Abstract
Recently, a novel species of the genus Borreliawas identified in Bothriocroton concolor and Ixodes holocyclus ticks from echidnas. Analyses of 16S rRNA and flaB genes identified three closely related genotypes of this bacterium (Borrelia sp. Aus A-C) that were unique and distinct from previously described borreliae. Phylogenetic analyses of flaB (763 bp), groEL (1537 bp), gyrB (1702 bp) and glpQ (874 bp) gene sequences and concatenated sequences (3585 bp) of three gene loci (16S rRNA, flaB and gyrB) were consistent with previous findings and confirm that this novel species of the genus Borrelia is more closely related to, yet distinct from, the Reptile-associated (REP) and Relapsing Fever (RF) groups. At the flaB locus, genotypes A, B and C shared the highest percentage sequence similarities (87.9, 88 and 87.9 %, respectively) with B.orrelia turcica (REP), whereas at the groEL and gyrB loci, these genotypes were most similar (88.2–89.4 %) to B.orrelia hermsii (RF). At the glpQ locus, genotypes A and B were most similar (85.7 and 85.4 % respectively) to Borrelia sp. Tortoise14H1 (REP). The presence of the glpQ gene, which is absent in the Lyme Borreliosis group spirochaetes, further emphasises that the novel species of the genus Borrelia characterized in the present study does not belong to this group. Phylogenetic analyses at multiple loci produced consistent topographies revealing the monophyletic grouping of this bacterium, therefore providing strong support for its species status. We propose the name ‘Candidatus Borrelia tachyglossi’, and hypothesize that this species of the genus Borrelia may be endemic to Australia. The pathogenic potential of this bacterium is not yet known.
Keywords: Australian ticks, Candidatus Borrelia tachyglossi, Spirochaetaceae, Bothriocroton concolor, Echidna
The family Spirochaetaceae is classified under the order Spirochaetales, belonging to the phylum Spirochaetes. This family consists of genera that are of concern to human health, such as Borrelia and Treponema [1], with common pathogenic species including ‘Treponema pallidum subsp. pallidum', the causative agent of syphilis worldwide [2], and ‘Treponema pallidum subsp. pertenue', the bacterium responsible for yaws [3]. The genus Borrelia is a member of the family Spirochaetaceae and through convention is divided into three major clades: Lyme disease/Borreliosis (LB) caused by members of the Borrelia burgdorferi sensu lato complex, the Relapsing Fever (RF) borreliae and the Reptile-associated (REP) borreliae [4]. The LB borreliae currently comprise over 20 recognized species including the primary Northern hemisphere Lyme-disease-causing agents Borrelia afzelii, Borrelia bavariensis, Borrelia burgdorferi sensu stricto, and Borrelia garinii, along with a newly described genospecies ‘Candidatus Borrelia mayonii’ that causes LB in the upper Midwestern USA [5–8]. Members of the LB group are vectored by hard ticks (family Ixodidae), with the pathogenic species commonly transmitted to humans and other animals by ticks within the Ixodes ricinus complex: Ixodes ricinus in Europe, Ixodes persulcatus in Europe and Asia, and Ixodes pacificus and Ixodes scapularis in USA [7]. These pathogenic spirochaetes are dependent on wildlife, particularly rodents and birds, which act as asymptomatic reservoir hosts that maintain their life cycles and transmission [9].
Spirochaetes within the RF group have been reported throughout a number of continents, including Africa [10], Eurasia [11] and North America [12]. These borreliae, in contrast to the LB group, are generally transmitted by soft ticks (family Argasidae), with the exceptions of Borrelia miyamotoi identified in I. persulcatus [13], I. ricinus [14], I. pacificus [15] and I. scapularis [16]; ‘Borrelia lonestari' in Amblyomma americanum [17]; Borrelia theileri in Rhipicephalus microplus [18]; and ‘Candidatus Borrelia texasensis’ in Dermacentor variabilis [19].
