Abstract
Objective(s):
Diabetes is a metabolic syndrome which is associated with the worldwide major public health problems. There are many natural compounds from the sea-market, as a valuable aquatic source, along with the variety of health and therapeutic benefits. In the present research, with respect to the traditional and ethnic uses of Sargassum oligocystum algae for healing of some diseases which have similar metabolic mechanism to the diabetes, its anti-diabetic effects in animal model was proposed.
Materials and Methods:
The animals (rat) were divided into the normal control, diabetic control, positive control and, the test groups. The test groups were gavaged with oral doses of 150 and 300 mg/kg of algae hydroalcoholic extracts. After 30 days of intervention the serum glucose, cholesterol, triglyceride, HDLC, LDLC, insulin, insulin resistance, β-cells function and, the histopathology of pancreatic tissue were evaluated.
Results:
In animals that were fed with algae extracts a significant decrease in the fasting blood glucose, triglyceride and HOMA-IR and an increase in the HOMA-B with no significant impacts on the insulin, cholesterol and HDL were observed. Also, the histopathology evaluations in the groups which were treated with algae extract revealed the regeneration and reconstitution of damaged pancreatic β-cells.
Conclusion:
The results give evidence that, the S. oligocystum algae extract has a healing effect on diabetes which can be considered as a new research prospect for the natural therapy of diabetes.
Keywords: Cholesterol, HDL, HOMA-IR, HOMA-B, Insulin resistance, Sargassum oligocystum
Introduction
Although only a few natural products from the sea-market are available as clinical drugs, potentially sea organisms have remained as a large source of natural drugs and unique bioactive compounds. Due to the diverse chemical compounds in marine organisms, more attention has been paid to the potential of the nutritional and pharmaceuticals aspects of marine products. Diabetes mellitus, as one of the most important public health concerns is a worldwide metabolic disease with fast-increasing prevalence (1). It is predicted that, by the year 2025 the incidence of diabetics will reach to the 300 million (2). Also, it has been established that, the diabetes is outcome of some oxidative stress mechanisms (3). Free radicals, particularly, the reactive oxygen species (ROS) are involved in the pancreatic β-cell degeneration and failure by the mediation of mortal cytokines. Usually, some protective action of antioxidant compounds on the diabetes is mainly due to the reduction or inhibition of ROS synthesis (4).
There are many natural-based compounds with effective anti-diabetic activities (5). Among them, the marine brown algae species are known as a rich source of extractable bioactive compounds including pigments, fucoidans and polyphenols which have positive impacts on the diabetes (6). The genus Sargassum oligocystum (7) is a brown macroalga (Sargassaceae, Fucales, Phaeo-phyceae) that is native to the shallow waters and coral reefs of tropical seas and oceans. It is one of the indigenous Sargassaceae families in the northern coast of the Persian Gulf which has been used in ethnic coastal populations. Many potential benefits of the Sargassum species such as antioxidant, hepatoprotective, cholines-terase inhibiton, neuroprotective, anticancer, antiinfla-mmatory, antiviral, antibacterial, antifungal properties were reported (8-11). Therefore, with respect to the aforementioned therapeutic values for S. oligocystum
and the shared metabolic pathways of diabetes with some of the mentioned diseases, its antidiabetic effect was proposed in animal model.
Materials and Methods
Algae preparation and extraction
The brown algae, S. oligocystum, samples were collected from the coastal waters of the Bushehr province, Iran and authenticated by the Agriculture and Natural Resources Research Centre, Bushehr and, the voucher was deposited at the herbarium. After washing, the algae were dried in a circulating oven at 45°C in dark to obtain the constant weight. The dried algae was grinded into fine powder and sieved to obtain a uniform size. Then, 10% (W/V) Hydroalcoholic extract (E) of algae powder in 70% ethanol was obtained by continuous shaking at 40°C for 24 hr. After filtration, residues were discarded and the filtrate was concentrated under vacuum to eliminate the solvent using a rotary evaporator apparatus (Laborota 4000, Heidolph, Germany) and reserved in dark bottles.
