Abstract
Objective(s):
Multiple sclerosis (MS) is considered as a chronic type of an inflammatory disease characterized by loss of myelin of CNS. Recent evidence indicates that Interleukin 17 (IL-17)-producing T helper cells (Th17 cells) population are increased and regulatory T cells (Treg cells) are decreased in MS. Despite extensive research in understanding the mechanism of Th17 and Treg differentiation, the role of microRNAs in MS is not completely understood. Thereby, as a step closer, we analyzed the expression profile of miR-9-5p and miR-106a-5p, and protein level of retinoic acid receptor (RAR)-related orphan receptor C (RORC; Th17 master transcription factor) as direct target of miR-106a-5p and forkhead box P3 (FOXP3; Treg master transcription factor) as indirect target of miR-9-5p in CD4+ T cells in two groups of relapsing and remitting in our relapsing-remitting MS (RR-MS) patients.
Materials and Methods:
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was utilized to assess the expression of miRNAs and mRNAs, in 40 RR-MS patients and 11 healthy individuals. Thus, FOXP3 and RAR-related orphan receptor γt (RORγt) was assessed in CD4+T-cells by flow cytometry. We also investigated the role of these miRNAs in Th17/Treg differentiation pathway through bioinformatics tools.
Results:
An up-regulation of miR-9-5p and down-regulation of miR-106a-5p in relapsing phase of MS patients were observed compared to healthy controls. RORC and FOXP3 were up-regulated in relapsing and remitting phases of MS, respectively.
Conclusion:
Expression pattern of miR-9-5p and miR-106a-5p and their targets suggest a possible inducing role of miR-9-5p and suppressing role of miR-106a-5p in differentiation pathway of Th17 cells during MS pathogenesis.
Keywords: MicroRNA, MiR-106a-5p, MiR-9-5p, Multiple sclerosis, Th17
Introduction
Multiple sclerosis (MS), a classical T cell–mediated autoimmune disease, is a prototype of systemic autoimmune disease, which is characterized by chronic inflammatory demyelinating of the central nervous system (CNS) (1, 2). Approximately 2.3 million individuals worldwide suffer from MS and 85% of these patients are classified as relapsing-remitting MS (RR-MS). This subtype is characterized by relapse that occurs about once a year and usually is followed by some degree of recovery termed remission. It has been estimated that up to 80% of these types of MS patients will develop secondary progressive MS, the other course of MS that has more intensive symptoms without any recovery. Approximately, 10% of MS individuals have been diagnosed with primary progressive MS and 5% with progressive relapsing MS (3).
Interleukin 17 (IL-17)-producing T helper cells (Th17 cells) are considered as the main effective cell lineage for pathogenesis of MS. This lineage is one of the CD4+ T cell subset playing critical roles in clearing bacterial and fungal infections that induces infla-mmation during autoimmune diseases (4, 5). Th17 cells are differentiated from naïve CD4+ T cell in the presence of IL-6, IL-23, and transforming growth factor-beta (TGF-β) cytokines (6-9) and are identified by producing IL-17, IL-21, and IL-22 (10). In addition, Th17 cells express unique transcription factors, including retinoic acid receptor (RAR)-related orphan receptor γt (RORγt) (11), RORα (12) and signal transducer and activator of transcription 3 (STAT3) (13). Regulatory T cell (Treg) is another subset of CD4+ T-cells that regulates immune system, maintains homeostasis, induces tolerance to self-antigens (14) and expresses high level of forkhead box P3 (FOXP3) (15), which is induced by TGF-β signaling pathway (16).
MicroRNAs (miRNAs) are an important class of endogenous non-coding RNAs that post-transcriptionally regulate expression of about one-third of all protein-coding genes. MiRNAs suppress expression of target genes by binding to 3′-UTR of their mRNA, and either destabilize them or inhibit protein translation (17). MiRNAs are implicated in a variety of cellular processes including differentiation, self-renewal, proliferation, metabolism, apoptosis (18, 19) and several diseases such as cancers (20) and autoimmune disorders (21).
Here we investigated expression levels of miR-9-5p and miR-106a-5p in CD4+ T cells of RR-MS patients. Previously, two studies reported expression of miR-9-5p in inactive lesion and plasma of MS-patient and only one study showed down-regulation of miR-106a-5p in whole blood of MS-patient. Hence, this is the first report on assessment of the expression levels of miR-9-5p and miR-106a-5p in CD4+ T cells of MS-patients in relapsing and remitting phases compared to control group. Furthermore, bioinformatics tools were utilized to predict function of these miRNAs in pathways of Th17 and Treg differentiation.