The third major clade of this genus, the REP borreliae, was identified after the discovery of Borrelia turcica in Hyalomma aegyptium ticks collected from tortoises in Turkey [20, 21], followed by subsequent addition of REP-related species of the genus Borrelia identified in various reptiles [4, 22]. While the LB and RF spirochaetes consist of zoonotic pathogens and are of significant public health concern in many countries, the pathogenicity and zoonotic potential of the REP group are not yet known.
Although LB borreliae have never been identified in Australian ticks, wildlife or people [23], two RF borreliae are recognized: B. theileri and Borrelia anserina that are transmitted by Rhipicephalus (Boophilus) australis [24, 25] and Argas persicus [26, 27], respectively. In addition, ‘Borrelia queenslandica' was the first species of the genus Borrelia to be reported from native long-haired rats, Rattus villosissimus, in north-west Queensland, Australia. While the soft tick, Ornithodorus gurneyi, was considered to be the vector of this species due to its presence in the region, transmission experiments were not successful, and molecular characterization was never conducted to reliably identify the species of the genus Borrelia [28, 29].
Sequence analysis of multiple loci offers the advantage over morphological characterization of being highly discriminatory, therefore serving as a reliable method for accurate identification, characterization and population, and epidemiological analyses in numerous bacterial studies [30, 31]. This technique was first used on Borrelia burgdorferi in 2008 [32] and has become an increasingly common technique for taxonomic and epidemiological studies of this genus [33–36].
Recently, a novel species of the genus Borrelia was detected in a number of echidna ticks, Bothriocroton concolor, collected [37]. An additional representative was detected in an Ixodes holocyclus tick [38]. Based on the 16S rRNA (1097 bp) and flagellin (flaB, 400 bp) gene phylogenetic analyses, this species of the genus Borrelia formed a distinct clade from other well-described borreliae, indicating this organism, designated ‘Borrelia sp. Aus’, to be unique [37]. In the present study, we conducted sequence analyses of the flaB, groEL, glpQ and gyrB genes, in addition to the 16S rRNA and short flaB loci reported previously [37], to study the relationship of this novel species to other of the genus Borrelia. Phylogenetic analysis confirmed its species status, and we hereby propose to designate this species as ‘Candidatus Borrelia tachyglossi’.
This study was conducted under the compliance of the Australian Code for the Responsible Conduct of Research, 2007 and Australian Code for the Care and Use of Animals for Scientific Purposes, 2013. Tick collection was carried out opportunistically with the approval from the Murdoch University Animal Ethics Committee.
Genomic DNA was extracted previously from 97 Bothriocroton concolor ticks, with 38 (39 %) ticks testing positive at the Borrelia-specific flaB locus [37]. These 38 Borrelia-positive ticks were included in this study. Nested- and hemi-nested PCRs were conducted using primers targeting the housekeeping genes flaB (763 bp), groEL (1537 bp), glpQ (874 bp) and gyrB (1702 bp) (Table S1, available in the online Supplementary Material). Primers were designed to amplify short fragments of each gene with overlapping regions in order to obtain maximum coverage of the genes analysed for accurate characterization. PCR cycling conditions were as described by Loh et al. [37] (see Table S1 for respective annealing temperatures), with the exception of groEL primers: initial denaturation at 95 °C for 5 mins, 35 cycles of denaturation at 95 °C for 30 s, annealing at 48 °C for 40 s, extension at 72 °C for 2 mins, and a final extension at 72 °C for 7 mins. Amplification products of the targeted DNA products were electrophoresed in 1–2 % agarose gel with blue light using safe (Invitrogen), and positive samples were purified and sent for Sanger sequencing.
DNA sequences generated at flaB, groEL, glpQ and gyrB loci were aligned and analysed together with sequences representing species of the genus Borrelia retrieved from GenBank. All sequences were aligned using mafft v7.017 [39] and then refined using muscle [40]. The best-fit model for each locus was assessed using mega6 [41] and was selected based on the Bayesian Information Criterion (BIC). Bayesian phylogenetic reconstructions using sequence alignments of all four loci were generated using MrBayes 3.2.6 [42], and concatenated phylogenetic reconstructions using concatenated sequence alignments were produced using the CIPRES Science Gateway V.3.3 [43]. GTR and HKY substitution models were selected, with gamma categories of five, MCMC length of 1 100 000, burn-in length of 10 000 and subsampling frequency of 200.
