Abstract
Objective(s):
Antrodia cinnamomea (AC) is found with anti-inflammatory and immunomodulatory biological activities. In this study, we investigated the anti-hepatitis effect of the emulsified AC extract from RO water or supercritical fluid CO2 with ethanol co-solvent extract methods of AC preparations.
Materials and Methods:
Five groups of eight to ten weeks male rats with a count of ten for each group were studied to evaluate the protection of two kinds of AC extract from hepatic injury. Acute liver injury of rats was induced by injecting 40% carbon tetrachloride (CCl4) 1 mg/kg intraperitoneally. Positive and negative control groups rats were perfused with CCl4 or isotonic saline, respectively. Experimental groups received oral administration once/day of AC preparations before CCl4 treatment: water AC extract (WAE group), or emulsified AC extract from supercritical fluid extraction (EAE group) for 5 days, and sacrificed on the 6th day and the blood and liver samples were collected under chloral hydrate anesthesia. The anti-inflammatory, antioxidant markers, and relevant signaling pathways were measured (AST, ALT, ROS, IL-1, IL-6, NO, and COX-2, MAPKs, and caspase-3).
Results:
EAE at 50 mg/kg significantly decreased the serum AST, ALT, IL-1, IL-6, NO, and ROS levels. Both extracts reduced the activation of p-ERK in the liver samples, but EAE inhibited COX-2 and caspase-3 protein expression better than WAE. The EAE ameliorated CCl4-induced hepatic injury significantly; as compared with WAE and the positive control.
Conclusion:
The hepatoprotection of EAE could be attributed to the antioxidant and anti-inflammatory effects of Antrodia.
Keywords: Antioxidant, Anti-inflammatory, Antrodia cinnamomea, Carbon tetrachloride, Hepatoprotection, MAPKs
Introduction
The liver is an important organ for uptake, metabolism, conjugation, and excretion of various endogenous and foreign substances. Chronic hepatitis or exposure to toxins leads to liver fibrosis and necrosis. This process eventually leads to hepatoma (1, 2). Antrodia cinnamomea (AC), is a unique mushroom species for indigenous people in Taiwan to ameliorate liver disorders from excessive alcohol consumption. Currently, AC is known to have diverse biological activities, including anti-hypertensive, anti-hyperlipid, anti-inflammatory, antioxidative, anti-tumor, and immunomodulating effects (3).
Active components of AC have been isolated from the mushroom or cultivated fruiting bodies and fermented mycelia including benzenoids, diterpenes, triterpenoids, steroids, maleic/succinic acid derivatives, and poly-saccharides (4-7). Antroquinonol and ethanolic extracts of AC mycelia, enhance HO-1 and Nrf-2 activation via MAPKs in vitro and in vivo (4). Antcin C, a steroid-like compound isolated from the AC fruiting bodies, protects hepatic cells from free radical-induced oxidative stress and cell death via Nrf2-dependent induction of antioxidant genes in vitro and in vivo. (5). Antrodan, a protein-bound polysaccharide isolated from AC mycelia, exhibits significant anti-inflammatory bioactivity in vitro (7). These studies show that AC antioxidants ameliorate acute hepatic injury in various models.
It has been reported that the drug formulated in a self-microemulsifying drug delivery system (SMEDDS), can self-improve its water solubility, dissolution rate, and bioavailability (8), it contacts with gastrointestinal fluid and forms an emulsion with the aid of gastro-intestinal motility. The preparation of emulsified AC extract (EAE) from supercritical fluid extraction would be better for hepatoprotection than the water extract of AC (WAE), presumably. Therefore, both extracts were compared in the carbon tetrachloride (CCl4)-induced acute liver injury. The protective mechanism was further compared for cytokines, reactive oxygen species (ROS) or reactive nitrogen species (RNS) production, as well as related signaling pathways.
Materials and Methods
Reagents
CCl4 was obtained from Sigma-Aldrich (St. Louis, MO, USA), Anti-phospho-p38, ERK, and JNK MAPKs, COX-2, caspase-3, and β-actin antibodies were purchased from Abcam (Cambridge, UK) and secondary anti-rabbit immunoglobulin G, conjugated to alkaline phosphatase from Jackson ImmunoResearch (Philadelphia, PA, USA).