Animal treatments
Fifty-four Wistar male rats (200-250 g) were purchased from the Laboratory Animals Cultivation and Breeding Center, Jundishapour University of Medical Sciences, Ahwaz, Iran. The animal handling and protocols were approved by the Animal Ethics Committee of Bushehr University of Medical Sciences (Permission No: Et/Anim/1395.66). For adaptation purposes, the animals were housed for a week under standard conditions in the animal house (22°C, 60% humidity, and 12 hr light/ dark cycle) and were free access to diet and water. Then, the animals were randomized into the normal control, positive control, diabetic control and, the test groups (containing 9 rats in each). The positive control was fed by gavages a 14 mg/kg dose of metformin and the test groups were fed with doses of 150 and 300 mg/kg per animal body weight of algae hydroalcoholic extracts (E-150 and E-300) over the 30 days of intervention.
Diabetes inducing
For experimental diabetes inducing, an IP injection of 1.2% streptozotocin (Enzo Life Sciences, USA), in normal saline at a single dose of 60 mg/kg of body weight of animals was used (12). After 12 hours fasting the blood samples were taken from the tail vein to confirm the diabetes induction on the third day of post-streptozotocin injection. The animals with fasting blood glucose of 12 mM/l or above were considered to be hyperglycemic and included in the next experiments.
Blood and tissue sampling
After 30 days of intervention, the animals were kept fasting for 12 hr and the blood samples were taken from the tail after an intramuscular injection of 50 mg/kg of anesthetizing mixture (containing 10% ketamine and 2% xylazine) (13). Blood samples were centrifuged at 3000× rpm for 10 min and the serum was taken for insulin, glucose, lipids and lipoproteins assays. Also, for evaluation of morphologic and morphometric parameters of β-cells, the pancreatic sections were sampled and fixed in 10% v/v formalin in 0.01 M phosphate buffered saline. Then, the samples were dehydrated in alcohol, embedded in paraffin and 3 µm sections were prepared. The tissue sections were stained with hematoxylin and eosin (H&E) and, the microscopic images were taken using a light microscope equipped with a digital camera (Moticam, model A-352, Netherlands & China) and the morphometric analysis performed by the Image tool version 3, software.
Biochemical assays
The blood glucose concentration was measured according to the glucose oxidase method (14) and serum insulin according to the protocol of the related rat insulin ELISA kit (Glory Science Co Ltd, China). The total serum cholesterol, triglyceride, and HDLC (high density-lipoprotein cholesterol) were measured according to the recommended instructions of the related kits (Parsazemoon Co, Tehran, Iran) using an autoanalyzer instrument (Vital Scientific, Spankeren, Netherlands). The LDLC (low density-lipoprotein cholesterol) concentration was calculated by the Friedewald equation (15).
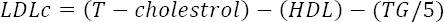
The LDLc and T- cholesterol are LDL- cholesterol and total cholesterol respectively.
Also, the insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) by the following equation (16):
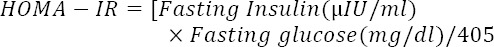
The steady state of pancreatic β-cell function was measured by calculating the homeostasis model assessment of β- cells (HOMA-B) using the following equation (17):
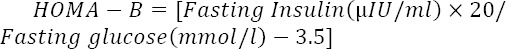
Statistical analysis
The biochemical data were analyzed using One- way ANOVA followed by the Tukey’s post-hoc test. The pancreatic morphometric data of pancreatic islets were analyzed by One-way ANOVA followed by Dunnett’s test. Data were expressed as median±SEM (median ± standard error of the mean) and statistically, the P-value ≤0.05 was considered as significant. For normalization of data which obtained from HOMA-B calculations, the Ln (HOMA-B) was expressed.