In the present study, Borrelia-specific flaB (763 bp), groEL (1537 bp), gyrB (1702 bp) and glpQ (874 bp) DNA sequences were successfully amplified and sequenced from 38 Borrelia-positive Bothriocroton concolor ticks described by Loh et al. [37]. Thirty samples were positive for all flaB fragments; 24 samples were positive for all glpQ fragments; 13 samples were positive for all groEL fragments; and 10 samples were positive for all gyrB fragments.
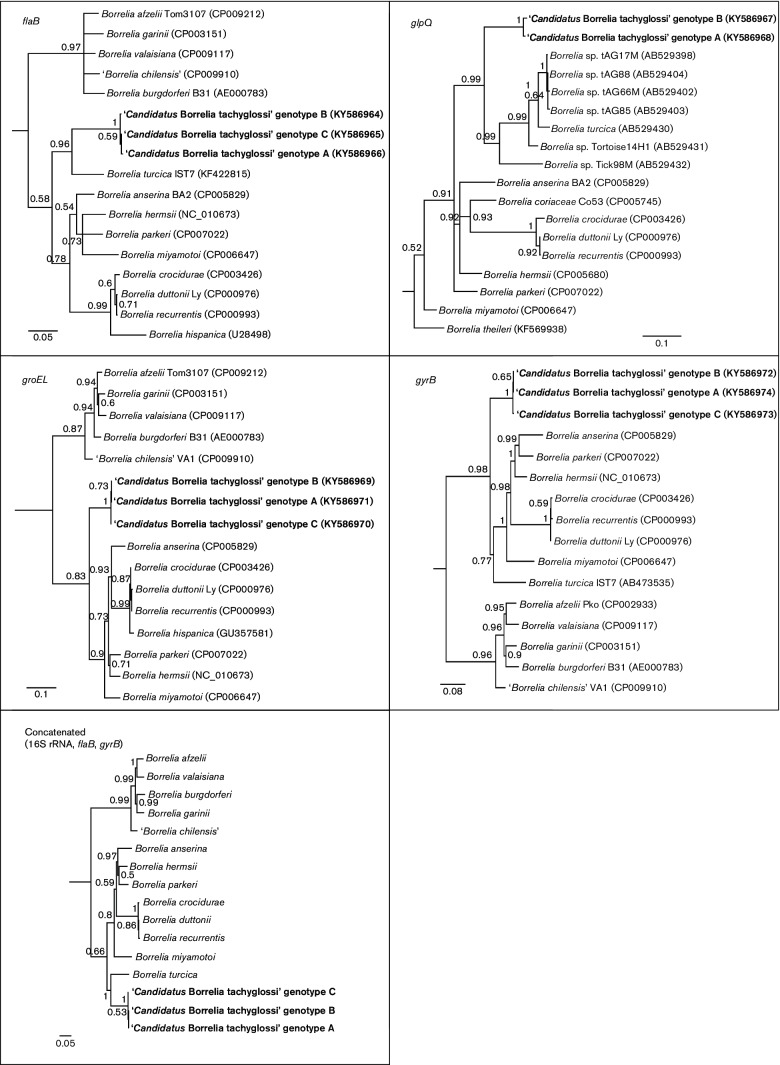
Previously, three closely related genotypes were distinguished in the Bothriocroton concolor ticks using the 16S rRNA gene sequences, tentatively given the designations Borrelia sp. Aus A, Borrelia sp. Aus B and Borrelia sp. Aus C [37]. However, in the present study, these genotypes are referred as ‘Candidatus Borrelia tachyglossi’ genotypes A, B and C, respectively. At the flaB locus, genotypes A, B and C consisted of nine, three and five identical samples, respectively. The flaB gene alignment (787 bp) between ‘Candidatus Borrelia tachyglossi’ genotypes and other described species of the genus Borrelia showed that the novel genotypes shared highest percentage sequence identities with Borrelia turcica from the REP group (87.9–88 %); similarities to the LB Borrelia group ranged from 82.1–83.2 %; with the least similarity to Borrelia hispanica from the RF group (79.8–80 %). The percentage identities within the ‘Candidatus Borrelia tachyglossi’ genotypes ranged from 99.6–99.9 %. The percentage nucleotide identities between ‘Candidatus Borrelia tachyglossi’ genotypes and Borreliaturcica (87.9–88 %) were higher than that between Borreliaturcica and Borrelia hermsii (86.7 %). In contrast, the percentage nucleotide identities between ‘Candidatus Borrelia tachyglossi’ genotypes and Borreliahermsii (84.6–84.8 %) were lower than that between Borreliaturcica and Borreliahermsii (86.7 %) (Table S2). Phylogenetic analyses of the flaB gene locus showed that the ‘Candidatus Borrelia tachyglossi’ genotypes clustered most closely with Borreliaturcica with a high posterior probability (Fig. 1).
