pharmaceutical worlds to address major challenges for drug discovery: to identify new drug-target interactions, to match existing drugs to new uses, and to evaluate for toxicity and efficacy in vivo, all at a scale that keeps up with medical need.
Drug discovery follows two principal strategies: target-based and phenotype-based screening (89). Target-focused drug discovery has become the center of investment in today’s pharmaceutical industry due to the ultra-high-throughput screening capabilities on purified protein target assays (388). This target-focused approach follows a defined sequence of steps: target discovery, high-throughput screening in vitro, in silico-assisted toxicology studies in cells and preclinical models, and ultimately clinical trials (255) (Figure 9). An increasingly applied alternative strategy is phenotypic screening which aims to identify compounds that exert a desired physiological effect without a priori knowledge of the target (90). The use of phenotypic screens in cellular systems such as immortalized cell lines, primary tissues, and induced pluripotent stem cells have evolved rapidly alongside emerging technologies in robotics, high-content imaging, image processing, and automated analysis (31). However, both target-focused and phenotypic strategies still face the challenge of a lengthy development phase (89) (Figure 9).
FIGURE 9.
Zebrafish assist in all drug discovery and development phases. Fundamental and preclinical research using zebrafish generate new targets and help to validate predicted targets in vivo. Cornerstones of the traditional R&D pipeline including lead optimization, toxicology testing, and long-term efficacy as well as safety studies are being developed in preclinical zebrafish assays. Drug repurposing can match existing drugs with potentially new clinical indications through screening in disease models. Incorporating whole-organism testing into traditional R&D pipelines early in the process can help critical decision-making and avoid late-stage failure of new chemical entities.
Over 80 chemical screens over the past 16 yr attest to the zebrafish’s utility in whole-organism, phenotype-based drug discovery (226, 319). Zebrafish cell populations or molecular pathways can be monitored at stages when zebrafish larvae are still small enough to be placed in 96-well plates. Simple addition of chemicals to larval medium then allows one to screen large numbers of chemicals by the phenotypic response of the larvae in each well (131). The similarities of zebrafish and human genes suggest that many targets are in principle conserved (156, 226) (Table 2).
In this section, we will highlight zebrafish strategies that complement traditional R&D pipelines. In some cases, as discussed in the previous section, zebrafish disease models can be used to discover clinically approved drugs that suppress the pathological progress of the disease, and thereby dramatically reduce the time from preclinical to clinical studies (Figures 9 and 10). We summarize current challenges including the relatively low throughput compared with in vitro screens while highlighting recent progress to leverage robotics and automated image analysis software to narrow down the gap between in vivo and in vitro throughput capacities.
FIGURE 10.
From human disease to therapies using zebrafish. In many cases, disease models can be used to develop screening paradigms for suppressor molecules of pathological hallmarks. This emerging concept has been shown to facilitate the transition from preclinical to clinical trials, in particular in areas of unmet clinical need through screening of clinically approved drug collections. Advantages and disadvantages of available chemical libraries are summarized in the box.
A. Drug Discovery in Zebrafish Leveraging Organismal Complexity
Along the drug development pipeline, the filtering of hit compounds based on in vivo efficacy and toxicity is still considered as one of the most challenging decisions (255). Involving whole-organism biology early on can provide a better understanding of difficult-to-predict variables such as bioavailability, biotransformation, xenobiotic defense mechanisms, as well as homeostatic loops across tissues that abolish or even potentiate the efficacy of a drug, or that may contribute to toxicity or off-target effects. However, early in vivo testing of new chemicals is limited by practical, cost, and ethical concerns to only test a handful of hit compounds in mice or rats.
In addition to evaluating efficacy and toxicity, a clear strength of whole-organism screening is the ability to reveal effects of compounds on cells outside the specific tissue of interest. Whereas cellular screens can only capture cell-autonomous responses to drugs, whole-organism screens can discover molecules that act through a cell type different from the affected cell population via hormonal, nervous, immunological or paracrine mechanisms. This concept is of particular importance for situations where resident cell types have to replace, repair, or remove cells that do not function properly. Examples are immune cells that orchestrate removal of cells while stimulating niche-resident stem cells to replace tissue in response to injury (25, 323).
Most zebrafish screens are carried out during the first week after fertilization, while the animals are still small, transparent, and do not require feeding (319). Several phenotypic readouts and screening strategies exist: wild-type embryos can be used to perform behavior-related readouts (189), anatomic readouts, physiological readouts such as heart rate or blood flow (219, 302), or readouts that can be visualized by simple staining like in situ hybridization (276). However, most screens use readouts that are based on transgenic fluorescent or bioluminescence reporter systems. Using bioluminescence allows a relatively quick assessment of promoter activity using conventional plate reader instruments, but requires a functional promoter of interest (13, 124). Fluorescence-based readouts allow high-content visually derived information, but are often of lower throughput, need longer lead-time for transgenic generation, and require more optimization of image acquisition and data handling. Once a model is up and running, it can be used to screen many different chemical libraries (see Table 9).
Table 9.
Selection of available chemical libraries
| Type | Name | Provider | Size | Description |
|---|---|---|---|---|
| Bioactives | TocrisMini | Tocris | 1,180 | Drugs, screening hits, and experimental small molecules with known bioactivity. Off-the-shelf repurchasing of large quantities. |
| Bioactives | SigmaLopac | Sigma | 1,280 | Drugs, screening hits, and experimental small molecules with known bioactivity. Off-the-shelf repurchasing of large quantities. |
| Bioactives | Prestwick Chemical Library | Prestwick | 1,280 | Approved drugs by FDA, EMA, and other agencies. Off-the-shelf repurchasing of large quantities. |
| Bioactives | Bioactive Compound Library | SelleckChem | 2,100 | Some FDA approved drugs, natural products, and other molecules with known bioactivity. Off-the-shelf repurchasing of large quantities. |
| FDA-approved drugs | FDA-approved Drug Library | SelleckChem | 978 | FDA-approved drugs. Off-the-shelf repurchasing of large quantities. |
| Targeted library | Screen-Well Compound Libraries | Enzo | >2,500 total | Selection of libraries containing bioactive lipids, Nuclear Receptor Ligands, GPCR ligands, among others. |
| Targeted library | Screening Libraries | Cayman Chemicals | NA | Selection of libraries containing epigenetics, kinase and stem cell modulators, bioactive lipids, prostaglandins |
| Plant crude extracts | TimTec | NA | Fractions of plant extracts. Difficult to identify active ingredient(s); potential reproducibility issues. | |
| Pure natural products | Natural Product library | TimTec | 800 | Single compounds derived from the natural space; potentially expensive to purchase larger quantities or even impossible to extract sufficient amounts for preclinical studies. |
| Pure natural products | Natpure | Charles Rivers | 14,100 | Single compounds derived from the natural space; potentially expensive to purchase larger quantities or even impossible to extract sufficient amounts for preclinical studies. |
| Pure natural products | Natural Compound Library | Chromadex | 1,850 | Single compounds derived from the natural space; potentially expensive to purchase larger quantities or even impossible to extract sufficient amounts for preclinical studies. |
| Plant crude extracts | Botanical Extract Libraries | Chromadex | >1,600 | Fractions of plant extracts. Difficult to identify active ingredient(s); potential reproducibility issues. |
| Pure natural products | Screen-Well Natural Product library | Enzo | 502 | Single compounds derived from the natural space; potentially expensive to purchase larger quantities or even impossible to extract sufficient. |
| Plant crude extracts | Greenpharma Extract Library GPEL | GreenPharma | NA | Fractions of plant extracts. Difficult to identify active ingredient(s); potential reproducibility issues. |
| Human Endogenous Metabolites | Human Endogenous Ligand Library GPLL | GreenPharma | 400 | Metabolite-like human endogenous ligands. |
| Diversity | NExT diversity libraries | National Institute of Health | 80,000 | Large libraries with potential for novelty; unknown bioactivity; expensive to repurchase or synthetize compounds. |
| Diversity | AXXDIV2.0 | AXXAM | 12,000, 48,000, or 180,000 | Large libraries with potential for novelty; unknown bioactivity; expensive to repurchase or synthesize compounds. |
| Diversity | Express-Pick Collection, Core Library Stock | Chembridge | Custom; >1,000,000 | Large libraries with potential for novelty; unknown bioactivity; expensive to repurchase or synthesize compounds. |
| Diversity | Exquiron | Custom; >250,000 | Large libraries with potential for novelty; unknown bioactivity; expensive to repurchase or synthesize compounds. | |
| Diversity | TimTec | TimTec | Custom; >>600,000 | Large libraries with potential for novelty; unknown bioactivity; expensive to repurchase or synthesize compounds. |
One of the first screens on a zebrafish disease model showed that out of 5,000 screened compounds, two molecules were able to suppress aortic coarctation, a rare pediatric malformation, and to restore blood circulation in gridlock/hey2 mutants (302). With time many other screens followed, and the sophistication of disease models as well as the screening readouts evolved at a fast pace (Table 8). For example, Asimaki et al. (13) modeled arrhythmogenic cardiomyopathy by overexpressing the pathogenic 2057del2 plakoglobin variant specifically in cardiac muscle. These transgenic zebrafish show abnormal cardiac physiology by 48 hpf and develop profound cardiac hypertrophy and arrhythmia by 5 wk of age, mirroring organ-wide human disease phenotypes in a way that an in vitro or cell culture models would not. In an elegant screen a luciferase reporter line for natriuretic peptide b Tg(nppb:luc) was used as a surrogate to monitor cardiomyocyte dysfunction at a scale that allowed the authors to carry out a high-throughput screen. Subsequently, a small molecule, SB216763, was identified that reversed arrhythmic contractions, likely through modulating cellular trafficking of components of intercalated disks and thereby restoring cell-cell connectivity while sparing the organism of obvious off-target toxicity (13).
Table 8.
Representative chemical screens to identify disease modifier compounds
| Read-out | Description | Key Findings Reported | Reference Nos. |
|---|---|---|---|
| Cardiovascular | |||
| Suppression of an aortic coarctation phenotype in hey2 mutants | 5,000 small molecules screened for compounds that restored blood circulation in hey2 mutants. | The first zebrafish screen to report the suppression of a disease phenotype in vivo through organismal phenotypic screening. | 302 |
| Rescue of doxorubicin-induced cardiotoxicity | Suppression of cardiac function defects induced by doxorubicin. 3,000 small molecules screened. | Identification of Visnagin, a small molecule suppressor of cardiotoxic effects of doxorubicin that does not interfere with its chemotherapeutic effects. | 219 |
| Restoration of rhythmic heart contractions | Screen for compounds that restored coordinated heart beats in mutants that display Ca2+ extrusion defects. | A small molecule, efsevin, potentiates VDAC2-mediated Ca2+ uptake into mitochondria. | 357 |
| Restoration of normal cardiac rhythmicity in kcnh6 mutants, a model of Long QT Syndrome Type 2 | 1,200 molecules screened visually for compounds that suppress a 2:1 atrioventricular block. | Flurandrenolide and 2-MMP shorten the ventricular action potential and suppress the Long QT syndrome phenotype. | 298 |
| Bioluminescence readout of a cardiac stress-signal in a disease model for arrhythmogenic cardiomyopathy | 4,200 molecules screened for suppression of a nppb:luc signal in zebrafish overexpressing mutant 2057del2 plakoglobin. | A small molecule, SB216763, prevented heart failure and reduced mortality. | 13 |
| Suppression of hyaloid vessel angiogenesis in Tg(fli1:EGFP) zebrafish | Angiogenesis inhibitor screen carried out in zebrafish; hits were further tested for suppression of angiogenesis in mammalian cells and a mouse model of retinopathy. | Identification of Quininib, a small molecule inhibitor of the cysteiyl leukotriene receptors 1 and 2, that inhibits revascularization in a model of oxygen-induced retinopathy in mice. | 320 |
| Metabolism | |||
| Enzymatic measurements of larval glucose levels | Screen of 13,120 compounds for modulators of whole body glucose levels. | Identification of PTPMT1 as target of a novel glucose-lowering molecule, alexidine. | 268 |
| Bioluminescence-based read-out of hepatic gluconeogenesis | A pck1:luc2 reporter line used to screen 2,460 molecules for modulators of a gluconeogenic fasting response. | Translocator protein (TSPO) ligands induce fasting metabolism in zebrafish and mice. | 124 |
| Intestinal lipid absorption | A fatty acid labeled fluorophore dye was used to monitor intestinal lipid uptake from the medium into the gallbladder; 3840 small molecules screened. | Identification of known and several previously unknown small molecules that inhibit intestinal lipid absorption. | 58 |
| Regeneration of beta cells using a genetic cell-ablation system and fluorescently-labeled beta cells | Screen of 7,000 compounds for enhancers of beta cell regeneration. | Adenosine signaling promotes beta cell regeneration in zebrafish and mice. | 5 |
| Differentiation of beta cell progenitors | Screen of 3,131 compounds for induction of secondary islet formation from intrapancreatic duct progenitors without affecting global Notch signaling. | Identification of two activators of beta cell differentiation, MPA and DSF. | 327 |
| Beta cell numbers using a fluorescent reporter | 3,348 compounds screened at 6 concentrations in a rapid semi-automated process. | Development of a quantitative “high-throughput” screen in zebrafish with up to 200,000 organisms quantified in one day. | 412 |
B. Matching Old Drugs With New Diseases
Drug repurposing (also known as drug repositioning) refers to the identification of a novel indication for a drug that has previously been approved for human use by regulatory authorities. Repurposing can dramatically reduce drug development costs because the toxicology and safety profiles are already well defined. Zebrafish disease models screened with preapproved drug libraries for their ability to suppress a given disease phenotype are one step closer to clinical therapy. For example, the screens using kcnh6 mutants to find suppressors of the long QT phenotype mentioned above showed that flurandrenolide and dexamethasone, glucocorticoids for which there is vast clinical experience, efficiently shorten ventricular action potentials (226, 298).
Several libraries are available that contain clinically approved compounds, including those of the National Institutes of Health that are available to academic researchers at cost (Table 9). However, it is an oversimplified assumption that drug repurposing automatically facilitates clinical trials, because the clinically accepted safety and toxicology profile of a drug depends largely on its disease indication. In other words, a compound that extends survival and quality of life of a terminal cancer patient may be indicated in this case, but severe adverse effects may exclude it from application in a generally healthy patient population for a less severe disease. Nevertheless, drug repurposing is an interesting area in which zebrafish are likely to take center stage among patient-derived genomics data, disease modeling, and drug discovery (67, 226, 442).
C. Target Deconvolution
The path to identifying the target of a compound found in a phenotypic screen can be uncertain and time consuming. The choice of compound library therefore has an important impact on the likelihood of finding a drug-target match, which is particularly true for in vivo phenotypic screens that in most cases are low-to-medium throughput. For example, compound libraries that are rich in information on the chemical structures are most likely to have known targets (50). These “bioactive” libraries contain clinically approved drugs, drugs that have entered clinical trials but have failed for lack of efficacy or toxicity, and experimental molecules that have been discovered as relatively specific for a target of interest. On the other hand, screening libraries of unknown compounds increase the chance to find true novelty and also increase the chance to operate within an open patent landscape, if desired. Advantages of different compound libraries are summarized in Figure 10 and Table 9.
Several complementary strategies exist to identify the target of an unknown molecule. For example, in silico predictions can match chemical structures to protein pockets, or match the structure of an unknown molecule to that of a similar one that has a well-defined target; both approaches have been used successfully in the context of whole-organism screens (195, 206, 422). Some molecules can be synthesized and conjugated to a substrate for subsequent affinity-chromatography pull-down and identification of the target, without affecting the molecule’s native biological activity (127, 268). Lastly, compounds can be screened against large panels of assays that probe modulation of protein superfamilies, including kinases, phosphatases, and G protein-coupled receptors (GPCRs) to identify the target and its relative selectivity over related targets. For example, Williams et al. (425) identified a new molecule, eggmanone, which phenotypically echoed genetic deletions of the hedgehog pathway. By screening the lead compounds against a large panel of phosphodiesterases, the authors found eggmanone to selectively inhibit three distinct phosphodiesterases, including PDE4D3 with an IC50 of 72 nM. Counter-screening against a panel of 442 kinases, 158 GPCRs, and 21 phosphatases confirmed selectivity of the compounds to PDE4D3. Once a plausible target is identified, genome-editing approaches can be used in cell lines or in zebrafish to validate the dependence of the phenotypic readout on the target.
Another emerging concept to match a molecule of unknown structure to a target is “bar coding” to show that the unknown molecule performs similarly to modulating a matrix of readouts as a well-defined molecule (38, 189, 190, 239). Bar coding is especially helpful when the efficacy of a particularly potent molecule may in fact not be mediated by one target, but several synergistic targets—the basis of polypharmacology (38, 126, 239). Whole-organism screening is also well suited for a polypharmacology approach because the synergistically acting targets may function in different tissues. Of course, the possibility of undesired adverse effects increases with the number of bioactive targets, i.e., the “dirtier” a drug is, the more important it is to assess toxicological effects in whole-organism models early during its development.
D. Efficacy and Route of Administration
Zebrafish screens are commonly carried out at one concentration (in most cases at 10 µM), with the exception of a few semi-automated screens that were able to screen a titration range (412). The compounds of the stock plates are commonly dissolved in DMSO and reach final DMSO concentrations of 0.1–2% in the screening plate. The compound libraries contain chemicals with different physicochemical characteristics ranging from hydrophilic to relatively hydrophobic. As a result, it is difficult to predict whether a particular chemical enters into the zebrafish orally, topically, or through the gills. In addition, it is not possible to predict the concentrations within zebrafish tissues, in particular because measuring drug concentrations in whole larvae is often misleading as the chemicals frequently attach to the skin. To bypass this latter problem, a group developed a microneedle biopsy in the yolk sac followed by mass spectroscopy to determine whether the compound has truly been absorbed into the zebrafish (288). Another means to test whether a compound is orally available is to administer it to adult zebrafish via gavage or gelatin-based food delivery. Oral gavage of adult zebrafish can be performed on zebrafish that are anesthetized and mounted in an incised sponge (61, 71). An alternative, although less precise, is the preparation of a mix of low-melting gelatin mass (such as Gelly Belly, Florida Aqua Farms) and the compound of interest. The solidified gelatin can be cut into small pieces and fed to adult zebrafish (348). Both techniques greatly expand the use of zebrafish for preclinical studies as they allow long-term drug administration.