Results
In the positive control (fed with 14 mg/kg of metformin) and the test groups (fed with 150 and 300 mg/kg doses of algae extract), the serum glucose and HOMA-IR were reduced but, an increase in the HOMA-B level was observed when compared to the control groups. (Table 1).
Table 1.
The serum insulin, glucose, HOMA-IR and HOMA-B of diabetic rats after 30 days of intervention
| Animal groups | LDLc (mg/dl) | HDLc (mg/l) | Cholesterol (mg/l) | Triglyceride (mg/dl) |
|---|---|---|---|---|
| Normal control | 14.33±1.8 | 30.33±2.67 | 50.83±4.78 | 39.83±2.71 |
| Diabetic control | 22.83±4.06 | 41.18±4.94 | 69.16±13.69 | 65.4±4.30* |
| Positive control | 19.1±4.89 | 35.62±6.13 | 65.25±13.46 | 43.5 ±10.01** |
| E-150 | 21.5±1.96 | 41.87±4.41 | 71.5±8.37 | 47.66±5.89** |
| E-300 | 20.12±2.62 | 44.68±6.28 | 70.55±7.58 | 45.4±4.41** |
E-150, E-300; the test groups which were fed with algae hydroalcoholic extracts at doses of 150 and 300 mg/kg of animal body weight respectively. Positive control, the group which was fed with 14mg/kg dose of metformin; HOMA-IR, the homeostasis model assessment of insulin resistance. HOMA-B, the homeostasis model assessment of β-cell function. *Significance, when compared to the normal control group. ** Significance, when compared to the diabetic control group. Ln (HOMA.B), the natural Logarithms of HOMA-B index; (P-value ≤0.05)
The serum triglyceride level in the test groups (which were fed with 150 and 300 mg/kg doses of algae extracts) and in positive control (fed with 14 mg/kg of metformin) were decreased compared to the control groups, while no differences in the LDL and cholesterol levels were observed (Table 2).
Table 2.
The Lipid profiles of diabetic rats after 30 days of intervention
| Animal groups | LDLc (mg/dl) | HDLc (mg/dl) | Cholesterol (mg/dl) | Triglyceride (mg/dl) |
|---|---|---|---|---|
| Normal control | 14.33±1.8 | 30.33±2.67 | 50.83±4.78 | 39.83±2.71 |
| Diabetic control | 22.83±4.06 | 41.18±4.94 | 69.16±13.69 | 65.4±4.30* |
| Positive control | 19.1±4.89 | 35.62±6.13 | 65.25±13.46 | 43.5 ±10.01** |
| E-150 | 21.5±1.96 | 41.87±4.41 | 71.5±8.37 | 47.66±5.89** |
| E-300 | 20.12±2.62 | 44.68±6.28 | 70.55±7.58 | 45.4±4.41** |
E-150, E-300; the test groups which were fed with algae hydroalcoholic extract at doses of 150 and 300 mg/kg of animal body weight respectively. Positive control, the group which was fed with 14mg/kg dose of metformin.
Significant, when compared to the normal control group.
Significance, when compared to the diabetic control group; (P-value ≤0.05)
For histopathology evaluations the morphologic parameters of pancreatic tissue, exocrine (Serous acinus) and the islets of the Langerhans were considered (Figure 1). In the diabetic control group, the morphology of exocrine tissue was normal, though the islets of the Langerhans were degenerated. By evaluation of the pancreatic sections in the test groups, a significant regeneration in the β-cells after 30 days of interventions was observed. The 300 mg/kg of hydroalcoholic extract showed a significant positive effect on the regeneration of β-cells.
Figure 1.
