Abstract
Objective(s):
When the nerve is injured near its entrance to the muscle belly, we cannot perform conventional methods. One useful method in such a situation is neurotization surgery. In this study, Bone marrow mesenchymal stem cells (BMSCs) implanted into the paralyzed muscle after neurotization surgery. These cells can stimulate axon growth and motor endplate formation, also prevent muscle atrophy.
Materials and Methods:
Thirty-six adult male Sprague-Dawley rats were randomized into six groups: intact group, sham surgery group, control group, DMEM group, cell+DMEM group, denervated group. The motor nerve of the lateral head of gastrocnemius muscle was cut, and the proximal portion of the severed nerve was transplanted to the proximal third of the muscle paralysis. BMSCs with/or DMEM was injected into the site of injury. All animals were evaluated by withdrawal reflex latency (WRL), electromyography, muscle weight, histology and immunohistochemistry.
Results:
The WRL difference between the control and cell+DMEM groups at weeks 4 and 12 post-operation was statistically significant (P<0.05). The mean number of motor end plates in cell+DMEM group was more than control group (P<0.05). At 12 weeks post-operation, the difference of the mean nerve conduction velocity (NCV) between cell treated group and sham surgery groups were not statistically significant (P>0.05). In weeks 4 and 12 post-operation, the mean fiber diameters in cell+DMEM group were more than control group (P<0.05).
Conclusion:
The results of this study demonstrate that transplantation of BMSCs after neurotization surgery, prevent muscle atrophy and improve muscle function.
Keywords: Bone marrow mesenchymal-, stem cells, Motor end plate, Nerve regeneration, Neurotization, Rat model
Introduction
Peripheral nerve regeneration is a serious clinical problem (1). These injuries are the result of trauma, accident and surgery can cause paresis in human and therefore impact on beauty of person, employment opportunities and quality of life individuals (2). Researchers use many techniques like, epineurial suture, nerve autograft and nerve guidance channels for treatment of peripheral nerve injuries (3). Neurotization (reinnervation of muscular fibers) is one of the techniques used for to face various problems, including: repair of nerve, implantation of nerve in the muscle (neuro-muscular), and muscular neurotization (4). When the nerve is injured near its entrance to the muscle belly, conventional methods of nerve repair will be useless, because of the distal stump of the injured nerve was not observed. One of the best methods in such a situation is direct neurotization (5). In this method, the proximal end of severed nerve is directly placed into the paralyzed muscle and the recovery of muscle function assessed (6). Researchers use many neurotrophic factors (7) and extracellular matrix molecules to help axonal sprouting and establishment of motor end plate between nerve and muscle fibers in order to prevent muscle atrophy (8). In some studies, for inducing axon growth and neovascularization, researchers used cell transplantation method after neurotization surgery (7, 9). Mesenchymal stem cells (MSCs) are multi-potent progenitor cells (10) that scientists used for sciatic nerve (8), and spinal cord injury repair (11). BMSCs have a high potential in nerve regeneration via secretion of nerve growth factor, brain-derived growth factor and glial cell-derived growth factor (7, 12). Furthermore, these cells can secrete extracellular matrix molecules like laminin, fibronectin and collagen I and IV, which stimulate axon growth and motor end plate formation, also BMSCs prevent muscle atrophy after denervation (13, 14). In many studies, use of BMSCs and neurotrophic factors for peripheral nerve regeneration. Although, scientists use of direct neurotization in order to prevent muscle atrophy, but researchers do not study on the effect of BMSCs after direct neurotization. So this study designs for evaluating the impact of BMSCs on recovery of skeletal muscle after direct neurotization in the rat as a laboratory model.
Materials and Methods
Animal
Thirty-six adult male Wistar rats (200-250 g) were selected and kept in the same condition with 12 hr light and 12 hr darkness. Animals randomly divided into six groups: intact group, sham surgery group, control group (direct neurotization without injection), DMEM group (direct neurotization+DMEM injected), cell+DMEM group (direct neurotization and BMSCs+DMEM injected), denervated group. The experimental procedures were approved by the Ethical Committee of Urmia University of Medical Sciences.
Isolation and culture of bone marrow stem cells
Isolation and culture of BMSCs were, according to a previously published method (15). Briefly, from 1-month- old Wistar rats by flushing femurs and tibias under sterile conditions with Dulbecco Modified Eagle Medium (DMEM), 10% fetal bovine serum (FBS) and 1% penicillin streptomycin (Sigma Aldrich). Cells were then plated in 75 cm2 culture flasks. After 3 days, nonadherent cells were removed along with the culture medium, and only adherent cells were further cultured in complete medium for four passages. Then the cells from several flasks were pooled, and number of cells was estimated by counting in a Burker's chamber. The final concentration was adjusted to about 1×106 cells/50 µl. In order to identify BMSCs into the muscle, 72 hr before transplantation, Bromodeoxyuridine (Brdu, sigma, 3 μg/ml) were added to the medium of the flask. The percentage of BrdU-labeled BMSCs was 95%. BMSCs identity is confirmed on the basis of morphological criteria and plastic adherence.