Preparation of AC extracts
Supercritical fluid extraction of AC fruiting body from solid-state culture was done, using CO2 mixed with a constant amount of ethanol co-solvent (10% of CO2 volume) at temperatures 50°C and pressure of 350 bar. The triterpenoid contents of AC fruiting body extracts were 0.11, 0.30, 0.20, 0.26, and 0.07% for Antcin A, B, C, H, and K, respectively. EAE was prepared as nine grams of pasty extract, yielded from 100 g AC after evaporating with ethanol and mixing well with 1 g salad oil (1 mg/ml), and stored at 4°C until it was used. WAE was prepared by autoclaving 40 g AC in 1200 ml RO water at 1.5 atmospheric pressure, 120°C for 2 hr. The extract was filtered, freeze-dried, adjusted to 40 ml with water (10 mg/ml), and stored at 4°C until used.
Treatment of animals
Male Sprague-Dawley rats (300-400 g), obtained from the National Laboratory Animal Center (Taipei, Taiwan), were maintained in the Animal Center of the Hungkuang University (Taichung, Taiwan). The animal studies were performed following the guidelines of the Guidebook for the Care and Use of Laboratory Animals (2002), published by the Chinese Society of Animal Science in Taiwan. Five groups of rats with a count of ten for each group were used to assess the protection of two AE extracts from hepatic injury. Rats were injected intra-peritoneally, with 40% CCl4 (1 mg/kg) to induce acute liver injury. Positive and normal controls were rats treated with and without CCl4, respectively. Treated groups received oral administration of A. cinnamomea preparations before CCl4 injection. Blood and liver samples were collected from animals after adminis-tering chloral hydrate (400 mg/kg, IP.) anesthesia. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), reactive oxygen species (ROS), interleukin (IL)-1, IL-6, nitric oxide (NO) were determined as follows:
Cytokine and liver function assays
Serum levels of IL-1β, and IL-6 were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). Hepatic injury was assessed by measuring serum ALT and AST with an automatic blood analyzer (Hitachi High-Technologies, Tokyo, Japan).
NO assay
Nitrite was measured as NO, using the Griess test. Briefly, a serum sample was reacted with an equal volume of Griess reagent (0.1% naphthylethylene diamine and 1% sulfanilamide (1:1) in H3PO4) in 96-well plates for 10 min. The absorbance at 540 nm was measured in a microplate reader.
ROS generation
ROS was measured with 2,7-dichlorodihydrofluo-rescein diacetate (H2DCF-DA). Additionally, H2DCF-DA was dissolved in methanol and deacetylated in serum mixed with 10 mM H2DCF for 10 min in the dark. The reaction solution was plated in 96-well plates and monitored on a Fluoroskan Ascent Fluorometer (Labsystems Oy, Helsinki, Finland) using an excitation wavelength of 485 nm and an emission wavelength of 538 nm.
Western blot
Rat liver tissues were homogenized in ice-cold lysis buffer (1:10, weight/volume), containing 20 mmol/l 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (pH 7.2), 1% Triton X-100, 10% glycerol, 1 mM PMSF (phenylmethylsulfonyl fluoride), 10 mg/ml leupeptin, and 10 mg/mL aprotinin. This solution was centrifuged at 10,000× g for 30 min at 4°C. Thereafter, 50 mg of protein was run on an 8% or 10% sodium dodecyl sulfate polyacrylamide gel and transferred onto nitrocellulose membranes (NEN Life Sciences, Boston, MA, USA) at 1.2 A for 3 hr. The membranes were blocked in 5% milk in Tris buffer saline with Tween-20. The membrane was then incubated with each polyclonal rabbit antibodies (p-p38, p-ERK, and p-JNK MAPKs; COX-2, caspase-3, β-actin) and diluted 1:1000 in blocking buffer. Membranes were incubated with secondary anti-rabbit immunoglobulin G, conju-gated to alkaline phosphatase (1:3000) and detected with alkaline phosphatase substrate solution (5-bromo -4-chloro-3-indolyl-phosphate/nitroblue tetrazolium; Kirkegaard & Perry, Baltimore, MD, USA).