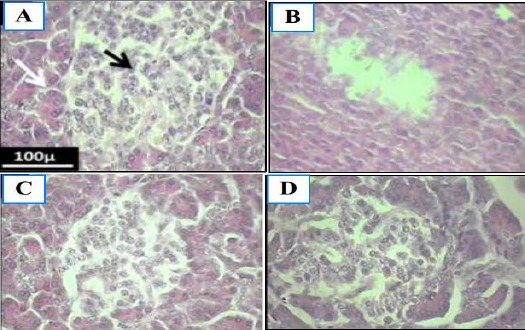
The photomicrograph of pancreatic tissue in diabetic rats. A and B, the normal control and diabetic control groups respectively. C and D, the test groups which were fed with 150 and 300 mg/kg of algae hydroalcoholic extracts respectively. Black arrows, the exocrine sinuses; White arrows, the Langerhans islets (H & E Staining; 400 ×)
The quantitative parameters such as pancreatic area and the percent of β-cells regeneration were assayed on 30th day of animal treatments. It was found, an increase in the islets area and the percent of regenerated pancreas in the test groups (Table 3).
Table 3.
The pancreatic tissue morphometric of diabetic rats after 30 days of intervention
| Animal groups | Morphometric | |
|---|---|---|
| Islets area (μm2) | % β-Cells regeneration | |
| Normal control | 45236 ±25.23 | -- |
| Diabetic control | 15913 ±17.13 | 8% ±1.03 |
| E-150 | 34125 ±48.02* | 80% ±4.07* |
| E-300 | 44125 ±52.31* | 82% ±7.09* |
E-150, E-300; the groups which were fed with 150 and 300 mg/kg doses of animal body weight of algae hydroalcoholic extracts respectively.
Significant, when compared to the diabetic control group; (P-value ≤0.05)
Discussion
Diabetes mellitus is a metabolic syndrome that is diagnosed by hyperglycemia, due to the impaired insulin secretion, insulin activity or both (17). The induced diabetes mellitus in rats by streptozotocin is associated with adverse changes in plasma lipids and lipoproteins profile (18). It was reported that, some marine algae species, have anti-inflammatory (19) and antidiabetic effects (20). Therefore, in the present study, the S. oligocystum hydroalcoholic effects on sugar, lipid profiles and histopathology of pancreatic tissue in diabetic rats was proposed. The results showed, the algae hydroalcoholic extracts caused a decrease in the blood sugar and the HOMA-IR, while increasing in the HOMA-B. The fucoidans, which many researchers have reported its effects on the inhibition of adipogenesis in 3T3-L1 cells, are sulfated hetero-polysaccharides that have been extracted from some brown algae (21) that regulates the glucose homeostasis (22) and, the lipids profiles in animals (23). Also, it was reported that fucoidans, polyphenols and flavonoids from a variety of other Sargassum species, including S. thumbergii, S. honeri, S. ringgoldianum, S. siliquastrum, S. graminifoliumand S. kjellmanianum, have an anti α-glucosidal activity which can be used in diabetes treatment (24). Accordingly, in our study a significant (P-value ≤0.05) decrease in serum glucose, triglyceride and HOMA-IR were observed in diabetic rats which were fed with S. oligocystum extracts. This may be related to the presence of such bioactive materials in S. oligocystum extract. Also, the anti-obesity effect of brown algae, P. binghamiae, was reported (25). The long-term effects of Lamina japonica on the obesity, insulin sensitivity, chronic inflammation of adipose tissue, reducing the adipose tissue inflammation and insulin resistance in high-fat diet mice were reported (26). As we know, the diabetes can be induced by oxidizing agents, such as free radicals and reactive oxygen species (ROS), leading to the lipid peroxidation, DNA break, and protein oxidation (27). Therefore, in our study, the decrease in triglyceride level may be related to the presence of one or more of the aforementioned anti-inflammatory mechanisms. On the other hand, the antioxidant compounds which may occur in S. oligocystum extract (28), can inhibit the production or scavenge such reactive species and induce the anti-diabetic properties. In the present study, the histopathology results of pancreas and, increment in the HOMA-B index indicates a positive impact of S. oligocystum on the proliferation of pancreatic β-cells in diabetic rats. The bioactive compounds of S. oligocystum, depending on the species, harvesting time, and especially, the extraction procedure, can be varied. For example, it was reported, the algae hydroalcoholic extracts contains about 70-80% of polyphenol compounds, but, the water extract was poor (25). In fact, many algae extracts in addition to having the protein, vitamins, minerals, fibers, and unsaturated fatty acids, were identified as a rich source of polyphenols and polysaccharides (29) with useful antioxidant and anti-diabetic properties. According to our results, it can be predicted that the protective effect of S. oligocystum extract on the regeneration of β-cells is mainly due to its high polyphenols contents. As shown in the photomicrographs of pancreatic sections, the islet cells were degenerated following exposure to the streptozotocin, while they were recovered and proliferated after treatment with algae extracts during 30 days of intervention. This proliferative effect was more at 300 mg/kg dose of S. oligocystum hydroalcoholic extract. In addition, quantitative results, such as Islets area and Islet cells number have shown the positive impacts of S. oligocystum algae extract on diabetic rats (Table 3).
