Abstract
Objective(s):
Enterotoxigenic Escherichia coli (ETEC) is an important cause of diarrheal disease in humans, particularly in children under 5 years and travelers in developing countries.
To our knowledge, no vaccine is licensed yet to protect against ETEC infection. Like many Gram-negative pathogens, ETEC can secrete outer membrane vesicles (OMVs). These structures contain various immunogenic virulence proteins such as LT and therefore can be used as vaccine candidates. In this study we attempted to isolate the OMVs of ETEC cultivated at different temperatures and evaluate their immunogenicity and protective efficacy in a murine model of infection.
Materials and Methods:
OMVs was purified from bacterial supernatant by ultracentrifugation. OMVs were encapsulated in chitosan nanoparticles prepared by ionic gelation method within a layer of Eudragit L100 for oral delivery. Female BALB/c mice of 9 weeks’ old were immunized by parenteral injection and oral administration with free and encapsulated OMVs obtained from bacteria cultivated at 37°C and 42°C. The serum samples were collected and the antibody titers were measured by an enzyme-linked immunosorbent assay (ELISA).
Results:
The protein concentrations of OMVs were 3.47 mg/ml and 2.46 mg/ml for bacteria grown at 37°C and 42°C respectively. OMVs loaded into nanoparticles (NP-OMVs) were homogeneous and spherical in shape, with a size of 532 nm. The encapsulation efficiency of NP was 90%. Mice immunized with OMVs, inhibited the ETEC colonization in their small intestine and induced production of antibodies against LT toxin.
Conclusion:
The results obtained in this research place OMVs among promising candidates to be used for vaccination.
Keywords: Chitosan nanoparticle, Enterotoxigenic –, Escherichia coli, Outer membrane vesicle, Virulence factors
Introduction
Enterotoxigenic Escherichia coli (ETEC) belongs to a diverse group of pathogens causing diarrhea. ETEC strains have several virulence factors like heat labile enterotoxin (LT), heat-stable enterotoxin (ST) and colonization factors (CFs). Following adherence and colonization in intestine, either or both LT or ST are expressed, resulting in diarrheal disease (1, 2). ETEC is one of the major causes of diarrhea in children under five years in endemic areas, leading to about 300000 to 500000 deaths per year (3-5). It is also the main cause of traveler’s diarrhea especially in military personnel traveling to endemic areas (6). Vaccination plays an important role in prevention of the infection and hence improving public health. Despite cumber some efforts of investigators on developing vaccine against ETEC, there is still no effective ETEC vaccine available in the market. Therefore research for development of an efficient vaccine against ETEC is needed (7-9).
Like all Gram-negative bacteria, ETEC is capable of producing outer membrane vesicles (OMVs), non-viable bubbles of bilayer membrane with a diameter of 20 to 250nm. OMV-mediated delivery of toxins and other virulence factors to host cells has been reported for several pathogens including members of the Enterobacteriaceae (10).
OMVs contain multiple putative virulence factors and immune modulating proteins, suggesting their ability to act as important candidates for producing vaccine against ETEC (11, 12). OMVs based vaccines have been developed for a number of Gram negative bacteria including Neisseria meningitidis, Helicobacter pylori, and Vibrio cholera (13-15). OMVs have been shown to elicit antibodies against multiple bacterial antigens and provide protection in animal models of infections (16). OMVs isolated from N. meningitides serogroup B in the presence of detergents, is shown to be safe and immunogenic in humans, and is used as a vaccine to control an epidemic of meningococcal meningitis in New Zealand (17, 18).
LT is one of the major virulence factors in ETEC. Immunity against LT is predominantly directed toward the B subunit of LT (LTB) which has 80 percent similarity to CTB of cholera toxin in three-dimensional structure and function (19, 20). Secretion of LT enterotoxin is associated with the release of OMVs and ETEC secreted vesicles are rich in LT toxin (14).
OMVs secretion is also considered as a critical bacterial response to different environmental stresses. To counteract environmental effects, bacteria activate stress sensors, which leads to the change in the transcriptional profile and downstream products including bacterial envelope composition (21, 22). Therefore, environmental conditions can affect OMVs production and cause significant changes in the amount and composition of secreted OMVs.
Oral administration of vaccine for mucosal immunity and systemic immune stimulation is the most effective and convenient route, when the gastrointestinal tract is the bacterial target. Problems related with oral drug delivery are, harsh effect of extremely acidic condition of stomach and low transit time of drug in the gastrointestinal tract (23, 24). Mucoadhesive materials, like chitosan can overcome these problems by providing more access and better absorption of materials in the intestine. Chitosan is deacetylated chitin polymer, commercially available in different forms in the market. The main problem lying with chitosan nanoparticles (NPs) is its low resistance to acidic pH (25, 26).
