Abstract
Purpose
In our previous study, Ag+-loaded TiO2 and Ag+-loaded SiO2 coatings for tracheal intubation were prepared to prevent ventilator-associated pneumonia (VAP), but the antimicrobial targets and the underlying mechanisms of TiO2 and Ag-TiO2 (Ag+) are still unclear. We attempted to elucidate the antimicrobial activity and potential mechanisms against Staphylococcus aureus.
Methodology
The study tested the TiO2 and Ag+ bacteriostatic activity against S. aureus strains by MIC assays and S. aureus growth curves, lesion in the membranes by surface hydrophobicity tests, conductivity tests and measurements of DNA and RNA contents in S. aureus cultures, and investigated the inhibition of soluble protein and nucleic acid synthesis by measurements of soluble protein content, fluorescent intensity and nucleic acid content of living S. aureus.
Results
The MIC values of TiO2 and Ag+ were 1.6 mg ml−1 and 5.781 µg ml−1. TiO2 and Ag+ could inhibit the growth of S. aureus. After treatment with TiO2 and Ag+, the surface hydrophobicity was significantly reduced, the conductivity of cultures increased, and DNA and RNA content in cultures showed no obvious changes. The expressions of soluble proteins and nucleic acid contents of living S. aureus were reduced after treatment with TiO2 and Ag+.
Conclusion
TiO2 and Ag+ could cause slight lesion in the membrane to affect S. aureus membrane permeability, but not decomposition of membrane. Moreover, TiO2 and Ag+ could lead to reduced expression of soluble protein by inhibiting the synthesis of nucleic acids, thereby further inhibiting the growth of S. aureus.
Keywords: TiO2, Ag+, Staphylococcusaureus, bactericidal mechanism, effector targets
Introduction
TiO2 is one of the most important semiconducting oxides because of its photocatalytic activity, conservative properties, low cost, low toxicity and stability in response to light illumination [1] However, TiO2 has a high band gap energy (3.2 eV), which limits its wide applications in the visible light range of the solar spectrum [2, 3]. Additionally, the generation of reactive oxygen species by TiO2 is also inhibited by its low electron transfer rate to oxygen and high rate of electron–hole recombination [4]. To enhance the generation of reactive oxygen species by TiO2 nanomaterials under conditions of visible light irradiation, noble metals such as Pd, Pt, Au and Ag have been used to modify TiO2 [5–11].
Among these noble metals, Ag has attracted significant attention for doping TiO2 to inactivate bacteria because of its excellent broad-spectrum antibacterial properties, heat resistance, good dispersion and low drug resistance [12].
We prepared antimicrobial films that contained Ag-TiO2 and Ag-SiO2 polyethylene endotracheal tubes that had excellent antimicrobial properties against ventilator-associated pneumonia (VAP) [13]. Zhou et al. found that Ag+-TiO2 nanobelts exhibited greater photocatalytic degradation activity against methyl orange than TiO2 because of the greater amounts of reactive oxygen species that are generated [14]. Because the inactivation of bacterial cells shows a correlation with the photocatalytic decomposition of organic matter, nanobelts with Ag+-deposited TiO2 are expected to have improved bactericidal properties under conditions of visible light irradiation [15]. Recently, the bactericidal mechanism and biosafety of Ag+ was investigated by researchers. Heidenau et al. found that an Ag+ concentration of 3.5 µM was not cytotoxic to mouse L929 fibroblasts [16]. Baldi et al. found that an Ag+ concentration between 30 and 70 µM affected both cell viability and morphology [17]. Jin et al. found that, for both Gram-negative Escherichia coli ATCC 15597 and Gram-positive Bacillus subtilis, the antimicrobial effects of Ag+ were greater under light conditions compared with those under dark conditions [18]. Tan et al. found that Ag nanoparticles exhibited efficient microbicidal activity against Staphylococcus aureus, but did not significantly affect the viability of osteoblast cells (MC3T3-E1) [19]. Shi et al. found that, compared with TiO2-SiO2, Ag-TiO2-SiO2 exhibited higher photocatalytic activity and antibacterial efficiency after uniform Ag nanoparticles were loaded onto TiO2 [20]. In response to an Ag concentration of 0.6 mg ml−1, the antibacterial rates against Escherichia coli and S. aureus were 93.41 and 97.37 %, respectively. Jin et al. proposed that the potential antibacterial mechanisms of Ag+ were as follows: first, Ag+ can be easily adsorbed onto the surface of E. coli and affect the outer membrane permeability and fluidity; second, Ag+(silver nanoparticles with carbon dots doped with sulfur) with a smaller size (7.3±1.0 and 6.1±0.8 nm) can permeate the membrane of E. coli to interact with DNA and components of the respiratory chain; and third, Ag+ release can cause E. coli death [21]. However, as Ag nanoparticles are ionic antibacterial implant materials, the best way to achieve good bactericidal or anti-adhesion properties of medical devices without harming normal tissues or cells remains a common subject of debate.
