Abstract
Objective(s):
To investigate the effect of miR-103 on the angiogenesis of ischemic stroke rats via targeting vascular endothelial growth factor (VEGF) at the molecular level.
Materials and Methods:
Rat models had received the middle cerebral artery occlusion (MCAO) or sham operation before grouping, and cell models of oxygen-glucose deprivation (OGD) were performed. FITC-dextran, matrigel, and Trans-well assays were used to evaluate the vascular density, tube formation, and cell migration respectively. The expression levels of miR-103 and VEGF were detected by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting. Dual-luciferase assay was used for analyzing the targeting relationship between miR-103 and VEGF.
Results:
We found the reduced miR-103 in rats after MCAO. Down-regulating miR-103 with the miR-103 inhibitor enhanced VEGF, ameliorated the neurological scores, decreased infarct volume, and increased vascular density in rats after MCAO. Besides, in OGD human umbilical vein endothelial cells (HUVECs), inhibition of miR-103 could promote the increase of tube length and the migration of cells. Additionally, we found that miR-103 could directly target VEGF and thereby lead to the down-expression of VEGF. Meanwhile, si-VEGF could reverse the effect of miR-103 inhibitor on angiogenesis in rats subjected to MCAO.
Conclusion:
Inhibition of miR-103 could promote ischemic stroke angiogenesis and reduce infarction volume via enhancing VEGF, which provides a new target for the clinical treatment of ischemic stroke.
Keywords: Infarction, Ischemia, Middle cerebral artery, MIRN103 microRNA, Stroke, Vascular endothelial-growth factor A
Introduction
Stroke, a well-known devastating disease with significant morbidity and mortality, as well as the high rates of disability and recurrence, seriously threatens to human health and quality of life (1). As reported, stroke is globally credited as the second and fifth leading cause of death for people older than 60 years and aged 15-59 years, respectively (2). Clinically, there are two main categories of stroke including ischemic (result from lack of blood flow) and hemorrhagic (due to bleeding), with the former accounting for 60%-80% (3). To complicate matters, the incidence rate of ischemic stroke in China is still rising by 8.7% year by year (4), making the prevention and treatment of ischemic stroke a matter of great urgency. Of note, previous studies have demonstrated that promoting angiogenesis after ischemic stroke can facilitate the function recovery, helping to directly restoring the oxygen and nutrient supply in damaged brain tissues (5, 6). Apart from that, patients with the higher density of the new capillaries in the injured area after cerebral ischemia had a better prognosis and a lower mortality in clinical trials over the past decades (7). Therefore, improving angiogenesis of ischemic areas has been regarded as one of the effective treatment for stroke (8).
MicroRNAs (miRNAs), as a series of non-coding RNAs with regulatory function, can combine specifically with the 3’-untranslated region (UTR) of mRNA to form silencing complex, and thereby exerting functions via inhibiting the translation or promoting the degradation of mRNA (9). Mounting evidence showed that miRNAs are involved in vascular development and vascular diseases by the modulation of the key angiogenesis biomarkers in physiological and pathological conditions (10). In recent years, studies regarding the regulation of a variety of miRNAs in the angiogenesis after ischemic stroke are increasing, such as miR-210 (11), miR-493(12), and miR-107(13), which has been reported to affect the progression and prognosis of ischemic stroke. MiR-103 belongs to the miR-103/107 family located on human chromosome 5 and it is found to be widely distributed in many kinds of human tissues, including liver, placenta, brain, stomach, lung, etc. (14), which exhibited regulatory effects on cell proliferation, angiogenesis, lipid metabolism, as well as anti-inflammatory (15). In the study by Wilson R et al., miR-103 could control the production of inflammatory cytokines into the tumor microenvironment through targeting TREX1, thus aggravating DNA damage and inhibiting angiogenesis in vitro and in vivo (16). According to the finding by Chen et al., the expression of miR-103 is modulated by hypoxia inducible factor 1α, which can target argonaute 1 (AGO1) to promote the formation of tumor vessels (17). Moreover, after searching on a target gene prediction website, we found vascular endothelial growth factor (VEGF) to be the target gene of miR-103. Although it has also been proved that VEGF can effectively affect the angiogenesis and vascular density after ischemic stroke (18), the specific mechanism of miR-103 and VEGF affecting ischemic stroke has not been clearly elucidated.
Therefore, middle cerebral artery occlusion (MCAO) model and oxygen-glucose deprivation (OGD) model are constructed to simulate the in vivo cerebral infarction environment, aiming to investigate whether miR-103 can target VEGF to regulate the angiogenesis of ischemic stroke in rats.