E. Toxicology
Several important studies have been carried out to date that illustrate how zebrafish can be leveraged to detect the adverse toxic effects of some small molecules (63, 115, 222, 275). A seminal study found that thalidomide exerts teratogenic effects during zebrafish development that resemble those found in human newborns whose mothers were treated with the drug (under the brand name Contergan) (165). Thalidomide was widely prescribed in Europe in the 1950s and 1960s as a sedative or anti-emetic during pregnancy. More than 10,000 children were born with severe upper and lower limb malformations because of potent teratogenic effects that occurred in humans, but were not observed in mice or rats (294). Interestingly, zebrafish treated with low levels of thalidomide exhibited dose-dependent growth defects during limb formation.
A thalidomide-binding protein, Cereblon (CRBN), was identified, and crbn loss-of-function in zebrafish mimicked the limb outgrowth defects seen with thalidomide treatment (165). Importantly, the limb defects in thalidomide-treated zebrafish embryos could be rescued by injecting mRNA encoding a mutated protein unable to bind thalidomide. In contrast, wild-type mRNA was unable to rescue the toxic effects, suggesting that CRBN is the direct target of thalidomide teratogenicity. These results are significant because they show that drug treatments in zebrafish can reveal teratogenic effects that may be overlooked when focusing teratogenicity studies exclusively on rodent models. In addition, the genetic tractability of zebrafish greatly facilitates the distinction of therapeutically relevant targets from secondary targets that mediate toxicity, raising the possibility to use this model to develop new generation drugs that are specific for the primary target.
Clinically, the benefits of some drugs outweigh their toxic effects, and these drugs remain an important therapeutic choice. One such example is doxorubicin, a chemotherapeutic of the anthracycline class that is used to treat malignancies, including breast cancer and lymphomas, but causes cardiomyopathy as an important adverse effect. Recently, zebrafish were leveraged to identify a potential adjuvant treatment that reverts doxorubicin-induced cardiomyopathy (219). First, the authors established a screening paradigm in which doxorubicin-induced cardiotoxicity could be scored by visually assessing contractility and blood circulation of live zebrafish larvae. Among 3,000 screened compounds, 8 were identified as chemicals that efficiently protected from cardiac dysfunction. The authors further evaluated two of the hit compounds, visnagin and diphenylurea, which showed highly potent cardioprotective effects. Importantly, these compounds did not interfere with the chemotherapeutic effects of doxorubicin in zebrafish and mouse xenotransplantation models of leukemia and breast cancer, respectively.
Whereas these and other examples constitute a proof of concept that some clinically relevant toxic or teratogenic effects can be similar between zebrafish and humans, it is less clear how widely zebrafish can be used for unbiased toxicity screening of new chemical entities. Any drug discovery pipeline would benefit tremendously from the ability to reliably predict toxic effects. Such a comprehensive forward toxicity profiling remains a major challenge for the future, and the zebrafish model promises to be part of the solution (111, 226).
F. Scaling-Up the Throughput
Although the exact definition of high-throughput screening (HTS) is not carved in stone, 10,000–100,000 compound tests per day is commonly considered to be at HTS scale. Common zebrafish screens average around 2,000–5,000 compounds in total, and in most cases at a single concentration. The largest screen published to date tested 30,000 small molecules over several months to identify compounds that interfere with signaling pathways critical for embryonic development (425).
A recent screen studying the formation of secondary islets in the pancreas was able to improve the automation of zebrafish handling, drug administration, and the quantification of the reporter to scale up the throughput to test ~3,000 compounds in 16 replicates and at 6 different concentrations, cumulating to 500,000 zebrafish exposed to chemicals (412). The titration of dosage allowed determination of dose-response curves and width of the therapeutic window, thereby adding valuable information to preselect the best hits before validating the compounds in counter screens. Despite this progress, the overall throughput is largely limited by several factors, including the number of embryos that can be produced, the selection of healthy individuals from a pool of wild-type or fluorescently labeled reporter zebrafish, the loading into microtiter plates, and ultimately the complexity of the readout.
In a different approach, a system was developed to inject Tuberculosis marinum bacteria into the yolk of developing embryos, followed by an automated “health check,” distribution of embryos into 96-well plates, and automated analysis of fluorescence intensity of the bacterial content (42). This approach can be combined with chemical screening to identify new regulators of bacterial replication within a host organism. Instead of microorganisms, the same setup can be used to inject morpholinos to knock down genes with sufficient throughput to screen on a transient loss-of-function model.
Other efforts are geared towards automating image analysis in zebrafish by addressing the challenge of orienting the larvae and visualizing fluorescently labeled structures in three dimensions (293, 309). An instrument named the VAST BioImager mounts on a confocal or fluorescent microscope, loads a zebrafish larva in a capillary tube, and moves it along the tube. The setup can be programmed to apply pattern-recognition software for automated, user-defined positioning and image capture.
So far, automated efforts have been focused on probing preselected chemical collections with mostly known bioactive effects and a high proportion of clinically approved drugs. The goals are of chemical genetic interest, for example, to dissect a signaling pathway or discover a lead compound (90). The restriction of the chemical collection to bioactive targets and the mostly academic perspective on what is considered a hit compound limits to some extent the necessity for HTS. Nevertheless, drug discovery approaches in zebrafish will benefit from an increasing level of sophistication, and efforts are underway to increase the throughput of compounds tested and data points that can be analyzed.
V. SUMMARY AND CONCLUSIONS
Zebrafish have developed at a breathtaking pace towards being a principal animal model in biomedical research. Studies of several zebrafish disease models have shown the high relevance of this approach, and some of the first drugs discovered in zebrafish are now in clinical trials further underscoring the translational impact of this model. Reverse genetic techniques have recently been added to the suite of genetic manipulations that can be performed easily, space- and cost-efficiently in zebrafish, opening up indefinite possibilities to design preclinical disease models. Many practical and technical considerations go into generating a new disease model, as summarized in Figure 4. Physiologists and clinicians within the zebrafish community can help with genetic design and rigorous phenotyping of an appropriate model. Generating gene knockout models for newly identified disease-associated SNPs will remain a cornerstone of zebrafish research in the years to come.
Several questions remain for the future. How much clinical relevance can we extract from studying a relatively simple, mostly monogenic, disease modeled in zebrafish? Certainly, finding solutions for complex diseases whose progression is defined by the confluence of environment, behavioral, nutritional, genetic, and epigenetic factors is extremely challenging. However, on the clinical side of biomedical research, a trend has emerged towards improving the resolution of human disease phenotyping, subcategorizing disease, and reducing population complexity to better translate knowledge from preclinical models to humans. This trend is known as personalized medicine, and the power of zebrafish genetics in conjunction with pharmacology will likely help better define personalized medicine approaches and the design of proof-of-concept studies in subpopulations of a disease.
Conducting preclinical research with a reliable impact on human disease is a scientific challenge. With this challenge comes the responsibility to not over-sell or mislead as patients anxiously await for breakthroughs that promise to improve the quality of life. Failures in translational research undermine trust and ultimately consent to perform trials. Thus, with the genetic and phenotyping capabilities currently available to the zebrafish community, rigorous research design is a must. Zebrafish are still frequently used to complement human genetics studies with morpholino experiments to “confirm” a biased hypothesis through assays that at best distantly resemble human biology, like yolk sac consumption as a readout for adipose tissue plasticity or pericardial edema for any kind of heart condition. The zebrafish community has the potential to meet the challenge for rigorous preclinical research, given its ability to generate hundreds or even thousands of gene knockouts and to systematically profile their phenotypes to relate them to disease conditions.
To date, the capacity for in-depth phenotyping when it comes to physiology and tissue-specific biochemistry still lags behind the level of resolution in rodents. However, rather than competing with preclinical rodent models, zebrafish provide a powerful complementary platform. With increasing depth of phenotyping paved by nanotechnology solutions and sophistication of high-throughput and high-content screening efforts, we expect the zebrafish to further improve its position as a prime preclinical animal model, and to benefit human health.
GRANTS
We thank the Max Planck Society for financial support. R. Arnaout was supported by National Heart, Lung, and Blood Institute Grant K08HL125945 and American Heart Association Grant 15GPSPG238300004.
DISCLOSURES
P. Gut is an employee of Nestlé Institute of Health Sciences, part of Nestlé Group. S. Reischauer, R. Arnaout, and D. Y. R. Stainier have no conflicts of interest, financial or otherwise.
ACKNOWLEDGMENTS
We apologize to the authors whose papers we could not reference due to space limitations.
Present addresses: P. Gut, Nestlé Institute of Health Sciences, EPFL Innovation Park, Bâtiment H, 1015 Lausanne, Switzerland (e-mail: Philipp.Gut@rd.nestle.com); S. Reischauer, Max Planck Institute for Heart and Lung Research, Bad Nauheim, Germany (e-mail: Sven.Reischauer@mpi-bn.mpg.de); R. Arnaout, Cardiovascular Research Institute and Div. of Cardiology, Dept. of Medicine, Univ. of California San Francisco, San Francisco, CA (e-mail: rima.arnaout@ucsf.edu).
Address for reprint requests and other correspondence: D. Y. R. Stainier, Max Planck Institute for Heart and Lung Research, Dept. of Developmental Genetics, Ludwigstrasse 43, D-61231 Bad Nauheim, Germany (e-mail: Didier.Stainier@mpi-bn.mpg.de).
REFERENCES
- 1.Alvarez Y, Chen K, Reynolds AL, Waghorne N, O’Connor JJ, Kennedy BN. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis Model Mech 3: 236–245, 2010. doi: 10.1242/dmm.003772. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N.. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev 13: 2713–2724, 1999. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JL, Carten JD, Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol 101: 111–141, 2011. doi: 10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KV, Jürgens G, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42: 779–789, 1985. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- 5.Andersson O, Adams BA, Yoo D, Ellis GC, Gut P, Anderson RM, German MS, Stainier DY. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab 15: 885–894, 2012. doi: 10.1016/j.cmet.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreux PA, Williams EG, Koutnikova H, Houtkooper RH, Champy MF, Henry H, Schoonjans K, Williams RW, Auwerx J. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell 150: 1287–1299, 2012. doi: 10.1016/j.cell.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki Y, Niihori T, Banjo T, Okamoto N, Mizuno S, Kurosawa K, Ogata T, Takada F, Yano M, Ando T, Hoshika T, Barnett C, Ohashi H, Kawame H, Hasegawa T, Okutani T, Nagashima T, Hasegawa S, Funayama R, Nagashima T, Nakayama K, Inoue S, Watanabe Y, Ogura T, Matsubara Y. Gain-of-function mutations in RIT1 cause Noonan syndrome, a RAS/MAPK pathway syndrome. Am J Hum Genet 93: 173–180, 2013. doi: 10.1016/j.ajhg.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong GA, Liao M, You Z, Lissouba A, Chen BE, Drapeau P. Homology directed knockin of point mutations in the zebrafish tardbp and fus genes in ALS using the CRISPR/Cas9 system. PLoS One 11: e0150188, 2016. doi: 10.1371/journal.pone.0150188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouzi M, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci USA 104: 11316–11321, 2007. doi: 10.1073/pnas.0702724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaout R, Reischauer S, Stainier DY. Recovery of adult zebrafish hearts for high-throughput applications. J Vis Exp 12: 94, 2014. doi: 10.3791/52248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science 330: 971–974, 2010. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- 12.Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, Kishimoto Y, Hibi M, Kawakami K. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci USA 105: 1255–1260, 2008. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kléber AG, Saffitz JE. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med 6: 240ra74, 2014. doi: 10.1126/scitranslmed.3008008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auer TO, Del Bene F. CRISPR/Cas9 and TALEN-mediated knock-in approaches in zebrafish. Methods 69: 142–150, 2014. doi: 10.1016/j.ymeth.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Austin-Tse C, Halbritter J, Zariwala MA, Gilberti RM, Gee HY, Hellman N, Pathak N, Liu Y, Panizzi JR, Patel-King RS, Tritschler D, Bower R, O’Toole E, Porath JD, Hurd TW, Chaki M, Diaz KA, Kohl S, Lovric S, Hwang DY, Braun DA, Schueler M, Airik R, Otto EA, Leigh MW, Noone PG, Carson JL, Davis SD, Pittman JE, Ferkol TW, Atkinson JJ, Olivier KN, Sagel SD, Dell SD, Rosenfeld M, Milla CE, Loges NT, Omran H, Porter ME, King SM, Knowles MR, Drummond IA, Hildebrandt F. Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am J Hum Genet 93: 672–686, 2013. doi: 10.1016/j.ajhg.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek JS, Fang L, Li AC, Miller YI. Ezetimibe and simvastatin reduce cholesterol levels in zebrafish larvae fed a high-cholesterol diet. Cholesterol 2012: 564705, 2012. doi: 10.1155/2012/564705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bainbridge MN, Davis EE, Choi WY, Dickson A, Martinez HR, Wang M, Dinh H, Muzny DM, Pignatelli R, Katsanis N, Boerwinkle E, Gibbs RA, Jefferies JL. Loss of function mutations in NNT are associated with left ventricular noncompaction. Circ Cardiovasc Genet 8: 544–552, 2015. doi: 10.1161/CIRCGENETICS.115.001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldessari D, Mione M. How to create the vascular tree? (Latest) help from the zebrafish. Pharmacol Ther 118: 206–230, 2008. doi: 10.1016/j.pharmthera.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Barut BA, Zon LI. Realizing the potential of zebrafish as a model for human disease. Physiol Genomics 2: 49–51, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2: 371–382, 2007. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker JR, Deo RC, Werdich AA, Panàkovà D, Coy S, MacRae CA. Human cardiomyopathy mutations induce myocyte hyperplasia and activate hypertrophic pathways during cardiogenesis in zebrafish. Dis Model Mech 4: 400–410, 2011. doi: 10.1242/dmm.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG II, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC. In vivo genome editing using a high-efficiency TALEN system. Nature 491: 114–118, 2012. doi: 10.1038/nature11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature 483: 531–533, 2012. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 24.Beis D, Bartman T, Jin SW, Scott IC, D’Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, Baier H, Tallafuss A, Bally-Cuif L, Chen JN, Stainier DY, Jungblut B. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132: 4193–4204, 2005. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- 25.Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep 14: 1062–1072, 2013. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benz A, Kossack M, Auth D, Seyler C, Zitron E, Juergensen L, Katus HA, Hassel D. miR-19b regulates ventricular action potential duration in zebrafish. Sci Rep 6: 36033, 2016. doi: 10.1038/srep36033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard-Kargar C, Ktorza A. Endocrine pancreas plasticity under physiological and pathological conditions. Diabetes 50, Suppl 1: S30–S35, 2001. doi: 10.2337/diabetes.50.2007.S30. [DOI] [PubMed] [Google Scholar]
- 28.Blum M, De Robertis EM, Wallingford JB, Niehrs C. Morpholinos: Antisense and Sensibility. Dev Cell 35: 145–149, 2015. doi: 10.1016/j.devcel.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326: 1509–1512, 2009. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 30.Bonetti M, Paardekooper Overman J, Tessadori F, Noël E, Bakkers J, den Hertog J. Noonan and LEOPARD syndrome Shp2 variants induce heart displacement defects in zebrafish. Development 141: 1961–1970, 2014. doi: 10.1242/dev.106310. [DOI] [PubMed] [Google Scholar]
