Abstract
Objective(s):
Scutellarin (Scu) is the main effective constituent of Erigeron breviscapus which has anti-oxidant, anti-apoptotic, anti-inflammatory and other therapeutic properties. The purpose of this study was to investigate the protective effect of Scu on myocardial infarction (MI) induced by isoprenaline (ISO).
Materials and Methods:
The rats were subcutaneously injected with ISO (45 mg/kg) on the first day, then single tail-intravenously injected with different doses of Scu (10 mg/kg, 20 mg/kg, 40 mg/kg) for 7 consecutive days. The protective effect of Scu on ISO-induced MI was evaluated by measuring markers of heart injury in serum, levels of lipid peroxidation, and antioxidants in heart tissue, observing pathological changes of tissue, and detecting quantified expression of apoptotic-related family members and inflammation.
Results:
Compared with the model group, the concentration of troponin T (CTn-T) and troponin I (CTn-I), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) in the serum all decreased in the Scu high dose group. The activities of superoxide dismutase (SOD), catalase (CAT), and the content of reduced glutathione (GSH) in heart increased, and the content of malondialdehyde (MDA) and inducible nitric oxide synthase (iNOS) decreased. In addition, the histopathologic aspects showed that pathological heart change was found in the model group, and was reduced to varying degrees in the Scu group. Moreover, the expression of Bax, P53, Caspase3, Caspase9, cytochrome C, NGAL, NFκB, IL-1β and IL-6 in the heart decreased, while the expression of Bcl2 increased.
Conclusion:
Scu could reduce the degree of MI induced by ISO by improving the antioxidant, anti-apoptotic, and anti-inflammatory capacities of the body.
Keywords: Apoptosis, Inflammation, Isoprenaline, Myocardial infarction, Oxidative stress, Scutellarin
Introduction
Myocardial infarction (MI) is one of the most widely spread manifestations of cardiovascular disease, which is associated with imbalance between coronary blood supply and myocardial demand (1). MI is a major public health concern and the leading cause of morbidity and mortality in the Western world, even in China. So far, morbidity and mortality due to MI have reached epidemic proportions, accounting for 16.7 million deaths/year worldwide (2). Furthermore, MI can also cause other obvious symptoms, such as myocardial fibrosis (3) and cardiac hypertrophy (4). The specific mechanism involving MI have proved to be associated with oxidative stress (5), apoptosis (6), and inflammation (7). Previous studies have demonstrated that oxidative stress could participate in myocardial structural damage in cardiac hypertrophy (8). In addition, increasing evidence has indicated that the level or activities of endogenous antioxidants get altered in the myocardium in response to oxidative stress condi-tions (9). The activities of some antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX) decreased (10), which can lead to added lipid peroxidation. The increase of lipid peroxides could lead to the formation of malondialdehyde (MDA), increase free radical production and decrease in antioxidant status (5). Under the condition of oxidative stress, excessive production of reactive oxygen species (ROS) allows the release of pro-apoptotic factors from the mitochondria into the cytosol and thereby activating signs of endoplasmic reticulum stress and apoptotic cell death (11). Several lines of evidence suggest that inflammation is a key process involved in mediating myocardial tissue damage after an ischemic event (12). Neutrophils infiltrate the infarcted area where they can promote myocardial cell damage through the release of proteolytic enzymes, a myriad of inflammatory cytokines and chemokines, and the production of ROS (9). Cytokines and chemokines, as well as ROS, eventually cause the loss of organ function (13).
Traditional Chinese Medicines (TCMs) and their preparations have been widely used to prevent and treat diseases for thousands of years in China and other countries (14). So far, in order to combat MI and related disease, researchers have found that TCMs with antioxidant activity such as Vitex negundo (15), Aegle marmelos (16), Salvia miltiorrhiza (10), Anemarrhena asphodeloides Bunge (17) have cardio-protective effects. Erigeron breviscapus (vant.), a traditional Chinese medicinal plant, has a long history of medicinal use in Chinese medicine (18). In general, E. breviscapus is extensively used in clinics to treat ischemic cardio-cerebral vascular diseases (19). Scutellarin (Scu) is a flavonoid glycoside that is the main effective constituent of E. breviscapine (20). Scu could significantly improve hemodynamics, micro-circulation and blood flow, dilate blood vessels, reduce the blood platelet count, activate K+ channels and block Ca2+ channels, and increase cerebral blood flow, etc (21). In clinics, oral and injectable pharmaceuticals of Scu have been used for the treatment of myocardial ischemia, focal cerebral ischemia, angina pectoris, coronary heart disease, acute cerebral infarction, and paralysis induced by cerebrovascular diseases (22). Therefore, in China and some other Asian countries, due to the distinguished efficacy of Scu in the clinical therapy, Scu research has become a hot topic in recent years.
Many studies have been carried out on cardioprotective effect of E. breviscapus. These studies have shown that E. breviscapine can significantly reduce MI size, and increase the serum concentration of Ca2+, Mg2+, Zn2+, and SOD in dog models of myocardial ischemia-reperfusion (23). In rabbit models, breviscapine inhibited cell apoptosis and inflammatory injury, which was modulated by decreasing the expression of cytochrome C, Bax, caspase-3, Tumor Necrosis Factor (TNF-α) and nuclear factor kappa B (NFκB), increasing the expression of the B-cell lymphoma-2 (Bcl-2) and Bcl-2/Bax ratio (24). In addition, E. breviscapus has a significant protective function in acute myocardial infarction (AMI) patients with reperfusion injury, which can effectively decrease the serum levels of interleukin- 6 (IL-6), TNF-α and MDA, and increase the levels of SOD and nitric oxide (NO) in serum (25). Parallel results are also found in rat models (26). Similarly, Scu can also decrease the infarction size and inhibit apoptosis of myocardial cells in AMI dog model. Furthermore, dose-response relationship of Scu is better than that of E. breviscapine (27). Researchers have shown that Scu has a wide range of therapeutic properties, such as anti-apoptotic (28), anti-oxidant (29), anti-inflammatory (30), and other effects. Neuroprotection of Scu was investigated in neurotoxicity induced by β-amyloid peptide (Aβ) in the rat brain (28). The results indicated that Scu can attenuate the neurotoxicity of Aβ, by decreasing the levels of IL-6, interleukin-1 (IL-1β), and TNF-α, decreasing the percentage of apoptotic neurons, and increasing the levels of SOD and monoamine oxidase (MAO). In addition, researchers (29) have investigated the cardioprotective effect of Scu on ischemia-reperfusion (I/R) in rat cardiomyocytes H9C2. The results indicated that Scu significantly protected cardiomyocytes from I/R injury-induced oxidative stress and apoptosis. This study demonstrates the protective role of Scu in ischemic heart disease. Therefore, we can also study the protective effect of Scu on MI rat model through oxidative stress, apoptosis, and inflammation-related pathways.