The preparation and theoretical use of TiO2 and Ag+ antibacterial agents have been studied by our research group for many years [11, 22]. In this present study, we aimed to fabricate TiO2 and Ag+ antibacterial agents and investigate their bactericidal mechanisms. We attempted to elucidate the antimicrobial activity and potential mechanisms of TiO2 and Ag+ as antibacterial agents against S. aureus by determining their antimicrobial activity of the MIC values and S. aureus growth curve, conductivity, and macromolecular substances in culture medium, and by measuring the soluble proteins and nucleic acids of S. aureus after treatment with antibacterial agents.
Methods
Reagents and materials
2,3,5-triphenyltetrazolium chloride (TTC), hexadecane, 4′,6-diamidino-2-phenylindole (DAPI), hexadecane, protein marker and distilled water were of guaranteed reagent quality. The TiO2 stock solution (aged 60 days) was prepared according to previously published methods [22]. Ag+ stock solution was prepared so that the Ag-TiO2 mesoporous materials were dispersed into the water at a mass concentration of 5 wt% [11]. S. aureus ATCC 25923 was obtained from Hangzhou 100 Biotechnology. The biosafety cabinet and enzyme marker were produced by Thermo. The fluorescence microscope was produced by Olympus. Vertical electrophoresis apparatus was produced by Tanon. The conductivity meter was produced by Shanghai Dapu Instrument Co. The UV spectrophotometer was produced by Shanghai Xinmao Instrument Co. The decolorization table was produced by Shanghai Huxi Instrument Co.
Reagent preparation
Broth medium
Beef extract (3 g), peptone (10 g) and NaCl (5 g). All salts were dissolved in 800 ml distilled water, the pH was adjusted to 7.2–7.4, and then more distilled water was added to achieve a total volume of 1000 ml.
PBS buffer
PBS buffer contained Na2HPO4 (14.6 g), NaH2PO4 (1.48 g) and NaCl (43.83 g). All salts were dissolved in 1 l distilled water, and water was added to achieve a final volume of 5 l. The pH was adjusted to 7.2–7.4 with NaOH and stored at room temperature after autoclave sterilization.
Experimental groups
(1) Control; (2) TiO2 groups: 0.1, 0.2, 0.4, 0.8 or 1.6 mg ml−1; (3) Ag+ groups: 0.723, 1.445, 2.891, 5.781 or 11.560 µg ml−1.
MIC assay
A few S. aureus were picked up from agar/slant culture medium and inoculated in broth medium at 37 °C, 120 r.p.m. for 24 h. The final concentration of cells was adjusted to 106–107 c.f.u. ml−1 with broth medium. Then, 1.5 ml of the bacterial culture was inoculated on 12-well plates in which 10 µl serial dilutions of TiO2 or Ag+ were added. Plates were incubated at 37 °C, 120 r.p.m. for 24 h. Subsequently, 150 µl of 0.2 % TTC was added to each well. Changes in colour were observed after incubation at 37 °C, 120 r.p.m. for 4 h in the dark. Each group included triple technical replicates.