Surgical procedure
Rats were anesthetized using intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). A longitudinal incision was made on the posterior surface of the left thigh, and the tibial, common peroneal, and sural nerves were identified. The operation was terminated at this point in the sham-surgery group. The motor nerve of the lateral head of gastrocnemius muscle was cut, and the proximal portion of the severed nerve was transplanted to the proximal third of the muscle paralysis. A single 10/0 nylon suture was used to stabilize the epineurium of nerve stump to epimysium (5). In the control group, direct neurotization were done without injection. In DMEM group, direct neurotization were done and 50 μl DMEM were injected into the lateral head of the gastrocnemius muscle. In cell+DMEM group, direct neurotization was done and BMSCs+DMEM (approximately 1×106 cells in 50 μl) were injected into the lateral head of gastrocnemius muscle (16). In denervated group, the nerve of the lateral head of gastrocnemius muscle cut and the entrance of gluteus maximus muscle. After neurotization surgery, the cells that marked with Brdu were injected into the lateral head of the gastrocnemius muscle.
Neurobehavioral study
Four and 12 weeks post-operation, sensory func-tional recovery was evaluated by measuring withdrawal reflex (WRL). WRL was defined as time that rat withdraw hind paw because of heat. For this purpose, each rat was wrapped in a surgical towel, and then the affected hind paw positioned on a hot plate (DID SABZ Instrument Inc, Model DS8310) set in 56°C, the time was measured from the onset of contact to withdrawal of the hind paw, with a stopwatch. The maximum time for this neurobehavioral test was 12 sec to avoid skin damage to the foot (17).
Electromyography
After WRL evaluation, the animals in each group were subjected to electromyograpy studies using Narco bio-system (USA). During the test, their body temperature was kept constant between 36.5-37 °C using a temperature control unit (Narco, USA). Under intraperitoneally urethane anesthesia (1 g/kg), the left sciatic nerve (operated side) was re-exposed by incision of the previous surgical site in the mid-thigh level. Stimulating electrodes were placed in the tibial nerve, and a recording electrode was inserted into the gastrocnemius muscle. The difference in electro-myography latency, amplitude and distance between the proximal and distal stimulation sites was measured to calculate nerve conduction velocity (18).
Muscle weight
After neurobehavioral and electromyography assessment, gastrocnemius muscle completely deta-ched from the bone, the macroscopic appearance of neurotized muscle was evaluated, and wet weight of muscle was measured by digital scale (19).
Histological examination
The gastrocnemius muscles were fixed in 10 % formalin solution, and cross sections of the reinnerva-ted muscles stained with Hematoxylin-Eosin (H-E). The diameter of muscle fibers was measured with calibrated eyepiece. Methylene blue staining were used to examine and count the number of motor end plate. So, that, sections were taken to include the transplanted nerve and attached muscle such that the nerve branches and nerve terminals were parallel with along axis of the muscle (20).
Immunohistochemistry examination
Immunohistochemical staining was applied to detect cells derived from MSCs at 4 and 12 weeks post-operation. Briefly, a series of adjacent 6 μm thick sections (25 μm interval) from each block were incubated in 50% formaldehyde (Merck, Germany), 2×SSC (standard sodium citrate: 0.3 M NaCl, and 0.03M sodium citrate) at 65°C for 2 hr, washed for 10 min with 2×SSC at room temperature. They were then incubated in 2 M HCl (Merck, Germany) at 37°C for 30 min, rinsed in 0.1M boric Acid (Merck, Germany) for 10 min, washed in PBS, and incubated with mouse anti-BrdU monoclonal antibody (Sigma Aldrich) at 4°C overnight. After rinsing 3 times in PBS for 10 min, the sections were incubated overnight in the dark at 4°C with Rhodamine conjugated secondary antibody (1: 100), washed in PBS, covered with a cover slip, and were studied under a light microscope (21).
Statistical analysis
All data were analyzed by using a one-way analysis of variance (ANOVA) followed by the Turkey’s test. P<0.05 were considered to be statistically significant. All calculations were conducted using SPSS 16.0 software.
Results
Two rats died in the perioperative period (probably because of overdosing of the anesthetic drug).