Isoprenaline (ISO), a β-adrenoceptor agonist is a catecholamine drug widely used in the treatment of allergic emergencies, status asthmatic, bronchial asthma, ventricular bradycardia, cardiac arrest, and glaucoma (31). However, ISO can also induce side effects and cause obvious adverse reactions, which has been reported to produce MI in large doses (14). After auto-oxidation, ISO generates highly reactive free radicals, alters tissue defense systems including chemical scavengers or antioxidant molecules and the antioxidant enzymes catalase, superoxide dismutase, glutathione peroxidase, glutathione-S-transferase, and excessive accumulation of lipids in the heart tissue (32). Researchers have found ISO-induced myocardial disorders in the heart of experimental animals similar to those seen in human myocardial ischemia (33). Therefore, ISO has been widely used as a well-standardized model to produce MI in rats, in order to study the beneficial effects of many drugs on the processing of MI.
In the present study, we aim to investigate the protective role of Scu against ISO-induced MI in rats, and its functionary mechanism was also elucidated based on the antioxidant, anti-apoptotic, and anti-inflammatory activity of Scu.
Materials and Methods
Reagents
Scutellarin (purity 98%) was purchased from JingZhu Co Ltd, Nanjing, China. The assay kits for MDA (A003-1), CAT (A007-1), inducible nitric oxide synthase (iNOS) (A014-1), glutathione (GSH) (A006-2), SOD (A001-3), BCA (A045-4) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and pentobarbital sodium, isoprenaline were purchased from Sigma–Aldrich Co, St Louis, MO, USA. The ELISA kits for troponin I (CTn-I) (ml7091103), troponin T (CTn-T) (ml7090091), lactate dehydrogenase (LDH) (m8009110), aspartate aminotransferase (AST) (ml5078923) were purchased from Enzyme-linked Biotechnology Co. Ltd, (Shanghai). Salvia miltiorrhiza injection was purchased from Sichuan Shenghe Co Ltd.
Experimental animals
Animal experiments were approved by the Animal Care and Use Committee of the College of Animal Science, Fujian Agriculture and Forestry University, and the experiment was performed according to regulations and guidelines established by this committee. Thirty-six healthy adult male Sprague Dawley rats (weighing 180–200 g) were purchased from Shanghai Sikelai experimental animal Co Ltd. During the period of feeding SD rats, animals were acclimated to 22 ± 1°C and humidity was 65±10% RH, maintained under the light-dark cycle of 12 hr, free access to clean water and commercial food.
Experimental design
Before commencement of experiments, the rats were fed adaptively for 5–7 days and divided into six groups of six animals each and treated as follows:
(1) Group 1 (G1), the control group, the rats were subcutaneously injected with normal saline (10 ml/kg) on the first day and then tail-intravenously injected with saline (10 ml/kg) for 7 consecutive days.
(2) Group 2 (G2), model group, according to the experiments carried out by Radhiga Thangaiyan et. al. (34) and Zhang Weili et. al. (35), with some modifications; the rats were subcutaneously injected with ISO (45 mg/kg) on the first day and then tail-intravenously injected with saline (10 ml/kg) for 7 consecutive days.
(3) Group3 (G3), the positive control group, the rats were subcutaneously injected with ISO (45 mg/kg) on the first day and then tail-intravenously injected with S. miltiorrhiza injection (10 mg/kg) for 7 consecutive days.
(4) Groups 4–6 (G4-6), according to the experiments carried out by researchers (19, 36, 37), with some modifications, the rats were subcutaneously injected with ISO (45 mg/kg) on the first day, and then tail-intravenously injected with Scu (10, 20, 40 mg/kg, respectively) for 7 consecutive days. Here, Scu was dispersed in saline by sonication for 10 min to obtain a homogeneous suspension, similar to the experimental method of Liu Qingfei et. al. (38).
The rats were anesthetized with pentobarbital sodium and sacrificed on the ninth morning. Blood was collected and heart tissue was removed immediately, cleaned with ice saline, and dried with filter paper. The heart tissue was immediately frozen in liquid nitrogen and stored at -80°C for the determination and analysis of the following indicators.
A flowchart of the experiments involved in this study is shown in Figure 1.
Figure 1.
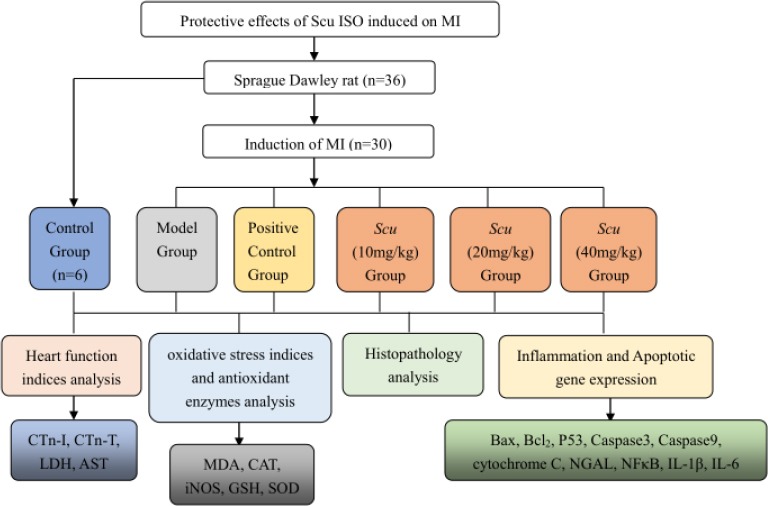
Flow chart demonstrating the experimental approach
Determination of heart function indexes
After blood collection, serum was separated by centrifugation at 4°C at the speed of 3000 rpm for 10 min (39, 40). The activity of AST, LDH, and concentrations of CTn-I and CTn-T of the obtained serum were determined with the Elisa kit according to the manufacturer’s instructions.
Determination of oxidative stress indexes and antioxidant enzymes in heart tissue
0.1 g heart tissue was taken from -80°C refrigerator, and after precooling physiological saline was added at the ratio of 1:9. A high throughput tissue grinder was used to prepare the homogenate. Then the homogenate was centrifuged at 4°C at the speed of 3000 rpm for 10 min. The 10% homogenate protein concentration of the supernatant was determined by Nanjing BCA protein concentration kit. The activities of SOD and CAT and contents of MDA, iNOS, and GSH in the heart homogenate supernatant were determined according to the commercially available diagnostic kit instructions.
Histopathological examination of heart (HE staining)
Heart tissue was soaked in 4% poly formaldehyde solution and fixed for 24 hr, processed and paraffin embedded as per the standard protocol. Sections of 5 μm thickness were cut, deparaffinized, dehydrated, and stained with hematoxylin and eosin (H&E) for the estimation of pathological changes of heart by an optical microscope and photographed using a microscope digital camera.