S. aureus growth curves
The S. aureus cells were incubated to the log-phase of growth. Next, 2 % inoculum was transferred to fresh broth medium with 0.5 MIC TiO2 or Ag+ and incubated at 37 °C, 120 r.p.m. Samples were obtained and the OD value was determined at 580 nm at 4 h intervals. Each group included triple technical replicates. S. aureus growth curves after TiO2 or Ag+ treatment were plotted as OD values on the vertical axis compared with time on the horizontal axis. Each group included triple technical replicates.
Surface hydrophobicity of S. aureus
S. aureus cells were incubated until they reached the log-phase of growth. Then, 2 % inoculum was transferred to fresh broth medium with 0.5 MIC TiO2 or Ag+ and incubated at 37 °C, 120 r.p.m. Next, 2 ml samples were transferred into 5 ml tubes and, after incubation for 0, 2, 8 or 16 h, they were centrifuged at 5000 r.p.m. (Allegra X-15R; Bechman) for 10 min. Cell pellets were washed twice with 0.1 M KNO3 (pH 6.2) and resuspended with the same buffer. Cell suspensions were adjusted to absorbance A400=0.4 (i.e. A0=0.4); 10 mM sodium phosphate buffer solution served as a blank control. Additionally, 0.2 ml hexadecane was added into the adjusted cell suspensions. Samples were mixed for 2 min using a vortex after a 10 min static incubation. Aqueous phases were transferred and the A400 (A1) absorbance values were measured until the two phases of the mixture became completely separated. Bacterial surface hydrophobicity was indicated by the absorption rate. Bacterial absorption rate =(1−A1/A0)×100 %. Each group included triple technical replicates.
Conductivity tests of S. aureus cultures
S. aureus cells were incubated until they reached the log-phase of growth. Next, 2 % inoculum was transferred to fresh broth medium with 0.5 MIC TiO2 or Ag+ and then incubated at 37 °C, 120 r.p.m. Thereafter, 5 ml samples were harvested after incubation for 0, 2, 4, 6 and 8 h, which were each centrifuged at 4000 r.p.m. for 10 min. Cell conductivity was determined with a conductivity meter after 20-fold dilutions of supernatants. Each group included triple technical replicates.
Measurements of DNA and RNA contents in cultures of S. aureus
S. aureus cells were incubated until they reached the log-phase of growth. Then, 2 % of the inoculum was transferred into fresh broth medium and was again incubated until the culture reached log-phase at 37 °C, 120 r.p.m. Cells were collected, washed twice with PBS and resuspended at an appropriate concentration to yield a cell suspension. A final concentration of 0.5 MIC TiO2 or Ag+ was added to the prepared suspension. After treatment for 0, 2, 4, 6 or 8 h, 3 ml of the suspension was collected and centrifuged at 4000 r.p.m. (Allegra X-15R; Bechman) for 10 min. Absorption values of DNA and RNA in the supernatant were measured at 260 nm using an ultraviolet spectrophotometer. Each group included triple technical replicates.
Measurement of soluble protein content in S. aureus
S. aureus cells were incubated until they reached the log-phase of growth. Then, 2 % of the inoculum was transferred into fresh broth medium with 0.5 MIC TiO2 or Ag+ and incubated at 37 °C, 120 r.p.m. Suspensions were harvested after incubation for 12 and 16 h, and 0.1 g cells were centrifuged at 4000 r.p.m. (Allegra X-15R; Bechman) for 10 min. Pellets were collected and resuspended in 40 µl sterile water. The sample buffer was mixed with the sample at a volume ratio of 1 : 4, and the mixture was heated in boiling water for 7 min and then centrifuged at 4000 r.p.m. (Allegra X-15R; Bechman) for 10 min. Soluble proteins in the supernatant were assessed by SDS-PAGE. Each group included triple technical replicates.