Eudragit is a copolymer of methacrylate and acid methacrylic. Eudragit L100 can resists the acidic pH and is completely soluble at physiological pH, which makes it a suitable candidate to protect cargo molecules and their safely transit from stomach (27).
In the present work, OMVs from ETEC were isolated at 2 different temperatures of 37°C and 42°C. The protective efficacy of OMVs either in their free form or encapsulated with chitosan and Eudragit were tested in the murine model after immunization by intradermal and oral routes.
Materials and Methods
Preparation of OMVs
OMVs were prepared based on the method described by Delbaz et al (28). In brief, 5 ml of overnight bacterial culture was inoculated to 500 ml of Luria broth (LB) medium (Difco, UK). The bacterial cultures were incubated at two different tempera-tures of 37°C and 42°C with a shaking condition of 150 rpm. The bacteria were inactivated by adding 5% phenol in end of logarithmic phase (OD600 = 1) of the growth. OMVs were extracted with 0.1M Tris-HCl, pH 8.6 containing 10 mM ethylenediamine tetra acetic acid (EDTA(and 0.5% w/v deoxycholate (Sigma, Germany) as a detergent. Cell fragments were sedimented by centrifugation at 18,000×g for 90 min and the supernatant was further centrifuged at 125,000 × g for 2 hr. The pallet containing OMVs was suspended in 15 ml distilled water containing 3% sucrose and stored at 4°C. The protein content of OMVs was measured using modified Lowery method (31).
To study the size and shape of OMVs with transmission electron microscopy (TEM), 0.1 ml of aforementioned OMV suspension was placed on copper formvar/carbon-coated grids and allowed to adsorb for 5 min. Grids were then washed with 1 ml of distilled water and blotted with whatman filter paper (Sigma, Germany). For negative staining, grids were treated with 2% uranyl acetate (Merck, Germany) in ddH2O for 1 min, air-dried and viewed with TEM (Zeiss - EM10C - 80 KV). Electron micrographs were recorded at magnification of 63000×.
Protein content of OMVs were analyzed on 12% SDS-PAGE (Criterion XT, Bio Rad Laboratories, CA) with the discontinuous buffer system of Laemmli and gels stained with coomassie blue (Merck, Germany).
Preparation of chitosan –TPP particles
The OMV nanoparticles were prepared by ionic gelation between positively charged chitosan and negatively charged tri-polyphosphate (TPP; Sigma, Germany). To obtain 5 mg/ml concentration, 25 mg of chitosan (medium molecular mass, degree of deacetylation about 80% Sigma-Aldrich, USA), was dissolved in 5ml of 2.0% acetic acid aqueous solution under stirring at room temperature. The pH of the resulting solution was adjusted to 5.5 using aqueous sodium hydroxide solution. One ml of OMVs (1 mg/ml) was added to 750 µl of chitosan solution under stirring condition at 4°C. TPP was dissolved in water at room temperature to obtain 2 mg/ml concentration. To prepare nanoparticles, 500 µl of TPP solution (2 mg/ml) was added slowly to the chitosan solution containing OMVs, with continuous stirring at 4°C. The solution was centrifuged at 14,000 × g for 10 min at 4°C. The final ratio of chitosan/TPP in nanoparticles was 3:1. Nanoparticles without OMVs, to be used as a control, were prepared by the same procedure.
OMV-chitosan nanoparticles were further coated within EudragitL100 (Sigma, Germany) using electro-static interaction. 2 mg of Eudragit was dissolved in 15 ml acetone and ethanol (1:1 ratio) and 0.2 mg of OMV-chitosan NPs, containing almost 50 μg OMV, was drip dropped into organic solvent under stirring condition. Paraffin with 1% span was then added to the mixture. The organic phase of resultant water in oil emulsion was left to evaporate on stirrer (IKA, RH, Germany). The remnant was centrifuged at 9500 × g for 30 min.
Particle size, zeta potential and encapsulation efficiency determination
The z-average particle size, distribution and poly dispersity index (PDI) of the chitosan-TPP nano-particles were measured at 25°C by dynamic light scattering (DLS) on a high performance particle sizer (HPPS-5001, Malvern, UK). The zeta potential of nanoparticles was measured by Zetasizer 3000 HAS (Malvern, UK). Mean values were obtained from the analysis of three different batches, each measured three times. Encapsulation efficiency (EE) was calculated.