- 31.Boutros M, Heigwer F, Laufer C. Microscopy-based high-content screening. Cell 163: 1314–1325, 2015. doi: 10.1016/j.cell.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Bowen ME, Henke K, Siegfried KR, Warman ML, Harris MP. Efficient mapping and cloning of mutations in zebrafish by low-coverage whole-genome sequencing. Genetics 190: 1017–1024, 2012. doi: 10.1534/genetics.111.136069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest 117: 2337–2346, 2007. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brand M, Heisenberg CP, Jiang YJ, Beuchle D, Lun K, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, van Eeden FJ, Nüsslein-Volhard C. Mutations in zebrafish genes affecting the formation of the boundary between midbrain and hindbrain. Development 123: 179–190, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Breslow JL. Mouse models of atherosclerosis. Science 272: 685–688, 1996. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 36.Brown DR, Samsa LA, Qian L, Liu J. Advances in the study of heart development and disease using zebrafish. J Cardiovasc Dev Dis 3: 13, 2016. doi: 10.3390/jcdd3020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brugada R, Hong K, Dumaine R, Cordeiro J, Gaita F, Borggrefe M, Menendez TM, Brugada J, Pollevick GD, Wolpert C, Burashnikov E, Matsuo K, Wu YS, Guerchicoff A, Bianchi F, Giustetto C, Schimpf R, Brugada P, Antzelevitch C. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation 109: 30–35, 2004. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 38.Bruni G, Rennekamp AJ, Velenich A, McCarroll M, Gendelev L, Fertsch E, Taylor J, Lakhani P, Lensen D, Evron T, Lorello PJ, Huang XP, Kolczewski S, Carey G, Caldarone BJ, Prinssen E, Roth BL, Keiser MJ, Peterson RT, Kokel D. Zebrafish behavioral profiling identifies multitarget antipsychotic-like compounds. Nat Chem Biol 12: 559–566, 2016. doi: 10.1038/nchembio.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger A, Lindsay H, Felker A, Hess C, Anders C, Chiavacci E, Zaugg J, Weber LM, Catena R, Jinek M, Robinson MD, Mosimann C. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 143: 2025–2037, 2016. doi: 10.1242/dev.134809. [DOI] [PubMed] [Google Scholar]
- 40.Bussmann J, Schulte-Merker S. Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development 138: 4327–4332, 2011. doi: 10.1242/dev.068080. [DOI] [PubMed] [Google Scholar]
- 41.Cao J, Navis A, Cox BD, Dickson AL, Gemberling M, Karra R, Bagnat M, Poss KD. Single epicardial cell transcriptome sequencing identifies Caveolin 1 as an essential factor in zebrafish heart regeneration. Development 143: 232–243, 2016. doi: 10.1242/dev.130534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho R, de Sonneville J, Stockhammer OW, Savage ND, Veneman WJ, Ottenhoff TH, Dirks RP, Meijer AH, Spaink HP. A high-throughput screen for tuberculosis progression. PLoS One 6: e16779, 2011. doi: 10.1371/journal.pone.0016779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39: e82, 2011. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nüsslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development 123: 293–302, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Chernyavskaya Y, Ebert AM, Milligan E, Garrity DM. Voltage-gated calcium channel CACNB2 (β2.1) protein is required in the heart for control of cell proliferation and heart tube integrity. Dev Dyn 241: 648–662, 2012. doi: 10.1002/dvdy.23746. [DOI] [PubMed] [Google Scholar]
- 46.Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin JE, Shaw RM, Martin GR, Stainier DY. Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci USA 107: 14662–14667, 2010. doi: 10.1073/pnas.0909432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol 6: e109, 2008. doi: 10.1371/journal.pbio.0060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Childs S, Weinstein BM, Mohideen MA, Donohue S, Bonkovsky H, Fishman MC. Zebrafish dracula encodes ferrochelatase and its mutation provides a model for erythropoietic protoporphyria. Curr Biol 10: 1001–1004, 2000. doi: 10.1016/S0960-9822(00)00653-9. [DOI] [PubMed] [Google Scholar]
- 49.Cho CH, Kim SS, Jeong MJ, Lee CO, Shin HS. The Na+ -Ca2+ exchanger is essential for embryonic heart development in mice. Mol Cells 10: 712–722, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ Jr. A clinical drug library screen identifies astemizole as an antimalarial agent. Nat Chem Biol 2: 415–416, 2006. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- 51.Chu CY, Chen CF, Rajendran RS, Shen CN, Chen TH, Yen CC, Chuang CK, Lin DS, Hsiao CD. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS One 7: e36474, 2012. doi: 10.1371/journal.pone.0036474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung WS, Andersson O, Row R, Kimelman D, Stainier DY. Suppression of Alk8-mediated Bmp signaling cell-autonomously induces pancreatic beta-cells in zebrafish. Proc Natl Acad Sci USA 107: 1142–1147, 2010. doi: 10.1073/pnas.0910205107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev Cell 15: 738–748, 2008. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung WS, Stainier DY. Intra-endodermal interactions are required for pancreatic beta cell induction. Dev Cell 14: 582–593, 2008. doi: 10.1016/j.devcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark KJ, Balciunas D, Pogoda HM, Ding Y, Westcot SE, Bedell VM, Greenwood TM, Urban MD, Skuster KJ, Petzold AM, Ni J, Nielsen AL, Patowary A, Scaria V, Sivasubbu S, Xu X, Hammerschmidt M, Ekker SC. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat Methods 8: 506–512, 2011. doi: 10.1038/nmeth.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clark KJ, Urban MD, Skuster KJ, Ekker SC. Transgenic zebrafish using transposable elements. Methods Cell Biol 104: 137–149, 2011. doi: 10.1016/B978-0-12-374814-0.00008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clay H, Coughlin SR. Mechanical vessel injury in zebrafish embryos. J Vis Exp 96: e52460, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clifton JD, Lucumi E, Myers MC, Napper A, Hama K, Farber SA, Smith AB III, Huryn DM, Diamond SL, Pack M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS One 5: e12386, 2010. doi: 10.1371/journal.pone.0012386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 332: 1519–1523, 2011. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collaborators GBD, Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, Brauer M, Burnett R, Cercy K, Charlson FJ, Cohen AJ, Dandona L, Estep K, Ferrari AJ, Frostad JJ, Fullman N, Gething PW, Godwin WW, Griswold M, Hay SI, Kinfu Y, Kyu HH, Larson HJ, Liang X, Lim SS, Liu PY, Lopez AD, Lozano R, Marczak L, Mensah GA, Mokdad AH, Moradi-Lakeh M, Naghavi M, Neal B, Reitsma MB, Roth GA, Salomon JA, Sur PJ, Vos T, Wagner JA, Wang H, Zhao Y, Zhou M, Aasvang GM, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulle AM, Abera SF, Abraham B, Abu-Raddad LJ, Abyu GY, Adebiyi AO, Adedeji IA, Ademi Z, Adou AK, Adsuar JC, Agardh EE, Agarwal A, Agrawal A, Kiadaliri AA, Ajala ON, Akinyemiju TF, Al-Aly Z, Alam K, Alam NKM, Aldhahri SF, Aldridge RW, Alemu ZA, Ali R, Alkerwi A, Alla F, Allebeck P, Alsharif U, Altirkawi KA, Martin EA, Alvis-Guzman N, Amare AT, Amberbir A, Amegah AK, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson BO, Antonio CAT, Anwari P, Ärnlöv J, Artaman A, Asayesh H, Asghar RJ, Assadi R, Atique S, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Azzopardi P, Bacha U, Badawi A, Bahit MC, Balakrishnan K, Barac A, Barber RM, Barker-Collo SL, Bärnighausen T, Barquera S, Barregard L, Barrero LH, Basu S, Batis C, Bazargan-Hejazi S, Beardsley J, Bedi N, Beghi E, Bell B, Bell ML, Bello AK, Bennett DA, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beyene AS, Bhala N, Bhansali A, Bhatt S, Biadgilign S, Bikbov B, Bisanzio D, Bjertness E, Blore JD, Borschmann R, Boufous S, Bourne RRA, Brainin M, Brazinova A, Breitborde NJK, Brenner H, Broday DM, Brugha TS, Brunekreef B, Butt ZA, Cahill LE, Calabria B, Campos-Nonato IR, Cárdenas R, Carpenter DO, Carrero JJ, Casey DC, Castañeda-Orjuela CA, Rivas JC, Castro RE, Catalá-López F, Chang J-C, Chiang PP-C, Chibalabala M, Chimed-Ochir O, Chisumpa VH, Chitheer AA, Choi J-YJ, Christensen H, Christopher DJ, Ciobanu LG, Coates MM, Colquhoun SM, Manzano AGC, Cooper LT, Cooperrider K, Cornaby L, Cortinovis M, Crump JA, Cuevas-Nasu L, Damasceno A, Dandona R, Darby SC, Dargan PI; GBD 2015 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1659–1724, 2016. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Collymore C, Rasmussen S, Tolwani RJ. Gavaging adult zebrafish. J Vis Exp 11: 78, 2013. doi: 10.3791/50691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cox AG, Hwang KL, Brown KK, Evason KJ, Beltz S, Tsomides A, O’Connor K, Galli GG, Yimlamai D, Chhangawala S, Yuan M, Lien EC, Wucherpfennig J, Nissim S, Minami A, Cohen DE, Camargo FD, Asara JM, Houvras Y, Stainier DY, Goessling W. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol 18: 886–896, 2016. doi: 10.1038/ncb3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cox AG, Saunders DC, Kelsey PB Jr, Conway AA, Tesmenitsky Y, Marchini JF, Brown KK, Stamler JS, Colagiovanni DB, Rosenthal GJ, Croce KJ, North TE, Goessling W. S-nitrosothiol signaling regulates liver development and improves outcome following toxic liver injury. Cell Reports 6: 56–69, 2014. doi: 10.1016/j.celrep.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cruz-Garcia L, Schlegel A. Lxr-driven enterocyte lipid droplet formation delays transport of ingested lipids. J Lipid Res 55: 1944–1958, 2014. doi: 10.1194/jlr.M052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Culp P, Nüsslein-Volhard C, Hopkins N. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc Natl Acad Sci USA 88: 7953–7957, 1991. doi: 10.1073/pnas.88.18.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DY. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236: 1025–1035, 2007. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- 67.Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J, Pelus LM, Desponts C, Chen YB, Rezner B, Armand P, Koreth J, Glotzbecker B, Ho VT, Alyea E, Isom M, Kao G, Armant M, Silberstein L, Hu P, Soiffer RJ, Scadden DT, Ritz J, Goessling W, North TE, Mendlein J, Ballen K, Zon LI, Antin JH, Shoemaker DD. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122: 3074–3081, 2013. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401, 2006. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 69.Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, Grunwald DJ. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet 8: e1002861, 2012. doi: 10.1371/journal.pgen.1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dalgin G, Prince VE. Differential levels of Neurod establish zebrafish endocrine pancreas cell fates. Dev Biol 402: 81–97, 2015. doi: 10.1016/j.ydbio.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dang M, Henderson RE, Garraway LA, Zon LI. Long-term drug administration in the adult zebrafish using oral gavage for cancer preclinical studies. Dis Model Mech 9: 811–820, 2016. doi: 10.1242/dmm.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delaunay F, Thisse C, Marchand O, Laudet V, Thisse B. An inherited functional circadian clock in zebrafish embryos. Science 289: 297–300, 2000. doi: 10.1126/science.289.5477.297. [DOI] [PubMed] [Google Scholar]
- 73.Dellefave LM, Pytel P, Mewborn S, Mora B, Guris DL, Fedson S, Waggoner D, Moskowitz I, McNally EM. Sarcomere mutations in cardiomyopathy with left ventricular hypertrabeculation. Circ Cardiovasc Genet 2: 442–449, 2009. doi: 10.1161/CIRCGENETICS.109.861955. [DOI] [PubMed] [Google Scholar]
- 74.Deo RC, Musso G, Tasan M, Tang P, Poon A, Yuan C, Felix JF, Vasan RS, Beroukhim R, De Marco T, Kwok PY, MacRae CA, Roth FP. Prioritizing causal disease genes using unbiased genomic features. Genome Biol 15: 534, 2014. doi: 10.1186/s13059-014-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dina C, Bouatia-Naji N, Tucker N, Delling FN, Toomer K, Durst R, Perrocheau M, Fernandez-Friera L, Solis J, Le Tourneau T, Chen M-H, Probst V, Bosse Y, Pibarot P, Zelenika D, Lathrop M, Hercberg S, Roussel R, Benjamin EJ, Bonnet F, Lo SH, Dolmatova E, Simonet F, Lecointe S, Kyndt F, Redon R, Le Marec H, Froguel P, Ellinor PT, Vasan RS, Bruneval P, Markwald RR, Norris RA, Milan DJ, Slaugenhaupt SA, Levine RA, Schott J-J, Hagege AA, Jeunemaitre X; PROMESA investigators; MVP-France; Leducq Transatlantic MITRAL Network . Genetic association analyses highlight biological pathways underlying mitral valve prolapse. Nat Genet 47: 1206–1211, 2015. doi: 10.1038/ng.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding Y, Liu W, Deng Y, Jomok B, Yang J, Huang W, Clark KJ, Zhong TP, Lin X, Ekker SC, Xu X. Trapping cardiac recessive mutants via expression-based insertional mutagenesis screening. Circ Res 112: 606–617, 2013. doi: 10.1161/CIRCRESAHA.112.300603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding Y, Long PA, Bos JM, Shih YH, Ma X, Sundsbak RS, Chen J, Jiang Y, Zhao L, Hu X, Wang J, Shi Y, Ackerman MJ, Lin X, Ekker SC, Redfield MM, Olson TM, Xu X. A modifier screen identifies DNAJB6 as a cardiomyopathy susceptibility gene. JCI Insight 1: 14, 2016. doi: 10.1172/jci.insight.88797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding Y, Sun X, Huang W, Hoage T, Redfield M, Kushwaha S, Sivasubbu S, Lin X, Ekker S, Xu X. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res 109: 658–669, 2011. doi: 10.1161/CIRCRESAHA.111.248260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong PD, Munson CA, Norton W, Crosnier C, Pan X, Gong Z, Neumann CJ, Stainier DY. Fgf10 regulates hepatopancreatic ductal system patterning and differentiation. Nat Genet 39: 397–402, 2007. doi: 10.1038/ng1961. [DOI] [PubMed] [Google Scholar]
- 80.Dong PD, Provost E, Leach SD, Stainier DY. Graded levels of Ptf1a differentially regulate endocrine and exocrine fates in the developing pancreas. Genes Dev 22: 1445–1450, 2008. doi: 10.1101/gad.1663208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 10: 252–256, 2000. doi: 10.1016/S0959-437X(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 82.Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science 346: 1258096, 2014. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 83.Doyle EL, Booher NJ, Standage DS, Voytas DF, Brendel VP, Vandyk JK, Bogdanove AJ. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res 40: W117–W122, 2012. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Amacher SL. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol 26: 702–708, 2008. doi: 10.1038/nbt1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Driever W, Fishman MC. The zebrafish: heritable disorders in transparent embryos. J Clin Invest 97: 1788–1794, 1996. doi: 10.1172/JCI118608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 123: 37–46, 1996. [DOI] [PubMed] [Google Scholar]
- 87.Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish 7: 205–213, 2010. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ebert AM, Hume GL, Warren KS, Cook NP, Burns CG, Mohideen MA, Siegal G, Yelon D, Fishman MC, Garrity DM. Calcium extrusion is critical for cardiac morphogenesis and rhythm in embryonic zebrafish hearts. Proc Natl Acad Sci USA 102: 17705–17710, 2005. doi: 10.1073/pnas.0502683102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eder J, Sedrani R, Wiesmann C. The discovery of first-in-class drugs: origins and evolution. Nat Rev Drug Discov 13: 577–587, 2014. doi: 10.1038/nrd4336. [DOI] [PubMed] [Google Scholar]
- 90.Eggert US. The why and how of phenotypic small-molecule screens. Nat Chem Biol 9: 206–209, 2013. doi: 10.1038/nchembio.1206. [DOI] [PubMed] [Google Scholar]
- 91.Eisen JS, Weston JA. Development of the neural crest in the zebrafish. Dev Biol 159: 50–59, 1993. doi: 10.1006/dbio.1993.1220. [DOI] [PubMed] [Google Scholar]
- 92.Eliceiri BP, Gonzalez AM, Baird A. Zebrafish model of the blood-brain barrier: morphological and permeability studies. Methods Mol Biol 686: 371–378, 2011. doi: 10.1007/978-1-60761-938-3_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elo B, Villano CM, Govorko D, White LA. Larval zebrafish as a model for glucose metabolism: expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J Mol Endocrinol 38: 433–440, 2007. doi: 10.1677/JME-06-0037. [DOI] [PubMed] [Google Scholar]
- 94.Falcinelli S, Picchietti S, Rodiles A, Cossignani L, Merrifield DL, Taddei AR, Maradonna F, Olivotto I, Gioacchini G, Carnevali O. Lactobacillus rhamnosus lowers zebrafish lipid content by changing gut microbiota and host transcription of genes involved in lipid metabolism. Sci Rep 5: 9336, 2015. doi: 10.1038/srep09336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Falcinelli S, Rodiles A, Unniappan S, Picchietti S, Gioacchini G, Merrifield DL, Carnevali O. Probiotic treatment reduces appetite and glucose level in the zebrafish model. Sci Rep 6: 18061, 2016. doi: 10.1038/srep18061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang L, Green SR, Baek JS, Lee SH, Ellett F, Deer E, Lieschke GJ, Witztum JL, Tsimikas S, Miller YI. In vivo visualization and attenuation of oxidized lipid accumulation in hypercholesterolemic zebrafish. J Clin Invest 121: 4861–4869, 2011. doi: 10.1172/JCI57755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S, Miller YI. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. J Biol Chem 285: 32343–32351, 2010. doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang L, Miller YI. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic Biol Med 53: 1411–1420, 2012. doi: 10.1016/j.freeradbiomed.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK, Halpern ME. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science 292: 1385–1388, 2001. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- 100.Field HA, Dong PD, Beis D, Stainier DY. Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol 261: 197–208, 2003. doi: 10.1016/S0012-1606(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 101.Flynn EJ III, Trent CM, Rawls JF. Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J Lipid Res 50: 1641–1652, 2009. doi: 10.1194/jlr.M800590-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foglia MJ, Cao J, Tornini VA, Poss KD. Multicolor mapping of the cardiomyocyte proliferation dynamics that construct the atrium. Development 143: 1688–1696, 2016. doi: 10.1242/dev.136606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Foglia MJ, Poss KD. Building and re-building the heart by cardiomyocyte proliferation. Development 143: 729–740, 2016. doi: 10.1242/dev.132910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foley JE, Maeder ML, Pearlberg J, Joung JK, Peterson RT, Yeh JR. Targeted mutagenesis in zebrafish using customized zinc-finger nucleases. Nat Protoc 4: 1855–1867, 2009. doi: 10.1038/nprot.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fraher D, Sanigorski A, Mellett NA, Meikle PJ, Sinclair AJ, Gibert Y. Zebrafish embryonic lipidomic analysis reveals that the yolk cell is metabolically active in processing lipid. Cell Reports 14: 1317–1329, 2016. doi: 10.1016/j.celrep.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 106.Freedman LP, Inglese J. The increasing urgency for standards in basic biologic research. Cancer Res 74: 4024–4029, 2014. doi: 10.1158/0008-5472.CAN-14-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 108.Fuentes F, Reynolds E, Lewellis SW, Venkiteswaran G, Knaut H. A plasmid set for efficient bacterial artificial chromosome (BAC) transgenesis in zebrafish. G3 (Bethesda) 6: 829–834, 2016. doi: 10.1534/g3.115.026344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gaiano N, Amsterdam A, Kawakami K, Allende M, Becker T, Hopkins N. Insertional mutagenesis and rapid cloning of essential genes in zebrafish. Nature 383: 829–832, 1996. doi: 10.1038/383829a0. [DOI] [PubMed] [Google Scholar]