Neurobehavioral study
Withdrawal reflex latency (WRL) was significantly lower in the cell transplantation group than in the denervation group 4 and 12 weeks post-operative and the difference were significant (P<0.05). Four
Weeks post-operative, WRL was significantly improved in the cell transplantation group, while the difference between cell transplantation and DMEM groups was not identified (P>0.05). However, all WRL values were significantly more than those seen in the intact and sham surgery groups (P<0.05). At 12 weeks post-operation, although WRL in the intact and sham surgery groups were lower than cell+DMEM group, but the difference was not statistically significant (P>0.05) (Figure 1).
Figure 1.
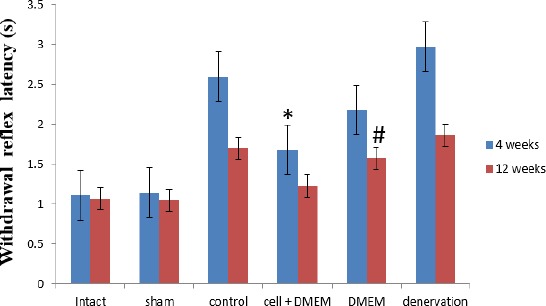
Withdrawal reflex latency (s) at 4 and 12 weeks after surgery, (± SEM)
* The significant statistical difference between cell+DMEM and control groups at 4 and 12 weeks post-operation, (P<0.02)
# The significant statistical difference between DMEM and cell+DMEM groups at 12 weeks post-operation, (P<0.05)
Muscle weight
4 weeks post-operative, the mean weight of muscles in all experimental groups reached its lowest value but there were significant statistical differences between the cell+DMEM group (1.6±0.27) and denervation group (0.8±0.22) (P<0.001). The result showed that there was a significant difference between the intact and sham surgery groups in comparison with other experimental groups (P<0.01). Twelve weeks post-operative, although the muscle mass in the cell+DMEM (2.1±0.31) and DMEM (1.89±0.97) were lower than the intact and sham surgery group (2.3±0.34, 2.3±0.33, respectively), but the difference was not significant (P>0.05).
Electromyography study
Electromyography stimulation studies indicated that the nerve conduction velocity (NCV) of the animals in the cell+DMEM group improved over time. Foure weeks post-operation, there was a significant difference between the intact and sham surgery groups in comparison with other experimental groups (P<0.002). Twelve weeks post-operative, the differences were identified between cell+DMEM in comparison with DMEM, denervation, and control groups (P<0.05). In the cell+DMEM group, the measured values were lower than those measured in the intact and sham surgery groups, but the differences were not statistically significant (P>0.05) (Figure 2).
Figure 2.
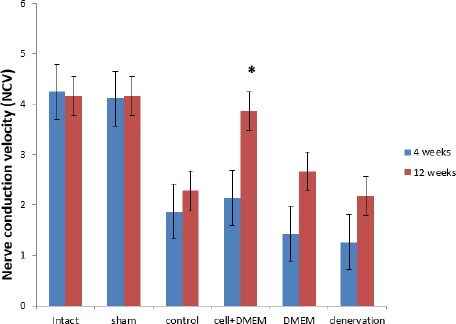
Nerve conduction velocity (NCV) at 4 and 12 weeks post-operative, (± SEM)
* The significant statistical difference between cell+DMEM in comparison with control, DMEM, and denervation groups at 12 weeks post-operation, (P<0.05)
Histological and immunohistochemical assessment
The mean muscle fiber diameters were measured 4 and 12 weeks post–operative in a cross-section. Some of them rounded, others polygonal shaped. Small branches of nerve were evident in the cross-section of the muscles. 4 weeks post–operative, the result indicated that the mean muscle fiber diameters in all experimental groups were lower than intact group and the differences were statistically significant (P<0.02) (Figure 3A). In the later period of reinnervation (12 weeks post-operation) most of the fibers were large and oval shaped in the cell+DMEM group. The result showed that preservation of muscle structure with less fiber atrophy in the cell+DMEM group, and the mean muscle fiber diameters in cell+DMEM group in comparison with control and denervated groups were significant differences (P<0.05). Although the mean muscle fiber diameters in cell+DMEM group were more than the DMEM group, but this difference was not significant (Figure 3-A). Twelve weeks post-operation, in the denervated muscle group can observe significant atrophy of muscle, marked by a reduction in the fiber size and an increase in connective tissue (Figure 3B).
Figure 3.