Materials and Methods
Preparation of blood samples
In this study, we tested 40 RR-MS patients including 20 in relapsing phase and 20 in remitting phase referring to MS-Clinic of Al-Zahra Hospital (Isfahan) and diagnosed based on McDonald criteria in MS (22) by a neurologist. Furthermore, 11 healthy individuals without any infections and allergic diseases within the same age as MS-patients participated voluntarily in this study. Ten milliliter of blood samples were drawn into EDTA containing tubes from the patients upon signing an informed consent. Furthermore, all patients with relapsing MS were new cases who had not received any immunomodulatory drug, whereas all remitting phase patients were treated with β- interferon (CinnoVex ™). Therefore to minimize the treatment effect, sampling from patients in remitting phase was carried out a week after previous injection, prior to the next receiving β- interferon. Meanwhile, all study protocols, consent forms and ethical issues were approved by Review Board of Royan Institute for Biotechnology (Project Id. No. 91000582).
PBL and CD4+ T-cells isolation
At the first step, the peripheral blood lymphocytes (PBLs) were isolated by density gradient lymphodex (Inno-train, USA) according to manufacturer’s protocol. Afterward, CD4+ cells were separated from other lymphocytes in samples using MACS CD4+ T cell isolation kit (Miltenyi Biotech, Germany). This kit utilizes an indirect magnetic labeling system and can isolate CD4+ cells by depleting non-CD4+ T cells (negative selection). Indirect magnetic labeling of non-CD4+ T cells was performed with a cocktail of biotin-conjugated monoclonal antibodies and anti-biotin monoclonal antibodies. Therefore, labeled cells retained on the magnetic field of a MACS separator, while the unlabeled T helper cells passed through the column.
Intracellular staining and flow cytometry
CD4+ T cells, isolated from PBL samples, were fixed in 4% Paraformaldehyde/phosphate-buffered saline (PBS) for 30 min at 4 °C. Then, cells were washed with PBS before incubation with Triton 0.2% for 20 min at 4 °C. Finally, cell surface and intracellular staining was performed. The antibodies used against CD4, FOXP3, and RORγt were: Mouse IgG2b K Isotype Control Alexa Fluor 488, Anti-Human CD4 Alexa Fluor 488, Mouse IgG1 K Isotype Control PE, Rat IgG2a K Isotype Control PE, Anti-Human FOXP3 PE and Anti-Human/Mouse RORγ(t) PE; (all were purchased from eBioscience, USA). A FACSCalibur and CellQuest software (BD Biosciences, USA) were used for flow cytometry.
RNA extraction and DNase treatment
RNA was purified from the isolated CD4+ T-cells using TRIzol reagent (Ambion, USA) according to the manufacturer’s protocol. RNA yield and A260/280 ratio were determined by Nano Drop spectrometer (Thermo Scientific, USA). RNase-free DNase (TaKaRa, Japan) treatment of total RNA was performed to eliminate any potential contamination with genomic DNA.
cDNA synthesis and real-time PCR
For quantitative analysis of RNA expression, we performed RT-qPCR. cDNA synthesis was carried out using two commercial kits: “PARSGENOME MiR-Amp kit” (Parsgenome, Iran) was used for miR-9-5p, miR-106a-5p and U48 cDNA synthesis, and “RevertAid First Strand cDNA Synthesis Kit” (Thermo Scientific, USA) was used for RORγt and FOXP3 cDNA synthesis. Data that obtained for the expression of miRNAs were normalized to the expression of U48 snRNA, which was previously confirmed as an appropriate reference gene (23). On the other hand, mRNA expression assessments were performed by comparing the expression of 18s rRNA as reference. RT-qPCRs were carried out under the following conditions: 95°C for 5 min followed by 40 cycles of 95°C for 5 sec, 61°C for 20 sec and 72°C for 30 sec in an ABI PRISM 7500 (Applied Biosystems, USA), using SYBR premix ExTaq II (TaKaRa, Japan) kit. All real-time PCR reactions were performed in triplicate.