Materials and Methods
Ethics statement
This animal experiment got the approval of the Ethics Committee of Affiliated Hospital of Hebei University. All procedures in experiments were conducted in obedience to the guidelines put forward by the National Institutes of Health (19).
Construction rat models of MCAO
Healthy male Sprague-Dawley (SD) rats of SPE (specific pathogen free) clean level weighing (200±20) g were provided by the Center for Animal Experiment in the Medical College of Wuhan University. The MCAO rat model was established by using modified suture-occlusion method (20) as follows: intraperitoneal injection of 10% chloral hydrate (360 mg/kg) was given to rats for anesthetization, an incision was made on the neck of rats right at the median position, and a nylon thread coated with paraffin was inserted into the internal carotid artery and fixed appropriately. After 2 hr of occlusion, the thread would be removed to restore blood perfusion. During the operation, the anus temperature of rats should be maintained at (37.0±0.5)°C. After operation, rats should be kept in a cage with clean, dry pad, and sufficient food and water, and their body temperature should be maintained at about 37°C.
Grouping and treatment of rats
The rats were divided into 4 groups with 10 in each group. (1) Sham group: Sham-operated rats were with separated blood vessel but without thread insertion; (2) MCAO group: Rats were given miR-103 negative control (NC) scramble by intracerebroventricular injection about 5 min after MCAO; (3) MCAO+miR-103 mimic group; (4) MCAO+miR-103 inhibitor group: Rats were given 10 μg of miR-103 mimic, and miR-103 inhibitor by intracerebroventricular injection about 5 min after MCAO respectively. Scrambled miRNA, and miR-103 mimic/inhibitor were purchased from Shanghai GenePharma Co, Ltd, which were wrapped using PEG liposomes before administration. At the 24 hr after MCAO, the brain tissues of rats were taken out immediately after decapitation and frozen in liquid nitrogen at -80°C for later use.
Neurological scores assessment and infarct volume measurement
The neurological function was assessed based on the five-point behavioral rating scale described in a previous report (21) in rats after MCAO for 24 hr. The cryopreserved brain tissues were taken out to slice into 5 pieces of serial coronal sections of 2 mm in thickness, which were immediately placed into 2% 2, 3, 5-triphenyltetrazolium chloride (TTC, Sigma) solution and stained for 30 min at room temperature and in darkness. Next, the sections were fixed for 24 hr in 4% paraformaldehyde solution at 4°C. The normal brain tissues should be stained red, while infarcted tissues stained white. The infarction area was measured with the image analysis software (Scion Image) and the infarct volume was calculated as follows: the cerebral infarct volume = the infarction area × thickness/2(22).
Quantitative real-time polymerase chain reaction (qRT-PCR)
The total RNA of tissues was extracted by following the instructions on the extraction kit (TakaRa, Japan). The concentration of the total RNA was detected with a NanoDrop-1000 spectrophotometer (NanoDrop Tech, Wilmington, DE). RNA samples had a ratio of A260/ A280 equal to or better than 1.8, indicating high purity. Based on the gene sequences published on the database Genbank, we used the software Primer 5.0 to design primers needed in this study (Table 1). The primers for PCR amplification were synthesized by Sangon Biotech (Shanghai) Co, Ltd. The reverse transcription PCR of total RNA was performed in accordance with the TaqMan MicroRNA Assay kit (Applied Biosystems; Life Technologies). PCR reaction conditions were as belows: pre-denaturation for 10 min at 95°C, followed by 35 cycles of denaturation for 30 sec at 94°C, 30 sec at 58°C, and 35 sec at 72°C, and finally terminating extending for 10 min at 2°C. U6 and GAPDH were taken as the internal reference genes for miR-103 and VEGF respectively. The relative expression level of target gene was calculated by using 2ΔΔCt (23).
Table 1.