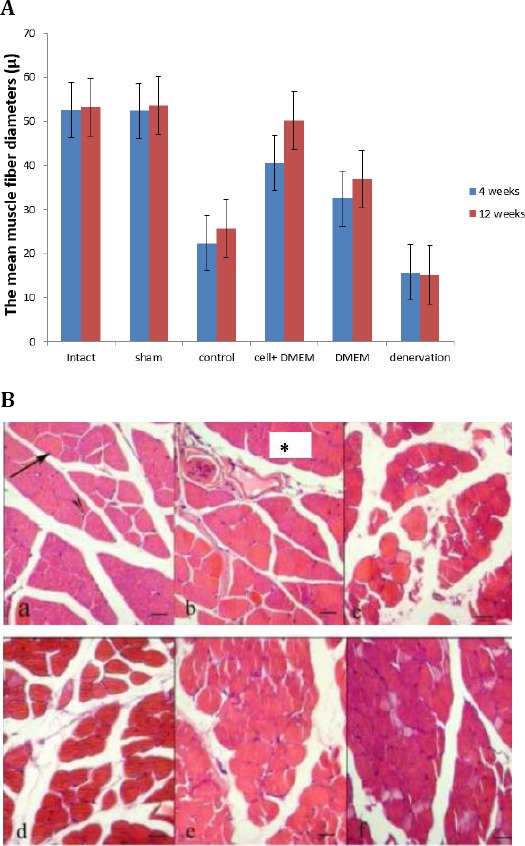
A. The mean muscle fiber diameters (µM) at 4 and 12 weeks post-operation, (±SEM). *The significant statistical difference between cell+DMEM in comparison with control, and denervation groups at 12 weeks post-operation, (P<0.05). B. Cross-section of the rat gastrocnemius muscle stained with hematoxylin and eosin (H&E), showing fiber morphology after reinnervation. (a): normal group, (b): sham surgery group, (c): denervation group, (d): control group, (e): DMEM group, (f): cell+DMEM group. Muscle fiber diameter and nucleus of muscle fiber (black arrows). The section was taken from the lateral head of gastrocnemius muscle 12 weeks afrer neurotization surgery (Scale bar 50 µm)
After methylene blue staining, the number of motor end plate with a reinnervated axon was counted. The result indicated that after 4 weeks, the number of motor end plate in experimental groups was significantly lower than intact groups (P<0.002). Twelve weeks post-operation, the mean number of motor end plates in cell+DMEM group was more than DMEM, control, and denervated groups and the differences were significant (P<0.03). The result showed that although the mean number of motor end plates in cell+DMEM group was lower than intact and sham surgery groups, but there were no statistical differences between these groups (P>0.05) (Figure 4). Immuno-histochemical study showed that the scattered Brdu-positive cells were detected in muscle. The most of the Brdu-positive cells were located near the motor end plates (Figure 5).
Figure 4.
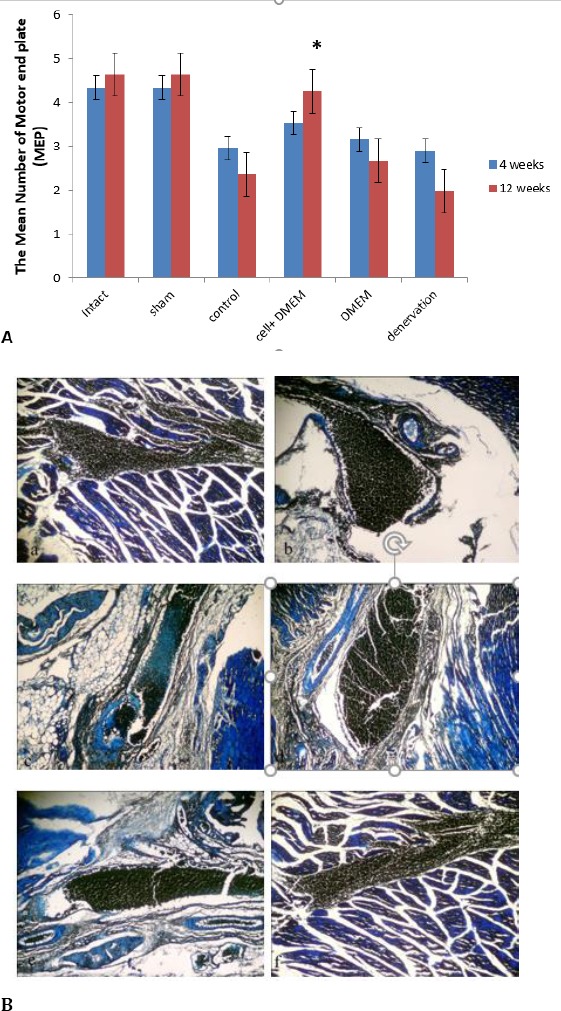
A. The mean number of motor end plate (NEP) at 4 and 12 weeks post-operation, (±SEM). * The significant statistical difference between cell+DMEM in comparison with control, DMEM, and denervation groups at 12 weeks post-operation, (P<0.05). B. Longitudinal-section of the rat gastrocnemius muscle stained with methylene blue, showing motor end plate after reinnervation. (a): normal group, (b): sham surgery group, (c): denervation group, (d): control group, (e): DMEM group, (f): cell+DMEM group. Muscle fiber diameter and nucleus of muscle fiber (black arrows). The section was taken from the lateral head of gastrocnemius muscle 12 weeks after neurotization surgery
Figure 5.