Antcin assay
Antcin was determined by liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method for the Antcins A, B, C, H, and K, in the extract of AC fruiting body. Quantitative LC-MS/MS analysis was performed using an Agilent 1100 HPLC system (Agilent Technologies), coupled to an API 4000 triple quadrupole tandem mass spectrometer (Applied Biosystem, Foster City, CA, USA). Chromatographic separation was performed on a Poroshell 120 C18 column (100 × 3.0 mm I.D., 2.7 μm; Agilent, CA, USA).
The mobile phase consisted of 0.2% formic acid aqueous solution (solution A), and acetonitrile (solution B), and a gradient elution program was set as follows: solution A, 70-50% (0-7.5 min), 50-40% (7.5-10.8 min), 40-0% (10.8-19 min), 0-40% (19-21 min), 40-50% (21-25 min), and 50-70% (25-30 min). The column temperature was fixed at 22 °C, the flow rate was set 0.5 ml min−1, and the injection volume was 10 μl. The electrospray negative mode was selected as an ion source for Antcins A, B, C, H, and K detection. The positive electrospray mode was selected as an ion source for 4,7-dimethoxy-5-methyl-1,3-benzodioxole detection. The quantification was performed in multiple reactions monitoring (MRM). The optimized ESI source parameters were as follows: ion spray voltage, −4500 V for negative mode and 4500 V for positive mode; nitrogen nebulizer gas pressure, 12 psi; nitrogen curtain gas pressure, 10 psi; heater temperature, 450°C, and collision activated dissociation (CAD) gas, 6 psi. The precursor-to-product ion transitions were m/z 453/409, m/z 467/408, m/z 469/425, m/z 485/413, and m/z 487/407 for antcin A, antcin B, Antcin C, antcin H, and antcin K respectively. All data acquisition and processing were performed using Analyst 1.4.1 software (AB SCIEX, Concord, ON, Canada). Antcin assay was done by ABM International Lab Inc (Pingtung, Taiwan).
Histopathology
Liver tissues were fixed with a 10% formaldehyde solution overnight and hematoxylin and eosin (H&E) stained for examination.
Statistical analysis
All data were expressed as mean + SD. For single variable comparisons, Student t-test was used. For multiple variable comparisons, data were analyzed with a one-way analysis of variance, using Dunnett’s test. A P<0.05 was considered statistically significant.
Results
Serum AST and ALT concentrations
Since antioxidants ameliorate acute hepatic injury in various models (4-6), we thus compared the effect of two AC preparations on the acute hepatic injury model. Serum AST and ALT concentrations in response to CCl4 stress and Antrodia cinnamomea (AC) extract treatment. The serum AST and ALT concentrations of rats subjected to 40% carbon tetrachloride (CCl4, 1 mg/kg), were measured by the presence of two kinds of AC extract from hepatic injury. The result showed that the EAE at 50 mg/kg significantly reduced serum ALT and AST levels from CCl4-induced rats (Figure 1). However, there was no significant effect of WAE on ALT and AST levels. Data were presented as the mean±SD. And *P<0.05 as compared to the CCl4 group.
Figure 1.
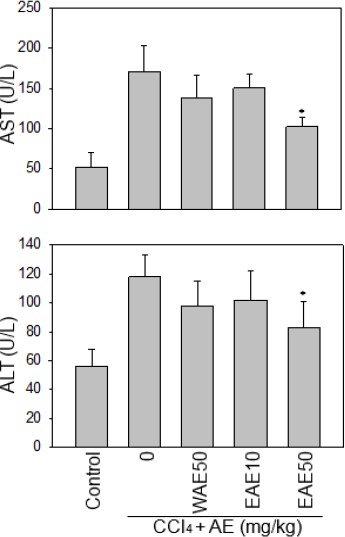
Serum AST and ALT concentrations in response to CCl4 stress and the Antrodia cinnamomea (AC) extract treatment. The result showed that EAE at 50 mg/kg significantly reduced serum ALT and AST levels from CCl4-induced rats. Data are presented as the mean±SE. *:P <0.05 as compared to the CCl4 group
Measurment of IL-1, and IL-6 levels in serum
We further evaluated the effect of AC extract on the induced acute liver injury of rats. The inflammatory cytokine, IL-1 and IL-6 levels in the serum were determined using ELISA kits (R&D Systems). The EAE, reduced serum IL-1 and IL-6 levels in a dose-dependent manner, in CCl4-induced rats (Figure 2).