Animal immunization
Nine-week old BALB/c mice of approximately 20 g weight were separated in randomized groups of 5 animals each. Out of three groups of animals for immunization, the first group was immunized with 10 µg of OMVs via subcutaneous route, the second and third groups were orally fed with 50 µg of free and encapsulated OMVs respectively. Control groups were administered with normal saline. Blood samples were collected from the eyes of anesthetized mice 5 days after the 2nd, 3rd and 4th injections/ oral admirations.
Fecal IgA of orally immunized mice was extracted by homogenizing of 1 g of fecal samples in 500µl of PBS containing 0.05% (w/v) of sodium azide. The homogenate was centrifuged at 5000 × g for 20 min at 4°C and supernatant was transferred to the sterile tube. PMSF (10 µl/ml sample) was added as a protease inhibitor and the mixture was centrifuged at 13,000 × g for 20 min at 4°C and supernatant containing IgA was stored in -20°C for further studies.
Determination of anti-OMVs IgG and IgA responses
The antibody response was measured by an enzyme-linked immunosorbent assay (ELISA). In brief, 2 µgof OMVs were coated with coating buffer (60 mM carbonate buffer, pH 9.6) in 96-well microtiter plates (MaxiSorb; Nunc, Wiesbaden, Germany). Each wells were blocked with100µl of 3% bovine serum albumin (BSA; Sigma, Germany) for 2 hr at 4°C. 100 µl of serially diluted sera were added to wells and incubated overnight at 4°C. Class specific goat anti-mouse IgG/IgA (Sigma) was added after washing with PBS containing 0.05 % Tween 20 (PBS-T) buffer, and incubated for 2 hr at 37°C. OPD as a substrate were added to wells at room temperature and OD490nm was measured with an ELISA reader (Sunrise remote; Tecan-Austria, Groeding, Austria).
For detection of LT in OMVs, ltb gene previously was cloned in pET28a and recombinant LTB protein expressed in BL21DE3 and purified with NI-NTA affinity chromatography. In ELISA, 2.5 µg recom-binant of LTB was coated in each well as an antigen and immunized mice sera was used as a primary antibody. BSA was coated as control and the ELISA was carried out as stated above.
ETEC binding inhibition assay
In order to check the inhibitory effect of immunized mice sera on attachment of ETEC to intestinal epithelium, HT-29 cells (Cell Bank, Pasteur Institute of Iran) were grown in Dulbecco’s modified eagle media (DMEM; Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco). The cells were poured to bottom of 24-well plates at a concentration of 8 × 104 cells per well and grown to near confluence at 37°C in a 5% CO2 atmosphere. ETEC cells at 105 CFU/ml concentration was pretreated with 40 µl of immunized and non-immunized mice sera and incubated for 30 min at room temperature. The mixture was added to HT-29 cells and incubated for 1 hr at room temperature. After washing with PBS, the cells were trypsinized and cultured on LB agar to measure the amount of bacteria attached to the HT-29 cells.
Toxin neutralization assay
To assess the ability of serum samples of immunized mice in neutralizing the LT toxin, ileal loop assay was performed (29). Briefly, mice were fasted for 24 hr before surgery and then were anesthetized by intraperitoneal injection of Nembutal (60 mg/kg body weight). The abdomen was open up and small intestine was divided into loops of 3 cm length. ETEC bacteria at 1 × 108 CFU/ml concentration was incubated with mouse serum for 30 min and the mixture was added to each loop created in the intestine. The loop injected with PBS was kept as a control. After injection of the loops, the abdomen was closed and the animals were sacrificed 18 hr later by pentobarbital and ileal loops were exteriorized. The ratio of fluid accumulation against loop length (g/cm) was calculated as an index of enterotoxigenicity.
Statistical analysis
Statistical analysis was performed using SPSS software version 22. The results were expressed as mean±SD. Data were statistically analyzed by ANOVA test with P<0.05 considered as significant difference.
Results
Characterization of purified OMVs
The protein concentrations of OMVs obtained from bacteria cultured at 37°C and 42°C were 3.47 mg/ml and 2.46 mg ml-1 respectively. OMVs produced at different temperatures also showed different pattern on SDS-PAGE (Figure 1b) Images obtained by TEM, showed vesicle size in the range of 20-50 nm. Vesicles were spherical with intensive surface (Figure 1a).
Figure 1.