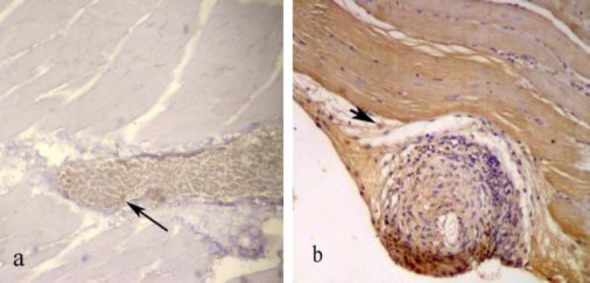
Immunohistochemical study of cross-section to the main axis of the gastrocnemius muscle 4 (a) and 12 (b) weeks post-operation. The scattered Brdu-positive cells were detected in muscle. The Most of the Brdu-positive cells were located near the motor end plate
Discussion
The finding of this study demonstrated that BMSCs significantly improved the recovery of the paralyzed muscle in rats with a direct nerve implantation.
Several studies have demonstrated that direct neurotization of an injured proximal nerve stump can grow into the paralyzed muscle and establish functional connections (motor end plates) (5). In our study, it seemed that the transplantation of adult BMSCs compared with DMEM, control, and denervated groups enhance nerve repair after direct neurotization. Bone marrow provides a source of hematopoietic and nonhematopoietic stem cells (22). The precursors of nonhemattopoietic tissues are referred to as BMSCs, their multipotentiality for differentiation, and their possible use for both cell and gene therapy (23). A previous study demonstrated that the intravenous injection of BMSCs significantly improved the recovery of hind limb motor function in rats with a spinal compression lesion (24). Several investigations about transplantation of adult BMSCs directly into the adult rat brain and spinal cord showed that reduces
functional deficits associated with stroke, traumatic brain injury, and spinal cord injury (25).
BMSCs can differentiate into a variety of cell types including myoblasts (26), adipocytes (27), osteoblasts (28), and chondroblasts (29), when placed into different microenvironments. In addition, the adult rat and human BMSCs can differentiate into neuronal phenotypes in vitro (30), and leads to extensive remyelination (31).
In the present study, the mean weight of muscles was significantly greater for the cell+DMEM group vs. the control group, but this difference was not significant in the DMEM group. As for skeletal muscle, denervation due to nerve injury or surgical division (e.g. for free muscle transfer) leads to muscle atrophy, as evidenced by a reduction in the number and size of muscle fibers, with preservation of residual connective tissue (6). So that muscle weight would expect to correlate with the degree of innervations of a muscle (32). A denervated muscle mass can lose up to two-thirds of its mass within 1 month (33). On the other hand, in this study, the mean muscle fiber diameter (µm) was significantly greater for the cell+DMEM group vs. the control group, but this difference was not significant in the DMEM group. BMSCs produce muscle when implanted in the appropriate tissue in vivo, and may serve to replenish lost cells; moreover, they contain a variety of cells that secrete substances that may lead to recovery (26). In addition, DMEM contains amino acids and vitamins, as well as additional supplementary component that may lead to recovery, but the mechanisms or factors that promote reduced deficit are still unknown (34). BMSCs secrete cytokines such as interleukins (35), nerve growth factor (NGF), brain-derived neuro-trophic factor (BDNF), and vascular endothelial cell growth factor (VEGF) (36). It has also been reported that BMSCs stimulate glial cells to produce neuro-trophic factors (37).
In the present study, the withdrawal reflex latency was greater for the control and DMEM group vs. the cell+DMEM group. Our results are in agreement with the findings of Urdzíková et al. (2006) who found improved behavioral parameters after spinal cord injury in BMSCs group in comparison with the control saline treated group (24). In addition, BMSCs can differentiate into neuron-like cells and glial (38). The glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo (39).
There are several reasons supporting the use of MSCs in recovery of the paralyzed muscle with a direct nerve implantation: easy to isolate from the bone marrow that can be obtained under local anesthesia, capable of rapid proliferation in culture, may serve to replenish lost cells (34), act by immune modulation (40), have self-renewal capacity and multi-potentiality (41), capacity to pass through the blood-brain barrier (42), moreover, they contain a variety of cells that secrete substances that may lead to recovery (34).