Furthermore, to check out the accuracy of amplifica-tions, we included a negative control in each run by eliminating the cDNA sample in the tube.
T/A cloning and PAGE
In order to evaluate primer specificity, RT-qPCR products were run on 12% Polyacrylamide Gel Electrophoresis (PAGE) as well as T/A cloning into the pTZ57R/T vector (Thermo Scientific) for further sequencing.
Statistical analysis
Data were analyzed with Statistical Program for Social Sciences (SPSS) software version 22.0 (IBM SPSS Inc., Chicago, USA) and GraphPad Prism 6 (GraphPad software, Inc., USA). Meanwhile, analysis of variance (ANOVA) test was used for statistical analyses. Finally, P≤0.05 was considered significant.
Signaling pathway analysis
In order to perform molecular enrichment analysis of miR-9-5p and miR-106a-5p targetome and finding out the most related signaling pathways, online in-silico databases including miRWalk (24) and miRTarBase (25) were used to obtain predicted/validated targets of miR-9-5p and miR-106a-5p. Hence, expression of respective target genes in lymph node and thymus were investigated using Unigene database (http://www.ncbi.nlm.nih.gov/unigene/). Finally, selected targets of miR-9-5p and miR-106a-5p were imputed in the database for annotation, visualization, and integrated discovery (DAVID), online database to investigate signaling pathway analysis. Alternatively, TargetScan (http://www.targetscan.org/) and miRNA target-prediction software, RNAhybrid (version 2.1; http://bibiserv.techfak. uni-bielefeld.de/rnahybrid/) were used to search for the major binding sites for microRNAs within 3′-UTR sequences of target mRNAs.
Results
Demographic and clinical features of the participants
Demographic information of patients and control is presented in Table 1. Statistical analysis demonstrated no major difference between patients and control groups concerning gender (P=0.800), and age (P=0.125). (Supplementary Table A1 provides detailed information of patients).
Table 1.
Characteristics of patients and control individuals
| Relapsing MS | Remitting MS | Healthy Controls | |
|---|---|---|---|
| Participants number | 20 | 20 | 11 |
| Age | 32.4±1.3 | 36.44±1.6 | 33.7±1.5 |
| Sex: Female/Male | 15/5 | 14/6 | 7/4 |
| Receiving Drug | NA | IFN-β | NA |
Age is presented as year±SEM. ‘NA’ indicates ‘not applicable’
Up-regulation of miR-9-5p in relapsing phase compared to remitting phase and healthy individuals
RT-qPCR data indicated higher expression level of miR-9-5p in CD4+ T-cells of relapsing phase compared to remitting phase and healthy individuals. Of note that, no significant difference was observed between remitting phase and healthy individuals (Figure 1A).
Figure 1.
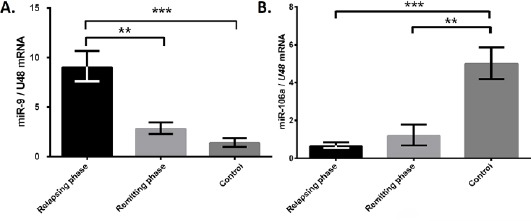
Up-regulation of miR-9-5p and down-regulation of miR-106a-5p in CD4+ T-cells of RR-MS patients
(A) The results of RT-qPCR showed higher miR-9-5p expression in relapsing phase of relapsing-remitting MS (RR-MS) (n=20) compared to remitting phase (n=20) and control (n=11) groups. (B) MiR-106a-5p was down-regulated in CD4+ cells of relapsing phase (n=20) and remitting phase (n=20) compared to healthy group (n=11). Bars represents the mean ± SD. ** P<0.01 and ***P <0.001.
Down-regulation of miR-106a-5p in relapsing phase compared to remitting phase and healthy individuals
The relative expression pattern of miR-106a-5p was investigated by RT-qPCR in three groups including relapsing phase (n=20), remitting phase (n=20), and healthy controls (n=11). Detailed analysis showed that CD4+ T-cells from patients with relapsing MS had significantly lower miR-106a-5p expression level compared to healthy group. However, this level of expression was to some extent lower in remitting phase of MS compared to healthy controls, but there was no significant difference between these two groups of patients (Figure 1B).