Figure 2.
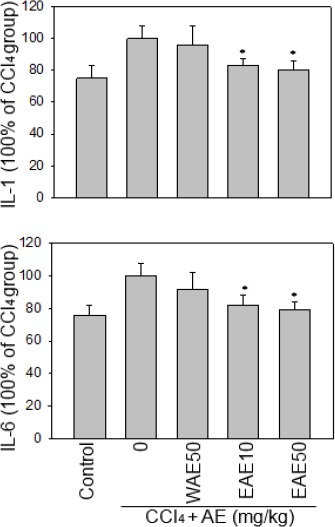
Effects of the Antrodia cinnamomea (AC) extract on serum IL-1 and IL-6 levels under CCl4 stress. The EAE dose-dependently reduced serum IL-1 and IL-6 levels from CCl4-induced rats. Data are presented as the mean±SE. *:P<0.05 as compared to the CCl4 group
NO assay
Levels of NO increased with CCl4-induced liver injury but were lower with the two kinds of AC extract treatment, suggesting that AC extract may protect rats from CCl4-induced liver injury. These results are consistent with the previous findings that; the reduction of IL-1, IL-6 and NO has a protective effect against CCl4-induced liver injury in animals.
ROS scavenging effect of EAE
ROS is necessary for normal physiological functions but also contribute to liver injury. We found that EAE was able to scavenge 40~50% of CCl4-induced serum ROS (Figure 4; *P<0.05), because ROS signals can trigger the intrinsic apoptosis pathway, EAE might reduce the apoptosis of hepatocytes under CCl4 stress by scavenging these free radicals. Our results showed that EAE could protect from liver injury by attenuating the increased serum ROS in the CCl4-induced rats.
Figure 3.
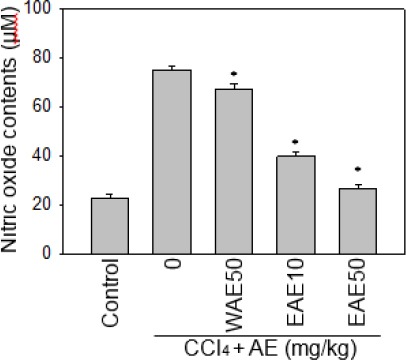
Effect of the Antrodia cinnamomea (AC) extract on serum nitric oxide level in rats with and without CCl4 stress. Levels of NO increased with CCl4-induced liver injury but were reduced with WAE or EAE. Data are expressed as the mean±SE.*:P<0.05 as compared to the CCl4 group
Figure 4.
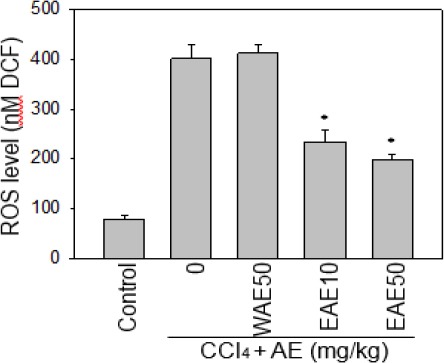
Effect of the Antrodia cinnamomea (AC) extract on serum ROS level under CCl4 stress. EAE was able to scavenge serum ROS generation of CCl4-induced rats. But, there is no scavenging activity with WEA. Data are expressed as the mean±SE. *:P<0.05 as compared to the CCl4 group
Histopathology analysis
Under CCl4 stress, liver cell degeneration increased significantly, in comparison with the control group in the histopathology of rats. Cell atrophy, irregular arrangement with degeneration were observed in the liver section of the CCl4 group (Figure 5B), but not in the control group (Figure 5A). The EAE group (Figure 5C) showed less cell degeneration and spotty necrosis than the PL group in 200 microscopic fields of the liver section. However, the WAE group (Figure 5D) showed much cells atrophy and hepatocellular degeneration than EAE group. The protection from liver injury was evident by attenuating the serum levels of IL-1, IL-6, NO, and ROS (Figs. 2-4; *P<0.05). These results were consistent with histopathology data; that EAE significantly reduced the CCl4-induced lesion in the liver (Figure 5).
Figure 5.