In this study, the BMSCs were transplanted directly into denervated gastrocnemious muscle. Our results are in agreement with the findings of Hocking et al. (2010), who reported that, the direct application of BMSCs on wounds which has the potential advantage of promoting regeneration via MSCs differentiation and BMSCs paracrine signaling (43). So that BMSCs acts as a chemoattractant for specific cell types to the wound (44). In addition, they may also regulate cell migration in response to injury (45).
In the present study, the mean number of motor end plates in the cell+DMEM group was significantly larger than the control and DMEM groups. A previous study reported that, when grafted the motor branch of the peroneal nerve of rabbit into the “a neural” zone of the lateral head of the gastrocnemius muscle, the motor function had recovered and motor end-plates were found in histological sections (46), So that function after neurotization of denervated muscles as well as histological evidence of new motor end-plate formation in a neural zone of the muscle (47).
Also, we found small number BrdU reactive MSCs cells in the cell+DMEM group survive in the muscle near to end plate. Li et al. (2000) showed that a small number (~0.5 %) of BrdU reactive cells that survive in the normal mouse brain might be because of the possibility that infused cells divide rapidly and consequently the label below levels of detection of BrdU (48). Transplanted stem cells can either replace missing populations of cells or rescue cells in the injured area by the production of interleukins and stem cell factor (36), that induced tissue plasticity, and neuroprotective factors (49). It has also been reported that BMSCs stimulate glial cells to produce neurotrophic factors such as NGF and BDNF (35, 37). These cytokines are known to be essential factors for the survival and differentiation of neuronal prog-enitor cells (24). BMSCs has the ability to produce extracellular matrix proteins such as; collagen I, collagen IV, fibronectin, and laminin (5, 50). Therefore, there is good evidence to support the hypothesis that transplantation of BMSCs may repair peripheral nerve injuries (51). In addition, Stem cells may help create a microenvironment that promoted axon extension gap (52). However, the mechanisms or factors that can be generated motor end plate are still unknown.
In the other hand, motor nerves are rarely purely motor, likewise, sensory nerves may not be purely sensory (53). The sensory fibers are traditionally thought to innervate mainly the intrafusal muscle spindles and Golgi tendon organs, and not the motor end plates (6). Also, it has been suggested that neutrally released acetylcholine may act as a neuromuscular trophic agent, reducing muscle atrophy and fibrosis by suppressing muscle collagen biosynthesis and reduction of lysosomal proteolysis (54).
In this study, the mean nerve conduction velocity (m/s) in the cell + DMEM group was significantly greater than in the control and DMEM groups. The decreased NCV is the effect of the loss of larger diameter fibers, a phenomenon reported in other traumatic nerve lesions. NCV has been shown to be dependent on axon diameter, myelination, and intermediate distance (55).
Conclusion
BMSCs effectively enhance the recovery of the paralyzed muscle in rats with a direct nerve implantation. However, the mechanisms or factors that promote reduced deficit are still not fully unknown.
Acknowledgment
This article was derived from an MSs thesis in the Urmia University of Medical Sciences. Funding for this research project was supported by grant no. 1655 from the Urmia University of Medical Sciences in Urmia, Iran.
References
- 1.Bellamkonda RV. Peripheral nerve regeneration:an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Mu L, Sobotka S, Su H. Nerve-muscle-endplate band grafting:a new technique for muscle reinnervation. Neurosurgery. 2011;69:208–224. doi: 10.1227/NEU.0b013e31822ed596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farjah GH, Heshmatian BM, Karimipour M, Saberi A. Using eggshell membrane as nerve guide channels in peripheral nerve regeneration. Iran J Basic Med Sci. 2013;16:901–905. [PMC free article] [PubMed] [Google Scholar]
- 4.Wehbe J, Chidiac R, Maalouf G. Neurotization by adjacent muscle. Pan Arab J Orth Trauma. 2004;8:69–76. [Google Scholar]
- 5.Bielecki M, Skowronski R, Skowronski J. A comparative morphological study of direct nerve implantation and neuromuscular pedicle methods in cross reinnervation of the rat skeletal muscle. Rocz Akad Med Bialymst. 2004;49:10–17. [PubMed] [Google Scholar]
- 6.Noordin S, Ahmed M, Rehman R, Ahmad T, Hashmi P. Neuronal regeneration in denervated muscle following sensory and muscular neurotization. Acta Orthop. 2008;79:126–133. doi: 10.1080/17453670710014879. [DOI] [PubMed] [Google Scholar]