Quantitative RT-PCR
Heart Bax, Bcl2, P53, Caspase3, Caspase9, cyto-chrome C, Neutrophil gelatinase-associated lipocalin (NGAL), NFκB, IL-1β and IL-6 genes expression were quantified using real-time PCR. Total RNA was isolated from tissue samples using TransZolup (Transgen, China) according to the manufacturer’s protocol. The purity and integrity of the total RNA were determined by spectrophotometry and agarose gel electrophoresis. The cDNA was prepared using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (Transgen, China) according to the manufacturer’s instructions. In brief, 0.5 μg of RNA with 1 μl of Anchored Oligo Primer, 10 μl of Reaction Mix, 1 μl of TransScript RT/RI Enzyme Mix, 1μl of gDNA Remover in a DEPC-treated tube were mixed. Nuclease-free water was added to a final volume of 20 μl. The reaction condition for reverse transcription was performed according to the manufacturer’s protocol.
Real-time quantitative PCR was conducted on the CFX384 Touch (BIO-RAD Laboratories, Inc) using SYBR premix Taq TM Kit (Takara, China). The housekeeping gene β-actin was used as a constitutive control for normalization. The primers for the target gene and β-actin were described as follows:
β-actin (111bp), forward: 5’–GAAGATCAAGATCATTGCTCC
T–3’,
reverse: 5’–TACTCCTGCTTGCTGATCCA–3’,
Bax (117bp), forward:5’–GGAGACACCTGAGCT GACCT–3’,
reverse: 5’–ATCCTCTGCAGCTCCATGTT–3’,
Bcl2 (120bp), forward:5’–AGGATTGTGGCCTTCTTT GA–3’,
reverse: 5’–CAGATGCCGGTTCAGGTACT–3’, P53 (119bp),
forward:5’–GCTTCGAGATGTTCCGAGAG–3’,
reverse: 5’–AGACTGGCCCTTCTTGGTCT–3’,
Caspase3 (147bp), forward:5’– GAGACAGACAGTGGAACT
GACGATG–3’,
reverse: 5’–GGCGCAAAGTGACTGGATGA–3’,
Caspase9 (113bp), forward:5’–AGCCAGATGCTGTCCCA TAC–3’,
reverse: 5’–ACCTGGGAAGGTGGAGTAGG–3’,
cytochrome C (140bp), forward:5’–CCTTTGTGGTGTT
GACCAGC–3’,
reverse: 5’– CCATGGAGGTTTGGTCCAGT–3’,
NGAL (123bp), forward:5’–ACATTCGTTCCAAGCTCC
AG–3’,
reverse: 5’– TGGCAAACTGGTCGTAGTCA–3’,
IL-1β (123bp), forward:5’–CAGCAGCATCTCGACAAG AG–3’,
reverse: 5’–AAAGAAGGTGCTTGGGTCCT–3’,
NFκB (119bp), forward:5’–GGGCTGACCTGAGTCTTCT G–3’,
reverse: 5’–GATAAGGAGTGCTGCCTTGC–3’,
IL-6 (126bp), forward:5’–AGTTGCCTTCTTGGGAC TGA–3’,
reverse: 5’–CCTCCGACTTGTGAAGTGGT–3’,
Amplification was performed in a final volume of 10 μl including 0.8 μl of cDNA template, 0.5 μl of forward primer, 0.5 μl of reverse primer, 5 μl of SYBR Green premix Taq TM, and 3.2 μl of nuclease-free water. The qPCR conditions were as follows: initial denaturation for 30 sec at 94°C, followed by 40 cycles annealing for 5 sec at 94°C, and 30 sec at 56°C with subsequent melting curve analysis, increasing the temperature from 65°C to 95°C. The relative quantification among sample mRNA expression levels was calculated relative to ß-actin gene mRNA levels using the 2△△Ct method.
Statistical analysis
SPSS 19.0 software (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. All values are expressed as mean ± SD. Statistical comparisons were made using Student’s t-test and one-way analysis of variance (ANOVA), followed by Tukey’s test. The level of significance was set at P<0.01.
Results
Protect effects of Scu on heart injury induced by ISO in rats
The results are shown in Table 1. When compared to G1, the values of CTn-I, CTn-T, LDH, and AST in the serum of G2 were significantly higher. Compared to the model group, Scu administration groups (G4-6) had effectively reduced heart damage induced by ISO. AST, LDH, CTn-T, and CTn-I values in the serum of G6 were significantly lower than those of G2. The results of G3 were similar to those of G4 and G5.
Table 1.
Effects of Scutellarin (Scu) on troponin T (CTn-T), troponin I (CTn-I), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) in rat serum with isoprenaline (ISO)-induced cardiotoxicity (n=6)
| Groups | CTn-I(pg/mL)a | CTn-T(ng/mL)a | LDH(IU/L)a | AST(U/L)a |
|---|---|---|---|---|
| G1(saline) | 194.16±39.63c | 267.39±17.68c | 5.52±0.62c | 43.23±2.85c |
| G2(isoprenaline) | 298.96±48.05b | 405.56±54.30b | 8.22±1.43b | 64.28±2.39b |
| G3(ISO+10mg/gS. miltiorrhiza) | 171.55±50.78c | 317.56±27.11c | 6.39±1.47 | 53.41±7.47bc |
| G4(ISO+10mg/kgScu) | 195.83±20.92c | 330.91±27.48bc | 6.60±1.23 | 47.89±10.57c |
| G5(ISO+20mg/kgScu) | 175.48±64.88c | 324.37±36.75bc | 6.14±1.30c | 46.40±1.43c |
| G6(ISO+40mg/kg Scu) | 167.36±28.42c | 280.95±29.70c | 5.13±0.71c | 46.32±3.81c |
Values represent mean±SD of six animals;
Significantly different from saline group (G1) P<0.01;
Significantly different from ISO group (G2) P<0.01
Effects of Scu on oxidative stress indexes and antioxidant enzymes in hearts of rats
According to Table 2, the activity of SOD, CAT, and content of GSH in heart tissue of G2 were significantly lower than those of G1, and the content of MDA and iNOS significantly increased. After Scu administration, the oxidative heart damage in rats significantly decreased. In G4-6, the activity of SOD, CAT, and content of GSH in heart tissue were significantly higher than those of the model group, and the activity was positively correlated with the dose of Scu. After administration of Scu, the MDA and iNOS contents in hearts of each administration group were significantly lower than those of the model group. The index of G3 was similar to those of G4 and G5. The results showed that Scu could increase the activity of antioxidant enzymes and decrease the oxidative stress induced by ISO.
Table 2.