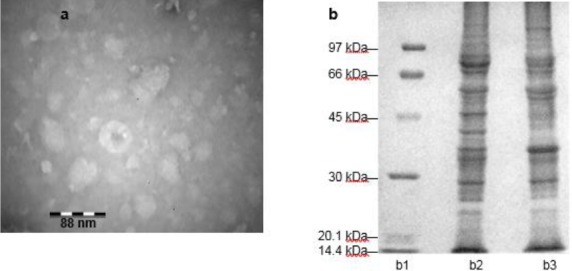
OMVs analysis: (a) The transmission electron microscopy image (magnification, 63000×) showing OMV (b) SDS-PAGE analysis of OMVs obtained from ETEC; b1. ladder b2. nOMV b3. sOMV
Abbreviations: nOMVs: Natural outer membrane vesicles, sOMVs: Stress outer membrane vesicles, ETEC: Enterotoxigenic Escherichia coli.
Characterization of nanoparticles
Zeta size analysis revealed the size of NP complexes to be 532±7 nm with PDI value of 0.29.
The charge of prepared nanoparticles was measured to be +23±0.2. Positive charge for nanoparticles is essential for their stability. The encapsulation efficiency of NP was 90% (Table 1).
Table 1.
Encapsulation efficiency
| sample | total protein (µg) | Absorbance of supernatants (OD595) | supernatants protein (µg) | EE% |
|---|---|---|---|---|
| nOMV | 500 | 0.048 | 25.76 | > 90 |
| sOMV | 500 | 0.056 | 38.14 | > 90 |
Percentage of OMVs loading into nanogels calculated by the formula given on methodology.
Abbreviations: nOMVs: Natural outer membrane vesicles, sOMVs: Stress outer membrane vesicles, OD: Optical Density, µg: Microgram, EE: Encapsulation efficiency
Serum and fecal antibody responses
Animals were monitored for 48 hr after vaccination and there was no unusual sign and animals were looking healthy. Since OMVs is widely associated with the LT toxin, therefore to evaluate the antibody production we performed ELISA with immunized mice sera using recombinant LT protein as an antigen. There was a significant difference in antibody titer of wells coated with LT compared to control (Figure 2).
Figure 2.
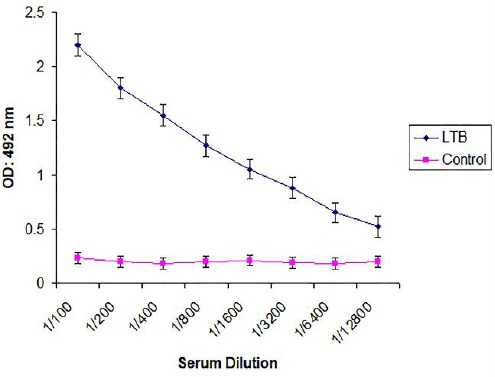
Serum immunoglobulin titers in immunized sera were measured against LTB as a component of OMVs. Abbreviations: LTB: Heat-labile enterotoxin B subunit, OD: Optical Density, nm: Nanometer
Mice elicited significant IgG and IgA antibodies in serum and fecal samples upon oral (IgG and IgA) and parenteral (IgG) immunization compared to control mice (P<0.05). The level of antibody titers was different between mice groups receiving OMVs in different forms. There was no significant difference between the antibody titers of mice receiving OMVs from bacteria cultivated at 42°C and 37°C. But statistically significant difference(P<0.05) were observed between antibody titer of the mice sera injected with OMVs or received non polymerized and polymerized OMVs orally (Figure 3). As shown in the Figure, lowest response was for orally given polymer free OMVs.
Figure 3.
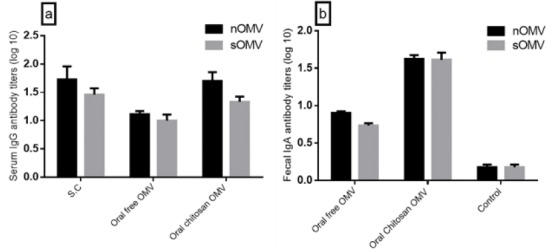
Serum and fecal anti-nOMV and sOMV antibody titers of mice immunized with different formulations. (A) Serum IgG titers after boost immunizations; (B) Fecal IgA titers after boost immunizations. Titers calculated as log 10 of last reciprocal dilution above cut-off. Error bars represent mean±SEM (n=3)
Abbreviations: nOMVs: Natural outer membrane vesicles, sOMVs: Stress outer membrane vesicles, SEM: Standard error of mean, IgG: Immunoglobulin G, IgA: Immunoglobulin A, S.C: Subcutaneous, log: Logarithm.
The rate of antibody titers declined gradually 42 days after the last treatment in all mice groups except those received encapsulated antigen orally. The sera of these animals retained high antibody titer even after 78 days of last antigen administration. IgA titer against encapsulated antigen was significantly higher than that of non-polymerized antigens (P<0.05).