Sequencing results
After cloning miR-9-5p, miR-106a-5p, and RNU48 real-time PCR products into the pTZ57R/T cloning vector, the recombinant pTZ57R/T vectors were sent sequencing and their results were compared to the sequences of miR-9-5p, miR-106a-5p and RNU48 existing in Gene bank; thereafter, obtained results determined that miR-9-5p, miR-106a-5p and RNU48 were specifically amplified.
Signaling pathway analysis of miR-9-5p and miR-106a-5p targetome suggested possible role of these miRNAs in differentiation of Th17 cells
Using miRTarBase and miRwalk databases, 197 and 12 validated and predicted targets for miR-9-5p and 60 and 15 validated and predicted targets for miR-106a-5p were collected, respectively (Table A.2 and A.3). All the predicted targets by miRWalk were confirmed in at least five prediction databases. Additionally, validated mRNA targets collected from miRTarBase were supported by experimental evidences including RT-qPCR, Western blotting and reporter assay analysis. In the next step, we found out that 100 targets of validated and 11 predicted targets of miR-9-5p and 42 validated targets and 12 predicted targets of miR-106a-5p were expressed in lymph nodes and thymus by using Unigene database.
Imputing Entrez IDs of selected miR-9-5p and miR-106a-5p targetome into a functional annotation tool, DAVID, determined association between imputed genes and several KEGG signaling pathways including pathway in cancer, TGF-β signaling pathway (supplementary Figure A1), Jak-STAT signaling pathway, and cell cycle (Table 2). The interaction of some predicted targets with miRNAs, which may have critical roles in Th17 differentiation, were analyzed by TargetScan and RNAhybrid databases. According to TargetScan, protein inhibitor of activated STAT3 (PIAS3) mRNA has one conserved site for interacting with miR-9-5p similar to RAR-related orphan receptor C (RORC), which has one conserved site for pairing to miR-106a-5p. In addition, STAT3 has two conserved sites and one poorly conserved site as target for miR-106a-5p (Figure 2).
Table 2.
Top relevant KEGG signaling pathways with miR-9-5p and miR-106a-5p targetome (DAVID database)
| miR-9-5p | miR-106a-5p | ||||
|---|---|---|---|---|---|
| Rank | KEGG pathway | genes in the pathway | P-value | genes in the pathway | P-value |
| 1 | Pathway in cancer | 12 | 3.7E-5 | 13 | 8.1E-8 |
| 3 | Pancreatic cancer | 4 | 2.0E-2 | 6 | 4.5E-5 |
| 4 | TGF-beta signaling pathway | 6 | 1.1E-4 | ||
| 5 | Cell cycle | 5 | 6.1E-4 | ||
| 6 | Jak-STAT signaling pathway | 5 | 3.5E-2 |
Figure 2.
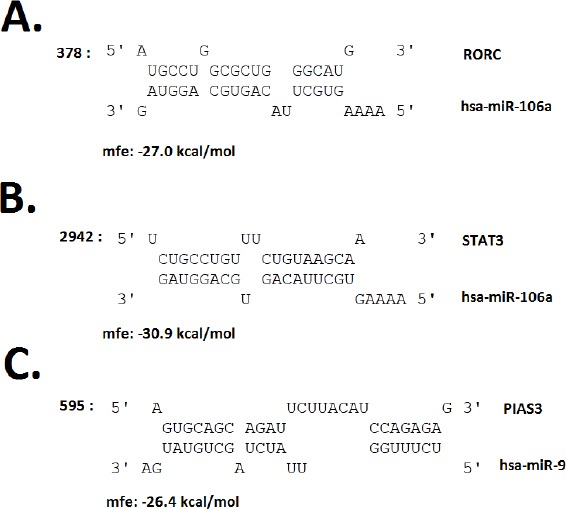
Potential miRNA–mRNA interactions for miR-9-5p and miR-106a-5p besides their targets.
(A, B) MiR-106a-5p, retinoic acid receptor (RAR)-related orphan receptor C (RORC) and signal transducer and activator of transcription 3 (STAT3) mRNA 3′ UTR major interference sites (C) miR-9-5p and protein inhibitor of activated STAT3 (PIAS3) mRNA 3′ UTR major interference sites. (mfe: minimum free energy)
RORC expression elevated in CD4+ T cells in contrast to FOXP3
We used RT-qPCR to investigate mRNA level of RORC and FOXP3. As were expected, mRNA level of RORC, a validated target of miR-106a-5p based on miRTarBase, increased in relapsing phase of MS, while FOXP3 had higher level of expression in healthy controls compared to relapsing phase patients (Figure 3). To investigate more about RORγt and FOXP3, flow cytometry analysis was performed. Expression level of RORγt was significantly higher in relapsing phase compared to remitting phase and healthy group. In opposite, FOXP3 expression elevated in remitting phase in comparison with two other groups (Figure 4).