Fig. 1.
Phylogenetic reconstructions based on flaB, groEL, glpQ and gyrB gene sequences, and concatenated gene sequences of ‘Candidatus Borrelia tachyglossi’ genotypes A, B, and C identified in Bothriocroton concolor ticks from echidnas. Brachyspirapilosicoli (AY241832), Treponema pallidum (NZ_CP010566), Escherichia coli (X56907) and Spirochaeta lutea (JNUP01000064) were used as outgroups for each gene, respectively. Sequences determined in this study are indicated in bold type. Node labels represent posterior probabilities. Bars, substitutions per nucleotide position.
At the glpQ locus, genotypes A and B consisted of eight and two identical samples respectively. The amplification of this gene was not successful for genotype C. The glpQ nucleotide alignment (947 bp) between ‘Candidatus Borrelia tachyglossi’ genotypes exhibited 83.9–85.7 % similarity with the REP species of the genus Borrelia and lower similarities (80.6–84.8 %) with the RF species of the genus Borrelia. The percentage similarity within ‘Candidatus Borrelia tachyglossi’ genotypes was 98.6 %. Phylogenetic analysis confirmed the closer relationship of the ‘Candidatus Borrelia tachyglossi’ genotypes from the present study with the REP Borrelia group (100 % bootstrap support) (Fig. 1). The percentage nucleotide identities between ‘Candidatus Borrelia tachyglossi’ genotypes and Borrelia turcica (84.1–84.2 %) and Borrelia coriaceae (RF) (84.1–84.8 %) were higher than that between Borrelia turcica and Borrelia coriaceae (83.3 %) (Table S3).
At the groEL locus, ‘Candidatus Borrelia tachyglossi’ genotypes A and B were identical, and both shared 99.9 % similarities with ‘Candidatus Borrelia tachyglossi’ genotype C. Nucleotide alignment (1540 bp) between ‘Candidatus Borrelia tachyglossi’ genotypes and other described species of the genus Borrelia showed that the novel isolates had the least similarity with the LB group (83.9–85 %) and were most similar to Borrelia hermsii from the RF group (89.3–89.4 %). Phylogenetic analysis of the groEL locus showed that ‘Candidatus Borrelia tachyglossi’ genotypes from the present study clustered with the RF group with strong posterior probability support (Fig. 1). The percentage nucleotide identities between ‘Candidatus Borrelia tachyglossi’ genotypes and Borreliahermsii (89.3–89.4 %) were higher than that between Borreliahermsii and Borreliaburgdorferi (84.8 %); whereas the percentage identity between ‘Candidatus Borrelia tachyglossi’ genotypes and Borreliaburgdorferi (84.3 %) was slightly lower (Table S4). The genetic distance between ‘Candidatus Borrelia tachyglossi’ and Borreliaturcica at this locus remains to be determined until the groEL gene is characterized in Borreliaturcica.