- 110.Gallardo VE, Varshney GK, Lee M, Bupp S, Xu L, Shinn P, Crawford NP, Inglese J, Burgess SM. Phenotype-driven chemical screening in zebrafish for compounds that inhibit collective cell migration identifies multiple pathways potentially involved in metastatic invasion. Dis Model Mech 8: 565–576, 2015. doi: 10.1242/dmm.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gamse JT, Gorelick DA. Mixtures, metabolites, and mechanisms: understanding toxicology using zebrafish. Zebrafish 13: 377–378, 2016. doi: 10.1089/zeb.2016.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geisler R, Rauch G-J, Baier H, van Bebber F, Bross L, Dekens MPS, Finger K, Fricke C, Gates MA, Geiger H, Geiger-Rudolph S, Gilmour D, Glaser S, Gnügge L, Habeck H, Hingst K, Holley S, Keenan J, Kirn A, Knaut H, Lashkari D, Maderspacher F, Martyn U, Neuhauss S, Neumann C, Nicolson T, Pelegri F, Ray R, Rick JM, Roehl H, Roeser T, Schauerte HE, Schier AF, Schönberger U, Schönthaler H-B, Schulte-Merker S, Seydler C, Talbot WS, Weiler C, Nüsslein-Volhard C, Haffter P. A radiation hybrid map of the zebrafish genome. Nat Genet 23: 86–89, 1999. doi: 10.1038/12692. [DOI] [PubMed] [Google Scholar]
- 113.Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. eLife 4: 4, 2015. doi: 10.7554/eLife.05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Genge CE, Davidson WS, Tibbits GF. Adult teleost heart expresses two distinct troponin C paralogs: cardiac TnC and a novel and teleost-specific ssTnC in a chamber- and temperature-dependent manner. Physiol Genomics 45: 866–875, 2013. doi: 10.1152/physiolgenomics.00074.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gibert Y, Sassi-Messai S, Fini JB, Bernard L, Zalko D, Cravedi JP, Balaguer P, Andersson-Lendahl M, Demeneix B, Laudet V. Bisphenol A induces otolith malformations during vertebrate embryogenesis. BMC Dev Biol 11: 4, 2011. doi: 10.1186/1471-213X-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA III, Smith HO, Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329: 52–56, 2010. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 117.Goessling W, Stainier DY. Endoderm specification and liver development. Methods Cell Biol 134: 463–483, 2016. doi: 10.1016/bs.mcb.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 118.Grabher C, Joly JS, Wittbrodt J. Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol 77: 381–401, 2004. doi: 10.1016/S0091-679X(04)77021-1. [DOI] [PubMed] [Google Scholar]
- 119.Griffin A, Parajes S, Weger M, Zaucker A, Taylor AE, O’Neil DM, Müller F, Krone N. Ferredoxin 1b (Fdx1b) is the essential mitochondrial redox partner for cortisol biosynthesis in zebrafish. Endocrinology 157: 1122–1134, 2016. doi: 10.1210/en.2015-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grunwald DJ, Streisinger G. Induction of recessive lethal and specific locus mutations in the zebrafish with ethyl nitrosourea. Genet Res 59: 103–116, 1992. doi: 10.1017/S0016672300030317. [DOI] [PubMed] [Google Scholar]
- 121.Guan J, Mishra S, Qiu Y, Shi J, Trudeau K, Las G, Liesa M, Shirihai OS, Connors LH, Seldin DC, Falk RH, MacRae CA, Liao R. Lysosomal dysfunction and impaired autophagy underlie the pathogenesis of amyloidogenic light chain-mediated cardiotoxicity. EMBO Mol Med 6: 1493–1507, 2014. doi: 10.15252/emmm.201404190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gupta V, Poss KD. Clonally dominant cardiomyocytes direct heart morphogenesis. Nature 484: 479–484, 2012. doi: 10.1038/nature11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gut P. Targeting mitochondrial energy metabolism with TSPO ligands. Biochem Soc Trans 43: 537–542, 2015. doi: 10.1042/BST20150019. [DOI] [PubMed] [Google Scholar]
- 124.Gut P, Baeza-Raja B, Andersson O, Hasenkamp L, Hsiao J, Hesselson D, Akassoglou K, Verdin E, Hirschey MD, Stainier DY. Whole-organism screening for gluconeogenesis identifies activators of fasting metabolism. Nat Chem Biol 9: 97–104, 2013. doi: 10.1038/nchembio.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature 502: 489–498, 2013. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- 126.Gut P, Verdin E. Rejuvenating SIRT1 activators. Cell Metab 17: 635–637, 2013. doi: 10.1016/j.cmet.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 127.Gutierrez A, Pan L, Groen RW, Baleydier F, Kentsis A, Marineau J, Grebliunaite R, Kozakewich E, Reed C, Pflumio F, Poglio S, Uzan B, Clemons P, VerPlank L, An F, Burbank J, Norton S, Tolliday N, Steen H, Weng AP, Yuan H, Bradner JE, Mitsiades C, Look AT, Aster JC. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest 124: 644–655, 2014. doi: 10.1172/JCI65093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Haeussler M, Schönig K, Eckert H, Eschstruth A, Mianné J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, Joly JS, Concordet JP. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol 17: 148, 2016. doi: 10.1186/s13059-016-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, Kelsh RN, Furutani-Seiki M, Vogelsang E, Beuchle D, Schach U, Fabian C, Nüsslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 123: 1–36, 1996. [DOI] [PubMed] [Google Scholar]
- 130.Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nüsslein-Volhard C. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development 123: 95–102, 1996. [DOI] [PubMed] [Google Scholar]
- 131.Hao J, Williams CH, Webb ME, Hong CC. Large scale zebrafish-based in vivo small molecule screen. J Vis Exp 30: 46, 2010. doi: 10.3791/2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harrison MR, Bussmann J, Huang Y, Zhao L, Osorio A, Burns CG, Burns CE, Sucov HM, Siekmann AF, Lien CL. Chemokine-guided angiogenesis directs coronary vasculature formation in zebrafish. Dev Cell 33: 442–454, 2015. doi: 10.1016/j.devcel.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hassel D, Dahme T, Erdmann J, Meder B, Huge A, Stoll M, Just S, Hess A, Ehlermann P, Weichenhan D, Grimmler M, Liptau H, Hetzer R, Regitz-Zagrosek V, Fischer C, Nürnberg P, Schunkert H, Katus HA, Rottbauer W. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat Med 15: 1281–1288, 2009. doi: 10.1038/nm.2037. [DOI] [PubMed] [Google Scholar]
- 134.Hassel D, Scholz EP, Trano N, Friedrich O, Just S, Meder B, Weiss DL, Zitron E, Marquart S, Vogel B, Karle CA, Seemann G, Fishman MC, Katus HA, Rottbauer W. Deficient zebrafish ether-à-go-go-related gene channel gating causes short-QT syndrome in zebrafish reggae mutants. Circulation 117: 866–875, 2008. doi: 10.1161/CIRCULATIONAHA.107.752220. [DOI] [PubMed] [Google Scholar]
- 135.Hein SJ, Lehmann LH, Kossack M, Juergensen L, Fuchs D, Katus HA, Hassel D. Advanced echocardiography in adult zebrafish reveals delayed recovery of heart function after myocardial cryoinjury. PLoS One 10: e0122665, 2015. doi: 10.1371/journal.pone.0122665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heisenberg CP, Brand M, Jiang YJ, Warga RM, Beuchle D, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes involved in forebrain development in the zebrafish, Danio rerio. Development 123: 191–203, 1996. [DOI] [PubMed] [Google Scholar]
- 137.Helker CS, Schuermann A, Karpanen T, Zeuschner D, Belting HG, Affolter M, Schulte-Merker S, Herzog W. The zebrafish common cardinal veins develop by a novel mechanism: lumen ensheathment. Development 140: 2776–2786, 2013. doi: 10.1242/dev.091876. [DOI] [PubMed] [Google Scholar]
- 138.Helker CS, Schuermann A, Pollmann C, Chng SC, Kiefer F, Reversade B, Herzog W. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. eLife 4: e06726, 2015. doi: 10.7554/eLife.06726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356: 494–499, 1992. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 140.Henke K, Bowen ME, Harris MP. Identification of mutations in zebrafish using next-generation sequencing. Curr Protoc Mol Biol 104: 13, 2013. [DOI] [PubMed] [Google Scholar]
- 141.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science 326: 294–298, 2009. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 12: 551–564, 2011. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hesselson D, Anderson RM, Beinat M, Stainier DY. Distinct populations of quiescent and proliferative pancreatic beta-cells identified by HOTcre mediated labeling. Proc Natl Acad Sci USA 106: 14896–14901, 2009. doi: 10.1073/pnas.0906348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hill JH, Franzosa EA, Huttenhower C, Guillemin K. A conserved bacterial protein induces pancreatic beta cell expansion during zebrafish development. eLife 5: e20145, 2016. doi: 10.7554/eLife.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hisano Y, Sakuma T, Nakade S, Ohga R, Ota S, Okamoto H, Yamamoto T, Kawahara A. Precise in-frame integration of exogenous DNA mediated by CRISPR/Cas9 system in zebrafish. Sci Rep 5: 8841, 2015. doi: 10.1038/srep08841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ho SY, Lorent K, Pack M, Farber SA. Zebrafish fat-free is required for intestinal lipid absorption and Golgi apparatus structure. Cell Metab 3: 289–300, 2006. doi: 10.1016/j.cmet.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hoage T, Ding Y, Xu X. Quantifying cardiac functions in embryonic and adult zebrafish. Methods Mol Biol 843: 11–20, 2012. doi: 10.1007/978-1-61779-523-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hoage T, Sun X, Xu X. Functions of the Wnt/β-catenin pathway in an anemia-induced zebrafish model of cardiomyopathy are location dependent. Biochem Biophys Res Commun 415: 490–496, 2011. doi: 10.1016/j.bbrc.2011.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodatsu A, Konno T, Hayashi K, Funada A, Fujita T, Nagata Y, Fujino N, Kawashiri MA, Yamagishi M. Compound heterozygosity deteriorates phenotypes of hypertrophic cardiomyopathy with founder MYBPC3 mutation: evidence from patients and zebrafish models. Am J Physiol Heart Circ Physiol 307: H1594–H1604, 2014. doi: 10.1152/ajpheart.00637.2013. [DOI] [PubMed] [Google Scholar]
- 150.Hoegg S, Brinkmann H, Taylor JS, Meyer A. Phylogenetic timing of the fish-specific genome duplication correlates with the diversification of teleost fish. J Mol Evol 59: 190–203, 2004. doi: 10.1007/s00239-004-2613-z. [DOI] [PubMed] [Google Scholar]
- 151.Holtzinger A, Evans T. Gata5 and Gata6 are functionally redundant in zebrafish for specification of cardiomyocytes. Dev Biol 312: 613–622, 2007. doi: 10.1016/j.ydbio.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hoshijima K, Jurynec MJ, Grunwald DJ. Precise editing of the zebrafish genome made simple and efficient. Dev Cell 36: 654–667, 2016. doi: 10.1016/j.devcel.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Houbrechts AM, Delarue J, Gabriëls IJ, Sourbron J, Darras VM. Permanent deiodinase type 2 deficiency strongly perturbs zebrafish development, growth, and fertility. Endocrinology 157: 3668–3681, 2016. doi: 10.1210/en.2016-1077. [DOI] [PubMed] [Google Scholar]
- 154.Housden BE, Muhar M, Gemberling M, Gersbach CA, Stainier DY, Seydoux G, Mohr SE, Zuber J, Perrimon N. Loss-of-function genetic tools for animal models: cross-species and cross-platform differences. Nat Rev Genet 18: 24–40, 2017. doi: 10.1038/nrg.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503, 2013. [Erratum in Nature. 505: 248, 2014]. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hruscha A, Krawitz P, Rechenberg A, Heinrich V, Hecht J, Haass C, Schmid B. Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 140: 4982–4987, 2013. doi: 10.1242/dev.099085. [DOI] [PubMed] [Google Scholar]
- 158.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278, 2014. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DY, Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev 26: 282–293, 2012. doi: 10.1101/gad.180968.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huisken J, Stainier DY. Selective plane illumination microscopy techniques in developmental biology. Development 136: 1963–1975, 2009. doi: 10.1242/dev.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hurley IA, Mueller RL, Dunn KA, Schmidt EJ, Friedman M, Ho RK, Prince VE, Yang Z, Thomas MG, Coates MI. A new time-scale for ray-finned fish evolution. Proc Biol Sci 274: 489–498, 2007. doi: 10.1098/rspb.2006.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425: 633–637, 2003. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 163.Imrie D, Sadler KC. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev Dyn 239: 3013–3023, 2010. doi: 10.1002/dvdy.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Irion U, Krauss J, Nüsslein-Volhard C. Precise and efficient genome editing in zebrafish using the CRISPR/Cas9 system. Development 141: 4827–4830, 2014. doi: 10.1242/dev.115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science 327: 1345–1350, 2010. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 166.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, Duvigneau JC, Lindroos-Christensen J, Zapf TC, Amann S, Saluzzo S, Jantscher F, Stiedl P, Todoric J, Martins R, Oberkofler H, Müller S, Hauser-Kronberger C, Kenner L, Casanova E, Sutterlüty-Fall H, Bilban M, Miller K, Kozlov AV, Krempler F, Knapp S, Lumeng CN, Patsch W, Wagner O, Pospisilik JA, Esterbauer H. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 158: 25–40, 2014. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci USA 110: 13904–13909, 2013. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, Kim KW. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull 75: 619–628, 2008. doi: 10.1016/j.brainresbull.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 169.Jopling C, Sleep E, Raya M, Martí M, Raya A, Izpisúa Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464: 606–609, 2010. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Junker JP, Noël ES, Guryev V, Peterson KA, Shah G, Huisken J, McMahon AP, Berezikov E, Bakkers J, van Oudenaarden A. Genome-wide RNA Tomography in the zebrafish embryo. Cell 159: 662–675, 2014. doi: 10.1016/j.cell.2014.09.038. [DOI] [PubMed] [Google Scholar]
- 171.Jurczyk A, Roy N, Bajwa R, Gut P, Lipson K, Yang C, Covassin L, Racki WJ, Rossini AA, Phillips N, Stainier DY, Greiner DL, Brehm MA, Bortell R, diIorio P. Dynamic glucoregulation and mammalian-like responses to metabolic and developmental disruption in zebrafish. Gen Comp Endocrinol 170: 334–345, 2011. doi: 10.1016/j.ygcen.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Karanth S, Tran VM, Kuberan B, Schlegel A. Polyunsaturated fatty acyl-coenzyme As are inhibitors of cholesterol biosynthesis in zebrafish and mice. Dis Model Mech 6: 1365–1377, 2013. doi: 10.1242/dmm.013425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karanth S, Zinkhan EK, Hill JT, Yost HJ, Schlegel A. FOXN3 regulates hepatic glucose utilization. Cell Reports 15: 2745–2755, 2016. doi: 10.1016/j.celrep.2016.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karra R, Knecht AK, Kikuchi K, Poss KD. Myocardial NF-κB activation is essential for zebrafish heart regeneration. Proc Natl Acad Sci USA 112: 13255–13260, 2015. doi: 10.1073/pnas.1511209112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kawakami K, Shima A, Kawakami N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc Natl Acad Sci USA 97: 11403–11408, 2000. doi: 10.1073/pnas.97.21.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kelsh RN, Brand M, Jiang YJ, Heisenberg CP, Lin S, Haffter P, Odenthal J, Mullins MC, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Kane DA, Warga RM, Beuchle D, Vogelsang L, Nüsslein-Volhard C. Zebrafish pigmentation mutations and the processes of neural crest development. Development 123: 369–389, 1996. [DOI] [PubMed] [Google Scholar]
- 177.Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496: 494–497, 2013. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell 20: 397–404, 2011. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464: 601–605, 2010. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim SH, Speirs CK, Solnica-Krezel L, Ess KC. Zebrafish model of tuberous sclerosis complex reveals cell-autonomous and non-cell-autonomous functions of mutant tuberin. Dis Model Mech 4: 255–267, 2011. doi: 10.1242/dmm.005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim SH, Wu SY, Baek JI, Choi SY, Su Y, Flynn CR, Gamse JT, Ess KC, Hardiman G, Lipschutz JH, Abumrad NN, Rockey DC. A post-developmental genetic screen for zebrafish models of inherited liver disease. PLoS One 10: e0125980, 2015. doi: 10.1371/journal.pone.0125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 203: 253–310, 1995. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 183.Kimmel RA, Dobler S, Schmitner N, Walsen T, Freudenblum J, Meyer D. Diabetic pdx1-mutant zebrafish show conserved responses to nutrient overload and anti-glycemic treatment. Sci Rep 5: 14241, 2015. doi: 10.1038/srep14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kimura A, Ito-Satoh M, Hayashi T, Takahashi M, Arimura T. Molecular etiology of idiopathic cardiomyopathy in Asian populations. J Cardiol 37, Suppl 1: 139–146, 2001. [PubMed] [Google Scholar]