Fluorescence intensity measurements of S. aureus
S. aureus cells were incubated until they reached the log-phase of growth. Then, 2 % of the inoculum was transferred into the fresh broth medium with 0.5 MIC TiO2 or Ag+ and was incubated at 37 °C, 120 r.p.m. for 24 h. Aliquots of 10 µl per sample were diluted 200-fold. Subsequently, 100 µl diluted suspension was spotted on a microslide and stained with the same volume of DAPI staining solution. Mixtures were incubated without shaking in the dark for 10 min. Fluorescence intensity was measured using a fluorescence microplate reader and imaged. Each group included triple technical replicates.
Nucleic acid content measurements of S. aureus
S. aureus cells were incubated until they reached the log-phase of growth. Then, 2 % of the inoculum was transferred into the fresh broth medium with0.5 MIC TiO2 or Ag+ and was incubated at 37 °C, 120 r.p.m. Then, 50 µl aliquots were harvested after incubation for 12, 16, 20 and 24 h, and stained with 150 µl DAPI staining solution. Prepared samples were measured immediately after shaking for 10 min. The fluorescence parameters were 364 nm to measure the DNA content and 400 nm to measure the RNA content. Each group included triple technical replicates.
Statistical analysis
All data are presented as mean±sd. Differences between group means were assessed by ANOVA for multiple comparisons using spss 16.0. Values of P<0.05 were considered significant.
RESULTS
MIC values of TiO2 against S. aureus
For assessments of cell viability, a commonly used reagent is TTC, which is a white stain when oxidized. This dye can react with succinate dehydrogenases in living tissues to enter cells and produce the reduced molecule 2,3,5-triphenyltetrazolium formazan (TTF, which appears red (Fig. 1a). We found the culture colours became lighter as the concentration of TiO2 (Fig. 1b) or Ag+ (Fig. 1c) increased, and sometimes even became colourless. This finding suggested that TiO2 and Ag+ could inhibit the growth of S. aureus. Additionally, this inhibitory effect is increased proportionally with the addition of TiO2 and Ag+. As changes in colour occurred, the MIC values of TiO2 and Ag+ against S. aureus were 1.6 mg ml−1 and 5.781 µg ml−1, respectively.
Fig. 1.
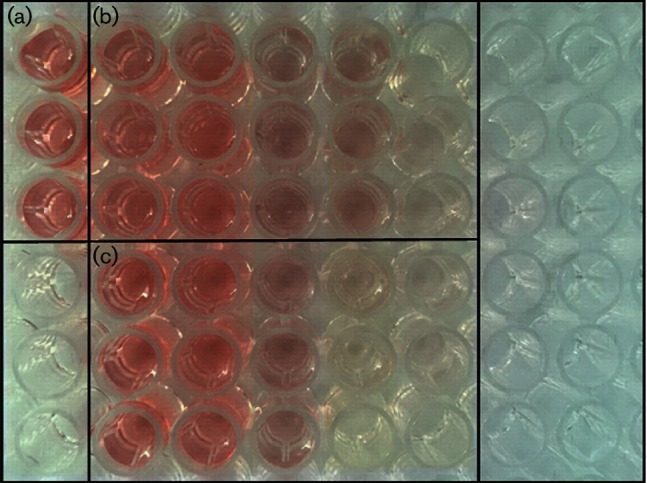
Determination of MIC values for TiO2 and Ag+ against S. aureus. (a) Control. (b) Groups treated with TiO2:0.1, 0.2, 0.4, 0.8 or 1.6 mg ml−1 from left to right. (c) Groups treated with Ag+: 0.723, 1.445, 2.891, 5.781 or 11.560 µg ml−1 from left to right.
Effects of TiO2 on S. aureus growth
Treatment with TiO2 or Ag+ could robustly inhibit the growth of S. aureus. As shown in Fig. 2, S. aureus in the control group rapidly entered log-phase growth after a short lag-phase and reached bacterial stationary phase after an incubation for 24 h. By contrast, the growth rate of S. aureus was significantly suppressed after the addition of either TiO2 or Ag+. The rates of inhibition yielded by either TiO2 or Ag+ against S. aureus were greatest after treatment for 16 h, which were 75.7 % (P<0.001) and 58.6 % (P<0.001), respectively.