At the gyrB locus, ‘Candidatus Borrelia tachyglossi’ genotypes A and B were identical. Nucleotide alignment (1712 bp) revealed that the ‘Candidatus Borrelia tachyglossi’ genotypes exhibited 80.8–82.0 %, 80.8–82.0 % and 84.8–88.2 % sequence similarity with the LB Borreliae, the RF group and Borrelia turcica, respectively. The percentage similarities within ‘Candidatus Borrelia tachyglossi’ genotypes ranged from 99.6 to 99.9 %. Phylogenetic analysis showed that ‘Candidatus Borrelia tachyglossi’ genotypes formed their own monophyletic clade, separate from Borrelia turcica and the RF group, with high bootstrap support (98 %) (Fig. 1). The percentage nucleotide identities between ‘Candidatus Borrelia tachyglossi’ genotypes and Borrelia turcica (87.8–88 %) and Borrelia hermsii (88.1–88.2 %) were higher than that between Borrelia turcica and Borrelia hermsii (86.7 %) (Table S5).
A Bayesian phylogenetic tree reconstructed using the concatenated alignment (3585 bp) consisting of three genes, 16S rRNA, flaB and gyrB, available for each of the main borreliae (Fig. 1), illustrated that the ‘Candidatus Borrelia tachyglossi’ genotypes from Bothriocroton concolor ticks grouped separately, with Borrelia turcica as the closest relative (91.1–91.2 % nucleotide identities) (Table S6). Likewise, the concatenated alignment (5154 bp), which excluded Borrelia turcica (REP), based on the four loci amplified in the present study (16S rRNA, flaB, groEL and gyrB) also produced a similar tree topology with ‘Candidatus Borrelia tachyglossi’ genotypes forming a monophyletic clade supported by high posterior probabilities (Fig. S1), sharing the highest percentage identities with Borrelia hermsii (90.3 %) (Table S7).
All phylogenetic trees reconstructed revealed similar topologies with the REP group species of the genus Borrelia as the closest sister clade. The recently established REP group has been detected in various reptiles from several countries and in ticks that parasitise them [4, 21]. The recent discovery of a novel species of the genus Borrelia in Amblyomma varenense collected from the reticulated python (Python reticulatus) showed that the species of the genus Borrelia identified clustered together with the REP-associated Borrelia group, along with Borrelia turcica, based on phylogenetic analyses of 16S rRNA and flaB genes [44]. However, the pathogenic potential of the species of the genus Borrelia belonging to the REP group is unknown.
A number of members within the genus Borrelia are well known to cause diseases in humans outside of Australia; nonetheless, borrelial tick-borne disease in humans still remains highly speculative and controversial in this country. ‘Candidatus Borrelia tachyglossi’ was first reported in 2016 [37], hence the urgency to further characterize this bacterium on the basis of multi-loci gene sequencing in order to confirm the taxonomic position of this new member in the genus Borrelia. Our results, based on sequence and phylogenetic characterization of multiple loci, provide conclusive evidence that ‘Candidatus Borrelia tachyglossi’, identified in Bothriocroton concolor ticks from echidnas, is distinct from other described species of the genus Borrelia and constitutes to a new clade in this genus.
Borrelial spirochaetes are well known to be associated closely with wildlife and utilize tick vectors to maintain a sylvatic life cycle [45, 46]. Australian wildlife are also known to be involved in spill-over of various zoonotic parasites [47]. Therefore, it is plausible that this bacterium is also likely to persist in the environment through circulation among native ticks and native mammalian (including marsupial) hosts. Unlike the Australian paralysis tick I. holocyclus, Bothriocroton concolor is a highly specialized tick, with echidnas as their primary host, and with a geographic distribution known only in Australia and Papua New Guinea [48]. ‘Candidatus Borrelia tachyglossi’ has previously been identified in one human-biting tick (I. holocyclus) removed from an echidna [38] and its prevalence in I. holocyclus or other human-biting ticks remains to be determined. The morphological characteristics and the pathogenicity of this bacterium are also unknown.
Description of ‘Candidatus Borrelia tachyglossi’
‘Candidatus Borrelia tachyglossi′ (ta.chy.glos′si. N.L. gen. n. tachyglossi of Tachyglossus aculeatus, the monotreme host of the ticks in which the bacterium was first identified]).