Primer sequences for quantitative real-time polymerase chain reaction (qRT-PCR)
| Gene | Forward 5’-3’ | Reverse 5’-3’ |
|---|---|---|
| miR-103 | TGATGCTGGTGCTAGAAGT | TCTCCACAGAACAGGCAAG |
| U6 | CTTCGGCAGCACATATACTAAAAT | CGCTTCACGAATTTGCGTGTCAT |
| VEGF | AACTTTCTGCTGTCTTGGGT | TCTCGATTGGATGGCAGTA |
| GADPH | ATGACTCTACCCACGGCAAG | TACTCAGCACCAGCATCACC |
Western blotting
The tissue proteins extracted were determined for concentration by following the instructions on the BCA Kit (Beyotime, Beijing). After the addition of loading buffer, proteins were boiled at 95°C for 10 min and 30 μg samples were loaded in each well. Next, 10% polyacrylamide gel electrophoresis (PAGE) was applied to isolate proteins, which were transferred to polyvinylidene fluoride (PVDF) membrane and closed in 5% bovine serum albumin (BSA) for 1 hr at room temperature. Then, primary antibody for VEGF (diluted by 1:500) (Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) and GADPH (diluted by 1:1000) (Sigma-Aldrich Chemical Company, St Louis MO, USA) were added for overnight reaction at 4°C. Next day, the membrane was washed with TBS Tween 20 (TBST) for 3 times/5 min before adding corresponding secondary antibody for 1 hr of incubation. After that, the membrane was washed again for 3 times/5 min, followed by development using chemiluminescence method, and GAPDH was the loading control.
Dual-luciferase reporter assay
To predict the binding site of miR-103 and VEGF 3’untranslated region (UTR), we used miRNA bioinformatics target prediction software TargetScan (http://ww.targetscan.org/vert-61/). The VEGF 3’UTR promoter sequence containing binding site for miR-103 was synthesized and VEGF 3’UTR wild type (WT) and VEGF 3’UTR mutant type (MUT) plasmids were constructed. There were four groups in this experiment: miR-103 mimics+VEGF-WT (WT+mimics group), miR-103 mimics+VEGF-MUT (MUT+mimics group), miR-103 NC+VEGF-WT (WT+NC group) and miR-103 NC+VEGF-MUT (MUT+NC group). HEK293T cells (purchased from American Type Culture Collection, ATCC) were inoculatedontothe 48-well plate appropriately. When cell confluence reached 80%-90%, Lipofectamine™ 2000 (Invitrogen) was applied fortransfection. 48 hr after transfection, the dual luciferase reporter assay kit (Promega Corporation, Madison, WI, USA) was used to test results.
Vascular density measurement by fluorescein-labelled dextran (FITC-dextran)
The vascular density of brain tissues around ischemic cortex of rats was measured by using FITC-dextran. The experimental rats were anesthetized by 10% chloral hydrate and injected slowly through a femoral vein with FITC-dextran (1 ml, 50 mg/ml, dissolved in sterile 0.9% NaCl solution). And 10 min later, brain tissues were soaked in 4% paraformaldehyde solution for 24 hr at 4°C. Next, tissues were embedded with OCT (optimum cutting temperature) and sliced into sections of 100 μm in thickness at -20°C with a cryotome (Leica Microsystems, Inc., Buffalo Grove, IL, USA). At last, a Confocal Laser Scanning Microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA) was used to observe the vascular density of brain tissues around the ischemic cortex.
Establishment and grouping of OGD cell models
Human umbilical vein endothelial cells (HUVECs) (purchased form ATCC) were cultured in medium supplemented with newborn bovine serum at 37°C in an incubator with 5%CO2. Cells were classified into four groups: Normoxia group (Cells were cultured in a conventional CO2 incubator); OGD group (Cells were placed in glucose-free medium and cultured in an anaerobic 5% CO2, 93% N2 incubator for 12 hr); OGD +miR-103 inhibitor group (Cells were transfected with miR-103 inhibitor before 12 hr of incubation in glucose-oxygen deprivation medium); and OGD +miR-103 inhibitor + siVEGF group (Cells were co-transfected with miR-103 inhibitor and siVEGF (purchased from Shanghai GenePharma Co, Ltd.) before 12 hr of culturing in glucose-oxygen deprivation medium). Transfection of cells was performed by following the instructions of Lipofectamine™ 2000 (Invitrogen).
HUVEC tube-formation assay
Pre-cooled Matrigel (BD Biosciences, USA) was loaded onto the 24-well plate for solidification at 37°C in 30 min. Then, HUVECs were digested, counted, and adjusted to the cell density of 4×105 cells/ml. Next, 100 μl of cell suspension was put into the matrigel-coated 96-well plate and cells were cultured at 37°C for 24 hr in a CO2 incubator. The formation of tubes was observed under a phase contrast microscope (Olympus) and photographed randomly under 100 fold microscope. The tube lengths were analyzed using Image-Pro Plus software, version 5.0 (BD Biosciences).