Fig. 2.
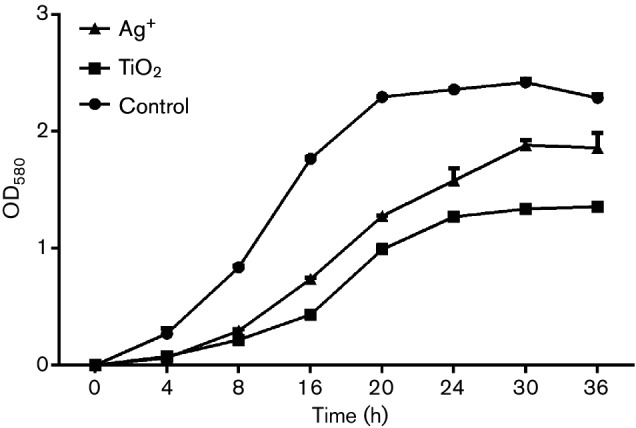
Effects of TiO2 and Ag+ on S. aureus growth curves.
Effects of TiO2 on the surface hydrophobicity of S. aureus
The bacterial cell surface is composed of both hydrophilic and hydrophobic sites, the latter mainly including lipopolysaccharides, lipids and proteins. Bacterial surface hydrophobicity is primarily dependent upon the ratio of proteins and polysaccharides, along with the types and orientations of carbohydrate and polysaccharides in the outer layer of cell. In this present study, we tested effects of TiO2 and Ag+ on bacterial surface hydrophobicity based on the hydrophobicity of hexadecane and bacterial absorption. As shown in Fig. 3, bacterial surface hydrophobicity was significantly reduced after treatment with either TiO2 or Ag+. Moreover, the effects of TiO2 on S. aureus surface hydrophobicity were higher than those of Ag+.
Fig. 3.
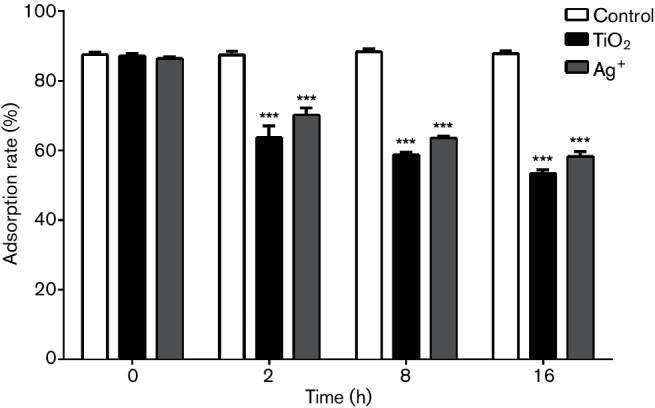
Adsorption rates of S. aureus subjected to different treatments (%) (***P<0.001 versus control).
Effects of TiO2 on the S. aureus membrane
Changes in bacterial membrane permeability can result in the leakage of intracellular small molecules, such as potassium ions and sodium, which can further alter extracellular conductivity [23]. When a bacterial membrane is heavily damaged, DNA and RNA macromolecules will escape from cells. As shown in Fig. 4, the conductivity of cultures increased after treatment with TiO2 or Ag+.
Fig. 4.