Binding assay of ETEC to HT-29 cells
The ability of anti-OMV antibodies collected from immunized mice to block the binding of ETEC bacteria to HT-29 cell lines was measured. Pretreatment of ETEC cells with anti-OMV antibodies blocked their binding to HT-29 cells. Treatment with antibodies raised against polymerized OMVs, produced at 37°C, showed highest inhibition of bacterial binding to HT-29 cells. Results are demonstrated in Table 2.
Table 2.
Binding inhibition assay
| Groups | Temp(°C) | Adherent Index |
|---|---|---|
| ETEC+Serum (S. C) | 37 | 2.3 |
| 42 | 3.2 | |
| ETEC+Serum (Oral Free OMVs) | 37 | 2.5 |
| 42 | 3.4 | |
| ETEC+Serum (Oral Encapsulated OMVs) | 37 | 2.1 |
| 42 | 1.8 |
The serum of mice groups receiving OMVs produced at 37°C have better performance in preventing adhesion in comparison to OMVs produced at 42°C Abbreviations: OMVs: Outer membrane vesicles, S.C: Subcutaneous.
Toxin neutralization assay
The inhibitory effect of anti-OMV antibody on LT-induced fluid accumulation was studied in the mice ileal loop assay (Figure 4). The fluid accumulation was not observed in mice ileal loops 18 hr post infection with ETEC treated with the immunized mice serum, whereas fluid accumulation was observed in loop inoculated with non-treated ETEC. Sera obtained from mice group, receiving polymerized vesicles of 37°C could inhibit LT toxin better than the other two groups. The results are summarized in Table 3.
Figure 4.
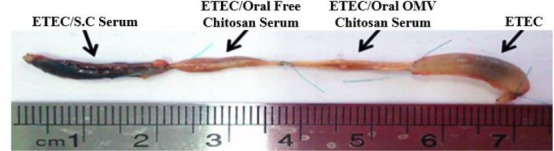
Mice ileal loop assay. Intestinal loops were inoculated with cell-free supernatants from ETEC, ETEC + serums
Abbreviations: S.C: Subcutaneous, ETEC: Enterotoxigenic Escherichia coli, OMVs: Outer membrane vesicles
Table 3.
Intestinal epithelial responses to ETEC and ETEC + serum
| Groups | Temp(°C) | weight(gr) |
|---|---|---|
| length(cm) | ||
| ETEC | 37 | 0.15 |
| 42 | 0.2 | |
| ETEC+ S. C serum | 37 | 0.03 |
| 42 | 0.06 | |
| ETEC+oral free OMV serum | 37 | 0.05 |
| 42 | 0.07 | |
| ETEC+oral encapsulated OMV serum | 37 | 0.02 |
| 42 | 0.05 |
Values obtained for the bacteria is larger than 0.1 and for bacteria + Serum is less than 0.1, indicating the inhibition of bacterial toxin (LTB) with antibody present in serum.
Abbreviations: ETEC: Enterotoxigenic Escherichia coli, Temp: Temperature, OMVs: Outer membrane vesicles, S.C: Subcutaneous.
Discussion
Design and development of mucosal vaccine against ETEC infection is one of the major problem that must be overcome (30, 31). Research attempts on vaccine production against ETEC mostly have been focused on a few immunogens such as LT toxin and colonization factors. Lack of sufficient protection by the LT toxoid or recombinant LTB and presence of various colonization factors, are some of the difficulties encountering the effective vaccine preparation against ETEC.
OMVs, due to the presence of several antigens and virulence factors in their structure, could potentially actuate for development of effective vaccine against ETEC strains (12, 32). In the present study, we focused on immunogenicity of OMV-associated antigens extracted at different environmental conditions. OMVs have ability to induce the immune response and thereby protect against enteric pathogens (10, 33). Capacity of OMV-based vaccines to stimulate a protective immune response has already been exploited against several bacterial pathogens like V. cholera and N. meningitidis (14, 34). Since naturally purified OMVs contain considerable amount of LPS, therefore to reduce the LPS content, we purified OMVs with deoxycholate detergent. Although LPS is an effective adjuvant but excessive activation of immune system due to high LPS content of antigens lead to septic shock. Thereby reducing endotoxicity of OMVs is an essential step towards their safe use as vaccine candidates. Norheim et al. reduced LPS content of OMVs from N. meningitides by detergent extractions (35). Van de Waterbeemd improved OMV based vaccine against N. meningitidis using genetically engineered strains and detoxified LPS (36).