Cell migration assay
The density of cell suspension was adjusted to 5×104/ml. Next, 2 ml cell suspension was added into the transwell, a 6-well plate was cultured for 12 hr in an incubator, and the basement membrane (Corning Company, USA) was taken out. After that, cotton swabs were used to wipe out the non-migrated cells from the transwell. Meanwhile, migrated cells were fixed for 15-20 min with 95% alcohol, stained for 10 min by hematoxylin, and observed under an optical microscope.
Statistical methods
All data were processed in the statistical software SPSS 21.0 (SPSS, Inc, Chicago, IL, USA). Data were presented by mean±standard deviation (x̄±s), which was analyzed with independent Student’s t-test between two groups, while among multiple groups were used by One-Way ANOVA and followed by Tukey post hoc tests for multiple comparisons. P<0.05 indicated that the difference was of statistical significance.
Results
Expression of miR-103 and VEGF in brain tissues of rats
According to the qRT-PCR and Western blotting, the miR-103 was down-regulated; whereas VEGF was up-regulated at the mRNA and protein expressions in the brain tissues from MCAO rats as compared to sham ones (all P<0.05). Meanwhile, the MCAO rats transfected with miR-103 mimic had an enhanced miR-103 and reduced VEGF (all P<0.05); however, rats in the MCAO+miR-103 inhibitor group showed completely opposite results (all P<0.05, Figure 1).
Figure 1.
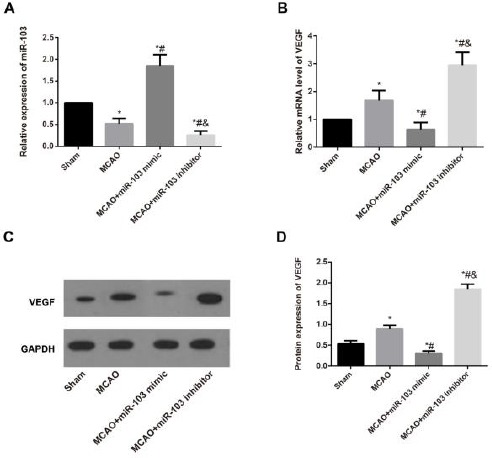
Expression of miR-103 and vascular endothelial growth factor (VEGF) detected by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting in the brain tissues of rats after middle cerebral artery occlusion (MCAO) in each group. A, The relative miR-103 expression in the brain tissues of rats after MCAO in each group measured by qRT-PCR; B-C, The relative mRNA level and protein expression of VEGF in the brain tissues of rats in each group detected by qRT-PCR and Western blotting; D. Comparison of VEGF protein expressions in rats from different groups. *, P<0.05 compared with Sham group; #, P<0.05 compared with MCAO group & P<0.05 compared with MCAO+miR-103 mimic group
Neurological scores assessment and infarct volume measurement
As shown in Figure 2, the neurological scores and the infarct volume were higher in the MCAO rats than the Sham ones (all P<0.05). In addition, the MCAO rats with the transfection of miR-103 mimic had an obviously elevation in neurological scores and infarct volume (all P<0.05), which were appreciably reduced in the MCAO rats transfected with miR-103 inhibitor (all P<0.05).
Figure 2.
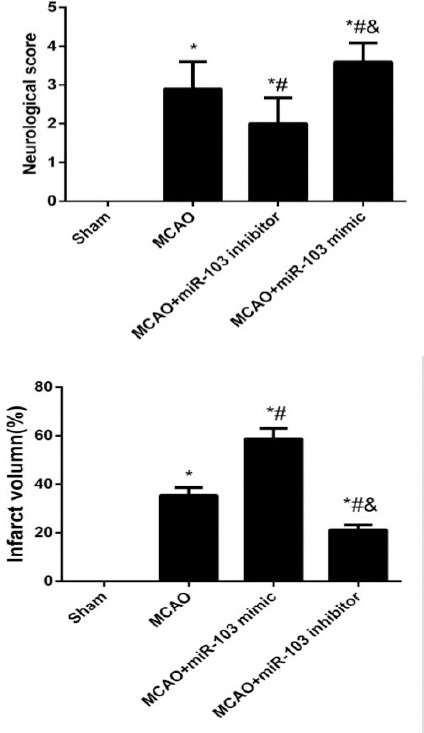
Comparison of the neurological scores and infarct volume of rats after middle cerebral artery occlusion (MCAO) in different groups. *P<0.05 compared with Sham group; #, P<0.05 compared with MCAO group; &, P<0.05 compared with MCAO+miR-103 mimic group
Vascular density measurement
The number of blood vessels and the vascular density from the rats in the MCAO and MCAO+miR-103 inhibitor groups were apparently elevated when compared with those in the Sham group (all P<0.05, Figure 3). Besides, with rats in the MCAO group as the baseline for comparison, the blood vessel number and the vascular density of rats were substantially and statistically higher in the MCAO+miR-103 inhibitor group, but significantly lower in the MCAO+miR-103 mimic group (all P<0.05).