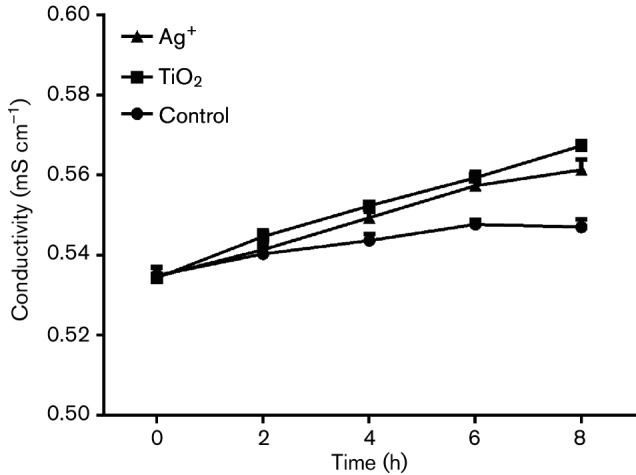
Changes in the conductivity of S. aureus in response to TiO2 or Ag+.
Furthermore, we assessed changes in the levels of DNA and RNA macromolecules when cells were treated with TiO2 or Ag+ to test for effects on membrane permeability in S. aureus. As shown in Fig. 5, the content of both DNA and RNA in S. aureus showed no obvious changes compared with those observed in the control group.
Fig. 5.
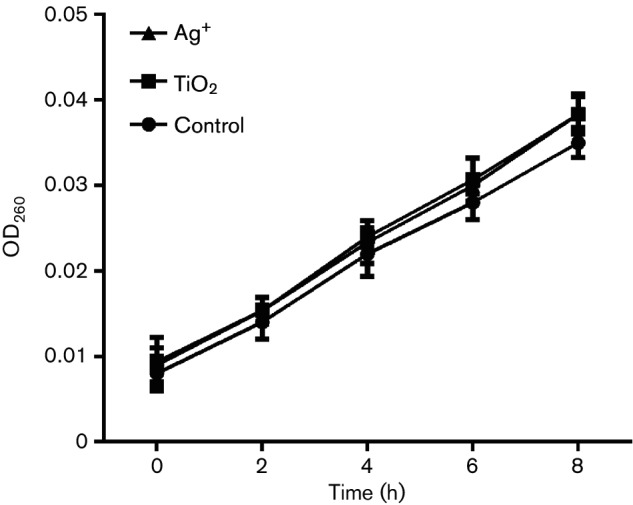
Changes in the OD260 of macromolecules from S. aureus in response to TiO2 or Ag+.
Together, these findings suggested that treatment with either TiO2 or Ag+ contributed to changes in membrane permeability in S. aureus. This caused slight lesion in the membrane, but not decomposition of membrane, and it did not cause extracellular DNA and RNA leakage.
Effects of TiO2 on the synthesis of soluble proteins in S. aureus
We used SDS-PAGE to determine the soluble protein content in S. aureus when cells were treated with TiO2 or Ag+. As shown in Fig. 6, the addition of TiO2 or Ag+ to the medium could suppress expression of soluble proteins in S. aureus. Moreover, the expression of proteins was further reduced after prolonged treatment with the effectors.
Fig. 6.
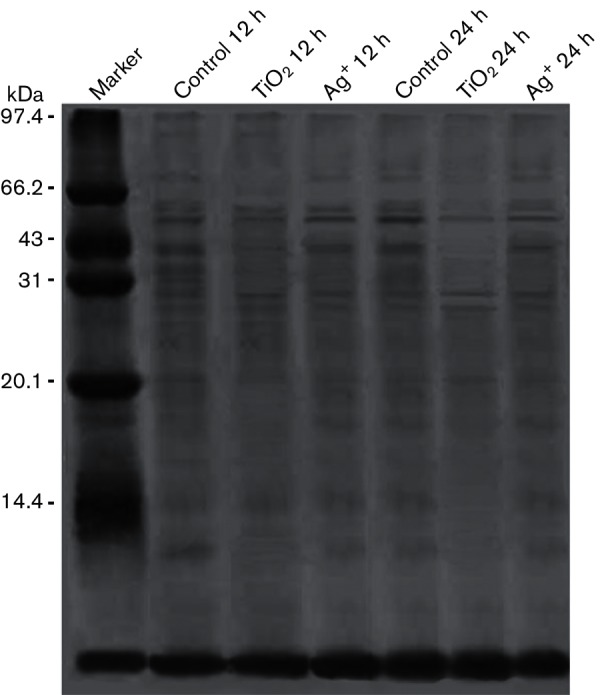
Soluble protein in S. aureus treated with TiO2 or Ag+.