Species can be differentiated from other borreliae based on sequence and phylogenetic analyses of five genomic loci (16S rRNA, flaB, groEL, gyrB and glpQ). Comparisons of the flaB gene sequences among the ‘Candidatus Borrelia tachyglossi’ genotypes showed two single nucleotide polymorphisms (SNPs) in genotype A at bases 485 and 519 (GenBank accession no. KY586966); and one SNP in genotype B at base 227 (KY586964). As for the groEL gene, analysis revealed two SNPs in genotype C at bases 656 and 1143 (KY586970). Analysis of the gyrB gene showed two SNPs in genotype B at bases 985 and 1416 (KY586972); and five SNPs in genotype C at bases 405, 757, 1075, 1093 and 1420 (KY586973). At the glpQ locus, genotype A (KY586968) and genotype B (KY586967) showed 12 base differences at bases 3, 43, 103, 112, 264, 515, 520, 530, 532, 702, 819 and 852.
The DNA G+C contents for 16S rRNA, flaB, groEL, gyrB and glpQ genes of ‘Candidatus Borrelia tachyglossi’ genotype A are 47.3, 40.9, 38.5, 33.8 and 34.1 mol%, respectively. The DA G+C contents for 16S rRNA, flaB, groEL, gyrB and glpQ genes of ‘Candidatus Borrelia tachyglossi’ genotype B are 47.2, 40.9, 38.5, 33.9 and 34.7 mol%, respectively. The DNA G+C contents for 16S rRNA, flaB, groEL, and gyrB genes of ‘Candidatus Borrelia tachyglossi’ genotype C are 47.2, 41, 38.5 and 34 mol%, respectively.
Funding information
This study was part-funded by the Australian Research Council (LP130100050), Bayer Healthcare, and Bayer Australia Ltd., and the School of Veterinary and Life Sciences Small Grants Scheme. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgements
The authors wish to acknowledge Frances Brigg and the Western Australian State Agriculture Biotechnology Centre for Sanger sequencing. The authors also acknowledge the veterinarians and veterinary nurses at the Australian Zoo Wildlife Hospital, Queensland, as well as the volunteers at Wagga Wagga, New South Wales, for the submission of tick samples.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: LB, lyme disease/Borreliosis; REP, reptile-associated; RF, relapsing Fever.
All sequences generated in this study were deposited in GenBank/EMBL/DDBJ under accession numbers KY586964-KY586974.
One supplementary figure and seven supplementary tables are available with the online Supplementary Material.
References
- 1.Baranton G, Old IG. The spirochaetes: a different way of life. Bull Inst Pasteur. 1995;93:63–95. doi: 10.1016/0020-2452(96)81485-0. [DOI] [Google Scholar]
- 2.Ma DY, Giacani L, Centurión-Lara A. The molecular epidemiology of Treponema pallidum subspecies pallidum. Sex Health. 2015;12:141–147. doi: 10.1071/SH14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitjà O, Marks M, Konan DJP, Ayelo G, Gonzalez-Beiras C, et al. Global epidemiology of yaws: a systematic review. Lancet Glob Health. 2015;3:e324. doi: 10.1016/S2214-109X(15)00011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takano A, Goka K, Une Y, Shimada Y, Fujita H, et al. Isolation and characterization of a novel Borrelia group of tick-borne borreliae from imported reptiles and their associated ticks. Environ Microbiol. 2010;12:134–146. doi: 10.1111/j.1462-2920.2009.02054.x. [DOI] [PubMed] [Google Scholar]
- 5.Margos G, Wilske B, Sing A, Hizo-Teufel C, Cao WC, et al. Borrelia bavariensis sp. nov. is widely distributed in Europe and Asia. Int J Syst Evol Microbiol. 2013;63:4284–4288. doi: 10.1099/ijs.0.052001-0. [DOI] [PubMed] [Google Scholar]
- 6.Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011;2:123–128. doi: 10.1016/j.ttbdis.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano A, Nakao M, Masuzawa T, Takada N, Yano Y, et al. Multilocus sequence typing implicates rodents as the main reservoir host of human-pathogenic Borrelia garinii in japan. J Clin Microbiol. 2011;49:2035–2039. doi: 10.1128/JCM.02544-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gern L, Humair P-F. Ecology of Borrelia burgdorferi sensu lato in Europe. In: Gray JS, Kahl O, Lane RS, Stanek G, editors. Lyme Borreliosis: Biology, Epidemiology and Control. Wallingford, UK: CABI Publishing; 2002. pp. 149–174. (editors) [Google Scholar]