Figure 3.
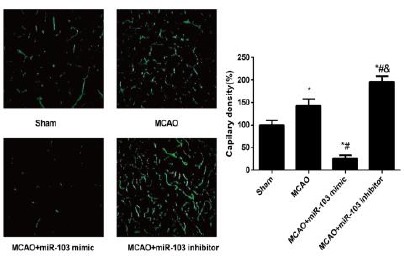
Vascular densities of rats in each group observed by a Confocal Laser Scanning Microscope. *P<0.05 compared with Sham group; #, P<0.05 compared with MCAO group; &, P<0.05 compared with MCAO+miR-103 mimic group
MiR-103 directly targets the 3’UTR of VEGF
The miRNAs target gene prediction website (http://www.microrna.org) was used to predict the target gene of miR-103, and found that miR-103 is complementary to the VEGF 3’-UTR region. As demonstrated by luciferase reporter gene assay, the relative activity of luciferase was remarkably reduced after the co-transfection of VEGF 3’UTR-WT and miR-103 mimic when compared with that of WT+NC group (P<0.05). On the other hand, no change in luciferase activity was observed in VEGF 3’UTR -MUT+miR-103 mimic and MUT+NC group systems (all P>0.05) (Figure 4), which suggested that VEGF was the target gene of miR-103.
Figure 4.
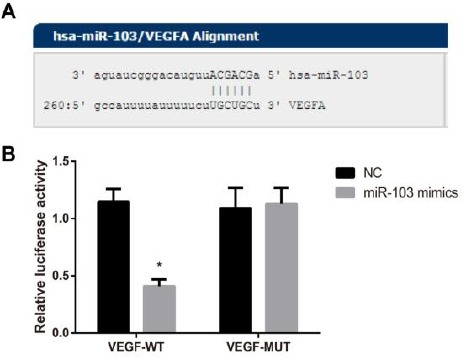
Vascular endothelial growth factor (VEGF) was confirmed to be the target gene of miR-103 by luciferase reporter gene assay. A, Putative miR-103 binding sites in the coding sequence region of VEGF 3’UTR; B, Luciferase activity analysis after con-transfection of VEGF 3’UTR-WT/-MUT plasmids with miR-103 mimic or miR-103 NC; *, P< 0.05 compared with NC group
Expression of miR-103 and VEGF in the OGD environment
We established the OGD model in vitro to simulate the cerebral infarction environment in vivo to detect the expressions of miR-103 and VEGF by qRT-PCR and Western blotting (Figure 5), and found that OGD-induced a significant reduction in miR-103 levels, but an elevation in the mRNA and protein expressions of VEGF in HUVECs (all P<0.05). Besides, HUVECs were transfected with miR-103 inhibitor and/or siVEGF for further observation. As compared to OGD group, expressions of miR-103 were declined apparently in the HUVECs from both OGD+miR-103 inhibitor and OGD+miR-103 inhibitor+siVEGF groups (all P<0.05). It is worthy of note that the mRNA and protein expression of VEGF was appreciably lower in HUVECs of the OGD +miR-103 inhibitor+siVEGF group than in cells of the OGD+miR-103 inhibitor group (all P<0.05).
Figure 5.

Expression of miR-103 and vascular endothelial growth factor (VEGF) in human umbilical vein endothelial cells (HUVECs) of each group in the oxygen-glucose deprivation (OGD) environment. A, The miR-103 expression in HUVECs of each group determined by qRT-PCR; B, The expression of VEGF mRNA in HUVECs of each group measured by qRT-PCR; C, The expression of VEGF protein in HUVECs of each group evaluated by Western blotting; D, Comparison of expression levels of VEGF protein in HUVECs of each group. *, P<0.05 compared with the Normoxia group; #, P<0.05 compared with the OGD group; &, P<0.05 compared with the OGD + miR-103 inhibitor group
MiR-103 regulates cell migration and tubule formation under OGD condition
As illustrated in Figure 6, the migrated cell number and the tube length were observed to be significantly increased in the OGD group in comparison with the Normoxia group (all P<0.05). In addition, as compared with the cells in the OGD group, the migrated cell number and the tube length were obviously enhanced in the HUVECs following the transfection of miR-103 inhibitor; in turn, this effect were markedly inhibited in the HUVECs transfected with miR-103 inhibitor and siVEGF (all P<0.05), which indicated that siVEGF can reverse the pro-angiogenesis effect of miR-103 inhibitor.