Figure 3.
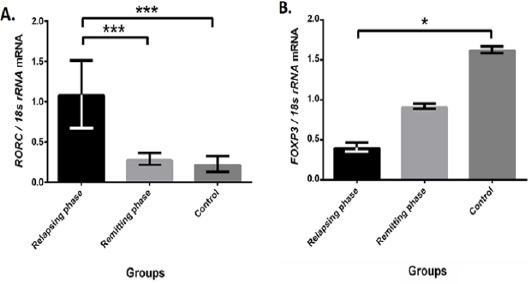
Evaluating expression levels of RORC and FOXP3
(A) Retinoic acid receptor (RAR)-related orphan receptor C (RORC), a miR-106a-5p target, up-regulated in relapsing phase (n=20) compared to remitting (n=20) and controls (n=11), whereas miR-106a-5p down-regulated. (B) Forkhead box P3 (FOXP3) as regulatory T cell (Treg) marker was higher in control samples. Bars represent the mean ± SD. ***P<0.001
Figure 4.
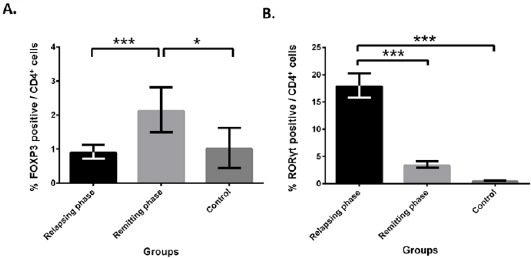
Flow cytometry data showed more RORγt positive cells in relapsing phase and more FOXP3 positive cells in remitting phase of multiple sclerosis
(A) Using Forkhead box P3 (FOXP3) intracellular marker revealed that there are more FOXP3 positive cells in remitting phase of MS (n=20) compared to relapsing phase (n=20) and control (n=11). (B) Flowcytometry using RAR-related orphan receptor γt (RORγt) marker indicated more Th17 in relapsing phase of relapsing-remitting MS (RR-MS) in comparison with two other groups. Bars represent the mean±SD. * P<0.05 and ***P<0.001
Discussion
Th17 cells are a subset of T cells, which involve in the pathogenesis of autoimmune disease, including MS (26). Therefore, factors that could influence Th17 cells differentiation have valuable potential for treatment of autoimmune diseases. In hope of evaluating the role of miRNAs in CD4+ T cells of MS patients (including relapsing and remitting phase patients) and healthy controls, two miRNAs including miR-9-5p and miR-106a-5p were chosen for further investigations. MiR-9-5p was previously reported to be up-regulated in inactive lesions of MS patients (27). Using miRNA PCR-array analysis, Gandhi et al. (28) discovered that the level of 3p arm of miR-9 was up-regulated in plasma of RR-MS patients. Microarray analysis showed down-regulation of miR-106a-5p in whole blood of MS patients (29). Similar to previous study, these two microRNAs were deregulated in other autoimmune disease. For instance, miR-9-5p and miR-106a-5p were deregulated in inflammatory bowel disease (30, 31). MiR-106-5p was found to be up-regulated in psoriasis (32). Here, for the first time, we evaluated miR-9-5p and miR-106a-5p expression levels in CD4+ T-cells of MS patients in relapsing and remitting phases separately. We found out that expression of miR-9-5p was up-regulated in CD4+ T-cells of patients in relapsing phase of RR-MS compared to the same parameters in patients with remitting form and healthy individuals, while miR-106a-5p showed lower level of expression in relapsing and remitting phase patients compared to healthy controls. The forkhead box O1 (FOXO1) and FOXO3 are two validated targets of miR-9-5p according to miRTarBase database. FOXO proteins have ability to bind to FOXP3 locus and control FOXP3 promoter activity and therefor induce FOXP3 expression (33). Thus, decreased expression level of FOXP3 could be due to down-regulation of FOXO1 and FOXO3, since both of these factors are required transcription factors for regulation of FOXP3 transcription. Meanwhile, FOXO1 and FOXO3 are assumed to be targets of miR-9-5p.