- 10.Trape JF, Diatta G, Arnathau C, Bitam I, Sarih M, et al. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida) PLoS One. 2013;8:e78473. doi: 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assous MV, Wilamowski A. Relapsing fever borreliosis in Eurasia—forgotten, but certainly not gone! Clin Microbiol Infec. 2009;15:407–414. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 12.Schwan TG, Raffel SJ, Schrumpf ME, Webster LS, Marques AR, et al. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg Infect Dis. 2009;15:1026–1031. doi: 10.3201/eid1507.090223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, et al. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for lyme disease in Japan. Int J Syst Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 14.Fraenkel CJ, Garpmo U, Berglund J. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J Clin Microbiol. 2002;40:3308–3312. doi: 10.1128/JCM.40.9.3308-3312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–123. doi: 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- 16.Scoles GA, Papero M, Beati L, Fish D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 17.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 18.Smith RD, Miranpuri GS, Adams JH, Ahrens EH. Borrelia theileri: isolation from ticks (Boophilus microplus) and tick-borne transmission between splenectomized calves. Am J Vet Res. 1985;46:1396–1398. [PubMed] [Google Scholar]
- 19.Lin T, Gao L, Seyfang A, Oliver JH. 'Candidatus Borrelia texasensis', from the American dog tick Dermacentor variabilis. Int J Syst Evol Microbiol. 2005;55:685–693. doi: 10.1099/ijs.0.02864-0. [DOI] [PubMed] [Google Scholar]
- 20.Güner ES, Hashimoto N, Kadosaka T, Imai Y, Masuzawa T. A novel, fast-growing Borrelia sp. isolated from the hard tick Hyalomma aegyptium in Turkey. Microbiology. 2003;149:2539–2544. doi: 10.1099/mic.0.26464-0. [DOI] [PubMed] [Google Scholar]
- 21.Güner ES, Watanabe M, Hashimoto N, Kadosaka T, Kawamura Y, et al. Borrelia turcica sp. nov., isolated from the hard tick Hyalomma aegyptium in Turkey. Int J Syst Evol Microbiol. 2004;54:1649–1652. doi: 10.1099/ijs.0.03050-0. [DOI] [PubMed] [Google Scholar]
- 22.Takano A, Fujita H, Kadosaka T, Konnai S, Tajima T, et al. Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with lyme disease and relapsing fever Borrelia spp. Environ Microbiol Rep. 2011;3:632–637. doi: 10.1111/j.1758-2229.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- 23.Chalada MJ, Stenos J, Bradbury RS. Is there a Lyme-like disease in Australia? Summary of the findings to date. One Health. 2016;2:42–54. doi: 10.1016/j.onehlt.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulhearn CR. A note on two blood parasites of cattle (Spirochaeta theileri and Bartonella bovis) recorded for the first time in Australia. Aust Vet J. 1946;22:118–119. doi: 10.1111/j.1751-0813.1946.tb06466.x. [DOI] [PubMed] [Google Scholar]
- 25.Callow LL, Hoyte HMD. Transmission experiments using babesia bigemina, Theileria mutans, Borrelia sp. and the cattle tick. Aust Vet J. 1961;37:381–390. doi: 10.1111/j.1751-0813.1961.tb03790.x. [DOI] [Google Scholar]
- 26.Gorrie CJR. Vaccination against spirochaetosis in fowls. Aust Vet J. 1950;26:308–315. doi: 10.1111/j.1751-0813.1950.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 27.Petney TN, Andrews RH, McDiarmid LA, Dixon BR. Argas persicus sensu stricto does occur in Australia. Parasitol Res. 2004;93:296–299. doi: 10.1007/s00436-004-1141-5. [DOI] [PubMed] [Google Scholar]
- 28.Pope JH, Carley JG. Isolation of Borrelia from native rats in north-west Queensland. Aust J Science. 1956;19:114. [Google Scholar]