Effects of Scutellarin (Scu) on superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), catalase (CAT), and inducible nitric oxide synthase (iNOS) in rat heart tissue with isoprenaline (ISO)-induced cardiotoxicity (n=6)
| Groups | SOD(U/mgprot)a | MDA(nmol/mgprot)a | GSH (μmol/gprot)a | CAT(U/mgprot)a | iNOS(U/mgprot)a |
|---|---|---|---|---|---|
| G1(saline) | 202.63±26.84c | 1.51±0.35c | 14.082±1.51c | 5.92±0.61c | 0.077±0.043c |
| G2(isoprenaline) | 140.85±16.97b | 4.15±0.65b | 7.53±1.11b | 2.80±1.03b | 0.27±0.048b |
| G3(ISO+10mg/gS.miltiorrhiza) | 224.80±60.25c | 1.74±0.63c | 12.18±3.86 | 5.06±1.22c | 0.12±0.030c |
| G4(ISO+10mg/kgScu) | 221.05±14.06c | 1.845±0.52c | 14.72±2.30c | 3.62±0.53b | 0.14±0.036bc |
| G5(ISO+20mg/kgScu) | 227.38±20.95c | 1.72±0.23c | 17.80±5.41c | 4.67±0.63c | 0.12±0.019c |
| G6(ISO+40mg/kg Scu) | 251.77±21.82c | 1.63±0.40c | 18.41±3.30c | 6.50±1.32c | 0.093±0.038c |
Values represent mean±SD of six animals;
Significantly different from saline group (G1) P<0.01;
Significantly different from ISO group (G2) P<0.01
Effects of Scu on the pathological changes of rat hearts
According to the HE staining of the heart tissue (Figure 2), the heart tissue showed the normal histology of the control group, being composed of muscle cells and cardiomyocytes, with one centrally placed nucleus. In the model group, cardiac muscle fiber was disorganized and marked by cellular injury, patchy necrosis, enlargement in the size of cardiomyocytes, and a large number of inflammatory cell infiltration. The damages of Scu administration groups were lower than those of the model group. The G6 had obvious reduction effects and the cells were arranged neatly.
Figure 2.
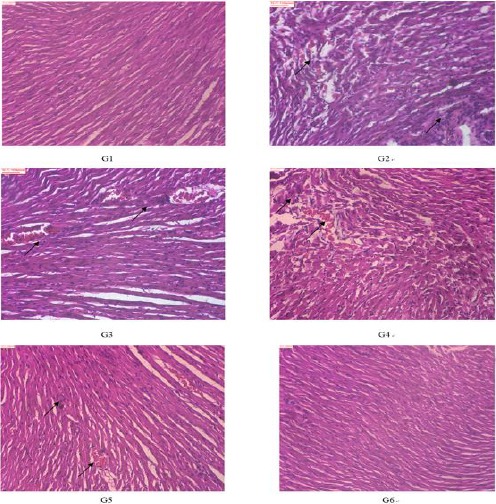
The effects of Scutellarin (Scu) on histological changes of heart in rats (100×, HE). Control group (G1), ISO-model group (G2), ISO+10 mg/kg S.miltiorrhiza group (G3), ISO+10 mg/kg Scu group. (G4), ISO+20 mg/kg Scu group (G5), ISO+40 mg/kg Scu group (G6). The arrows in figs indicate inflammatory cell infiltration and hemorrhage
Scu attenuated apoptosis in heart of rats
The effects of Scu on the mRNA expression of the apoptosis-associated gene in the heart induced by ISO are illustrated in Figure 3. The expression of Bax, P53, Caspase3, Caspase9, and cytochrome C in G2 was signifycantly higher than G1, and then gradually decreased in dose-dependently after administration of Scu. The results of G3 were similar to those of G4. The results showed that Scu could decrease expression of the apoptosis-associated gene in the heart induced by ISO.
Figure 3.
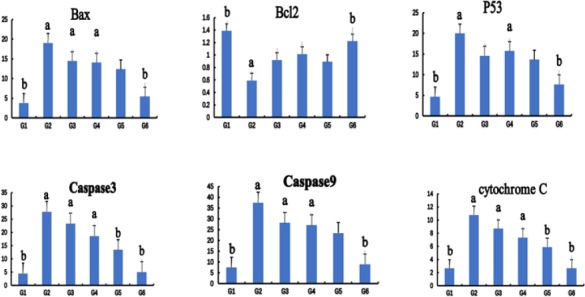
The effects of Scutellarin (Scu) on the mRNA expression of the apoptosis-associated gene in rat hearts. Control group (G1), ISO-model group (G2), ISO+10 mg/kg S. miltiorrhiza group (G3), ISO+10 mg/kg Scu group (G4), ISO+120 mg/kg Scu group (G5), ISO+40 mg/kg Scu group (G6). Each point is the mean±SD (n= 4). a or b: Significantly different from the control (G1) or ISO (G2) group, respectively, P<0.01 using ANOVA followed by Tukey’s test
Scu attenuated inflammation in rat hearts
The effects of Scu on the mRNA expression of inflammatory cytokines in heart induced by ISO are illustrated in Figure 4. The expression of Neutrophil gelatinase-associated lipocalin (NGAL), NFκB, IL-1β, and IL-6 in G2 was significantly higher than G1, and then gradually decreased dose-dependently after administration of Scu. The results of G3 were similar to those of G4. The results showed that Scu could decrease expression of inflammatory cytokines in the heart induced by ISO.
Figure 4.
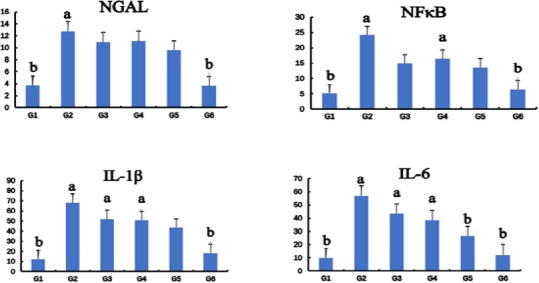
The effects of Scutellarin (Scu) on the mRNA expression of inflammation cytokine in rat hearts. Control group (G1), ISO-model group (G2), ISO+10 mg/kg S. miltiorrhiza group (G3), ISO+10 mg/kg Scu group (G4), ISO+120 mg/kg Scu group (G5), ISO+40 mg/kg Scu group (G6). Each point is the mean±SD (n=4). a or b: Significantly different from the control (G1) or ISO (G2) group, respectively, P<0.01 using ANOVA followed by Tukey’s test
Discussion
ISO can cause a certain degree of damage to the heart clinical indicators, which are related to cardiac myocytes damages. After MI, the levels of troponin in the blood were increased. Troponin complex consists of three subunits of troponin C (TnC), TnT and TnI, cardiac TnC is not specific for the heart and is not employed for the MI diagnosis (41). CTn-I and CTn-T are the most common indicators for the diagnosis of heart damage, and their elevation is an important indicator of heart damage in a laboratory test for detected MI (42). The LDH enzyme activity can reflect the tissue damage degree, which mainly presents in myocardium, skeletal muscle, liver, and kidneys (43). The concentrations that increase in the blood are indicative of cellular injury and inflammatory changes in tissues, particularly the heart (44). Hence, LDH was usually used to diagnose MI. Myocardium contains high concentrations of AST, which are released into the blood when heart tissue is damaged. Thus, AST may be used to evaluate MI. Our research showed that compared with G1, the CTn-I, CTn-T, LDH, and AST in the model group were significantly increased, indicating that ISO induced a certain degree of damage to the hearts of rats. Compared with the model group, these indicators of G4-G6 were significantly reduced, indicating that Scu could effectively reduce cardiotoxicity induced by ISO.