The pattern of SDS-PAGE analysis of the two OMVs purified at different conditions support our in vivo experiments. In response to heat shock, bacteria generally induce activation of stress sensors, a process known as “unfolded protein response” where Sigma 32 shifts RNA polymerase toward heat shock genes (21, 37). This leads to the changes in transcription profile and thereby resulting in modification of downstream processes of which the change in the composition of the bacterial envelope is obvious. This is because OMVs are secreted as a response to stress in order to remove the miss-folded proteins from bacteria and as bait to attract antimicrobial agents which bind to the cell surface (21, 37). Protein content and banding pattern of nOMV1s compared to sOMV2s and vesicles produced at different temperatures was different when analyzed on SDS PAGE. Mac Donald and Kuehn didn’t get any significant differences on the effects of temperature increases in OMV production (22). Antibodies raised against OMVs secreted at 37°C could prevent the attachment of bacteria to HT29 cells better than antibodies raised against OMVs of stress condition. This is may be due to demolished or dropped expression level of proteins involved in adhesion at temperature of 42°C.
In order to create a stronger immune response, most vaccines are administered along with adjuvant. ETEC derived OMV are rich in LT toxin with inherent adjuvant properties, therefore additional adjuvant is not required during vaccination (12, 16). The results of our ELISA conducted with recombinant B subunit of LT toxin confirmed the presence of LT in OMVs, further supporting OMVs as better immunogens (12).
Our findings are farther supported with previous studies where OMVs secreted by the ETEC could induce the production of specific antibodies against surface antigens (11, 12, 19).
Due to non-invasive, simplicity of administration and high efficacy of mucosal immunity in prevention of intestinal infections, oral route for OMVs adminis-tration was preferred. Similarity in the way of OMVs and pathogen arrival to intestine, also logically supports our oral administration for induction of the immune system against ETEC (38).
However, harsh condition of the gastrointestinal tract, especially the stomach, creates problems to the oral delivery. The problem can be overcome with encapsulation of OMVs in a suitable nano or micro particles and thereby protecting them from damaging effects of stomach acid (27, 39).
OMVs were encapsulated in chitosan nanoparticles for oral immunization of animals. Although chitosan, as non-toxic polymer, with biodegradability, bio-compatibility and low immunogenicity are suitable for drug delivery, but the main problem is the low resistance of this polymer in acidic pH (27, 39). Eudragit, the other polymer with protective effect against gastric acid is used along with chitosan. Badhana et al. (27), and Lee et al. (39) reported that encapsulated of immunogen with Eudragit induced stronger and prolonged immune responses compared to free immunogen (27, 39). Our results were in consistent with their findings. In this study polymerization was carried out by ionic gelation method and the EE of encapsulation was over 90%, compared to 86% and 87.7% reported by others (27, 39).
The IgG titers were highest in serum of mice injected with OMVs, followed by oral administration of polymerized and non-polymerized OMVs respect-tively. The antibody titers were declined 42 days after the last delivery in all mice groups except those received encapsulated antigen orally. Even 78 days after the last immunization, the antibody titer of polymerized OMV remained high compared to the other 2 groups. This can be attributed to the controll-ed release of antigens by polymeric nanoparticles (40). The level of IgA titers in polymerized OMV was higher than non-polymerized OMVs, indicating the preservation effect of polymer on antigen damaging in stomach.
The bio-adhesive nature of the chitosan which enhances the interaction of nanoparticles with the mucosal layer could be another reason for explaining the high antibody titer obtained against polymerized OMVs (41). Adsorption of nanoparticles to negatively charge epithelial cell surface in the gut can be increased due to high density of positive charges on chitosan. This leads to the better delivery of antigen to the target (40). In addition, antigens coated with chitosan increases its interaction with dendritic cells. Efficiency of immunization also enhances with the slow and controlled release of antigen which is more possible with polymerized antigen than the nacked one (40). The IgA titers of polymerized OMVs were higher than free OMVs.
Conclusion
The results of our study confirmed the potential value of chitosan nanoparticles containing ETEC OMVs offer a protection against ETEC infection in marine model. Therefore, encapsulation of OMVs triggering mucosal immunity can be a new approach of vaccination for effective neutralization of ETEC infection.
Acknowledgment
The authors wish to thank Deputy Research, Shahed University, Tehran, Iran for financial support. The results presented in this paper are part of a student’s thesis.