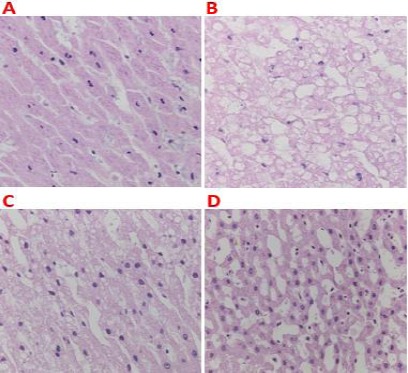
Protective effect of the Antrodia cinnamomea (AC) extract on CCl4-induced hepatic injury. Histopathology of liver slices from rats from (A) no treatment control group; (B) the CCl4 group, (C) the EAE group, and (D) the WAE group. The photographs show the liver section with 200× magnification
EAE inhibited CCl4-induced p-ERK MAPK, COX-2 and caspase-3 activation
The effects of AE preparations were further examined on CCl4-induced signaling pathways by Western blot assay. The result showed that both extracts reduced the p-ERK activation, and that EAE could inhibit COX-2, and caspase-3 levels significantly in the liver samples. EAE (50 mg) reduced the expression of the following proteins p-ERK (70 %), MAPK, caspase-3 (50%), and COX-2 (50%) respect-tively to the CCl4-induced liver cells of rats. This result is better than that in which, WAE (50 mg) reduced expression of p-ERK (50 %) MAPK, caspase-3 (20%), and COX-2 (20%), respectively (*P<0.05; Figure 6).
Figure 6.
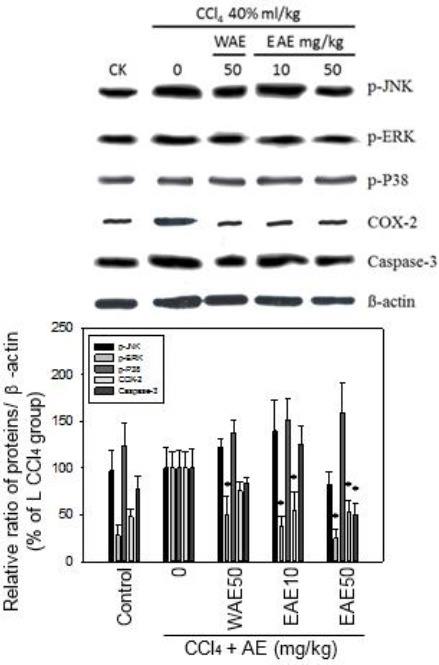
AC extract inhibited CCl4-induced phospho-ERK MAP kinase, COX-2 and caspase-3 under CCl4 stress. EAE and WEA both extracts reduced the p-ERK activation, and EAE inhibit COX-2, and caspase-3 levels better than WEA in the liver samples. Data are expressed as mean ± SEM of three independent experiments. *:P<0.05 as compared to CCl4 control
Discussion
The present results demonstrated that the EAE treatment (50 mg/kg), significantly decreased the serum AST, ALT, IL-1, IL-6, NO, and ROS levels. These data were consistent with the histopathology results. Both extracts reduced the activation of p-ERK signaling pathway, and only EAE inhibited COX-2, and caspase-3 protein levels. These results agreed with other reports and confirmed that active compounds such as Antcins from EAE treatment contributed to the hepatoprotection of the liver (5, 9).
Water extract of AC fruiting bodies has been shown to protect CCl4-induced chronic liver injury in ICR mice (10). Hsiao et al. (2003), reported that AE (250-1250 mg/kg) ameliorated the increase in plasma AST and ALT levels, and restored superoxide dismutase (SOD) activities, glutathione content and catalase activity of hepatic tissues dose-dependently (10). Water extract of AC mycelia, significantly inhibited a free radical initiator, 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced apoptotic cell death in the endothelial cells, with evidence shown by reduced DNA fragmentation, cytochrome c release, caspase-3 activation, and dysregulation of Bcl-2 and Bax. It also prevented the AAPH-induced reductions in SOD activity and protein levels (11). Thus, the water extract of AC fruiting bodies could prevent CCl4-induced chronic hepatotoxicity by scavenging the free radical formation or restoring antioxidant enzyme. However, our present result with low dose of WAE was not significant as compared with EAE in CCl4-induced acute hepatic injury.