In addition, from the perspective of histopatho-logy, the pathological changes of heart were found in the model group: cardiac muscle fibers were disorganized. Marked cellular injury, patchy necrosis, enlargement in the size of cardiomyocytes and accompaniment with a large number of inflammatory cell infiltration were observed in heart pathological sections. After administration of Scu, these lesions reduced by various degrees. It can be concluded that the administration of Scu to rats can effectively reduce the cardiotoxicity caused by ISO.
In the present study, the protective role of Scu in ISO-induced MI was studied by decreasing the levels of cardiac biomarkers related to MI and improving the pathological changes of heart. Previous researchers have demonstrated that MI has proven to be associated with oxidative stress (5), apoptosis (6), and inflammation (7). Therefore, the protective effect of Scu on MI can be attributed to the following mechanisms.
Research indicates that oxidative stress is one of the mechanisms of ISO-induced cardiotoxicity, and the oxidative stress mediated by ROS plays an important role in ISO cardiotoxicity (32). Once ISO enters the cell, it will generate highly cytotoxic free radicals and produce excessive ROS, resulting in loss of function and integrity of myocardial membranes (33). ISO could interact with the antioxidant system and affect the antioxidant system and antioxidant enzymes, leading to oxidative damage of heart tissues. There is an obvious positive correlation between the degree of oxidative stress and the severity of tissue damage (14).
In general, the free radicals produced are removed by the intracellular antioxidant enzymes such as SOD and CAT, thereby ensuring balanced generation and removal of free radicals (45). When the free radicals accumulate excessively in vivo and cannot be removed by the antioxidant enzymes in time, the balance between the free radicals and the body defense systems will be destroyed, so as to promote the process of lipid peroxidation and cause cell damage (46). The main product of lipid peroxidation process is MDA, so the MDA content of tissues can reflect the degree of lipid peroxidation and indirectly show the balance of free radical and antioxidant defense system (47). INOS is inducible nitric oxide synthase, involved in the synthesis of NO in vivo, when in the state of oxidative stress, the expression of iNOS increased, resulting in excessive NO production, while excessive NO easily led to lipid peroxidation. GSH, SOD, and CAT are the important defense lines to resist free radicals (48). They play an important role in the clearance of free radicals, the maintenance of free radical balance in vivo, the prevention of oxidative stress, and the accumulation of lipid peroxides (49). The tissue content of these enzymes can be used as an indicator of antioxidant capacity. The results showed that ISO can reduce the GSH content in hearts of rats, inhibit the activity of SOD and CAT, which can lead to the accumulation of lipid peroxidation products and body damage. In our study, Scu can reduce the toxicity of heart induced by ISO in rats effectively by improving the antioxidant capacity and free radical scavenging ability, reducing the degree of oxidative stress and lipid peroxidation.
Multiple lines of evidence demonstrate that cell death is important in the pathogenesis of MI. Researchers have investigated the apoptotic pathway in ISO-induced MI (34, 50). The results show that administration of ISO in rats resulted in upregulation of the expressions of myocardial pro-apoptotic signaling proteins, including Bax, caspase-3, caspase-9, cytochrome C, and P53, and down-regulation of the expression of anti-apoptotic Bcl-2. In the process of apoptosis, oxidative stress can influence mitochondria and lysosomes. Researchers speculate that mitochondria and lysosome destabilization resulting in apoptosis may involve processes including the activation of procaspases or other proapoptotic proteins, that is to say, Bcl-2 family proteins and caspases are checkpoints of the apoptosis pathways (51). Bax is an apoptosis promoter of Bcl-2 family, under the influence of apoptotic stimuli, such as oxidative stress, Bax could cause the release of cytochrome C and stimulate programmed cell death (52). Bcl-2 is an anti-apoptotic member of the Bcl-2 family, by scavenging oxygen-free radicals inside the cells and repressing the release of cytochrome C into the cytoplasm, which play important roles in the regulation of the apoptotic pathway (53). Caspase enzymes, including caspase-3 and caspase-9, play an essential role in the process of apoptosis (35). Cytochrome C released into the cytoplasm, by proteolytic cleavage activation of procaspase-9 and then after activating caspase-9, procaspase-3 and caspase-3 were activated (54). Active caspase-3 eventually causes the final-stage apoptosis (55). p53 is a pro-apoptotic protein, and the data of the current study designate an association between the expression of Bax, caspase-3, and p53 and a decrease in the expression of Bcl-2 (56). Researchers (57) also evaluated the correlation between apoptosis and MI. The results suggested that after the treatment with crocetin, the level of caspase-3 and Bax significantly decreased and Bcl-2 increased in the myocardial tissues of MI rats compared with the MI group. Our results indicated that Scu can reduce the apoptosis induced by ISO in rat hearts effectively by decreasing the expression of Bax, caspase-3, caspase-9, p53, and increasing the expression of Bcl-2. NGAL is also referred to as lipocalin-2 (Lcn-2), a member of the lipocalin family of proteins. Under normal conditions, the concentration of NGAL is very low in kidneys, heart, stomach, lungs, etc (58). However, the levels of NGAL maybe up-regulated in response to inflammation, intoxication, ischemia, and acute kidney injury (59). Recent reports demonstrated that NGAL is not only a biomarker for renal injury but also a potentially valuable early marker of MI (60). Recent studies have suggested that serum levels of NGAL in patients with chronic heart failure or AMI were significantly higher when compared with control subjects (61). Furthermore, growing evidence suggests that high plasma NGAL levels may also be associated with inflammation. Upregulation of NGAL can be a pro-inflammatory induction of cytokines IL-6 (62), interleukin-8 (IL-8) (63), IL-1β (64), and TNF-α (62). Consistent with previous research results, our experimental results show that Scu reduced the mRNA expression of NGAL.
In addition, increasing evidence indicates that ISO induces a myriad of inflammatory cytokines (22) and chemokines (12) including translocation of the redox-sensitive transcription factor NFκB from the cytosol to the nucleus. The transcription factor NFκB plays an important role in regulating the production of inflammatory cytokines and promoting transcription of target genes, IL-1β, and IL-6, resulting in inflammatory responses caused by activation of proinflammatory cytokines (65). IL-1β is the earliest cytokine-producing inflammatory reaction. It can stimulate the production of inflammatory mediators, chemotaxis of neutrophils, and other inflammatory cells into the lesion, which lead to a series of inflammations and tissue injury (66). The mRNA expression of IL-1β is positively related to the degree of inflammation and can be used as an indicator of the severity and efficacy of the disease (67). IL-1β inhibition is noteworthy for anti-inflammatory properties leading to inhibition of a cascade that activates NFκB, iNOS, and pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α (68). IL-6 is involved in several inflammatory processes such as neutrophils recruitment and maturation (13). Besides the direct activation and toxicity of vascular endothelial cells and inflammatory cells, IL-6 can catalyze and amplify inflammatory and toxic effects, resulting in tissue cell damage (69). Researchers (36) have investigated the hepatoprotective effect of Scu on diosbulbin B inducing liver injury in mice. The results indicated that Scu protects against the liver injury induced by diosbulbin B via inhibiting the NFκB signaling pathway, decreasing the increased serum levels of IL-6 and inhibiting the translocation of NFκB from the cytoplasm to the nucleus. Similarly, a study (30) also reported the anti-inflammatory activity of Scu in microglial cells via suppressing lipopolysaccharide (LPS)-induced mRNA expressions of IL-1β in BV-2 cells and primary microglia, inhibiting LPS-induced NFκB nuclear translocation. Consistent with previous research results, our experimental results showed that Scu reduced the mRNA expression of inflammation cytokines NFκB, IL-1β, and IL-6, in the heart.