Footnotes
OMVs obtained from bacteria cultured at 37 °C
OMVs obtained from bacteria cultured at 42 °C
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Nazarian S, Gargari SL, Rasooli I, Alerasol M, Bagheri S, Alipoor SD. Prevalent phenotypic and genotypic profile of enterotoxigenic Escherichia coli among Iranian children. Jpn J Infect Dis. 2014;67:78–85. doi: 10.7883/yoken.67.78. [DOI] [PubMed] [Google Scholar]
- 2.Qadri F, Svennerholm AM, Faruque ASG, Sack RB. Enterotoxigenic Escherichia coli in developing countries:epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev. 2005;18:465–483. doi: 10.1128/CMR.18.3.465-483.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nazarian S, Mousavi Gargari SL, Rasooli I, Amani J, Bagheri S, Alerasool M. An in silico chimeric multi subunit vaccine targeting virulence factors of enterotoxigenic Escherichia coli(ETEC) with its bacterial inbuilt adjuvant. J Microbiol Methods. 2012;90:36–45. doi: 10.1016/j.mimet.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Jiang ZD, Lowe B, Verenkar MP, Ashley D, Steffen R, Tornieporth N, et al. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay) J Infect Dis. 2002;185:497–502. doi: 10.1086/338834. [DOI] [PubMed] [Google Scholar]
- 5.Katz DE, DeLorimier AJ, Wolf MK, Hall ER, Cassels FJ, van Hamont JE, et al. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli(ETEC) CS6 antigen. Vaccine. 2003;21:341–346. doi: 10.1016/s0264-410x(02)00613-8. [DOI] [PubMed] [Google Scholar]
- 6.Steffen R, Castelli F, Dieter Nothdurft H, Rombo L, Jane Zuckerman N, et al. Vaccination against Enterotoxigenic Escherichia coli a Cause of Travelers Diarrhea. J Travel Med. 2005;12:102–107. doi: 10.2310/7060.2005.12207. [DOI] [PubMed] [Google Scholar]
- 7.Nazarian S, Gargari SL, Rasooli I, Hasannia S, Pirooznia N, et al. A PLGA-encapsulated chimeric protein protects against adherence and toxicity of enterotoxigenic Escherichia coli. Microbiol Res. 2014;169:205–212. doi: 10.1016/j.micres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Walker RI, Steele D, Aguado T Committee AHETE. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli(ETEC) disease. Vaccine. 2007;25:2545–2566. doi: 10.1016/j.vaccine.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren A, Bourgeois L, Carlin N, Clements J, Gustafsson B, Hartford M, et al. Safety and immunogenicity of an improved oral inactivated multivalent enterotoxigenic Escherichia coli(ETEC) vaccine administered alone and together with dmLT adjuvant in a double-blind, randomized, placebo-controlled phase I study. Vaccin. e2014;32:7077–7084. doi: 10.1016/j.vaccine.2014.10.069. [DOI] [PubMed] [Google Scholar]
- 10.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Review Microbiol. 2010;64:163–184. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy K, Bartels S, Qadri F, Fleckenstein JM. Enterotoxigenic Escherichia coli elicits immune responses to multiple surface proteins. Infect Immun. 2010;78:3027–3035. doi: 10.1128/IAI.00264-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy K, Hamilton DJ, Munson GP, Fleckenstein JM, et al. Outer membrane vesicles induce immune responses to virulence proteins and protect against colonization by enterotoxigenic Escherichia coli. Clin Vaccine immunol. 2011;18:1803–1808. doi: 10.1128/CVI.05217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop AL, Schild S, Patimalla B, Klein B, Camilli A. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect Immun. 2010;78:4402–4420. doi: 10.1128/IAI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O´Hallahan J, et al. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccin. e2009;27:B3–B12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 15.Keenan JI, Rijpkema SG, Durrani Z, Roake JA. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol Med Microbiol. 2003;36:199–205. doi: 10.1016/S0928-8244(03)00091-9. [DOI] [PubMed] [Google Scholar]
- 16.Acevedo R, Fernandez S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, et al. Bacterial outer membrane vesicles and vaccine applications. Front Immunol. 2014;25:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danzig L. Meningococcal vaccines. PediatrInfect Dis J. 2004;23:S285–S292. [PubMed] [Google Scholar]
- 18.Leitner DR, Zingl FG, Schild S. A glimpse on outer membrane vesicles as vaccine candidates. Vaccines Vaccin. 2015;6:293. [Google Scholar]