- 185.Kinkel MD, Eames SC, Philipson LH, Prince VE. Intraperitoneal injection into adult zebrafish. J Vis Exp 42: 2126, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Knöll R, Postel R, Wang J, Krätzner R, Hennecke G, Vacaru AM, Vakeel P, Schubert C, Murthy K, Rana BK, Kube D, Knöll G, Schäfer K, Hayashi T, Holm T, Kimura A, Schork N, Toliat MR, Nürnberg P, Schultheiss H-P, Schaper W, Schaper J, Bos E, Den Hertog J, van Eeden FJM, Peters PJ, Hasenfuss G, Chien KR, Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation 116: 515–525, 2007. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- 187.Koenighofer M, Hung CY, McCauley JL, Dallman J, Back EJ, Mihalek I, Gripp KW, Sol-Church K, Rusconi P, Zhang Z, Shi GX, Andres DA, Bodamer OA. Mutations in RIT1 cause Noonan syndrome - additional functional evidence and expanding the clinical phenotype. Clin Genet 89: 359–366, 2016. doi: 10.1111/cge.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell 32: 97–108, 2015. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, Macrae CA, Shoichet B, Peterson RT. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol 6: 231–237, 2010. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kokel D, Rennekamp AJ, Shah AH, Liebel U, Peterson RT. Behavioral barcoding in the cloud: embracing data-intensive digital phenotyping in neuropharmacology. Trends Biotechnol 30: 421–425, 2012. doi: 10.1016/j.tibtech.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kondrychyn I, Garcia-Lecea M, Emelyanov A, Parinov S, Korzh V. Genome-wide analysis of Tol2 transposon reintegration in zebrafish. BMC Genomics 10: 418, 2009. doi: 10.1186/1471-2164-10-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kupperman E, An S, Osborne N, Waldron S, Stainier DYR. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature 406: 192–195, 2000. doi: 10.1038/35018092. [DOI] [PubMed] [Google Scholar]
- 193.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236: 3088–3099, 2007. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 194.Lackey DE, Burk DH, Ali MR, Mostaedi R, Smith WH, Park J, Scherer PE, Seay SA, McCoin CS, Bonaldo P, Adams SH. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab 306: E233–E246, 2014. doi: 10.1152/ajpendo.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Laggner C, Kokel D, Setola V, Tolia A, Lin H, Irwin JJ, Keiser MJ, Cheung CY, Minor DL Jr, Roth BL, Peterson RT, Shoichet BK. Chemical informatics and target identification in a zebrafish phenotypic screen. Nat Chem Biol 8: 144–146, 2011. doi: 10.1038/nchembio.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lai YC, Chang WT, Lin KY, Liau I. Optical assessment of the cardiac rhythm of contracting cardiomyocytes in vitro and a pulsating heart in vivo for pharmacological screening. Biomed Opt Express 5: 1616–1625, 2014. doi: 10.1364/BOE.5.001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Langenbacher AD, Dong Y, Shu X, Choi J, Nicoll DA, Goldhaber JI, Philipson KD, Chen JN. Mutation in sodium-calcium exchanger 1 (NCX1) causes cardiac fibrillation in zebrafish. Proc Natl Acad Sci USA 102: 17699–17704, 2005. doi: 10.1073/pnas.0502679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Langheinrich U, Vacun G, Wagner T. Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol Appl Pharmacol 193: 370–382, 2003. doi: 10.1016/j.taap.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 199.Lauer M. A Look at Trends in NIH’s Model Organism Research. Bethesda, MD: NIH Extramural Nexus, 2016. [Google Scholar]
- 200.Law SH, Sargent TD. The serine-threonine protein kinase PAK4 is dispensable in zebrafish: identification of a morpholino-generated pseudophenotype. PLoS One 9: e100268, 2014. doi: 10.1371/journal.pone.0100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lawson ND. Reverse genetics in zebrafish: mutants, morphants, and moving forward. Trends Cell Biol 26: 77–79, 2016. doi: 10.1016/j.tcb.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 202.Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet 3: 674–682, 2002. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- 203.Lazic S, Scott IC. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev Biol 354: 123–133, 2011. doi: 10.1016/j.ydbio.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 204.Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, Giraldez AJ. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 503: 360–364, 2013. doi: 10.1038/nature12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Leibold S, Hammerschmidt M. Long-term hyperphagia and caloric restriction caused by low- or high-density husbandry have differential effects on zebrafish postembryonic development, somatic growth, fat accumulation and reproduction. PLoS One 10: e0120776, 2015. doi: 10.1371/journal.pone.0120776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lemieux GA, Keiser MJ, Sassano MF, Laggner C, Mayer F, Bainton RJ, Werb Z, Roth BL, Shoichet BK, Ashrafi K. In silico molecular comparisons of C. elegans and mammalian pharmacology identify distinct targets that regulate feeding. PLoS Biol 11: e1001712, 2013. doi: 10.1371/journal.pbio.1001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leshchiner I, Alexa K, Kelsey P, Adzhubei I, Austin-Tse CA, Cooney JD, Anderson H, King MJ, Stottmann RW, Garnaas MK, Ha S, Drummond IA, Paw BH, North TE, Beier DR, Goessling W, Sunyaev SR. Mutation mapping and identification by whole-genome sequencing. Genome Res 22: 1541–1548, 2012. doi: 10.1101/gr.135541.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li J, Casteels T, Frogne T, Ingvorsen C, Honoré C, Courtney M, Huber KV, Schmitner N, Kimmel RA, Romanov RA, Sturtzel C, Lardeau CH, Klughammer J, Farlik M, Sdelci S, Vieira A, Avolio F, Briand F, Baburin I, Májek P, Pauler FM, Penz T, Stukalov A, Gridling M, Parapatics K, Barbieux C, Berishvili E, Spittler A, Colinge J, Bennett KL, Hering S, Sulpice T, Bock C, Distel M, Harkany T, Meyer D, Superti-Furga G, Collombat P, Hecksher-Sørensen J, Kubicek S. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell 168: 86–100.e15, 2017. doi: 10.1016/j.cell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Li M, Page-McCaw P, Chen W. FGF1 mediates overnutrition-induced compensatory β-cell differentiation. Diabetes 65: 96–109, 2016. doi: 10.2337/db15-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Li MA, Pettitt SJ, Eckert S, Ning Z, Rice S, Cadiñanos J, Yusa K, Conte N, Bradley A. The piggyBac transposon displays local and distant reintegration preferences and can cause mutations at noncanonical integration sites. Mol Cell Biol 33: 1317–1330, 2013. doi: 10.1128/MCB.00670-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li P, Lahvic JL, Binder V, Pugach EK, Riley EB, Tamplin OJ, Panigrahy D, Bowman TV, Barrett FG, Heffner GC, McKinney-Freeman S, Schlaeger TM, Daley GQ, Zeldin DC, Zon LI. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature 523: 468–471, 2015. doi: 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8: 353–367, 2007. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- 213.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 7: 251–264, 2010. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 214.Lim S, Wang Y, Yu X, Huang Y, Featherstone MS, Sampath K. A simple strategy for heritable chromosomal deletions in zebrafish via the combinatorial action of targeting nucleases. Genome Biol 14: R69, 2013. doi: 10.1186/gb-2013-14-7-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lin S, Gaiano N, Culp P, Burns JC, Friedmann T, Yee JK, Hopkins N. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science 265: 666–669, 1994. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- 216.Little AG, Seebacher F. Thyroid hormone regulates cardiac performance during cold acclimation in zebrafish (Danio rerio). J Exp Biol 217: 718–725, 2014. doi: 10.1242/jeb.096602. [DOI] [PubMed] [Google Scholar]
- 217.Liu C, Gates KP, Fang L, Amar MJ, Schneider DA, Geng H, Huang W, Kim J, Pattison J, Zhang J, Witztum JL, Remaley AT, Dong PD, Miller YI. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis Model Mech 8: 989–998, 2015. doi: 10.1242/dmm.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu J, Bressan M, Hassel D, Huisken J, Staudt D, Kikuchi K, Poss KD, Mikawa T, Stainier DY. A dual role for ErbB2 signaling in cardiac trabeculation. Development 137: 3867–3875, 2010. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liu Y, Asnani A, Zou L, Bentley VL, Yu M, Wang Y, Dellaire G, Sarkar KS, Dai M, Chen HH, Sosnovik DE, Shin JT, Haber DA, Berman JN, Chao W, Peterson RT. Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase. Sci Transl Med 6: 266ra170, 2014. doi: 10.1126/scitranslmed.3010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Long L, Guo H, Yao D, Xiong K, Li Y, Liu P, Zhu Z, Liu D. Regulation of transcriptionally active genes via the catalytically inactive Cas9 in C. elegans and D. rerio. Cell Res 25: 638–641, 2015. doi: 10.1038/cr.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10: 686–690, 2013. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 222.Lorent K, Gong W, Koo KA, Waisbourd-Zinman O, Karjoo S, Zhao X, Sealy I, Kettleborough RN, Stemple DL, Windsor PA, Whittaker SJ, Porter JR, Wells RG, Pack M. Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med 7: 286ra67, 2015. doi: 10.1126/scitranslmed.aaa1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJL, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012. [Erratum in Lancet 381: 628, 2013]. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lucas ME, Müller F, Rüdiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development 133: 4015–4024, 2006. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- 225.Lundby A, Rossin EJ, Steffensen AB, Acha MR, Newton-Cheh C, Pfeufer A, Lynch SN, Olesen S-P, Brunak S, Ellinor PT, Jukema JW, Trompet S, Ford I, Macfarlane PW, Krijthe BP, Hofman A, Uitterlinden AG, Stricker BH, Nathoe HM, Spiering W, Daly MJ, Asselbergs FW, van der Harst P, Milan DJ, de Bakker PIW, Lage K, Olsen JV; QT Interval International GWAS Consortium (QT-IGC) . Annotation of loci from genome-wide association studies using tissue-specific quantitative interaction proteomics. Nat Methods 11: 868–874, 2014. doi: 10.1038/nmeth.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov 14: 721–731, 2015. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- 227.Maddison LA, Chen W. Nutrient excess stimulates β-cell neogenesis in zebrafish. Diabetes 61: 2517–2524, 2012. doi: 10.2337/db11-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Maddison LA, Joest KE, Kammeyer RM, Chen W. Skeletal muscle insulin resistance in zebrafish induces alterations in β-cell number and glucose tolerance in an age- and diet-dependent manner. Am J Physiol Endocrinol Metab 308: E662–E669, 2015. doi: 10.1152/ajpendo.00441.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Maeder ML, Thibodeau-Beganny S, Osiak A, Wright DA, Anthony RM, Eichtinger M, Jiang T, Foley JE, Winfrey RJ, Townsend JA, Unger-Wallace E, Sander JD, Müller-Lerch F, Fu F, Pearlberg J, Göbel C, Dassie JP, Pruett-Miller SM, Porteus MH, Sgroi DC, Iafrate AJ, Dobbs D, McCray PB Jr, Cathomen T, Voytas DF, Joung JK. Rapid “open-source” engineering of customized zinc-finger nucleases for highly efficient gene modification. Mol Cell 31: 294–301, 2008. doi: 10.1016/j.molcel.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, Burns CE, Burns CG, MacRae CA, Poss KD, Lee RT. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev Cell 34: 387–399, 2015. doi: 10.1016/j.devcel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods 10: 957–963, 2013. doi: 10.1038/nmeth.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Manfroid I, Delporte F, Baudhuin A, Motte P, Neumann CJ, Voz ML, Martial JA, Peers B. Reciprocal endoderm-mesoderm interactions mediated by fgf24 and fgf10 govern pancreas development. Development 134: 4011–4021, 2007. doi: 10.1242/dev.007823. [DOI] [PubMed] [Google Scholar]
- 233.Manfroid I, Ghaye A, Naye F, Detry N, Palm S, Pan L, Ma TP, Huang W, Rovira M, Martial JA, Parsons MJ, Moens CB, Voz ML, Peers B. Zebrafish sox9b is crucial for hepatopancreatic duct development and pancreatic endocrine cell regeneration. Dev Biol 366: 268–278, 2012. doi: 10.1016/j.ydbio.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Marín-Juez R, Jong-Raadsen S, Yang S, Spaink HP. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J Endocrinol 222: 229–241, 2014. doi: 10.1530/JOE-14-0178. [DOI] [PubMed] [Google Scholar]
- 235.Martin ED, Moriarty MA, Byrnes L, Grealy M. Plakoglobin has both structural and signalling roles in zebrafish development. Dev Biol 327: 83–96, 2009. doi: 10.1016/j.ydbio.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 236.Masca NG, Hensor EM, Cornelius VR, Buffa FM, Marriott HM, Eales JM, Messenger MP, Anderson AE, Boot C, Bunce C, Goldin RD, Harris J, Hinchliffe RF, Junaid H, Kingston S, Martin-Ruiz C, Nelson CP, Peacock J, Seed PT, Shinkins B, Staples KJ, Toombs J, Wright AK, Teare MD. RIPOSTE: a framework for improving the design and analysis of laboratory-based research. eLife 4: 05519, 2015. doi: 10.7554/eLife.05519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Matrone G, Maqsood S, Taylor J, Mullins JJ, Tucker CS, Denvir MA. Targeted laser ablation of the zebrafish larval heart induces models of heart block, valvular regurgitation, and outflow tract obstruction. Zebrafish 11: 536–541, 2014. doi: 10.1089/zeb.2014.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Matthews RP, Lorent K, Mañoral-Mobias R, Huang Y, Gong W, Murray IV, Blair IA, Pack M. TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase. Development 136: 865–875, 2009. doi: 10.1242/dev.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.McCarroll MN, Gendelev L, Keiser MJ, Kokel D. Leveraging large-scale behavioral profiling in zebrafish to explore neuroactive polypharmacology. ACS Chem Biol 11: 842–849, 2016. doi: 10.1021/acschembio.5b00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 353: aaf7907, 2016. doi: 10.1126/science.aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.McMenamin SK, Minchin JE, Gordon TN, Rawls JF, Parichy DM. Dwarfism and increased adiposity in the gh1 mutant zebrafish vizzini. Endocrinology 154: 1476–1487, 2013. doi: 10.1210/en.2012-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol 26: 695–701, 2008. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Michel M, Page-McCaw PS, Chen W, Cone RD. Leptin signaling regulates glucose homeostasis, but not adipostasis, in the zebrafish. Proc Natl Acad Sci USA 113: 3084–3089, 2016. doi: 10.1073/pnas.1513212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mickoleit M, Schmid B, Weber M, Fahrbach FO, Hombach S, Reischauer S, Huisken J. High-resolution reconstruction of the beating zebrafish heart. Nat Methods 11: 919–922, 2014. doi: 10.1038/nmeth.3037. [DOI] [PubMed] [Google Scholar]
- 245.Milan DJ, Giokas AC, Serluca FC, Peterson RT, MacRae CA. Notch1b and neuregulin are required for specification of central cardiac conduction tissue. Development 133: 1125–1132, 2006. doi: 10.1242/dev.02279. [DOI] [PubMed] [Google Scholar]
- 246.Milan DJ, Jones IL, Ellinor PT, MacRae CA. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am J Physiol Heart Circ Physiol 291: H269–H273, 2006. doi: 10.1152/ajpheart.00960.2005. [DOI] [PubMed] [Google Scholar]
- 247.Milan DJ, Kim AM, Winterfield JR, Jones IL, Pfeufer A, Sanna S, Arking DE, Amsterdam AH, Sabeh KM, Mably JD, Rosenbaum DS, Peterson RT, Chakravarti A, Kääb S, Roden DM, MacRae CA. Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization. Circulation 120: 553–559, 2009. doi: 10.1161/CIRCULATIONAHA.108.821082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 107: 1355–1358, 2003. doi: 10.1161/01.CIR.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 249.Miller AC, Obholzer ND, Shah AN, Megason SG, Moens CB. RNA-seq-based mapping and candidate identification of mutations from forward genetic screens. Genome Res 23: 679–686, 2013. doi: 10.1101/gr.147322.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, Dulay GP, Hua KL, Ankoudinova I, Cost GJ, Urnov FD, Zhang HS, Holmes MC, Zhang L, Gregory PD, Rebar EJ. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29: 143–148, 2011. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 251.Minchin JE, Dahlman I, Harvey CJ, Mejhert N, Singh MK, Epstein JA, Arner P, Torres-Vázquez J, Rawls JF. Plexin D1 determines body fat distribution by regulating the type V collagen microenvironment in visceral adipose tissue. Proc Natl Acad Sci USA 112: 4363–4368, 2015. doi: 10.1073/pnas.1416412112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Minchin JE, Rawls JF. In vivo analysis of white adipose tissue in zebrafish. Methods Cell Biol 105: 63–86, 2011. doi: 10.1016/B978-0-12-381320-6.00003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mishra S, Guan J, Plovie E, Seldin DC, Connors LH, Merlini G, Falk RH, MacRae CA, Liao R. Human amyloidogenic light chain proteins result in cardiac dysfunction, cell death, and early mortality in zebrafish. Am J Physiol Heart Circ Physiol 305: H95–H103, 2013. doi: 10.1152/ajpheart.00186.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Moens CB, Donn TM, Wolf-Saxon ER, Ma TP. Reverse genetics in zebrafish by TILLING. Brief Funct Genomics Proteomics 7: 454–459, 2008. doi: 10.1093/bfgp/eln046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Moller DE. Metabolic disease drug discovery- “hitting the target” is easier said than done. Cell Metab 15: 19–24, 2012. doi: 10.1016/j.cmet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 256.Morgan TH. Random segregation versus coupling in mendelian inheritance. Science 34: 384, 1911. doi: 10.1126/science.34.873.384. [DOI] [PubMed] [Google Scholar]
- 257.Moriarty MA, Ryan R, Lalor P, Dockery P, Byrnes L, Grealy M. Loss of plakophilin 2 disrupts heart development in zebrafish. Int J Dev Biol 56: 711–718, 2012. doi: 10.1387/ijdb.113390mm. [DOI] [PubMed] [Google Scholar]
- 258.Morrison MA, Zimmerman MW, Look AT, Stewart RA. Studying the peripheral sympathetic nervous system and neuroblastoma in zebrafish. Methods Cell Biol 134: 97–138, 2016. doi: 10.1016/bs.mcb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 260.Moss JB, Koustubhan P, Greenman M, Parsons MJ, Walter I, Moss LG. Regeneration of the pancreas in adult zebrafish. Diabetes 58: 1844–1851, 2009. doi: 10.2337/db08-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Moss LG, Caplan TV, Moss JB. Imaging beta cell regeneration and interactions with islet vasculature in transparent adult zebrafish. Zebrafish 10: 249–257, 2013. doi: 10.1089/zeb.2012.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–2223, 2012. [Erratum in Lancet 381: 628, 2013]. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 263.Mussolino C, Morbitzer R, Lütge F, Dannemann N, Lahaye T, Cathomen T. A novel TALE nuclease scaffold enables high genome editing activity in combination with low toxicity. Nucleic Acids Res 39: 9283–9293, 2011. doi: 10.1093/nar/gkr597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Muto A, Calof AL, Lander AD, Schilling TF. Multifactorial origins of heart and gut defects in nipbl-deficient zebrafish, a model of Cornelia de Lange Syndrome. PLoS Biol 9: e1001181, 2011. doi: 10.1371/journal.pbio.1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nagayoshi S, Hayashi E, Abe G, Osato N, Asakawa K, Urasaki A, Horikawa K, Ikeo K, Takeda H, Kawakami K. Insertional mutagenesis by the Tol2 transposon-mediated enhancer trap approach generated mutations in two developmental genes: tcf7 and synembryn-like. Development 135: 159–169, 2008. doi: 10.1242/dev.009050. [DOI] [PubMed] [Google Scholar]