Figure 6.
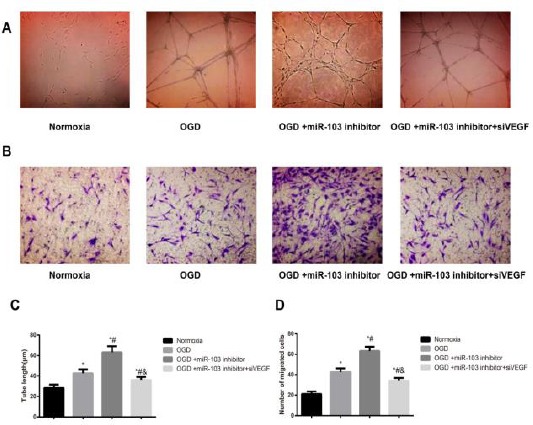
Cell migration and tube formation of human umbilical vein endothelial cells (HUVECs) in each group. A&C, The tube length in each group was measured by using a tube formation assay; B&D, The number of migrated cells were detected by Transwell assay; *, P<0.05 compared with the Normoxia group; #, P<0.05 compared with the OGD group; &, P<0.05 compared with the OGD+miR-103 inhibitor group
Discussion
We successfully constructed MCAO rat models in this study and found the down-regulation of miR-103 and the up-regulation of VEGF in the brain tissues of rats following ischemic brain injury. Besides, miR-103 and VEGF also showed similar expression patterns in the OGD-induced hypoxic HUVECs in vitro, further confirming our vivo experiment. As we know, miRNAs are abundant in the vascular system, which are believed to affect numerous complex physiological cellular processes, including angiogenesis (24). Also, a wide range of previous studies demonstrated that the expressions of various miRNAs altered following ischemia and hypoxia in certain organs, such as brain tissues (25, 26). For example, the miRNA Microarray analysis performed by Jeyaseelan et al. identified that miR-19b and miR-209 were found to be highly expressed, while miR-103 and miR-107 were decreased after MCAO in rats (27), which highlighted the important function of miRNAs in regulating the transcription and translation of mRNAs in the post-ischemic brain tissues. Besides, statistically down-regulated expression of miR-103/107 was observed in vivo and in vitro by Deng et al. in remodeled intrapulmonary vascular in hypoxia-induced pulmonary hypertension (28). On the other hand, VEGF, as a diffusible endothelial cell-specific mitogen and angiogenic factor, plays a regulatory role in physiological and pathological angiogenesis (29). In the cerebral ischemia, VEGF would be highly expressed to exert an acute neuroprotective effect to promote neurogenesis and cerebral angiogenesis, thereby alleviating the ischemic injury and improving neurological outcome (30). As such, numerous previous studies demonstrated that after cerebral ischemia, VEGF was elevated in ischemic injury areas, which would last for 3 to 7 days (18, 31). Therefore, the down-regulated miR-103 and up-regulated VEGF in ischemic stroke possibly be resulted from the natural self-defense mechanism of the body to restore the blood flow, as well as the oxygen and nutrient supplies to promote the angiogenesis to the ischemic brain tissues.
Angiogenesis is a physiological process that new blood vessels are formed from pre-existing vasculature after the proliferating, remodeling and sprouting of endothelial cells. In order to further investigate its regulation mechanism of miR-103 in the angiogenesis of ischemic stroke, miR-103 mimic and miR-103 inhibitor were given to MCAO rat models through intracerebroventricular injection, and found that miR-103 inhibitor can further increase the expression level of VEGF, enhance the neurological function score, reduce the infarct volume, and elevate the vascular density in brain tissues of MCAO rats. As indicated by Wang et al., knockdown of miR-103/107 could reduce necrosis in vitro and in vivo to regulate myocardial ischemia/reperfusion injury (32). Suppressing miR-103-1 in the study by Vinciguerra et al. could prevent NCX1 reduction after brain ischemia, thus ameliorating brain damage and neurological deficits (33), which added weight to the finding in our study. Notably, Yang and his group noted that exogenous VEGF administration to MCAO rats could induce angiogenesis in ischemic boundary, enhance the microvessel density and improve behavioral recovery of stroke rats (34). These findings suggested that inhibition of miR-103 may improve the outcome of stroke by up-regulating the expression of VEGF to promote angiogenesis.