- 19.Leitner DR, Lichtenegger S, Temel P, Zingl FG, Ratzberger D, Roier S, et al. A combined vaccine approach against Vibrio cholerae and ETEC based on outer membrane vesicles. Front Microbiol. 2015;6:823. doi: 10.3389/fmicb.2015.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez J, olmgren J. Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr Opin Immunol. 2005;17:388–398. doi: 10.1016/j.coi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology. 2014;160:2109–2121. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald IA, Kuehn MJ. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J Bacteriol. 2013;195:2971–2981. doi: 10.1128/JB.02267-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aziz MA, Midha S, Waheed SM, Bhatnagar R. Oral vaccines:new needs, new possibilities. Bioessays. 2007;29:591–604. doi: 10.1002/bies.20580. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Liu H, Zhang X, Qian F. Intranasal and oral vaccination with protein-based antigens:advantages, challenges and formulation strategies. Protein Cell. 2015;6:480–503. doi: 10.1007/s13238-015-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koide SS. Chitin-chitosan:properties, benefits and risks. Nutr Res. 1998;18:1091–1101. [Google Scholar]
- 26.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles:the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badhana S, Garud N, Garud A. Colon specific drug delivery of mesalamine using eudragit S100-coated chitosan microspheres for the treatment of ulcerative colitis. Int Curr Pharm J. 2013;2:42–48. [Google Scholar]
- 28.Delbaz SA, Siadat SD, Aghasadeghi MR, Piryaie M, Peerayeh SN, Mousavi SF, et al. Biological and immunological evaluation of Neisseria meningitidis serogroup A outer membrane vesicle as vaccine candidates. Jundishapur J Microbiol. 2013;6:e5007. [Google Scholar]
- 29.Sawasvirojwong S, Srimanote P, Chatsudthipong V, Muanprasat C, et al. An adult mouse model of Vibrio cholerae-induced diarrhea for studying pathogenesis and potential therapy of Cholera. PLoS Negl Trop Dis. 2013;7:e2293. doi: 10.1371/journal.pntd.0002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alerasol M, Mousavi Gargari SL, Nazarian S, Bagheri S, et al. Immunogenicity of a fusion protein comprising coli surface antigen 3 and labile B subunit of enterotoxigenic Escherichia coli. Iran Biomed J. 2014;18:212–218. doi: 10.6091/ibj.1344.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagheri S, Mousavi Gargari SL, Rasooli I, Nazarian S, Alerasol M. A CssA, CssB and LTB chimeric protein induces protection against Enterotoxigenic Escherichia coli. Braz J Infect Dis. 2014;18:308–314. doi: 10.1016/j.bjid.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy K, Hamilton D, Allen KP, Randolph MP, Fleckenstein JM, et al. The EtpA exoprotein of enterotoxigenic Escherichia coli promotes intestinal colonization and is aprotec tive antigen in an experimental model of murine infection. Infect Immun. 2008;76:2106–2112. doi: 10.1128/IAI.01304-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callico A, Fernandez S, Cabrera R, Acosta M, Aranguren Y, Fernandez Y. Proteoliposomes derived from Escherichia coil i nduced systemic and mucosal response. VacciMonitor. 2010;19:32. [Google Scholar]
- 34.Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008;76:4554–4563. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norheim G, Tunheim G, Naess LM, Kristiansen PA, Caugant DA, Rosenqvist E. An Outer Membrane Vesicle Vaccine for Prevention of Serogroup A and W_135 Meningococcal Disease in the African Meningitis Belt. Scand J Immunol. 2012;76:99–107. doi: 10.1111/j.1365-3083.2012.02709.x. [DOI] [PubMed] [Google Scholar]
- 36.Van de Waterbeemd B, Streefland M, Van der Ley P, Zomer B, Van Dijken H, Martens D. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccin. e2010;28:4810–4816. doi: 10.1016/j.vaccine.2010.04.082. [DOI] [PubMed] [Google Scholar]
- 37.Klimentova J, Stulik J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol Res. 2015;170:1–9. doi: 10.1016/j.micres.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Davitt CJ, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev. 2015;91:52–69. doi: 10.1016/j.addr.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Lee WJ, Cha S, Shin M, Jung M, Islam MA, Cho CS. Efficacy of thiolated eudragit microspheres as an oral vaccine delivery system to induce mucosal immunity against enterotoxigenic Escherichia coli in mice. Eur JPharm Biopharm. 2012;81:43–48. doi: 10.1016/j.ejpb.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 40.Camacho AI, Irache JM, de SJ, Sanchez-Gomez S, Gamazo C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccin. e2013;31:3288–3294. doi: 10.1016/j.vaccine.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 41.Elgadir MA, Uddin M, Ferdosh S, Adam A, Chowdhury AJK, Sarker M. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems:A review. J Food Drug Anal. 2015;23:619–629. doi: 10.1016/j.jfda.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]