Conclusion
The brown macroalgae, S. oligocystum, improves the diabetic by reducing the insulin resistance, decreasing glucose concentration and regeneration of pancreatic damaged β-cells. Therefore it can be considered as a subject for further research.
Acknowledgment
The authors wish to thank the driving team of Persian Gulf Marine Biotechnology Research Center and, the facilities offered by Vice-Chancellery for Research of Bushehr University of Medical Sciences, Bushehr, Iran are greatly appreciated.
References
- 1.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus:a review of current trends. Oman Med J. 2012;27:269–273. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes 1995–2025:prevalence, numerical estimates, and projections. Diabetes care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Desco M-C, Asensi M, Márquez R, Martínez-Valls J, Vento M, Pallardó FV, et al. Xanthine oxidase is involved in free radical production in type 1 diabetes. Diabetes. 2002;51:1118–1124. doi: 10.2337/diabetes.51.4.1118. [DOI] [PubMed] [Google Scholar]
- 4.Tabatabaie T, Vasquez-Weldon A, Moore DR, Kotake Y. Free radicals and the pathogenesis of type 1 diabetes. Diabetes. 2003;52:1994–1999. doi: 10.2337/diabetes.52.8.1994. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari P, Rahuja N, Kumar R, Lakshmi V, Srivastava MN, Agarwal SC, et al. Search for antihyperglycemic activity in few marine flora and fauna. Indian. J Sci Technol. 2008;1:1–5. [Google Scholar]
- 6.Lee S-H, Min K-H, Han J-S, Lee D-H, Park D-B, Jung W-K, et al. Effects of brown alga Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem Toxicol. 2012;50:575–582. doi: 10.1016/j.fct.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Montagne C. Plantes cellulaires. In 'Voyage au Pôle Sud et dans l'Océanie surles corvettes l'Astrolabe et la Zelée... pendant les années. 1837–1838–1839–1840, sous le commandement de MJ Dumont d'Urville. Botanique. 1845;1:20. [Google Scholar]
- 8.Yende SR, Harle UN, Chaugule BB. Therapeutic potential and health benefits of Sargassum species. Pharmacogn Rev. 2014;8:1–7. doi: 10.4103/0973-7847.125514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zandi K, Ahmadzadeh S, Tajbakhsh S, Rastian Z, Yousefi F, Farshadpour F, et al. Anticancer activity of Sargassum oligocystum water extract against human cancer cell lines. Eur Rev Med Pharmacol Sci. 2010;14:669–673. [PubMed] [Google Scholar]
- 10.Patel S. Therapeutic importance of sulfated polysaccharides from seaweeds:updating the recent findings. 3 Biotech. 2012;2:171–185. [Google Scholar]
- 11.Sarvestani FS, Esmaeili H, Ramavandi B. Modification of Sargassum angustifolium by molybdate during a facile cultivation for high-rate phosphate removal from wastewater:structural characterization and adsorptive behavior. 3 Biotech. 2016;6:251. doi: 10.1007/s13205-016-0570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasconcelos C, Maranhão H, Batista T, Carneiro E, Ferreira F, Costa J, et al. Hypoglycaemic activity and molecular mechanisms of Caesalpinia ferrea Martius bark extract on streptozotocin-induced diabetes in Wistar rats. J Ethnopharmacol. 2011;137:1533–1541. doi: 10.1016/j.jep.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Kawai S, Takagi Y, Kaneko S, Kurosawa T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp Anim. 2011;60:481–487. doi: 10.1538/expanim.60.481. [DOI] [PubMed] [Google Scholar]