- 7.Ritfeld GJ, Nandoe Tewarie RD, Vajn K, Rahiem ST, Hurtado A, Wendell DF, et al. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transplant. 2012;21:1561–1575. doi: 10.3727/096368912X640484. [DOI] [PubMed] [Google Scholar]
- 8.Pereira Lopes FR, Camargo de Moura Campos L, Dias Correa RJ, Jr, Balduino A, Lora S, Langone F. Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp Neurol. 2006;198:457–468. doi: 10.1016/j.expneurol.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Bhagavati S, Xu W. Isolation and enrichment of skeletal muscle progenitor cells from mouse bone marrow. Biochem Biophys Res Commun. 2004;318:119–124. doi: 10.1016/j.bbrc.2004.03.192. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz E, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC:The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 11.Cantinieaux D, Quertainmont R, Blacher S, Rossi L, Wanet T, Noël A, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats:an original strategy to avoid cell transplantation. PloS One. 2013;8:e69515. doi: 10.1371/journal.pone.0069515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dadon-Nachum M, Melamed E, Offen D. Stem cells treatment for sciatic nerve injury. Expert OpinBiol Ther. 2004;11:1591–1597. doi: 10.1517/14712598.2011.628933. [DOI] [PubMed] [Google Scholar]
- 14.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 15.Zarbakhsh S, Bakhtiyari M, Faghihi A, Joghataei MT, Mehdizadeh M, Khoei S, et al. The Effects of Schwann and bone marrow stromal stem cells on sciatic nerve injury in rat:A comparison of functional recovery. Cell J. 2012;14:39–46. [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda A, Hirata H, Akeda K, Morita A, Nagakura T, Tsujii M. Enhanced reinnervation after neurotization with Schwann cell transplantation. Muscle Nerve. 2005;31:229–234. doi: 10.1002/mus.20254. [DOI] [PubMed] [Google Scholar]
- 17.Konya D, Liao WL, Choi H, Yu D, Woodard MC, Newton KM, et al. Functional recovery in T13-L1 hemisected rats resulting from peripheral nerve rerouting:role of central neuroplasticity. Regen Med. 2008;3:309–327. doi: 10.2217/17460751.3.3.309. [DOI] [PubMed] [Google Scholar]
- 18.Farjah GH, Fazli F. The effect of chick embryo amniotic fluid on sciatic nerve regeneration of rats. Iran J Vet Res. 2015;16:167–171. [PMC free article] [PubMed] [Google Scholar]
- 19.Azizi S, Mohammadi R, Amini K, Fallah R, Karegar K. Bridging small-gap peripheral nerve defect using silicone rubber chamber in the rat sciatic nerve transection model. Vet Res Forum. 2012;1:107–115. [Google Scholar]
- 20.Isaacs J, Feher J, Shall M, Vota S, Fox MS, Mallu S, et al. Spita Effects of nandrolone on recovery after neurotization of chronically denervated muscle in a rat model. J Neurosurg. 2013;119:914–923. doi: 10.3171/2013.5.JNS121837. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N, Fujita A, Hsieh MM, Bonifacino A, Krouse AE, Metzger ME, et al. Bone marrow as a hematopoietic stem cell source for gene therapy in sickle cell disease (SCD):evidence from rhesus and SCD patients. Hum Gene Ther Clin Dev. 2017 doi: 10.1089/humc.2017.029. doi:10.1089/humc.2017.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prockop D. Heritable osteoarthritis. Diagnosis and possible modes of cell and gene therapy. Osteoarthritis Cartilage. 1999;7:364–366. doi: 10.1053/joca.1998.0213. [DOI] [PubMed] [Google Scholar]
- 24.Urdzíková L, Jendelová P, Glogarová K, Burian M, Hájek M, Syková E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- 25.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, et al. Spinal cord injury in rat:treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 26.He J, Teng X, Yu Y, Huang H, Ye W, Ding Y, et al. Injection of Sca-1+/CD45+/CD31+mouse bone mesenchymal stromal-like cells improves cardiac function in a mouse myocardial infarct model. Differentiation. 2013;86:57–64. doi: 10.1016/j.diff.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Vogel G. Can old cells learn new tricks? Science. 2000;287:1418–9. doi: 10.1126/science.287.5457.1418. [DOI] [PubMed] [Google Scholar]
- 28.Ozdal-kurt F, Tuglu I, Vatsnsever HS, Tong S, Sen BH, Deliloglu-Gurhan SI. The effect of different implant biomaterials on the behavior of canine bone marrow stromal cells during their differentiation into osteoblasts. Biotech Histochem. 2016;91:412–422. doi: 10.1080/10520295.2016.1183819. [DOI] [PubMed] [Google Scholar]