EAE had several advantages over WAE preparation for better hepatoprotection. This may be explained by a report that showed that SNEDDS of CoQ10 improves its water solubility, dissolution rate, and bioavailability on rat liver cirrhosis model as compared with CoQ10 powder (12). Upon administration, self-nanoemulsifying system comes in contact with gastrointestinal fluid and forms o/w nano-emulsion with the aid of gastrointestinal motility. This provides a large surface area for enhancing the drug release and absorption. The bioavailability of CoQ10 SNEDDS was increased in 2.1-fold compared with CoQ10 suspension after oral administration. ALT, AST, alkaline phosphatase, total protein, and albumin were significantly improved.
Biological membranes play critical roles in the homeostasis of all organisms because they segregate important activities between and within cells and tissues. Small hydrophobic molecules can partition across biological membranes, down to a concentration gradient but hydrophilic molecules generally require some sort of selective transport system to cross the lipid bilayer. Studies show that certain compounds can facilitate the transport of polar molecules across biological membranes (13, 14). Ergostane and lanostane tetracyclic triterpenoids are the major bioactive compounds in AE (15). Pure ergostanes such as antcins B, C, and H can easily pass through the intestinal Caco-2 cell monolayer, while lanostanes are not (16). Antcins H and K have a high polarity as compared with antcins C and B. However, all ergostanes from AE (antcins B, C, H, and K), show favorable permeability than the pure compounds. The self-dispersing lipid formulations (SDLFs), is one of the promising approaches to overcome the formulation difficulties of various hydrophobic/lipophilic drugs, and to improve the oral bioavailability of poorly absorbed drugs (17). Our results were consistent with these concepts that high concentrations of antcins C, H, and K were detected in the liver and other tissues from EAE-treated animals (Table 1).
Table 1.
Comparison of Antcins concentration between EAE/ WAE treatments
| Antcin C (ppm) | Antcin H (ppm) | Antcin K (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Con | EAE | WAE | Con | EAE | WAE | Con | EAE | WAE | |
| Liver | 0 | 1.1±0.4 | 0 | 0 | 1.0±0.3 | 0 | 0 | 3.0±0.2 | 0 |
| Intestine | 0 | 2.0±0.3 | 1.0 ± 0.1 | 0 | 6.0±0.2 | 0 | 0 | 15±0.5 | 0 |
| Stomach | 0 | 1.0±0.1 | 0 | 0 | 2.1±0.4 | 0 | 0 | 2.0±0.2 | 0 |
| Brain | 0 | 0 | 0 | 0 | 1.0±0.1 | 0 | 0 | 0 | 0 |
Con: control; emulsified AC extract: 50 mg/kg; Water extract of AC: 50 mg/kg
Recently, antcin K was reported to protect the N-nitrosodiethylamine (DEN)-induced liver inflammation, fibrosis and carcinogenesis in rats. The inhibition of DEN-enhanced hepatocellular inflammation is achieved through suppressing NF-κB, scavenging ROS activity and upregulating antioxidant defense mechanisms (18). Antcin C has been reported to protect hepatic cells from AAPH-induced cell death through the inhibition of ROS, lipid peroxidation, ALT/AST and GSH depletion (5). It was correlated with induction of antioxidant genes and SOD via transcriptional activation of Nrf2. In addition, antcin C down-regulates pro-apoptotic factors including, Bax, cytochrome c, capase 9, -4, -12, -3, and PARP. These evidence indicate that antcins C and K can protect liver cells from oxidative stress and cell death via anti-oxidative and anti-inflammatory mechanisms.
Conclusion
The presented data showed that emulsified AC preparation was more effective than WAE to ameliorate CCl4-induced acute hepatic injury by inhibition of cytokines, ROS, and NO through anti-oxidative and anti-inflammatory effects in rats. The protective mechanism was partially attributed to antcins through the suppression of p-ERK signaling pathway, and COX-2 and caspase-3.