In addition, we also established the OGD model of HUVECs in vitro to simulate the cerebral infarction environment in vivo and found that miR-103 inhibitor can elevate the expression of VEGF mRNA and protein in OGD-induced HUVECs, with the increased number of migrated cells and the length of tubes, to promote the generation of new vessels, which added weight to verify the results in vivo experiment. Similarly, miR-140-5p had an inhibitory effect on angiogenesis in the study by Sun et al., and inhibition of miR-140-5p can statistically improve the expression of its target gene VEGFA, thus enhancing the proliferation, invasion, tube formation of HUVECs, which could be partially related to the regulation of the target VEGFA (35). The finding by He et al. also revealed that the over-expression of miR-150 could specifically suppress the expression of VEGF, whereby reducing the vascular density of brain tissues around the infract area in MCAO rats and inhibiting the angiogenesis, proliferation and migration of BMVECs (36). By using gene chips, Zhao et al. found the obvious down-expression of many miRNAs in MACO rats, including miR-195, miR-103, miR-107, and highly-expressed miR-195 could inhibit angiogenesis by down-regulate its target gene VEGFA to block the migration and vascular formation of hypoxic HUVECs (3). Not surprisely, both the target gene prediction website and the luciferase assay in our study identified that VEGF was also to be the target gene of miR-103, and si-VEGF can reverse the pro-angiogenesis effect of miR-103 inhibitor.
Conclusion
Taken together, our study supported the notion that miR-103 was decreased but VEGF was increased in the rat MCAO models in vivo, and inhibition of miR-103 could effectively improve neurological scores and reduce infarct volume, as well as enhance the migration and tube formation of HUVECs in vitro, to promote angiogenesis by targeting VEGF. Therefore, miR-103 is expected to become a new therapeutic target for promoting angiogenesis after stroke.
Acknowledgment
This study was supported by a grant from The Department of Science and Technology of Hebei province (No. 07276101D-75).
References
- 1.Sun Y, Gui H, Li Q, Luo ZM, Zheng MJ, Duan JL, et al. MicroRNA-124 protects neurons against apoptosis in cerebral ischemic stroke. CNS Neurosci Ther. 2013;19:813–819. doi: 10.1111/cns.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei N, Xiao L, Xue R, Zhang D, Zhou J, Ren H, et al. MicroRNA-9 Mediates the Cell Apoptosis by Targeting Bcl2l11 in Ischemic Stroke. Mol Neurobiol. 2016;53:6809–6817. doi: 10.1007/s12035-015-9605-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhao WJ, Zhang HF, Su JY. Downregulation of microRNA-195 promotes angiogenesis induced by cerebral infarction via targeting VEGFA. Mol Med Rep. 2017;16:5434–5440. doi: 10.3892/mmr.2017.7230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Truelsen T, Bonita R. Epidemiological transition of stroke in China? Stroke. 2008;39:1653–1654. doi: 10.1161/STROKEAHA.107.510552. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Boada C, Domingues-Montanari S, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Chopp M. Neurorestorative treatment of stroke:cell and pharmacological approaches. NeuroRx. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages:the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21:1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Wei L, Keogh CL, Whitaker VR, Theus MH, Yu SP. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12:47–62. doi: 10.1016/j.pathophys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Prokopi M, Kousparou CA, Epenetos AA. The Secret Role of microRNAs in Cancer Stem Cell Development and Potential Therapy:A Notch-Pathway Approach. Front Oncol. 2014;4:389. doi: 10.3389/fonc.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascul Pharmacol. 2011;55:79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, et al. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem. 2012;370:45–51. doi: 10.1007/s11010-012-1396-6. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, He Q, Baral S, Mao L, Li Y, Jin H, et al. MicroRNA-493 regulates angiogenesis in a rat model of ischemic stroke by targeting MIF. FEBS J. 2016;283:1720–1733. doi: 10.1111/febs.13697. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Mao L, Gao Y, Baral S, Zhou Y, Hu B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep. 2015;5:13316. doi: 10.1038/srep13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peltier HJ, Latham GJ. Normalization of microRNA expression levels in quantitative RT-PCR assays:identification of suitable reference RNA targets in normal and cancerous human solid tissues. RNA. 2008;14:844–852. doi: 10.1261/rna.939908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research:a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol Genet Metab. 