- 266.Nair G, Hebrok M. Islet formation in mice and men: lessons for the generation of functional insulin-producing β-cells from human pluripotent stem cells. Curr Opin Genet Dev 32: 171–180, 2015. doi: 10.1016/j.gde.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26: 216–220, 2000. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 268.Nath AK, Ryu JH, Jin YN, Roberts LD, Dejam A, Gerszten RE, Peterson RT. PTPMT1 inhibition lowers glucose through succinate dehydrogenase phosphorylation. Cell Reports 10: 694–701, 2015. doi: 10.1016/j.celrep.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Naye F, Voz ML, Detry N, Hammerschmidt M, Peers B, Manfroid I. Essential roles of zebrafish bmp2a, fgf10, and fgf24 in the specification of the ventral pancreas. Mol Biol Cell 23: 945–954, 2012. doi: 10.1091/mbc.E11-08-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, Abdelilah S, Stainier DY, Driever W. Mutations affecting craniofacial development in zebrafish. Development 123: 357–367, 1996. [DOI] [PubMed] [Google Scholar]
- 271.Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, Nüsslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron 20: 271–283, 1998. doi: 10.1016/S0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 272.Ninov N, Borius M, Stainier DY. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 139: 1557–1567, 2012. doi: 10.1242/dev.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ninov N, Hesselson D, Gut P, Zhou A, Fidelin K, Stainier DY. Metabolic regulation of cellular plasticity in the pancreas. Curr Biol 23: 1242–1250, 2013. doi: 10.1016/j.cub.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nolte H, Hölper S, Housley MP, Islam S, Piller T, Konzer A, Stainier DY, Braun T, Krüger M. Dynamics of zebrafish fin regeneration using a pulsed SILAC approach. Proteomics 15: 739–751, 2015. doi: 10.1002/pmic.201400316. [DOI] [PubMed] [Google Scholar]
- 275.North TE, Babu IR, Vedder LM, Lord AM, Wishnok JS, Tannenbaum SR, Zon LI, Goessling W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc Natl Acad Sci USA 107: 17315–17320, 2010. doi: 10.1073/pnas.1008209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, Fitzgerald GA, Daley GQ, Orkin SH, Zon LI. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447: 1007–1011, 2007. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Novodvorsky P, Watson O, Gray C, Wilkinson RN, Reeve S, Smythe C, Beniston R, Plant K, Maguire R, M K Rothman A, Elworthy S, van Eeden FJ, Chico TJ, Rothman MKA, Elworthy S, van Eeden FJM, Chico TJA. klf2ash317 mutant zebrafish do not recapitulate morpholino-induced vascular and haematopoietic phenotypes. PLoS One 10: e0141611, 2015. doi: 10.1371/journal.pone.0141611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nurse P. Cyclin dependent kinases and cell cycle control (nobel lecture). ChemBioChem 3: 596–603, 2002. doi:. [DOI] [PubMed] [Google Scholar]
- 279.Nussbaum JM, Liu LJ, Hasan SA, Schaub M, McClendon A, Stainier DY, Sakaguchi TF. Homeostatic generation of reactive oxygen species protects the zebrafish liver from steatosis. Hepatology 58: 1326–1338, 2013. doi: 10.1002/hep.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nüsslein-Volhard C, Kluding H, Jürgens G. Genes affecting the segmental subdivision of the Drosophila embryo. Cold Spring Harb Symp Quant Biol 50: 145–154, 1985. doi: 10.1101/SQB.1985.050.01.020. [DOI] [PubMed] [Google Scholar]
- 281.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801, 1980. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 282.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, Rajput B, Robbertse B, Smith-White B, Ako-Adjei D, Astashyn A, Badretdin A, Bao Y, Blinkova O, Brover V, Chetvernin V, Choi J, Cox E, Ermolaeva O, Farrell CM, Goldfarb T, Gupta T, Haft D, Hatcher E, Hlavina W, Joardar VS, Kodali VK, Li W, Maglott D, Masterson P, McGarvey KM, Murphy MR, O’Neill K, Pujar S, Rangwala SH, Rausch D, Riddick LD, Schoch C, Shkeda A, Storz SS, Sun H, Thibaud-Nissen F, Tolstoy I, Tully RE, Vatsan AR, Wallin C, Webb D, Wu W, Landrum MJ, Kimchi A, Tatusova T, DiCuccio M, Kitts P, Murphy TD, Pruitt KD. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res 44: D733–D745, 2016. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Ober EA, Field HA, Stainier DY. From endoderm formation to liver and pancreas development in zebrafish. Mech Dev 120: 5–18, 2003. doi: 10.1016/S0925-4773(02)00327-1. [DOI] [PubMed] [Google Scholar]
- 284.Ocorr K, Fink M, Cammarato A, Bernstein S, Bodmer R. Semi-automated optical heartbeat analysis of small hearts. J Vis Exp 31: 1435, 2009. doi: 10.3791/1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, Umemoto N, Kuroyanagi J, Nishimura N, Tanaka T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol 10: 21, 2010. doi: 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Olsen AS, Sarras MP Jr, Leontovich A, Intine RV. Heritable transmission of diabetic metabolic memory in zebrafish correlates with DNA hypomethylation and aberrant gene expression. Diabetes 61: 485–491, 2012. doi: 10.2337/db11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Opitz R, Maquet E, Huisken J, Antonica F, Trubiroha A, Pottier G, Janssens V, Costagliola S. Transgenic zebrafish illuminate the dynamics of thyroid morphogenesis and its relationship to cardiovascular development. Dev Biol 372: 203–216, 2012. doi: 10.1016/j.ydbio.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 288.Ordas A, Raterink RJ, Cunningham F, Jansen HJ, Wiweger MI, Jong-Raadsen S, Bos S, Bates RH, Barros D, Meijer AH, Vreeken RJ, Ballell-Pages L, Dirks RP, Hankemeier T, Spaink HP. Testing tuberculosis drug efficacy in a zebrafish high-throughput translational medicine screen. Antimicrob Agents Chemother 59: 753–762, 2015. doi: 10.1128/AAC.03588-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Orr N, Arnaout R, Gula LJ, Spears DA, Leong-Sit P, Li Q, Tarhuni W, Reischauer S, Chauhan VS, Borkovich M, Uppal S, Adler A, Coughlin SR, Stainier DY, Gollob MH. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat Commun 7: 11303, 2016. doi: 10.1038/ncomms11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Otsuna H, Hutcheson DA, Duncan RN, McPherson AD, Scoresby AN, Gaynes BF, Tong Z, Fujimoto E, Kwan KM, Chien CB, Dorsky RI. High-resolution analysis of central nervous system expression patterns in zebrafish Gal4 enhancer-trap lines. Dev Dyn 244: 785–796, 2015. doi: 10.1002/dvdy.24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Paardekooper Overman J, Yi JS, Bonetti M, Soulsby M, Preisinger C, Stokes MP, Hui L, Silva JC, Overvoorde J, Giansanti P, Heck AJ, Kontaridis MI, den Hertog J, Bennett AM. PZR coordinates Shp2 Noonan and LEOPARD syndrome signaling in zebrafish and mice. Mol Cell Biol 34: 2874–2889, 2014. doi: 10.1128/MCB.00135-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pack M, Solnica-Krezel L, Malicki J, Neuhauss SC, Schier AF, Stemple DL, Driever W, Fishman MC. Mutations affecting development of zebrafish digestive organs. Development 123: 321–328, 1996. [DOI] [PubMed] [Google Scholar]
- 293.Pardo-Martin C, Chang TY, Koo BK, Gilleland CL, Wasserman SC, Yanik MF. High-throughput in vivo vertebrate screening. Nat Methods 7: 634–636, 2010. doi: 10.1038/nmeth.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med 5: 582–585, 1999. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 295.Parrie LE, Renfrew EM, Wal AV, Mueller RL, Garrity DM. Zebrafish tbx5 paralogs demonstrate independent essential requirements in cardiac and pectoral fin development. Dev Dyn 242: 485–502, 2013. doi: 10.1002/dvdy.23953. [DOI] [PubMed] [Google Scholar]
- 296.Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev 126: 898–912, 2009. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Patton EE, Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet 2: 956–966, 2001. doi: 10.1038/35103567. [DOI] [PubMed] [Google Scholar]
- 298.Peal DS, Mills RW, Lynch SN, Mosley JM, Lim E, Ellinor PT, January CT, Peterson RT, Milan DJ. Novel chemical suppressors of long QT syndrome identified by an in vivo functional screen. Circulation 123: 23–30, 2011. doi: 10.1161/CIRCULATIONAHA.110.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pérez-Victoria FJ, Schindler C, Magadán JG, Mardones GA, Delevoye C, Romao M, Raposo G, Bonifacino JS. Ang2/fat-free is a conserved subunit of the Golgi-associated retrograde protein complex. Mol Biol Cell 21: 3386–3395, 2010. doi: 10.1091/mbc.E10-05-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Perles Z, Moon S, Ta-Shma A, Yaacov B, Francescatto L, Edvardson S, Rein AJ, Elpeleg O, Katsanis N. A human laterality disorder caused by a homozygous deleterious mutation in MMP21. J Med Genet 52: 840–847, 2015. doi: 10.1136/jmedgenet-2015-103336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pestel J, Ramadass R, Gauvrit S, Helker C, Herzog W, Stainier DY. Real-time 3D visualization of cellular rearrangements during cardiac valve formation. Development 143: 2217–2227, 2016. doi: 10.1242/dev.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol 22: 595–599, 2004. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 303.Pham LN, Kanther M, Semova I, Rawls JF. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc 3: 1862–1875, 2008. doi: 10.1038/nprot.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Pisharath H, Rhee JM, Swanson MA, Leach SD, Parsons MJ. Targeted ablation of beta cells in the embryonic zebrafish pancreas using E. coli nitroreductase. Mech Dev 124: 218–229, 2007. doi: 10.1016/j.mod.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science 331: 1078–1080, 2011. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet 11: 710–722, 2010. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science 298: 2188–2190, 2002. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 308.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, Zeiher AM, Dimmeler S. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev 21: 2644–2658, 2007. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pulak R. Tools for automating the imaging of zebrafish larvae. Methods 96: 118–126, 2016. doi: 10.1016/j.ymeth.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 310.Quach HNB, Tao S, Vrljicak P, Joshi A, Ruan H, Sukumaran R, Varshney GK, LaFave MC, Burgess SM, Winkler C, Emelyanov A, Parinov S, Sampath K; Ds Screen Team . A multifunctional mutagenesis system for analysis of gene function in zebrafish. G3 (Bethesda) 5: 1283–1299, 2015. doi: 10.1534/g3.114.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Ramspacher C, Steed E, Boselli F, Ferreira R, Faggianelli N, Roth S, Spiegelhalter C, Messaddeq N, Trinh L, Liebling M, Chacko N, Tessadori F, Bakkers J, Laporte J, Hnia K, Vermot J. Developmental alterations in heart biomechanics and skeletal muscle function in desmin mutants suggest an early pathological root for desminopathies. Cell Reports 11: 1564–1576, 2015. doi: 10.1016/j.celrep.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 312.Ransom DG, Haffter P, Odenthal J, Brownlie A, Vogelsang E, Kelsh RN, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Mullins MC, Nüsslein-Volhard C. Characterization of zebrafish mutants with defects in embryonic hematopoiesis. Development 123: 311–319, 1996. [DOI] [PubMed] [Google Scholar]
- 313.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127: 423–433, 2006. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA 101: 4596–4601, 2004. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, Amo R, Kamisago M, Momma K, Katayama H, Nakagawa M, Fujiwara Y, Matsushima M, Mizuno K, Tokuyama M, Hirota H, Muneuchi J, Higashinakagawa T, Matsuoka R. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet 39: 1013–1017, 2007. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 316.Reinstein E, Orvin K, Tayeb-Fligelman E, Stiebel-Kalish H, Tzur S, Pimienta AL, Bazak L, Bengal T, Cohen L, Gaton DD, Bormans C, Landau M, Kornowski R, Shohat M, Behar DM. Mutations in TAX1BP3 cause dilated cardiomyopathy with septo-optic dysplasia. Hum Mutat 36: 439–442, 2015. doi: 10.1002/humu.22759. [DOI] [PubMed] [Google Scholar]
- 317.Reischauer S, Arnaout R, Ramadass R, Stainier DY. Actin binding GFP allows 4D in vivo imaging of myofilament dynamics in the zebrafish heart and the identification of Erbb2 signaling as a remodeling factor of myofibril architecture. Circ Res 115: 845–856, 2014. doi: 10.1161/CIRCRESAHA.115.304356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Reischauer S, Stone OA, Villasenor A, Chi N, Jin SW, Martin M, Lee MT, Fukuda N, Marass M, Witty A, Fiddes I, Kuo T, Chung WS, Salek S, Lerrigo R, Alsiö J, Luo S, Tworus D, Augustine SM, Mucenieks S, Nystedt B, Giraldez AJ, Schroth GP, Andersson O, Stainier DY. Cloche is a bHLH-PAS transcription factor that drives haemato-vascular specification. Nature 535: 294–298, 2016. doi: 10.1038/nature18614. [DOI] [PubMed] [Google Scholar]
- 319.Rennekamp AJ, Peterson RT. 15 years of zebrafish chemical screening. Curr Opin Chem Biol 24: 58–70, 2015. doi: 10.1016/j.cbpa.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Reynolds AL, Alvarez Y, Sasore T, Waghorne N, Butler CT, Kilty C, Smith AJ, McVicar C, Wong VH, Galvin O, Merrigan S, Osman J, Grebnev G, Sjölander A, Stitt AW, Kennedy BN. Phenotype-based discovery of 2-[(E)-2-(quinolin-2-yl)vinyl]phenol as a novel regulator of ocular angiogenesis. J Biol Chem 291: 7242–7255, 2016. doi: 10.1074/jbc.M115.710665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol 30: 460–465, 2012. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Roder K, Werdich AA, Li W, Liu M, Kim TY, Organ-Darling LE, Moshal KS, Hwang JM, Lu Y, Choi BR, MacRae CA, Koren G. RING finger protein RNF207, a novel regulator of cardiac excitation. J Biol Chem 289: 33730–33740, 2014. doi: 10.1074/jbc.M114.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, Goodell MA, Rando TA. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature 510: 393–396, 2014. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Rohner N, Perathoner S, Frohnhöfer HG, Harris MP. Enhancing the efficiency of N-ethyl-N-nitrosourea-induced mutagenesis in the zebrafish. Zebrafish 8: 119–123, 2011. doi: 10.1089/zeb.2011.0703. [DOI] [PubMed] [Google Scholar]
- 325.Rossi A, Kontarakis Z, Gerri C, Nolte H, Hölper S, Krüger M, Stainier DY. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524: 230–233, 2015. doi: 10.1038/nature14580. [DOI] [PubMed] [Google Scholar]
- 326.Rottbauer W, Just S, Wessels G, Trano N, Most P, Katus HA, Fishman MC. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev 19: 1624–1634, 2005. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Rovira M, Huang W, Yusuff S, Shim JS, Ferrante AA, Liu JO, Parsons MJ. Chemical screen identifies FDA-approved drugs and target pathways that induce precocious pancreatic endocrine differentiation. Proc Natl Acad Sci USA 108: 19264–19269, 2011. doi: 10.1073/pnas.1113081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Rubin N, Harrison MR, Krainock M, Kim R, Lien CL. Recent advancements in understanding endogenous heart regeneration-insights from adult zebrafish and neonatal mice. Semin Cell Dev Biol 58: 34–40, 2016. doi: 10.1016/j.semcdb.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ryan S, Willer J, Marjoram L, Bagwell J, Mankiewicz J, Leshchiner I, Goessling W, Bagnat M, Katsanis N. Rapid identification of kidney cyst mutations by whole exome sequencing in zebrafish. Development 140: 4445–4451, 2013. doi: 10.1242/dev.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development 132: 3561–3572, 2005. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- 331.Sakaguchi TF, Sadler KC, Crosnier C, Stainier DY. Endothelial signals modulate hepatocyte apicobasal polarization in zebrafish. Curr Biol 18: 1565–1571, 2008. doi: 10.1016/j.cub.2008.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, Curtin SJ, Blackburn JS, Thibodeau-Beganny S, Qi Y, Pierick CJ, Hoffman E, Maeder ML, Khayter C, Reyon D, Dobbs D, Langenau DM, Stupar RM, Giraldez AJ, Voytas DF, Peterson RT, Yeh JR, Joung JK. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat Methods 8: 67–69, 2011. doi: 10.1038/nmeth.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32: 347–355, 2014. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Sapp V, Gaffney L, EauClaire SF, Matthews RP. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology 60: 1581–1592, 2014. doi: 10.1002/hep.27284. [DOI] [PubMed] [Google Scholar]
- 335.Sarras MP Jr, Mason S, McAllister G, Intine RV. Inhibition of poly-ADP ribose polymerase enzyme activity prevents hyperglycemia-induced impairment of angiogenesis during wound healing. Wound Repair Regen 22: 666–670, 2014. doi: 10.1111/wrr.12216. [DOI] [PubMed] [Google Scholar]
- 336.Schaub M, Nussbaum J, Verkade H, Ober EA, Stainier DY, Sakaguchi TF. Mutation of zebrafish Snapc4 is associated with loss of the intrahepatic biliary network. Dev Biol 363: 128–137, 2012. doi: 10.1016/j.ydbio.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Scheid LM, Mosqueira M, Hein S, Kossack M, Juergensen L, Mueller M, Meder B, Fink RH, Katus HA, Hassel D. Essential light chain S195 phosphorylation is required for cardiac adaptation under physical stress. Cardiovasc Res 111: 44–55, 2016. doi: 10.1093/cvr/cvw066. [DOI] [PubMed] [Google Scholar]
- 338.Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development 123: 165–178, 1996. [DOI] [PubMed] [Google Scholar]
- 339.Schlegel A. Studying lipoprotein trafficking in zebrafish, the case of chylomicron retention disease. J Mol Med (Berl) 93: 115–118, 2015. doi: 10.1007/s00109-014-1248-9. [DOI] [PubMed] [Google Scholar]