As mentioned before, STAT3 is a master transcription factor for Th17 cells differentiation, thus its expression level should be elevated in MS patients. PIAS3, an inhibitor of activated STAT3, is a predicted target of miR-9-5p. So, we hypothesized that up-regulation of miR-9-5p may have led to down-regulation of PIAS3, resulting in increased level of STAT3 and finally increased population of Th17 cells as we expected to observe in MS-patients; however, additional experiments are needed to evaluate expression level of PIAS3 and confirm this hypothesis (34, 35).
In contrast to miR-9-5p, miR-106a-5p showed reduction in CD4+ T-cells of relapsing and remitting phases of RR-MS compared to healthy controls. MiRWalk database predicted STAT3 and RORC as miR-106a-5p targets by combining results of 10 prediction databases. As stated before, STAT3 is an essential regulator of Th17 cells and therefore it is reasonable to have elevated level in patient due to more Th17 cells.
RORC is another predicted target of miR-106a-5p. Its expression was significantly higher in relapsing phase of RR-MS patients in comparison with remitting phase and healthy controls. Splicing of RORC gene produces two isoforms, including RORγ and RORγt. RORγt is only expressed in lymphocytes and is lineage specific transcription factor for Th17 differentiation and this may account for elevated number of Th17 cells, especially in early stages of MS (36).
MiRTarBase database introduces RUNX1 (also known as AML1) as a validated target of miR-106a-5p. RUNX1 is one of the major members of RUNX family expressed in resting CD4+ T-cells, which could interact with FOXP3 through regions distinct from their DNA-binding domains. RUNX1 binds to the promoter of IL-2 in these cells. Similar to RORγt, RUNX1 could also bind to IL-17 promoter (37). This protein involves in Th17 or Treg differentiation through interacting with one of two master transcription factors RORγt or FOXP3, respectively. Findings suggest that elevated levels of STAT3, RORC and RUNX1 in CD4+ T cells, especially in Th17 cells from MS patients, result from decreased levels of miR-106a-5p, which target Th17 inducing genes. Our flow cytometry results confirmed the higher percentage of RORγt positive cells in relapsing phase in comparison with remitting phase and healthy controls. In contrast, the percentage of Foxp3 positive cells in remitting phase was higher compared to relapsing phase patients and healthy individuals. Foxp3 showed significant difference between remitting phase and relapsing phases at protein level, probably by reason of the fact that many genes are also regulated at the post-transcriptional level. Based on these observations, we hypothesized that up-regulation of miR-9-5p and down-regulation of miR-106a-5p in relapsing phase of MS compared to two other groups correlates closely with Th17 differentiation of naïve T-cells and severity of the disease. In other words, these miRNAs involve in Th17 cells induction and Treg cells inhibition through targeting genes as mentioned before.
Conclusion
In this study we have shown that transcription level of miR-9-5p increases, while miR-106a-5p displays down-regulation in CD4+ T cells of relapsing phase of RR-MS patients. In addition, we investigated expression level of RORC as a target of miR-106a-5p. Potential role of these two miRNAs in differentiation of Th17 and Treg cells were investigated through in-silico molecular enrichment analysis. These analyses suggested the inductive role of miR-9-5p and the suppressive role of miR-106a-5p in differentiation of Th17 cells by targeting same factors in several pathways such as Jak-STAT. To our knowledge, miRNAs are targets for therapy, thus we propose that miR-9-5p and miR-106a-5p could be used as two potential therapeutic targets for prevention, suppression or symptom reduction in MS patients. Nowadays, a few miRNAs have entered the preclinical and clinical stage for therapeutic use in human (38). Thus, utilization of antagomiR or mimic oligonucleotides would be possible to prevent the progress of autoimmune disorders such as MS.
Acknowledgment
We especially thank Al-Zahra Hospital, Isfahan, Iran, staffs and participated patients. The results described in this paper were parts of Maryam Majd and Aref Hosseini theses to obtain Master degree. This work was supported mostly by a grant-in-aid of research from Royan Institute for Biotechnology, Isfahan, Iran. [No. 91000582].
Conflict of interest
None of the authors has any conflicts of interest to disclose and all authors support submission to this journal.