Conclusion
In summary, the present study showed that Scu could reduce the damage of MI caused by ISO in rats, by improving the antioxidant, anti-apoptotic and anti-inflammatory capacity of the body. Therefore, Scu could serve as an important component in curing ischemic heart disease.
Acknowledgment
The authors gratefully acknowledge the financial support of the National Nature Science Foundation (No. 81303298 and 81202987) of China, the Modern Agro-industry Technology Research System (CARS-45-KXJ19), the Fujian Provincial Natural Science Foun-dation (2016J01371), Training program for excellent scientific research talents of young teachers in Fujian Province University (2017), the Fujian Agriculture and Forestry University Technological Innovation Special Fund (CXZX2017299), and the Fujian Agriculture and Forestry University Technology Development Fund (KF2015022).
References
- 1.Awada HK, Johnson NR, Wang Y. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction. J Control Release. 2015;207:7–17. doi: 10.1016/j.jconrel.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meena B, Anbu Rajan L, Anandan R. Protective effect of betaine on protein, glycoproteins and amino acids in isoprenaline-induced myocardial infarction in albino rats. Biomed Prev Nutr. 2014;4:403–409. [Google Scholar]
- 3.Chen SW, Tung YC, Jung SM, Chu Y, Lin PJ, Kao WW, et al. Lumican-null mice are susceptible to aging and isoproterenol-induced myocardial fibrosis. Biochem Biophys Res Commun. 2017;482:1304–1311. doi: 10.1016/j.bbrc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 4.Nagoor Meeran MF, Jagadeesh GS, Selvaraj P. Thymol attenuates altered lipid metabolism in β-adrenergic agonist induced myocardial infarcted rats by inhibiting tachycardia, altered electrocardiogram, apoptosis and cardiac hypertrophy. J Funct Foods. 2015;14:51–62. [Google Scholar]
- 5.Tang YN, He XC, Ye M, Huang H, Chen HL, Peng WL, et al. Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. J Ethnopharmacol. 2015;175:451–455. doi: 10.1016/j.jep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Suchal K, Malik S, Gamad N, Malhotra RK, Goyal SN, Bhatia J, et al. Kampeferol protects against oxidative stress and apoptotic damage in experimental model of isoproterenol-induced cardiac toxicity in rats. Phytomedicine. 2016;23:1401–1408. doi: 10.1016/j.phymed.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Allijn IE, Czarny BM, Wang X, Chong SY, Weiler M, da Silva AE, et al. Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J Control Release. 2017;247:127–133. doi: 10.1016/j.jconrel.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Haskova P, Jansova H, Bures J, Machacek M, Jirkovska A, Franz KJ, et al. Cardioprotective effects of iron chelator HAPI and ROS-activated boronate prochelator BHAPI against catecholamine-induced oxidative cellular injury. Toxicology. 2016;371:17–28. doi: 10.1016/j.tox.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raish M. Momordica charantia polysaccharides ameliorate oxidative stress, hyperlipidemia, inflammation, and apoptosis during myocardial infarction by inhibiting the NF-κB signaling pathway. Int J Biol Macromol. 2017;97:544–551. doi: 10.1016/j.ijbiomac.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 10.Geng ZH, Huang L, Song MB, Song YM. Protective effect of a polysaccharide from Salvia miltiorrhiza on isoproterenol (ISO)-induced myocardial injury in rats. Carbohydr Polym. 2015;132:638–642. doi: 10.1016/j.carbpol.2015.06.086. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda J, Okada M, Yamawaki H. T3 peptide, an active fragment of tumstatin, inhibits H2O2-induced apoptosis in H9c2 cardiomyoblasts. Eur J Pharmacol. 2017;807:64–70. doi: 10.1016/j.ejphar.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 12.Woudstra L, Biesbroek PS, Emmens RW, Heymans S, Juffermans LJ, van Rossum AC, et al. Lymphocytic myocarditis occurs with myocardial infarction and coincides with increased inflammation, hemorrhage and instability in coronary artery atherosclerotic plaques. Int J Cardiol. 2017;232:53–62. doi: 10.1016/j.ijcard.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 13.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 14.Long J, Gao M, Kong Y, Shen X, Du X, Son YO, et al. Cardioprotective effect of total paeony glycosides against isoprenaline-induced myocardial ischemia in rats. Phytomedicine. 2012;19:672–676. doi: 10.1016/j.phymed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 15.E MP, Mopuri R, Pulaganti M, Kareem MA, Islam MSK RD, et al. Molecular assessment of protective effect of Vitex negundo in ISO induced myocardial infarction in rats. Biomed Pharmacother. 2017;92:249–253. doi: 10.1016/j.biopha.2017.05.078. [DOI] [PubMed] [Google Scholar]
- 16.Krushna GS, Shivaranjani VL, Umamaheswari J, Srinivasulu C, Hussain SA, Kareem MA, et al. In vivo and molecular docking studies using whole extract and phytocompounds of Aegle marmelos fruit protective effects against Isoproterenol-induced myocardial infarction in rats. Biomed Pharmacother. 2017;91:880–889. doi: 10.1016/j.biopha.2017.04.115. [DOI] [PubMed] [Google Scholar]
- 17.Deng XY, Chen JJ, Li HY, Ma ZQ, Ma SP, Fu Q. Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem Biol Interact. 2015;240:22–28. doi: 10.1016/j.cbi.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Gao M, Huang W, Liu CZ. Separation of scutellarin from crude extracts of Erigeron breviscapus(vant.). Hand. Mazz. by macroporous resins. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;858:22–26. doi: 10.1016/j.jchromb.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 19.Chai L, Guo H, Li H, Wang S, Wang YL, Shi F, et al. Scutellarin and caffeic acid ester fraction, active components of Dengzhanxixin injection, upregulate neurotrophins synthesis and release in hypoxia/reoxygenation rat astrocytes. J Ethnopharmacol. 2013;150:100–107. doi: 10.1016/j.jep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Yao H, Huang X, Shi P, Lin Z, Zhu M, Liu A, et al. DPPH·luminol chemiluminescence system and its application in the determination of scutellarin in pharmaceutical injections and rat plasma with flow injection analysis. Luminescence. 2017;32:588–595. doi: 10.1002/bio.3225. [DOI] [PubMed] [Google Scholar]