- 340.Schlegel A. Studying non-alcoholic fatty liver disease with zebrafish: a confluence of optics, genetics, and physiology. Cell Mol Life Sci 69: 3953–3961, 2012. doi: 10.1007/s00018-012-1037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Schlegel A, Gut P. Metabolic insights from zebrafish genetics, physiology, and chemical biology. Cell Mol Life Sci 72: 2249–2260, 2015. doi: 10.1007/s00018-014-1816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Schlegel A, Stainier DY. Lessons from “lower” organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet 3: e199, 2007. doi: 10.1371/journal.pgen.0030199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Schlegel A, Stainier DY. Microsomal triglyceride transfer protein is required for yolk lipid utilization and absorption of dietary lipids in zebrafish larvae. Biochemistry 45: 15179–15187, 2006. doi: 10.1021/bi0619268. [DOI] [PubMed] [Google Scholar]
- 344.Schoenebeck JJ, Yelon D. Illuminating cardiac development: Advances in imaging add new dimensions to the utility of zebrafish genetics. Semin Cell Dev Biol 18: 27–35, 2007. doi: 10.1016/j.semcdb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Schönberger J, Wang L, Shin JT, Kim SD, Depreux FF, Zhu H, Zon L, Pizard A, Kim JB, Macrae CA, Mungall AJ, Seidman JG, Seidman CE. Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss. Nat Genet 37: 418–422, 2005. doi: 10.1038/ng1527. [DOI] [PubMed] [Google Scholar]
- 346.Schulte-Merker S, Stainier DY. Out with the old, in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development 141: 3103–3104, 2014. doi: 10.1242/dev.112003. [DOI] [PubMed] [Google Scholar]
- 347.Schulz N, Liu KC, Charbord J, Mattsson CL, Tao L, Tworus D, Andersson O. Critical role for adenosine receptor A2a in β-cell proliferation. Mol Metab 5: 1138–1146, 2016. doi: 10.1016/j.molmet.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Sciarra JB, Tyler A, Kolb A. A gelatin-based diet for oral dosing juvenile to adult zebrafish. LAS Pro 32–35, 2014. [Google Scholar]
- 349.Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods 4: 323–326, 2007. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- 350.Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DY. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat Genet 31: 106–110, 2002. doi: 10.1038/ng875. [DOI] [PubMed] [Google Scholar]
- 351.Seth A, Stemple DL, Barroso I. The emerging use of zebrafish to model metabolic disease. Dis Model Mech 6: 1080–1088, 2013. doi: 10.1242/dmm.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Shah AN, Davey CF, Whitebirch AC, Miller AC, Moens CB. Rapid reverse genetic screening using CRISPR in zebrafish. Nat Methods 12: 535–540, 2015. doi: 10.1038/nmeth.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Sharma P, Abbasi C, Lazic S, Teng AC, Wang D, Dubois N, Ignatchenko V, Wong V, Liu J, Araki T, Tiburcy M, Ackerley C, Zimmermann WH, Hamilton R, Sun Y, Liu PP, Keller G, Stagljar I, Scott IC, Kislinger T, Gramolini AO. Evolutionarily conserved intercalated disc protein Tmem65 regulates cardiac conduction and connexin 43 function. Nat Commun 6: 8391, 2015. doi: 10.1038/ncomms9391. [DOI] [PubMed] [Google Scholar]
- 354.Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, Wang L, Hodgkins A, Iyer V, Huang X, Skarnes WC. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nat Methods 11: 399–402, 2014. doi: 10.1038/nmeth.2857. [DOI] [PubMed] [Google Scholar]
- 355.Shih YH, Zhang Y, Ding Y, Ross CA, Li H, Olson TM, Xu X. Cardiac transcriptome and dilated cardiomyopathy genes in zebrafish. Circ Cardiovasc Genet 8: 261–269, 2015. doi: 10.1161/CIRCGENETICS.114.000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shima A, Himmelbauer H, Mitani H, Furutani-Seiki M, Wittbrodt J, Schartl M. Fish genomes flying. Symposium on Medaka Genomics. EMBO Rep 4: 121–125, 2003. doi: 10.1038/sj.embor.embor743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Shimizu H, Schredelseker J, Huang J, Lu K, Naghdi S, Lu F, Franklin S, Fiji HD, Wang K, Zhu H, Tian C, Lin B, Nakano H, Ehrlich A, Nakai J, Stieg AZ, Gimzewski JK, Nakano A, Goldhaber JI, Vondriska TM, Hajnóczky G, Kwon O, Chen JN. Mitochondrial Ca(2+) uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. eLife 4: 04801, 2015. doi: 10.7554/eLife.04801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, Strawbridge RJ, Pers TH, Fischer K, Justice AE, Workalemahu T, Wu JM, Buchkovich ML, Heard-Costa NL, Roman TS, Drong AW, Song C, Gustafsson S, Day FR, Esko T, Fall T, Kutalik Z, Luan J, Randall JC, Scherag A, Vedantam S, Wood AR, Chen J, Fehrmann R, Karjalainen J, Kahali B, Liu CT, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bragg-Gresham JL, Buyske S, Demirkan A, Ehret GB, Feitosa MF, Goel A, Jackson AU, Johnson T, Kleber ME, Kristiansson K, Mangino M, Mateo Leach I, Medina-Gomez C, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Stančáková A, Ju Sung Y, Tanaka T, Teumer A, Van Vliet-Ostaptchouk JV, Yengo L, Zhang W, Albrecht E, Ärnlöv J, Arscott GM, Bandinelli S, Barrett A, Bellis C, Bennett AJ, Berne C, Blüher M, Böhringer S, Bonnet F, Böttcher Y, Bruinenberg M, Carba DB, Caspersen IH, Clarke R, Daw EW, Deelen J, Deelman E, Delgado G, Doney AS, Eklund N, Erdos MR, Estrada K, Eury E, Friedrich N, Garcia ME, Giedraitis V, Gigante B, Go AS, Golay A, Grallert H, Grammer TB, Gräßler J, Grewal J, Groves CJ, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heikkilä K, Herzig KH, Helmer Q, Hillege HL, Holmen O, Hunt SC, Isaacs A, Ittermann T, James AL, Johansson I, Juliusdottir T, Kalafati IP, Kinnunen L, Koenig W, Kooner IK, Kratzer W, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lobbens S, Lorentzon M, Mach F, Magnusson PK, Mahajan A, McArdle WL, Menni C, Merger S, Mihailov E, Milani L, Mills R, Moayyeri A, Monda KL, Mooijaart SP, Mühleisen TW, Mulas A, Müller G, Müller-Nurasyid M, Nagaraja R, Nalls MA, Narisu N, Glorioso N, Nolte IM, Olden M, Rayner NW, Renstrom F, Ried JS, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Sennblad B, Seufferlein T, Sitlani CM, Vernon Smith A, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tayo BO, Thorand B, Thorleifsson G, Tomaschitz A, Troffa C, van Oort FV, Verweij N, Vonk JM, Waite LL, Wennauer R, Wilsgaard T, Wojczynski MK, Wong A, Zhang Q, Hua Zhao J, Brennan EP, Choi M, Eriksson P, Folkersen L, Franco-Cereceda A, Gharavi AG, Hedman AK, Hivert MF, Huang J, Kanoni S, Karpe F, Keildson S, Kiryluk K, Liang L, Lifton RP, Ma B, McKnight AJ, McPherson R, Metspalu A, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Olsson C, Perry JR, Reinmaa E, Salem RM, Sandholm N, Schadt EE, Scott RA, Stolk L, Vallejo EE, Westra HJ, Zondervan KT, Amouyel P, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Brown MJ, Burnier M, Campbell H, Chakravarti A, Chines PS, Claudi-Boehm S, Collins FS, Crawford DC, Danesh J, de Faire U, de Geus EJC, Dörr M, Erbel R, Eriksson JG, Farrall M, Ferrannini E, Ferrières J, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gieger C, Gudnason V, Haiman CA, Harris TB, Hattersley AT, Heliövaara M, Hicks AA, Hingorani AD, Hoffmann W, Hofman A, Homuth G, Humphries SE, Hyppönen E, Illig T, Jarvelin M-R, Johansen B, Jousilahti P, Jula AM, Kaprio J, Kee F, Keinanen-Kiukaanniemi SM, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuulasmaa K, Kuusisto J, Lakka TA, Langenberg C, Le Marchand L, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Musk AW, Möhlenkamp S, Morris AD, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Palmer LJ, Penninx BW, Peters A, Pramstaller PP, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schwarz PEH, Shuldiner AR, Staessen JA, Steinthorsdottir V, Stolk RP, Strauch K, Tönjes A, Tremblay A, Tremoli E, Vohl M-C, Völker U, Vollenweider P, Wilson JF, Witteman JC, Adair LS, Bochud M, Boehm BO, Bornstein SR, Bouchard C, Cauchi S, Caulfield MJ, Chambers JC, Chasman DI, Cooper RS, Dedoussis G, Ferrucci L, Froguel P, Grabe H-J, Hamsten A, Hui J, Hveem K, Jöckel K-H, Kivimaki M, Kuh D, Laakso M, Liu Y, März W, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sinisalo J, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Veronesi G, Walker M, Wareham NJ, Watkins H, Wichmann H-E, Abecasis GR, Assimes TL, Berndt SI, Boehnke M, Borecki IB, Deloukas P, Franke L, Frayling TM, Groop LC, Hunter DJ, Kaplan RC, O’Connell JR, Qi L, Schlessinger D, Strachan DP, Stefansson K, van Duijn CM, Willer CJ, Visscher PM, Yang J, Hirschhorn JN, Zillikens MC, McCarthy MI, Speliotes EK, North KE, Fox CS, Barroso I, Franks PW, Ingelsson E, Heid IM, Loos RJF, Cupples LA, Morris AP, Lindgren CM, Mohlke KL; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGCICBPInternational Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature 518: 187–196, 2015. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Siccardi AJ III, Garris HW, Jones WT, Moseley DB, D’Abramo LR, Watts SA. Growth and survival of zebrafish (Danio rerio) fed different commercial and laboratory diets. Zebrafish 6: 275–280, 2009. doi: 10.1089/zeb.2008.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784, 2007. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 361.Siu BL, Niimura H, Osborne JA, Fatkin D, MacRae C, Solomon S, Benson DW, Seidman JG, Seidman CE. Familial dilated cardiomyopathy locus maps to chromosome 2q31. Circulation 99: 1022–1026, 1999. doi: 10.1161/01.CIR.99.8.1022. [DOI] [PubMed] [Google Scholar]
- 362.Sivasubbu S, Balciunas D, Davidson AE, Pickart MA, Hermanson SB, Wangensteen KJ, Wolbrink DC, Ekker SC. Gene-breaking transposon mutagenesis reveals an essential role for histone H2afza in zebrafish larval development. Mech Dev 123: 513–529, 2006. doi: 10.1016/j.mod.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 363.Smith DL Jr, Barry RJ, Powell ML, Nagy TR, D’Abramo LR, Watts SA. Dietary protein source influence on body size and composition in growing zebrafish. Zebrafish 10: 439–446, 2013. doi: 10.1089/zeb.2012.0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Sogah VM, Serluca FC, Fishman MC, Yelon DL, Macrae CA, Mably JD. Distinct troponin C isoform requirements in cardiac and skeletal muscle. Dev Dyn 239: 3115–3123, 2010. doi: 10.1002/dvdy.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SC, Malicki J, Schier AF, Stainier DY, Zwartkruis F, Abdelilah S, Driever W. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development 123: 67–80, 1996. [DOI] [PubMed] [Google Scholar]
- 366.Song J, Ampatzis K, Björnfors ER, El Manira A. Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature 529: 399–402, 2016. doi: 10.1038/nature16497. [DOI] [PubMed] [Google Scholar]
- 367.Song Y, Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J 21: 2042–2049, 2007. doi: 10.1096/fj.06-7503com. [DOI] [PubMed] [Google Scholar]
- 368.Soroldoni D, Hogan BM, Oates AC. Simple and efficient transgenesis with meganuclease constructs in zebrafish. Methods Mol Biol 546: 117–130, 2009. doi: 10.1007/978-1-60327-977-2_8. [DOI] [PubMed] [Google Scholar]
- 369.Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol 12: 1215–1220, 2002. doi: 10.1016/S0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- 370.Stainier DY, Fouquet B, Chen JN, Warren KS, Weinstein BM, Meiler SE, Mohideen MA, Neuhauss SC, Solnica-Krezel L, Schier AF, Zwartkruis F, Stemple DL, Malicki J, Driever W, Fishman MC. Mutations affecting the formation and function of the cardiovascular system in the zebrafish embryo. Development 123: 285–292, 1996. [DOI] [PubMed] [Google Scholar]
- 371.Stainier DY, Kontarakis Z, Rossi A. Making sense of anti-sense data. Dev Cell 32: 7–8, 2015. doi: 10.1016/j.devcel.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 372.Stainier DY, Weinstein BM, Detrich HW III, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121: 3141–3150, 1995. [DOI] [PubMed] [Google Scholar]
- 373.Staudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu Rev Genet 46: 397–418, 2012. doi: 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Staudt DW, Liu J, Thorn KS, Stuurman N, Liebling M, Stainier DY. High-resolution imaging of cardiomyocyte behavior reveals two distinct steps in ventricular trabeculation. Development 141: 585–593, 2014. doi: 10.1242/dev.098632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Stemple DL. TILLING--a high-throughput harvest for functional genomics. Nat Rev Genet 5: 145–150, 2004. doi: 10.1038/nrg1273. [DOI] [PubMed] [Google Scholar]
- 376.Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL, Miller YI. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res 104: 952–960, 2009. doi: 10.1161/CIRCRESAHA.108.189803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Stoyek MR, Croll RP, Smith FM. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J Comp Neurol 523: 1683–1700, 2015. doi: 10.1002/cne.23764. [DOI] [PubMed] [Google Scholar]
- 378.Stoyek MR, Quinn TA, Croll RP, Smith FM. Zebrafish heart as a model to study the integrative autonomic control of pacemaker function. Am J Physiol Heart Circ Physiol 311: H676–H688, 2016. doi: 10.1152/ajpheart.00330.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291: 293–296, 1981. doi: 10.1038/291293a0. [DOI] [PubMed] [Google Scholar]
- 380.Stuart GW, McMurray JV, Westerfield M. Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development 103: 403–412, 1988. [DOI] [PubMed] [Google Scholar]
- 381.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 121: 2094–2101, 2011. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Sun L, Lien CL, Xu X, Shung KK. In vivo cardiac imaging of adult zebrafish using high frequency ultrasound (45-75 MHz). Ultrasound Med Biol 34: 31–39, 2008. doi: 10.1016/j.ultrasmedbio.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Sun P, Zhang Y, Yu F, Parks E, Lyman A, Wu Q, Ai L, Hu CH, Zhou Q, Shung K, Lien CL, Hsiai TK. Micro-electrocardiograms to study post-ventricular amputation of zebrafish heart. Ann Biomed Eng 37: 890–901, 2009. doi: 10.1007/s10439-009-9668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Sun X, Hoage T, Bai P, Ding Y, Chen Z, Zhang R, Huang W, Jahangir A, Paw B, Li YG, Xu X. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One 4: e6596, 2009. doi: 10.1371/journal.pone.0006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sun Z, Hopkins N. vhnf1, the MODY5 and familial GCKD-associated gene, regulates regional specification of the zebrafish gut, pronephros, and hindbrain. Genes Dev 15: 3217–3229, 2001. doi: 10.1101/gad946701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Sung YH, Jin Y, Kim S, Lee HW. Generation of knockout mice using engineered nucleases. Methods 69: 85–93, 2014. doi: 10.1016/j.ymeth.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 387.Suster ML, Kikuta H, Urasaki A, Asakawa K, Kawakami K. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol Biol 561: 41–63, 2009. doi: 10.1007/978-1-60327-019-9_3. [DOI] [PubMed] [Google Scholar]
- 388.Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov 10: 507–519, 2011. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 389.Tahara N, Brush M, Kawakami Y. Cell migration during heart regeneration in zebrafish. Dev Dyn 245: 774–787, 2016. doi: 10.1002/dvdy.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Talbot JC, Amacher SL. A streamlined CRISPR pipeline to reliably generate zebrafish frameshifting alleles. Zebrafish 11: 583–585, 2014. doi: 10.1089/zeb.2014.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Taylor MR, Hurley JB, Van Epps HA, Brockerhoff SE. A zebrafish model for pyruvate dehydrogenase deficiency: rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc Natl Acad Sci USA 101: 4584–4589, 2004. doi: 10.1073/pnas.0307074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118: 91–98, 2002. doi: 10.1016/S0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- 393.Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg H-P, Seidman JG, Seidman CE. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 77: 701–712, 1994. doi: 10.1016/0092-8674(94)90054-X. [DOI] [PubMed] [Google Scholar]
- 394.Towbin JA, McQuinn TC. Gridlock: a model for coarctation of the aorta? Nat Med 1: 1141–1142, 1995. doi: 10.1038/nm1195-1141. [DOI] [PubMed] [Google Scholar]
- 395.Trinh A, Hochgreb T, Graham M, Wu D, Ruf-Zamojski F, Jayasena CS, Saxena A, Hawk R, Gonzalez-Serricchio A, Dixson A, Chow E, Gonzales C, Leung HY, Solomon I, Bronner-Fraser M, Megason SG, Fraser SE. A versatile gene trap to visualize and interrogate the function of the vertebrate proteome. Genes Dev 25: 2306–2320, 2011. doi: 10.1101/gad.174037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Tsai CT, Hsieh CS, Chang SN, Chuang EY, Ueng KC, Tsai CF, Lin TH, Wu CK, Lee JK, Lin LY, Wang YC, Yu CC, Lai LP, Tseng CD, Hwang JJ, Chiang FT, Lin JL. Genome-wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat Commun 7: 10190, 2016. doi: 10.1038/ncomms10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tsuji N, Ninov N, Delawary M, Osman S, Roh AS, Gut P, Stainier DY. Whole organism high content screening identifies stimulators of pancreatic beta-cell proliferation. PLoS One 9: e104112, 2014. doi: 10.1371/journal.pone.0104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Turola E, Petta S, Vanni E, Milosa F, Valenti L, Critelli R, Miele L, Maccio L, Calvaruso V, Fracanzani AL, Bianchini M, Raos N, Bugianesi E, Mercorella S, Di Giovanni M, Craxì A, Fargion S, Grieco A, Cammà C, Cotelli F, Villa E. Ovarian senescence increases liver fibrosis in humans and zebrafish with steatosis. Dis Model Mech 8: 1037–1046, 2015. doi: 10.1242/dmm.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 174: 639–649, 2006. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene 22: 5792–5812, 2003. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- 401.van der Velden YU, Wang L, Zevenhoven J, van Rooijen E, van Lohuizen M, Giles RH, Clevers H, Haramis AP. The serine-threonine kinase LKB1 is essential for survival under energetic stress in zebrafish. Proc Natl Acad Sci USA 108: 4358–4363, 2011. doi: 10.1073/pnas.1010210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR. Can animal models of disease reliably inform human studies? PLoS Med 7: e1000245, 2010. doi: 10.1371/journal.pmed.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. eLife 4: 06489, 2015. doi: 10.7554/eLife.06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Varshney GK, Pei W, LaFave MC, Idol J, Xu L, Gallardo V, Carrington B, Bishop K, Jones M, Li M, Harper U, Huang SC, Prakash A, Chen W, Sood R, Ledin J, Burgess SM. High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome Res 25: 1030–1042, 2015. doi: 10.1101/gr.186379.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Varshney GK, Sood R, Burgess SM. Understanding and editing the zebrafish genome. Adv Genet 92: 1–52, 2015. doi: 10.1016/bs.adgen.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 406.Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 22: 306–311, 2016. [Erratum in Nat Med 22: 446, 2016]. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Venkatachalam AB, Thisse C, Thisse B, Wright JM. Differential tissue-specific distribution of transcripts for the duplicated fatty acid-binding protein 10 (fabp10) genes in embryos, larvae and adult zebrafish (Danio rerio). FEBS J 276: 6787–6797, 2009. doi: 10.1111/j.1742-4658.2009.07393.x. [DOI] [PubMed] [Google Scholar]