References
- 1.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 2.McFarland HF, Martin R. Multiple sclerosis:a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 3.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis:an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 5.Dong C. TH17 cells in development:an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 6.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17–producing CD4+effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 7.Lassmann H, Brück W, Lucchinetti C. Heterogeneity of multiple sclerosis pathogenesis:implications for diagnosis and therapy. Trends Mol Med. 2001;7:115–121. doi: 10.1016/s1471-4914(00)01909-2. [DOI] [PubMed] [Google Scholar]
- 8.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβin the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329–3341. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORαand RORγ. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J BIOL CHEM. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 14.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+regulatory T cells. Semin Immunol; 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Josefowicz SZ, Kas A, Chu T-T, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 16.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge:TGF-βinduces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 17.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Boehm M, Slack FJ. MicroRNA control of lifespan and metabolism. Cell Cycle. 2006;5:837–840. doi: 10.4161/cc.5.8.2688. [DOI] [PubMed] [Google Scholar]
- 19.Hatfield S, Ruohola-Baker H. microRNA and stem cell function. Cell Tissue Res. 2008;331:57–66. doi: 10.1007/s00441-007-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Negrini M, Ferracin M, Sabbioni S, Croce CM. MicroRNAs in human cancer:from research to therapy. J Cell Sci. 2007;120:1833–1840. doi: 10.1242/jcs.03450. [DOI] [PubMed] [Google Scholar]
- 21.Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32:189–194. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Organization WH. Atlas: Multiple sclerosis resources in the world 2008:WHO; 2008. [Google Scholar]
- 23.Fenoglio C, Cantoni C, De Riz M, Ridolfi E, Cortini F, Serpente M, et al. Expression and genetic analysis of miRNAs involved in CD4+cell activation in patients with multiple sclerosis. Neurosci Lett. 2011;504:9–12. doi: 10.1016/j.neulet.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk–database:prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Hsu S-D, Lin F-M, Wu W-Y, Liang C, Huang W-C, Chan W-L, et al. miRTarBase:a database curates experimentally validated microRNA–target interactions. Nucleic Acids Res. 2010;39:163–169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, et al. MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain. 2009;132:3342–3352. doi: 10.1093/brain/awp300. [DOI] [PubMed] [Google Scholar]
- 28.Gandhi R, Healy B, Gholipour T, Egorova S, Musallam A, Hussain MS, et al. Circulating microRNAs as biomarkers for disease staging in multiple sclerosis. Ann Neurol. 2013;73:729–740. doi: 10.1002/ana.23880. [DOI] [PubMed] [Google Scholar]
- 29.Cox MB, Cairns MJ, Gandhi KS, Carroll AP, Moscovis S, Stewart GJ, et al. MicroRNAs miR-17 and miR-20a inhibit T cell activation genes and are under-expressed in MS whole blood. PLoS One. 2010;5:e12132. doi: 10.1371/journal.pone.0012132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paraskevi A, Theodoropoulos G, Papaconstantinou I, Mantzaris G, Nikiteas N, Gazouli M. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Fasseu M, Tréton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5:e13160. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonkoly E, Wei T, Janson PC, Sääf A, Lundeberg L, Tengvall-Linder M, et al. MicroRNAs:novel regulators involved in the pathogenesis of psoriasis? Plos One. 2007;2:e610. doi: 10.1371/journal.pone.0000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouyang W, Beckett O, Ma Q, Paik J-h, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 34.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, et al. Specific inhibition of Stat3 signal transduction by PIAS3. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 35.Yagil Z, Nechushtan H, Kay G, Yang CM, Kemeny DM, Razin E. The enigma of the role of protein inhibitor of activated STAT3 (PIAS3) in the immune response. Trends Immunol. 2010;31:199–204. doi: 10.1016/j.it.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Jetten AM. Retinoid-related orphan receptors (RORs):critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2009;7:e003. doi: 10.1621/nrs.07003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu H, Djuretic I, Sundrud MS, Rao A. Transcriptional partners in regulatory T cells:Foxp3, Runx and NFAT. Trends Immunol. 2007;28:329–332. doi: 10.1016/j.it.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. MicroRNA therapeutics:Discovering novel targets and developing specific therapy. Perspect Clin Res. 2016;7:68–74. doi: 10.4103/2229-3485.179431. [DOI] [PMC free article] [PubMed] [Google Scholar]