Acknowledgment
The results described in this paper were part of student thesis. This work was supported by grants from the Yuanpei University of Medical Technology (102-COMP6023-04).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Moradpour D, Blum HE. Pathogenesis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2005;17:477–483. doi: 10.1097/00042737-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Che QM, Zhao X, Pu XP. Antifibrotic effects of chronic baicalein administration in a CCl4liver fibrosis model in rats. Eur J Pharmacol. 2010;631:53–60. doi: 10.1016/j.ejphar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Yue PY, Wong YY, Wong KY, Tsoi YK, Leung KS. Current evidence for the hepatoprotective activities of the medicinal mushroom Antrodia cinnamomea. Chin Med. 2013;8:21. doi: 10.1186/1749-8546-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar KJ, Chu FH, Hsieh HW, Liao JW, Li WH, Lin JC, et al. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136:168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Gokila Vani M, Kumar KJ, Liao JW, Chien SC, Mau JL, Chiang SS, et al. Antcin c from antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid Based Complement. Alternat Med. 2013;2013:296082. doi: 10.1155/2013/296082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han HF, Nakamura N, Zuo F, Hirakawa A, Yokozawa T, Hattori M. Protective effects of a neutral polysaccharide isolated from the mycelium of Antrodia cinnamomea on Propionibacterium acnes and lipopolysaccharide-induced hepatic injury in mice. Chem Pharm Bull (Tokyo) 2006;54:496–500. doi: 10.1248/cpb.54.496. [DOI] [PubMed] [Google Scholar]
- 7.Ker YB, Peng CC, Chang WL, Chyau CC, Peng RY. Hepatoprotective bioactivity of the glycoprotein, antrodan, isolated from Antrodia cinnamomea mycelia. PLoS One. 2014;9:e93191. doi: 10.1371/journal.pone.0093191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZQ, Liu Y, Zhao JH, Wang L, Feng NP. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int J Nanomedicine. 2012;7:1115–1125. doi: 10.2147/IJN.S28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu HW, Hua KF. Hepatoprotective effect of wheat-based solid-state fermented Antrodia cinnamomea in carbon tetrachloride-induced liver injury in rat. PLoS One. 2016;11:e0153087. doi: 10.1371/journal.pone.0153087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsiao G, Shen MY, Lin KH, Lan MH, Wu LY, Chou DS, et al. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003;51:3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 11.Hseu YC, Chen SC, Yech YJ, Wang L, Yang HL. Antioxidant activity of Antrodia camphorata on free radical-induced endothelial cell damage. J Ethnopharmacol. 2008;118:237–245. doi: 10.1016/j.jep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Balakrishnan P, Lee BJ, Oh DH, Kim JO, Lee YI, Kim DD, et al. Enhanced oral bioavailability of Coenzyme Q10by self-emulsifying drug delivery systems. Int J Pharm. 2009;374:66–72. doi: 10.1016/j.ijpharm.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Gordon G S, Moses A C, Silver R D, Flier J S, Carey MC. Nasal absorption of insulin:enhancement by hydrophobic bile salts. Proc Natl Acad Sci USA. 1985;82:7419–7423. doi: 10.1073/pnas.82.21.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowe CL, Mokhtarzadeh L, Venkatesan P, Babu S, Axelrod HR, Sofia MJ, et al. Design of compounds that increase the absorption of polar-molecules. Proc Natl Acad Sci USA. 1997;94:12218–12223. doi: 10.1073/pnas.94.22.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiao X, Wang Q, Ji S, Huang Y, Liu KD, Zhang ZX, Bo T, Tzeng YM, et al. Metabolites identification and multi-component pharmacokinetics of ergostane and lanostane triterpenoids in the anticancer mushroom Antrodia cinnamomea. J Pharm Biomed Anal. 2015;111:266–276. doi: 10.1016/j.jpba.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Qiao X, Qian Y, Li ZW, Tzen YM, Zhou DM, et al. Intestinal absorption of ergostane and lanostane triterpenoids from Antrodia cinnamomea using Caco-2 cell monolayer model. Nat Prod Bioprospect. 2015;5:237–246. doi: 10.1007/s13659-015-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50:179–188. doi: 10.1016/s0939-6411(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 18.Tien AJ, Chien CY, Chen YH, Lin LC, Chien CT. Fruiting bodies of Antrodia cinnamomea and its active triterpenoid, antcin K, ameliorates N-nitrosodiethylamine-induced hepatic inflammation, fibrosis and carcinogenesis in rats. Am J Chin Med. 2017;45:173–198. doi: 10.1142/S0192415X17500124. [DOI] [PubMed] [Google Scholar]