- 29.Lee B, Han L, Frank EH, Grodzinsky AJ, Ortiz C. Dynamic nanomechanics of individual bone marrow stromal cells and cell-matrix composites during chondrogenic. J Biomech. 2015;48:171–175. doi: 10.1016/j.jbiomech.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu J, Moochhala S, Moore XL, Ng KC, Tan MH, Lee LK, et al. Adult bone marrow cells differentiate into neural phenotypes and improve functional recovery in rats following traumatic brain injury. Neurosci Lett. 2006;398:12–17. doi: 10.1016/j.neulet.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 31.Inoue M, Honmou O, Oka S, Houkin K, Hashi K, Kocsis JD. Comparative analysis of remyelinating potential of focal and intravenous administration of autologous bone marrow cells into the rat demyelinated spinal cord. Glia. 2003;44:111–118. doi: 10.1002/glia.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vathana T, Nijhuis TH, Friedrich PF, Bishop AT, Shin AY. An experimental study to determine and correlate choline acetyltransferase assay with functional muscle testing after nerve injury. J Neurosurg. 2014;120:1125–1130. doi: 10.3171/2014.1.JNS122241. [DOI] [PubMed] [Google Scholar]
- 33.Dow DE, Carlson BM, Hassett CA, Dennis RG, Faulkner JA. Electrical stimulation of denervated muscles of rats maintains mass and force, but not recovery following grafting. Restor Neurol Neurosci. 2006;24:41–54. [PubMed] [Google Scholar]
- 34.Zhen G, Xue Z, Zhao J, Gu N, Tang Z, Xu, et al. Mesenchymal stem cell transplantation increases expression of vascular endothelial growth factor in papain-induced emphysematous lungs and inhibits apoptosis of lung cells. Cytotherapy. 2010;12:605–614. doi: 10.3109/14653241003745888. [DOI] [PubMed] [Google Scholar]
- 35.Delk NA, Farach-Carson MC. Interleukin-6:a bone marrow stromal cell paracrine signal that induces neuroendocrine differentiation and modulates autophagy in bone metastatic PCa cells. Autophagy. 2012;8:650–663. doi: 10.4161/auto.19226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Björklund LM, Sánchez-Pernaute R, Chung S, Andersson T, Chen IYC, McNaught KSP, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma. 2002;19:1609–1617. doi: 10.1089/089771502762300265. [DOI] [PubMed] [Google Scholar]
- 38.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neuroscie Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.JendelováP Herynek V, Urdzikova L, GlogarováK KroupováJ, Andersson B, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 40.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 41.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells:biology of adult mesenchymal stem cells:regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:1–10. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hocking AM, Gibran NS. Mesenchymal stem cells:paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells:Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 45.Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316:48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunelli G, Monini L. Direct muscular neurotization. J Hand Surg. 1985;10:993–997. doi: 10.1016/s0363-5023(85)80022-8. [DOI] [PubMed] [Google Scholar]
- 47.Chiu DT, Chen L, Spielholtz N, Beasley R. A comparative electrophysiological study on neurotisation in rats. J Hand Surg Br. 1991;16:505–510. doi: 10.1016/0266-7681(91)90104-v. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Choppm M, Chen J, Wang L, Gautam SC, Xu YX, et al. Interstriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Song S, Kamath S, Mosquera D, Zigova T, Sanberg P, Vesely D, et al. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp Neurol. 2004;185:191–197. doi: 10.1016/j.expneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Chen CJ, Ou YC, Liao SL, Chen WY, Chen SY, Wu CW, et al. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–453. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Wakao S, Hayashi T, Kitada M, Kohama M, Matsue D, Teramoto N, et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537–547. doi: 10.1016/j.expneurol.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Kang SB, Olson JL, Atala A, Yoo JJ. Functional recovery of completely denervated muscle:implications for innervation of tissue-engineered muscle. Tissue Eng Part A. 2012;18:1912–1920. doi: 10.1089/ten.tea.2011.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson JA, Bahattacharyya BJ, Vaden JH, Wilson JA, Icyuz M, Howard AD, et al. Motor and sensory deficits in the teetering mice result from mutation of the ESCRT component HGS. PLoS Genet. 2015;26:11–e1005290. doi: 10.1371/journal.pgen.1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Virtanen P, Tolonen U, Savolainen J, Takala T. Effect of reinnervation on collagen synthesis in rat skeletal muscle. J Appl Physiol. 1992;72:2069–2074. doi: 10.1152/jappl.1992.72.6.2069. [DOI] [PubMed] [Google Scholar]
- 55.Manganelli F, Pisciotta C, Reilly MM, Tozza S, Schenone A, Fabrizi GM, et al. Nerve conduction velocity in CMT1A:what else can we tell? Eur J Neurol. 2016;23:1566–1571. doi: 10.1111/ene.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]