- 21.Chu J, Zou C, Li C, Zhang J, Zhao Y, Xu M, et al. Determination of scutellarein in human plasma by enzymatic hydrolysis and liquid chromatograph-triple quadrupole tandem mass spectrometer analysis:Its use in determining the bioequivalence of scutellarin in Chinese volunteers. Eur J Integr Med. 2016;8:519–525. [Google Scholar]
- 22.Zhao S, Sun Y, Li X, Wang J, Yan L, Zhang Z, et al. Scutellarin inhibits RANKL-mediated osteoclastogenesis and titanium particle-induced osteolysis via suppression of NF-κB and MAPK signaling pathway. Int Immunopharmacol. 2016;40:458–465. doi: 10.1016/j.intimp.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Jiao ZY. Experimental research of the effect and mechanism of Dengzhanhua against myocardial ischemia of canines. Changchun: Jilin University; 2006. [Google Scholar]
- 24.Zhao GA, Tang RJ, Qi SL. Protective of effect breviscapine combined with ischemic preconditioning on myocardial cell apoptosis induced by myocardial ischemia reperfusion in rabbits. Chin Hosp Pharm J. 2010;30:1806–1808. [Google Scholar]
- 25.Wang YX, Yang J, Wang HB, Song HH. Effect of Erigeron breviscapus injection on myocardial ischemia reperfusion injury. J Hainan Med Univ. 2015;21:1188–1190. [Google Scholar]
- 26.Gong MY, Du C, Yuan BY. Effects of breviscapine on serum TNF-αand IL-6 in rats with myocardial ischemia reperfusion. Lishizhen Med Mater Med Res. 2013;24:1615–1616. [Google Scholar]
- 27.Lin L, Zou H, Chang J. Protective Effects of scutellarin and breviscapine on acute myocardial infarction. China Pharm. 2010;19:12–13. [Google Scholar]
- 28.Guo LL, Guan ZZ, Huang Y, Wang YL, Shi JS. The neurotoxicity of β-amyloid peptide toward rat brain is associated with enhanced oxidative stress, inflammation and apoptosis, all of which can be attenuated by scutellarin. Exp Toxicol Pathol. 2013;65:579–584. doi: 10.1016/j.etp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Yu J, Wu J, Qi F, Wang H, Wang Z, et al. Scutellarin protects cardiomyocyte ischemia-reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 2016;157:200–207. doi: 10.1016/j.lfs.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Wang H, Guo H, Kang L, Gao X, Hu L. Neuroprotection of Scutellarin is mediated by inhibition of microglial inflammatory activation. Neuroscience. 2011;185:150–160. doi: 10.1016/j.neuroscience.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Amano S, Arai M, Goto S, Togari A. Inhibitory effect of NPY on isoprenaline-induced osteoclastogenesis in mouse bone marrow cells. Biochim Biophys Acta. 2007;1770:966–973. doi: 10.1016/j.bbagen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Anandan R, Ganesan B, Obulesu T, Mathew S, Kumar RS, Lakshmanan PT, et al. Dietary chitosan supplementation attenuates isoprenaline-induced oxidative stress in rat myocardium. Int J Biol Macromol. 2012;51:783–787. doi: 10.1016/j.ijbiomac.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Zaafan MA, Zaki HF, El-Brairy AI, Kenawy SA. Protective effects of atorvastatin and quercetin on isoprenaline-induced myocardial infarction in rats. Bull Fac Pharm Cairo Univ. 2013;51:35–41. [Google Scholar]
- 34.Radhiga T, Rajamanickam C, Sundaresan A, Ezhumalai M, Pugalendi KV. Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie. 2012;94:1135–1142. doi: 10.1016/j.biochi.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Li Y, Ge Z. Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model. Biomed Pharmacother. 2017;93:376–382. doi: 10.1016/j.biopha.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 36.Niu C, Sheng Y, Yang R, Lu B, Bai Q, Ji L, et al. Scutellarin protects against the liver injury induced by diosbulbin B in mice and its mechanism. J Ethnopharmacol. 2015;164:301–308. doi: 10.1016/j.jep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Wang L, Li Y, Bai L, Xue M. Acute and subacute toxicological evaluation of scutellarin in rodents. Regul Toxicol Pharmacol. 2011;60:106–111. doi: 10.1016/j.yrtph.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 38.Liu Q, Shi Y, Wang Y, Lu J, Cong W, Luo G, et al. Metabolism profile of scutellarin in urine following oral administration to rats by ultra-performance liquid chromatography coupled to time-of-flight mass spectrometry. Talanta. 2009;80:84–91. doi: 10.1016/j.talanta.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 39.Tilyek A, Chai C, Hou X, Zhou B, Zhang C, Cao Z, et al. The protective effects of Ribes diacanthum Pall on cisplatin-induced nephrotoxicity in mice. J Ethnopharmacol. 2016;178:297–306. doi: 10.1016/j.jep.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Yang J, Wang LZ, Sun LR, Dong Q. Crocin attenuates cisplatin-induced liver injury in the mice. Hum Exp Toxicol. 2014;33:855–862. doi: 10.1177/0960327113511475. [DOI] [PubMed] [Google Scholar]
- 41.Body R, McDowell G, Carley S, Wibberley C, Ferguson J, Mackway-Jones K. A FABP-ulous 'rule out'strategy? Heart fatty acid binding protein and troponin for rapid exclusion of acute myocardial infarction. Resuscitation. 2011;82:1041–1046. doi: 10.1016/j.resuscitation.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Roos A, Hellgren A, Rafatnia F, Hammarsten O, Ljung R, Carlsson AC, et al. Investigations, findings, and follow-up in patients with chest pain and elevated high-sensitivity cardiac troponin T levels but no myocardial infarction. Int J Cardiol. 2017;232:111–116. doi: 10.1016/j.ijcard.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 43.Akila P, Asaikumar L, Vennila L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed Pharmacother. 2017;85:582–591. doi: 10.1016/j.biopha.2016.11.067. [DOI] [PubMed] [Google Scholar]
- 44.Peer PA, Trivedi PC, Nigade PB, Ghaisas MM, Deshpande AD. Cardioprotective effect of Azadirachta indica A. Juss. on isoprenaline induced myocardial infarction in rats. Int J Cardiol. 2008;126:123–126. doi: 10.1016/j.ijcard.2007.01.108. [DOI] [PubMed] [Google Scholar]