- 408.Vergauwen L, Benoot D, Blust R, Knapen D. Long-term warm or cold acclimation elicits a specific transcriptional response and affects energy metabolism in zebrafish. Comp Biochem Physiol A Mol Integr Physiol 157: 149–157, 2010. doi: 10.1016/j.cbpa.2010.06.160. [DOI] [PubMed] [Google Scholar]
- 409.Vergauwen L, Knapen D, Hagenaars A, De Boeck G, Blust R. Assessing the impact of thermal acclimation on physiological condition in the zebrafish model. J Comp Physiol B 183: 109–121, 2013. doi: 10.1007/s00360-012-0691-6. [DOI] [PubMed] [Google Scholar]
- 410.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2163–2196, 2012. [Erratum in Lancet. 381: 628, 2013]. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Walsh EC, Stainier DY. UDP-glucose dehydrogenase required for cardiac valve formation in zebrafish. Science 293: 1670–1673, 2001. doi: 10.1126/science.293.5535.1670. [DOI] [PubMed] [Google Scholar]
- 412.Wang G, Rajpurohit SK, Delaspre F, Walker SL, White DT, Ceasrine A, Kuruvilla R, Li RJ, Shim JS, Liu JO, Parsons MJ, Mumm JS. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic β-cell mass. eLife 4: 08261, 2015. doi: 10.7554/eLife.08261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412a.Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, Coggeshall M, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fraser MS, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Hay SI, Huynh C, Johnson CO, Kassebaum NJ, Kinfu Y, Kulikoff XR, Kutz M, Kyu HH, Larson HJ, Leung J, Liang X, Lim SS, Lind M, Lozano R, Marquez N, Mensah GA, Mikesell J, Mokdad AH, Mooney MD, Nguyen G, Nsoesie E, Pigott DM, Pinho C, Roth GA, Salomon JA, Sandar L, Silpakit N, Sligar A, Sorensen RJD, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, VanderZanden A, Vollset SE, Wanga V, Whiteford HA, Wolock T, Zoeckler L, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Abreu DMX, Abu-Raddad LJ, Abyu GY, Achoki T, Adelekan AL, Ademi Z, Adou AK, Adsuar JC, Afanvi KA, Afshin A, Agardh EE, Agarwal A, Agrawal A, Kiadaliri AA, Ajala ON, Akanda AS, Akinyemi RO, Akinyemiju TF, Akseer N, Lami FHA, Alabed S, Al-Aly Z, Alam K, Alam NKM, Alasfoor D, Aldhahri SF, Aldridge RW, Alegretti MA, Aleman AV, Alemu ZA, Alexander LT, Alhabib S, Ali R, Alkerwi A, Alla F, Allebeck P, Al-Raddadi R, Alsharif U, Altirkawi KA, Martin EA, Alvis-Guzman N, Amare AT, Amegah AK, Ameh EA, Amini H, Ammar W, Amrock SM, Andersen HH, Anderson BO, Anderson GM, Antonio CAT, Aregay AF, Ärnlöv J, Arsenijevic VSA, Artaman A, Asayesh H, Asghar RJ, Atique S, Avokpaho EFGA, Awasthi A, Azzopardi P, Bacha U, Badawi A, Bahit MC, Balakrishnan K, Banerjee A, Barac A, Barker-Collo SL, Bärnighausen T, Barregard L, Barrero LH, Basu A, Basu S, Bayou YT, Bazargan-Hejazi S, Beardsley J, Bedi N, Beghi E, Belay HA, Bell B, Bell ML, Bello AK, Bennett DA, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beyene AS, Bhala N, Bhalla A, Biadgilign S, Bikbov B, Abdulhak AAB, Biroscak BJ, Biryukov S, Bjertness E, Blore JD, Blosser CD, Bohensky MA, Borschmann R, Bose D, Bourne RRA, Brainin M, Brayne CEG, Brazinova A, Breitborde NJK, Brenner H, Brewer JD, Brown A, Brown J, Brugha TS, Buckle GC, Butt ZA, Calabria B, Campos-Nonato IR, Campuzano JC, Carapetis JR, Cárdenas R, Carpenter DO, Carrero JJ, Castañeda-Orjuela CA, Rivas JC, Catalá-López F, Cavalleri F, Cercy K, Cerda J, Chen W, Chew A, Chiang PP-C, Chibalabala M, Chibueze CE, Chimed-Ochir O, Chisumpa VH, Choi J-YJ, Chowdhury R, Christensen H, Christopher DJ, Ciobanu LG, Cirillo M, Cohen AJ, Colistro V, Colomar M, Colquhoun SM, Cooper C, Cooper LT, Cortinovis M, Cowie BC, Crump JA, Damsere-Derry J, Danawi H, Dandona R, Daoud F, Darby SC, Dargan PI, das Neves J, Davey G, Davis AC, Davitoiu DV, de Castro EF, de Jager P, Leo DD, Degenhardt L, Dellavalle RP, Deribe K, Deribew A, Dharmaratne SD, Dhillon PK, Diaz-Torné C, Ding EL, dos Santos KPB, Dossou E, Driscoll TR, Duan L, Dubey M, Duncan BB, Ellenbogen RG, Ellingsen CL, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Faghmous IDA, Fahimi S, Faraon EJA, Farid TA, Farinha CSS, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fereshtehnejad S-M, Fernandes JG, Fernandes JC, Fischer F, Fitchett JRA, Flaxman A, Foigt N, Fowkes FGR, Franca EB, Franklin RC, Friedman J, Frostad J, Fürst T, Futran ND, Gall SL, Gambashidze K, Gamkrelidze A, Ganguly P, Gankpé FG, Gebre T, Gebrehiwot TT, Gebremedhin AT, Gebru AA, Geleijnse JM, Gessner BD, Ghoshal AG, Gibney KB, Gillum RF, Gilmour S, Giref AZ, Giroud M, Gishu MD, Giussani G, Glaser E, Godwin WW, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gosselin RA, Gotay CC, Goto A, Gouda HN, Greaves F, Gugnani HC, Gupta R, Gupta R, Gupta V, Gutiérrez RA, Hafezi-Nejad N, Haile D, Hailu AD, Hailu GB, Halasa YA, Hamadeh RR, Hamidi S, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Havmoeller R, Heckbert SR, Heredia-Pi IB, Heydarpour P, Hilderink HBM, Hoek HW, Hogg RS, Horino M, Horita N, Hosgood HD, Hotez PJ, Hoy DG, Hsairi M, Htet AS, Htike MMT, Hu G, Huang C, Huang H, Huiart L, Husseini A, Huybrechts I, Huynh G, Iburg KM, Innos K, Inoue M, Iyer VJ, Jacobs TA, Jacobsen KH, Jahanmehr N, Jakovljevic MB, James P, Javanbakht M, Jayaraman SP, Jayatilleke AU, Jeemon P, Jensen PN, Jha V, Jiang G, Jiang Y, Jibat T, Jimenez-Corona A, Jonas JB, Joshi TK, Kabir Z, Kamal R, Kan H, Kant S, Karch A, Karema CK, Karimkhani C, Karletsos D, Karthikeyan G, Kasaeian A, Katibeh M, Kaul A, Kawakami N, Kayibanda JF, Keiyoro PN, Kemmer L, Kemp AH, Kengne AP, Keren A, Kereselidze M, Kesavachandran CN, Khader YS, Khalil IA, Khan AR, Khan EA, Khang Y-H, Khera S, Khoja TAM, Kieling C, Kim D, Kim YJ, Kissela BM, Kissoon N, Knibbs LD, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Kosen S, Koul PA, Koyanagi A, Krog NH, Defo BK, Bicer BK, Kudom AA, Kuipers EJ, Kulkarni VS, Kumar GA, Kwan GF, Lal A, Lal DK, Lalloo R, Lallukka T, Lam H, Lam JO, Langan SM, Lansingh VC, Larsson A, Laryea DO, Latif AA, Lawrynowicz AEB, Leigh J, Levi M, Li Y, Lindsay MP, Lipshultz SE, Liu PY, Liu S, Liu Y, Lo L-T, Logroscino G, Lotufo PA, Lucas RM, Lunevicius R, Lyons RA, Ma S, Machado VMP, Mackay MT, MacLachlan JH, Razek HMAE, Magdy M, Razek AE, Majdan M, Majeed A, Malekzadeh R, Manamo WAA, Mandisarisa J, Mangalam S, Mapoma CC, Marcenes W, Margolis DJ, Martin GR, Martinez-Raga J, Marzan MB, Masiye F, Mason-Jones AJ, Massano J, Matzopoulos R, Mayosi BM, McGarvey ST, McGrath JJ, McKee M, McMahon BJ, Meaney PA, Mehari A, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen AB, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mhimbira FA, Micha R, Millear A, Miller TR, Mirarefin M, Misganaw A, Mock CN, Mohammad KA, Mohammadi A, Mohammed S, Mohan V, Mola GLD, Monasta L, Hernandez JCM, Montero P, Montico M, Montine TJ, Moradi-Lakeh M, Morawska L, Morgan K, Mori R, Mozaffarian D, Mueller UO, Murthy GVS, Murthy S, Musa KI, Nachega JB, Nagel G, Naidoo KS, Naik N, Naldi L, Nangia V, Nash D, Nejjari C, Neupane S, Newton CR, Newton JN, Ng M, Ngalesoni FN, de Dieu Ngirabega J, Nguyen QL, Nisar MI, Pete PMN, Nomura M, Norheim OF, Norman PE, Norrving B, Nyakarahuka L, Ogbo FA, Ohkubo T, Ojelabi FA, Olivares PR, Olusanya BO, Olusanya JO, Opio JN, Oren E, Ortiz A, Osman M, Ota E, Ozdemir R, Pa M, Pain A, Pandian JD, Pant PR, Papachristou C, Park E-K, Park J-H, Parry CD, Parsaeian M, Caicedo AJP, Patten SB, Patton GC, Paul VK, Pearce N, Pedro JM, Stokic LP, Pereira DM, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Plass D, Platts-Mills JA, Polinder S, Pope CA, Popova S, Poulton RG, Pourmalek F, Prabhakaran D, Qorbani M, Quame-Amaglo J, Quistberg DA, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai RK, Rajavi Z, Rajsic S, Raju M, Rakovac I, Rana SM, Ranabhat CL, Rangaswamy T, Rao P, Rao SR, Refaat AH, Rehm J, Reitsma MB, Remuzzi G, Resnikoff S, Ribeiro AL, Ricci S, Blancas MJR, Roberts B, Roca A, Rojas-Rueda D, Ronfani L, Roshandel G, Rothenbacher D, Roy A, Roy NK, Ruhago GM, Sagar R, Saha S, Sahathevan R, Saleh MM, Sanabria JR, Sanchez-Niño MD, Sanchez-Riera L, Santos IS, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schaub MP, Schmidt MI, Schneider IJC, Schöttker B, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shaddick G, Shaheen A, Shahraz S, Shaikh MA, Shakh-Nazarova M, Sharma R, She J, Sheikhbahaei S, Shen J, Shen Z, Shepard DS, Sheth KN, Shetty BP, Shi P, Shibuya K, Shin M-J, Shiri R, Shiue I, Shrime MG, Sigfusdottir ID, Silberberg DH, Silva DAS, Silveira DGA, Silverberg JI, Simard EP, Singh A, Singh GM, Singh JA, Singh OP, Singh PK, Singh V, Soneji S, Søreide K, Soriano JB, Sposato LA, Sreeramareddy CT, Stathopoulou V, Stein DJ, Stein MB, Stranges S, Stroumpoulis K, Sunguya BF, Sur P, Swaminathan S, Sykes BL, Szoeke CEI, Tabarés-Seisdedos R, Tabb KM, Takahashi K, Takala JS, Talongwa RT, Tandon N, Tavakkoli M, Taye B, Taylor HR, Ao BJT, Tedla BA, Tefera WM, Have MT, Terkawi AS, Tesfay FH, Tessema GA, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tillmann T, Tirschwell DL, Tonelli M, Topor-Madry R, Topouzis F, Towbin JA, Traebert J, Tran BX, Truelsen T, Trujillo U, Tura AK, Tuzcu EM, Uchendu US, Ukwaja KN, Undurraga EA, Uthman OA, Dingenen RV, van Donkelaar A, Vasankari T, Vasconcelos AMN, Venketasubramanian N, Vidavalur R, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Wagner JA, Wagner GR, Wallin MT, Wang L, Watkins DA, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Westerman R, White RA, Wijeratne T, Wilkinson JD, Williams HC, Wiysonge CS, Woldeyohannes SM, Wolfe CDA, Won S, Wong JQ, Woolf AD, Xavier D, Xiao Q, Xu G, Yakob B, Yalew AZ, Yan LL, Yano Y, Yaseri M, Ye P, Yebyo HG, Yip P, Yirsaw BD, Yonemoto N, Yonga G, Younis MZ, Yu S, Zaidi Z, Zaki MES, Zannad F, Zavala DE, Zeeb H, Zeleke BM, Zhang H, Zodpey S, Zonies D, Zuhlke LJ, Vos T, Lopez AD, Murray CJL; GBD 2015 Mortality and Causes of Death Collaborators . Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1459–1544, 2016. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Wang J, Panáková D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138: 3421–3430, 2011. doi: 10.1242/dev.068601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Warren KS, Baker K, Fishman MC. The slow mo mutation reduces pacemaker current and heart rate in adult zebrafish. Am J Physiol Heart Circ Physiol 281: H1711–H1719, 2001. [DOI] [PubMed] [Google Scholar]
- 415.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O’Donoghue A, Spirito P, Matsumori A, Moravec CS, Seidman JG, Seidman CE. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med 332: 1058–1065, 1995. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 416.Weger BD, Weger M, Nusser M, Brenner-Weiss G, Dickmeis T. A chemical screening system for glucocorticoid stress hormone signaling in an intact vertebrate. ACS Chem Biol 7: 1178–1183, 2012. doi: 10.1021/cb3000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Weger M, Weger BD, Diotel N, Rastegar S, Hirota T, Kay SA, Strähle U, Dickmeis T. Real-time in vivo monitoring of circadian E-box enhancer activity: a robust and sensitive zebrafish reporter line for developmental, chemical and neural biology of the circadian clock. Dev Biol 380: 259–273, 2013. doi: 10.1016/j.ydbio.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 418.Weinstein BM, Schier AF, Abdelilah S, Malicki J, Solnica-Krezel L, Stemple DL, Stainier DY, Zwartkruis F, Driever W, Fishman MC. Hematopoietic mutations in the zebrafish. Development 123: 303–309, 1996. [DOI] [PubMed] [Google Scholar]
- 419.Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med 1: 1143–1147, 1995. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- 420.Wen D, Liu A, Chen F, Yang J, Dai R. Validation of visualized transgenic zebrafish as a high throughput model to assay bradycardia related cardio toxicity risk candidates. J Appl Toxicol 32: 834–842, 2012. doi: 10.1002/jat.2755. [DOI] [PubMed] [Google Scholar]
- 421.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 20: 103–118, 2014. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ, Langdon E, Tomlinson ML, Mosher J, Kaufman C, Chen F, Long HK, Kramer M, Datta S, Neuberg D, Granter S, Young RA, Morrison S, Wheeler GN, Zon LI. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature 471: 518–522, 2011. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Wiedenheft B, Sternberg SH, Doudna JA. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482: 331–338, 2012. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 424.Wienholds E, van Eeden F, Kosters M, Mudde J, Plasterk RH, Cuppen E. Efficient target-selected mutagenesis in zebrafish. Genome Res 13: 2700–2707, 2003. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Williams CH, Hempel JE, Hao J, Frist AY, Williams MM, Fleming JT, Sulikowski GA, Cooper MK, Chiang C, Hong CC. An in vivo chemical genetic screen identifies phosphodiesterase 4 as a pharmacological target for hedgehog signaling inhibition. Cell Reports 11: 43–50, 2015. doi: 10.1016/j.celrep.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wong S, Stephens WZ, Burns AR, Stagaman K, David LA, Bohannan BJ, Guillemin K, Rawls JF. Ontogenetic differences in dietary fat influence microbiota assembly in the zebrafish gut. MBio 6: e00687–e15, 2015. doi: 10.1128/mBio.00687-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wooderchak-Donahue WL, McDonald J, O’Fallon B, Upton PD, Li W, Roman BL, Young S, Plant P, Fülöp GT, Langa C, Morrell NW, Botella LM, Bernabeu C, Stevenson DA, Runo JR, Bayrak-Toydemir P. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet 93: 530–537, 2013. doi: 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Wu X, Kriz AJ, Sharp PA. Target specificity of the CRISPR-Cas9 system. Quant Biol 2: 59–70, 2014. doi: 10.1007/s40484-014-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Xu X, Meiler SE, Zhong TP, Mohideen M, Crossley DA, Burggren WW, Fishman MC. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat Genet 30: 205–209, 2002. doi: 10.1038/ng816. [DOI] [PubMed] [Google Scholar]
- 430.Yang J, Xu X. α-Actinin2 is required for the lateral alignment of Z discs and ventricular chamber enlargement during zebrafish cardiogenesis. FASEB J 26: 4230–4242, 2012. doi: 10.1096/fj.12-207969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol 15: 859–865, 1997. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- 432.Ye L, Robertson MA, Mastracci TL, Anderson RM. An insulin signaling feedback loop regulates pancreas progenitor cell differentiation during islet development and regeneration. Dev Biol 409: 354–369, 2016. doi: 10.1016/j.ydbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Yelon D, Horne SA, Stainier DY. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev Biol 214: 23–37, 1999. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 434.Yin C, Evason KJ, Maher JJ, Stainier DY. The basic helix-loop-helix transcription factor, heart and neural crest derivatives expressed transcript 2, marks hepatic stellate cells in zebrafish: analysis of stellate cell entry into the developing liver. Hepatology 56: 1958–1970, 2012. doi: 10.1002/hep.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Yu F, Li R, Parks E, Takabe W, Hsiai TK. Electrocardiogram signals to assess zebrafish heart regeneration: implication of long QT intervals. Ann Biomed Eng 38: 2346–2357, 2010. doi: 10.1007/s10439-010-9993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Yu HC, Coughlin CR, Geiger EA, Salvador BJ, Elias ER, Cavanaugh JL, Chatfield KC, Miyamoto SD, Shaikh TH. Discovery of a potentially deleterious variant in TMEM87B in a patient with a hemizygous 2q13 microdeletion suggests a recessive condition characterized by congenital heart disease and restrictive cardiomyopathy. Cold Spring Harb Mol Case Stud 2: a000844, 2016. doi: 10.1101/mcs.a000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Zada D, Tovin A, Lerer-Goldshtein T, Appelbaum L. Pharmacological treatment and BBB-targeted genetic therapy for MCT8-dependent hypomyelination in zebrafish. Dis Model Mech 9: 1339–1348, 2016. doi: 10.1242/dmm.027227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zhang X, Beebe T, Jen N, Lee CA, Tai Y, Hsiai TK. Flexible and waterproof micro-sensors to uncover zebrafish circadian rhythms: The next generation of cardiac monitoring for drug screening. Biosens Bioelectron 71: 150–157, 2015. doi: 10.1016/j.bios.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455: 627–632, 2008. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhu S, Russ HA, Wang X, Zhang M, Ma T, Xu T, Tang S, Hebrok M, Ding S. Human pancreatic beta-like cells converted from fibroblasts. Nat Commun 7: 10080, 2016. doi: 10.1038/ncomms10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Zhu X, Xu Y, Yu S, Lu L, Ding M, Cheng J, Song G, Gao X, Yao L, Fan D, Meng S, Zhang X, Hu S, Tian Y. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci Rep 4: 6420, 2014. doi: 10.1038/srep06420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Zon L. Translational research: the path for bringing discovery to patients. Cell Stem Cell 14: 146–148, 2014. doi: 10.1016/j.stem.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 4: 35–44, 2005. doi: 10.1038/nrd1606. [DOI] [PubMed] [Google Scholar]
- 444.Zou J, Tran D, Baalbaki M, Tang LF, Poon A, Pelonero A, Titus EW, Yuan C, Shi C, Patchava S, Halper E, Garg J, Movsesyan I, Yin C, Wu R, Wilsbacher LD, Liu J, Hager RL, Coughlin SR, Jinek M, Pullinger CR, Kane JP, Hart DO, Kwok PY, Deo RC. An internal promoter underlies the difference in disease severity between N- and C-terminal truncation mutations of Titin in zebrafish. eLife 4: e09406, 2015. doi: 10.7554/eLife.09406. [DOI] [PMC free article] [PubMed] [Google Scholar]