- 29.Carley JG, Pope JH. A new species of Borrelia (B. queenslandica) from Rattus villosissimus in Queensland. Aust J Exp Biol Med Sci. 1962;40:255–261. doi: 10.1038/icb.1962.29. [DOI] [PubMed] [Google Scholar]
- 30.Enright MC, Spratt BG. Multilocus sequence typing. Trends Microbiol. 1999;7:482–487. doi: 10.1016/S0966-842X(99)01609-1. [DOI] [PubMed] [Google Scholar]
- 31.Urwin R, Maiden MC. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 2003;11:479–487. doi: 10.1016/j.tim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Margos G, Gatewood AG, Aanensen DM, Hanincová K, Terekhova D, et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci USA. 2008;105:8730–8735. doi: 10.1073/pnas.0800323105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo A, Anda P, Escudero R, Larsson C, Bergstrom S, et al. Phylogenetic analysis of a virulent Borrelia species isolated from patients with relapsing fever. J Clin Microbiol. 2010;48:2484–2489. doi: 10.1128/JCM.00541-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwan TG, Anderson JM, Lopez JE, Fischer RJ, Raffel SJ, et al. Endemic foci of the tick-borne relapsing fever spirochete Borrelia crocidurae in Mali, West Africa, and the potential for human infection. PLoS Negl Trop Dis. 2012;6:e1924. doi: 10.1371/journal.pntd.0001924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacquot M, Bisseux M, Abrial D, Marsot M, Ferquel E, et al. High-throughput sequence typing reveals genetic differentiation and host specialization among populations of the Borrelia burgdorferi species complex that infect rodents. PLoS One. 2014;9:e88581. doi: 10.1371/journal.pone.0088581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jungnick S, Margos G, Rieger M, Dzaferovic E, Bent SJ, et al. Borrelia burgdorferi sensu stricto and Borrelia afzelii: population structure and differential pathogenicity. Int J Med Microbiol. 2015;305:673–681. doi: 10.1016/j.ijmm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Loh SM, Gofton AW, Lo N, Gillett A, Ryan UM, et al. Novel Borrelia species detected in echidna ticks, Bothriocroton concolor, in Australia. Parasit Vectors. 2016;9:339. doi: 10.1186/s13071-016-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gofton AW, Oskam CL, Lo N, Beninati T, Wei H, et al. Inhibition of the endosymbiont “Candidatus Midichloria mitochondrii” during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasit Vectors. 2015;8:345. doi: 10.1186/s13071-015-0958-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huelsenbeck JP, Ronquist F. MRBAYES: bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 43.Miller MA, Pfeiffer W, Schwartz T. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 November. New Orleans, LA: 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. p. 1–8.www.phylo.org/sub_sections/portal/cite.php [Google Scholar]
- 44.Trinachartvanit W, Hirunkanokpun S, Sudsangiem R, Lijuan W, Boonkusol D, et al. Borrelia sp. phylogenetically different from lyme disease- and relapsing fever-related Borrelia spp. in Amblyomma varanense from Python reticulatus. Parasit Vectors. 2016;9:359. doi: 10.1186/s13071-016-1629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakao M, Miyamoto K, Fukunaga M. Lyme disease spirochetes in japan: enzootic transmission cycles in birds, rodents, and Ixodes persulcatus ticks. J Infect Dis. 1994;170:878–882. doi: 10.1093/infdis/170.4.878. [DOI] [PubMed] [Google Scholar]
- 46.Oliver JH, Lin T, Gao L, Clark KL, Banks CW, et al. An enzootic transmission cycle of lyme borreliosis spirochetes in the southeastern United States. Proc Natl Acad Sci USA. 2003;100:11642–11645. doi: 10.1073/pnas.1434553100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson RC. Parasite zoonoses and wildlife: one health, spillover and human activity. Int J Parasitol. 2013;43:1079–1088. doi: 10.1016/j.ijpara.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts FHS. Australian Ticks. 2nd ed. Melbourne: CSIRO; 1970. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.