- 45.Bazmandegan G, Boroushaki MT, Shamsizadeh A, Ayoobi F, Hakimizadeh E, Allahtavakoli M. Brown propolis attenuates cerebral ischemia-induced oxidative damage via affecting antioxidant enzyme system in mice. Biomed Pharmacother. 2017;85:503–510. doi: 10.1016/j.biopha.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 46.Wojtunik-Kulesza KA, Oniszczuk A, Oniszczuk T, Waksmundzka-Hajnos M. The influence of common free radicals and antioxidants on development of Alzheimer's Disease. Biomed Pharmacother. 2016;78:39–49. doi: 10.1016/j.biopha.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 47.Kang L, Zhao H, Chen C, Zhang X, Xu M, Duan H. Sappanone A protects mice against cisplatin-induced kidney injury. Int Immunopharmacol. 2016;38:246–251. doi: 10.1016/j.intimp.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 48.da Costa MF, Liborio AB, Teles F, Martins Cda S, Soares PM, Meneses GC, et al. Red propolis ameliorates ischemic-reperfusion acute kidney injury. Phytomedicine. 2015;22:787–795. doi: 10.1016/j.phymed.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Cagin YF, Erdogan MA, Sahin N, Parlakpinar H, Atayan Y, Polat A, et al. Protective effects of apocynin on cisplatin-induced hepatotoxicity in rats. Arch Med Res. 2015;46:517–526. doi: 10.1016/j.arcmed.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 50.Othman AI, Elkomy MM, El-Missiry MA, Dardor M. Epigallocatechin-3-gallate prevents cardiac apoptosis by modulating the intrinsic apoptotic pathway in isoproterenol-induced myocardial infarction. Eur J Pharmacol. 2017;794:27–36. doi: 10.1016/j.ejphar.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Gupta M, Sharma P, Mazumder AG, Patial V, Singh D. Dwindling of cardio damaging effect of isoproterenol by Punica granatum L. peel extract involve activation of nitric oxide-mediated Nrf2/ARE signaling pathway and apoptosis inhibition. Nitric Oxide. 2015;50:105–113. doi: 10.1016/j.niox.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Sahu BD, Anubolu H, Koneru M, Kumar JM, Kuncha M, Rachamalla SS, et al. Cardioprotective effect of embelin on isoproterenol-induced myocardial injury in rats:possible involvement of mitochondrial dysfunction and apoptosis. Life Sci. 2014;107:59–67. doi: 10.1016/j.lfs.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Chang JW, Hwang HS, Kim YS, Kim HJ, Shin YS, Jittreetat T, et al. Protective effect of Artemisia asiatica(Pamp.). Nakai ex Kitam ethanol extract against cisplatin-induced apoptosis of human HaCaT keratinocytes:Involvement of NF-kappa B- and Bcl-2-controlled mitochondrial signaling. Phytomedicine. 2015;22:679–688. doi: 10.1016/j.phymed.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Yue C, Chen J, Hou R, Tian W, Liu K, Wang D, et al. The antioxidant action and mechanism of selenizing Schisandra chinensis polysaccharide in chicken embryo hepatocyte. Int J Biol Macromol. 2017;98:506–514. doi: 10.1016/j.ijbiomac.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Im GJ, Chang J, Lee S, Choi J, Jung HH, Lee HM, et al. Protective role of edaravone against cisplatin-induced ototoxicity in an auditory cell line. Hear Res. 2015;330:113–118. doi: 10.1016/j.heares.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Khan R, Khan AQ, Qamar W, Lateef A, Tahir M, Rehman MU, et al. Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis:probable role of p38MAPK and p53. Toxicol Appl Pharmacol. 2012;258:315–329. doi: 10.1016/j.taap.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Li Y, Ge Z. Cardiaprotective effect of crocetin by attenuating apoptosis in isoproterenol induced myocardial infarction rat model. Biomed Pharmacother. 2017;93:376–382. doi: 10.1016/j.biopha.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 58.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palazzuoli A, Beltrami M, Pellegrini M, Nuti R. Natriuretic peptides and NGAL in heart failure:does a link exist? Clin Chim Acta. 2012;413:1832–1838. doi: 10.1016/j.cca.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 60.Gouweleeuw L, Naude PJ, Rots M, DeJongste MJ, Eisel UL, Schoemaker RG. The role of neutrophil gelatinase associated lipocalin (NGAL) as biological constituent linking depression and cardiovascular disease. Brain Behav Immun. 2015;46:23–32. doi: 10.1016/j.bbi.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 61.Frogoudaki A, Andreou C, Parissis J, Maniotis C, Nikolaou M, Rizos I, et al. Clinical and prognostic implications of plasma NGAL and NT-proBNP in adult patients with congenital heart disease. Int J Cardiol. 2014;177:1026–1030. doi: 10.1016/j.ijcard.2014.09.134. [DOI] [PubMed] [Google Scholar]
- 62.Hamzic N, Blomqvist A, Nilsberth C. Immune-induced expression of lipocalin-2 in brain endothelial cells:relationship with interleukin-6, cyclooxygenase-2 and the febrile response. J Neuroendocrinol. 2013;25:271–280. doi: 10.1111/jne.12000. [DOI] [PubMed] [Google Scholar]
- 63.Eilenberg W, Stojkovic S, Piechota-Polanczyk A, Kaun C, Rauscher S, Groger M, et al. Neutrophil gelatinase-associated lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg. 2016;51:623–631. doi: 10.1016/j.ejvs.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 64.Borkham-Kamphorst E, Drews F, Weiskirchen R. Induction of lipocalin-2 expression in acute and chronic experimental liver injury moderated by pro-inflammatory cytokines interleukin-1βthrough nuclear factor-κB activation. Liver Int. 2011;31:656–665. doi: 10.1111/j.1478-3231.2011.02495.x. [DOI] [PubMed] [Google Scholar]
- 65.Ethiraj P, Veerappan K, Samuel S, Sivapatham S. Interferon βimproves the efficacy of low dose cisplatin by inhibiting NF-κB/p-Akt signaling on HeLa cells. Biomed Pharmacother. 2016;82:124–132. doi: 10.1016/j.biopha.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 66.Talwar H, Bauerfeld C, Bouhamdan M, Farshi P, Liu Y, Samavati L. MKP-1 negatively regulates LPS-mediated IL-1βproduction through p38 activation and HIF-1αexpression. Cell Signal. 2017;34:1–10. doi: 10.1016/j.cellsig.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rao Z, Wang S, Wang J. Peroxiredoxin 4 inhibits IL-1beta-induced chondrocyte apoptosis via PI3K/AKT signaling. Biomed Pharmacother. 2017;90:414–420. doi: 10.1016/j.biopha.2017.03.075. [DOI] [PubMed] [Google Scholar]
- 68.Bueno-Silva B, Kawamoto D, Ando-Suguimoto ES, Casarin RCV, Alencar SM, Rosalen PL, et al. Brazilian red propolis effects on peritoneal macrophage activity:nitric oxide, cell viability, pro-inflammatory cytokines and gene expression. J Ethnopharmacol. 2017 doi: 10.1016/j.jep.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 69.Nojiri T, Hosoda H, Kimura T, Tokudome T, Miura K, Takabatake H, et al. Protective effects of ghrelin on cisplatin-induced nephrotoxicity in mice. Peptides. 2016;82:85–91. doi: 10.1016/j.peptides.2016.06.003. [DOI] [PubMed] [Google Scholar]