- 14.Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–161. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Song Y, Manson JE, Tinker L, Howard BV, Kuller LH, Nathan L, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women the Women's Health Initiative Observational Study. Diabetes care. 2007;30:1747–1752. doi: 10.2337/dc07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franz MJ. Medical nutrition therapy for diabetes mellitus and hypoglycemia of nondiabetic origin. Krause's food. 2008 [Google Scholar]
- 18.Choi JS, Yokozawa T, Oura H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med. 1991;57:208–211. doi: 10.1055/s-2006-960075. [DOI] [PubMed] [Google Scholar]
- 19.Han YR, Ali M, Woo MH, Jung HA, Choi JS. Anti-Diabetic and anti-inflammatory potential of the edible brown alga Hizikia Fusiformis. J Food Biochem. 2015;39:417–428. [Google Scholar]
- 20.Kang M-C, Wijesinghe W, Lee S-H, Kang S-M, Ko S-C, Yang X, et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type ІІdiabetes in db/db mouse model. Food Chem Toxicol. 2013;53:294–298. doi: 10.1016/j.fct.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Kim K-J, Lee O-H, Lee B-Y. Fucoidan, a sulfated polysaccharide, inhibits adipogenesis through the mitogen-activated protein kinase pathway in 3T3-L1 preadipocytes. Life Sci. 2010;86:791–797. doi: 10.1016/j.lfs.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Kim K-J, Yoon K-Y, Lee B-Y. Fucoidan regulate blood glucose homeostasis in C57BL/KSJ m+/+db and C57BL/KSJ db/db mice. Fitoterapia. 2012;83:1105–1109. doi: 10.1016/j.fitote.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Thomes P, Rajendran M, Pasanban B, Rengasamy R. Cardioprotective activity of Cladosiphon okamuranus fucoidan against isoproterenol induced myocardial infarction in rats. Phytomedicine. 2010;18:52–57. doi: 10.1016/j.phymed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Shan X, Liu X, Hao J, Cai C, Fan F, Dun Y, et al. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int J Biol Macromol. 2016;82:249–255. doi: 10.1016/j.ijbiomac.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Kang S-I, Kim M-H, Shin H-S, Kim H-M, Hong Y-S, Park J-G, et al. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 preadipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J Nutr Biochem. 2010;21:1251–1257. doi: 10.1016/j.jnutbio.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Oh J-H, Kim J, Lee Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr Res Pract. 2016;10:42–48. doi: 10.4162/nrp.2016.10.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma B, Salunke R, Balomajumder C, Daniel S, Roy P. Anti-diabetic potential of alkaloid rich fraction from Capparis decidua on diabetic mice. J Ethnopharmacol. 2010;127:457–462. doi: 10.1016/j.jep.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Duan X-J, Zhang W-W, Li X-M, Wang B-G. Evaluation of antioxidant property of extract and fractions obtained from a red algae Polysiphonia urceolata. Food Chem. 2006;95:37–43. [Google Scholar]
- 29.MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev. 2007;65:535–543. doi: 10.1301/nr.2007.dec.535-543. [DOI] [PubMed] [Google Scholar]