2007;91:209–217. doi: 10.1016/j.ymgme.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson R, Espinosa-Diez C, Kanner N, Chatterjee N, Ruhl R, Hipfinger C, et al. MicroRNA regulation of endothelial TREX1 reprograms the tumour microenvironment. Nat Commun. 2016;7:13597. doi: 10.1038/ncomms13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, et al. Hypoxia-responsive miRNAs target argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123:1057–1067. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist. 1996;39:199. 208-11. [PubMed] [Google Scholar]
- 20.Yang M, Wang S, Hao F, Li Y, Tang H, Shi X. NMR analysis of the rat neurochemical changes induced by middle cerebral artery occlusion. Talanta. 2012;88:136–144. doi: 10.1016/j.talanta.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Shi H, Sun BL, Zhang J, Lu S, Zhang P, Wang H, et al. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol Disord Drug Targets. 2013;12:381–391. doi: 10.2174/1871527311312030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zhang K, Zhao L, Tang J, Gao L, Wei Z. Anti-inflammatory effects of vinpocetine on the functional expression of nuclear factor-kappa B and tumor necrosis factor-alpha in a rat model of cerebral ischemia-reperfusion injury. Neurosci Lett. 2014;566:247–251. doi: 10.1016/j.neulet.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 23.Wang R, Tian S, Wang HB, Chu DP, Cao JL, Xia HF, et al. MiR-185 is involved in human breast carcinogenesis by targeting Vegfa. FEBS Lett. 2014;588:4438–4447. doi: 10.1016/j.febslet.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Pedrioli DM, Karpanen T, Dabouras V, Jurisic G, van de Hoek G, Shin JW, et al. miR-31 functions as a negative regulator of lymphatic vascular lineage-specific differentiation in vitro and vascular development in vivo. Mol Cell Biol. 2010;30:3620–3634. doi: 10.1128/MCB.00185-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang G, Zhou R, He X, Shi Z, Huang M, Yu J, et al. Expression levels of microRNA-199 and hypoxia-inducible factor-1 alpha in brain tissue of patients with intractable epilepsy. Int J Neurosci. 2016;126:326–334. doi: 10.3109/00207454.2014.994209. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Ji B, Cheng B, Chen J, Bai B. Neuroprotection of microRNA in neurological disorders (Review) Biomed Rep. 2014;2:611–619. doi: 10.3892/br.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 28.Deng B, Du J, Hu R, Wang AP, Wu WH, Hu CP, et al. MicroRNA-103/107 is involved in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells by targeting HIF-1beta. Life Sci. 2016;147:117–124. doi: 10.1016/j.lfs.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Kazemi-Lomedasht F, Behdani M, Pooshang Bagheri K, Habibi Anbouhi M, Abolhassani M, Khanahmad H, et al. Expression and purification of functional human vascular endothelial growth factor-a121;the most important angiogenesis factor. Adv Pharm Bull. 2014;4:323–328. doi: 10.5681/apb.2014.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margaritescu O, Pirici D, Margaritescu C. VEGF expression in human brain tissue after acute ischemic stroke. Rom J Morphol Embryol. 2011;52:1283–1292. [PubMed] [Google Scholar]
- 32.Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. 2015;117:352–363. doi: 10.1161/CIRCRESAHA.117.305781. [DOI] [PubMed] [Google Scholar]
- 33.Vinciguerra A, Formisano L, Cerullo P, Guida N, Cuomo O, Esposito A, et al. MicroRNA-103-1 selectively downregulates brain NCX1 and its inhibition by anti-miRNA ameliorates stroke damage and neurological deficits. Mol Ther. 2014;22:1829–1838. doi: 10.1038/mt.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JP, Liu HJ, Liu XF. VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg. 2010;23:149–155. doi: 10.3109/08941930903469482. [DOI] [PubMed] [Google Scholar]
- 35.Sun J, Tao S, Liu L, Guo D, Xia Z, Huang M. miR1405p regulates angiogenesis following ischemic stroke by targeting VEGFA. Mol Med Rep. 2016;13:4499–4505. doi: 10.3892/mmr.2016.5066. [DOI] [PubMed] [Google Scholar]
- 36.He QW, Li Q, Jin HJ, Zhi F, Suraj B, Zhu YY, et al. MiR-150 regulates poststroke cerebral angiogenesis via Vascular Endothelial Growth Factor in Rats. CNS Neurosci Ther. 2016;22:507–517. doi: 10.1111/cns.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]