Effects of TiO2 on nucleic acid synthesis in S. aureus
DAPI is a fluorescent stain that can strongly bind to DNA and RNA, and it is often used to detect nucleic acids. Its fluorescence intensity is positively correlated with the nucleic acid content. In the present study, we studied the effects of TiO2 and Ag+ on nucleic acid content in S. aureus by measuring the fluorescence intensity of cells that had been treated with DAPI. As shown in Fig. 7(a–c), the fluorescence intensity of groups treated with TiO2 or Ag+ was decreased compared with the control group. Further analysis showed that the fluorescence of both DNA and RNA were significantly decreased in the treated groups (both P<0.001), as shown in Tables 1 and 2 and Fig. 8(a, b).
Fig. 7.
Viability staining of S. aureus treated with TiO2 or Ag+ for 24 h using DAPI (×200). (a) Control. (b) Groups treated with TiO2. (c) Groups treated with Ag+. Bars, 100 µm.
Table 1. DNA content of S. aureus treated with TiO2 and Ag+.
| DNA | 12 h | 16 h | 20 h | 24 h |
|---|---|---|---|---|
| Control | 310.67±16.56 | 322.67±21.55 | 324.67±12.66 | 324.67±23.50 |
| TiO2 | 173.67±18.61 | 156.67±10.02 | 186.00±10.82 | 177.00±8.72 |
| Ag+ | 244.00±12.49 | 217.33±7.64 | 228.00±9.54 | 249.67±15.04 |
Table 2. RNA content of S. aureus treated with TiO2 and Ag+.
| RNA | 12 h | 16 h | 20 h | 24 h |
|---|---|---|---|---|
| Control | 75.33±6.51 | 94.00±7.00 | 122.33±15.53 | 140.00±14.93 |
| TiO2 | 38.00±3.61 | 51.00±3.00 | 53.00±2.65 | 67.33±4.04 |
| Ag+ | 49.67±3.06 | 71.00±3.61 | 78.33±3.06 | 79.00±6.24 |
Fig. 8.
DNA and RNA content of S. aureus treated with TiO2 or Ag+. (a) DNA content of S. aureus treated with TiO2 or Ag+. (b) RNA content of S. aureus treated with TiO2 or Ag+. **P<0.01 versus control; ***P<0.001 versus control.
DISCUSSION
VAP is a common serious complication in patients with tracheal intubation. The bacteria associated with VAP are almost all antibiotic-resistant strains, such as S. aureus, E. coli and Pseudomonas aeruginosa. TiO2 has a strong redox ability and stable chemical properties and bactericidal effect by photocatalysis which can decompose bacteria, viruses and fungi. The sterilizing rate is 99.00 % in no light and 99.99 % in UV light irradiation [24]. Ag+, with contact antibacterial action, is the most powerful antibacterial material in inorganic antibacterial materials. In order to effectively control the VAP caused by infection after tracheal intubation, we prepared Ag+-loaded TiO2 and Ag+-loaded SiO2 coatings, which could effectively inhibit bacterial growth, such as S. aureus, E. coli and P. aeruginosa [25] and had good biocompatibility [26]. In order to apply TiO2 and Ag+ in medical instruments, the underling mechanisms of TiO2 and Ag+ antibacterial effects were explored in the study.
It was found that both TiO2 and Ag+ could inhibit the growth of S. aureus. Moreover, TTC staining analysis indicated that the MIC of TiO2 was 1.6 mg ml−1, while that of Ag+ was 5.781 µg ml−1. Therefore, with the same dose, Ag+had stronger antibacterial activity than TiO2. We used these 0.5 MIC concentrations as working concentrations to explore the mechanisms of TiO2 and Ag+ against S. aureus. Our findings indicated that TiO2 and Ag+ utilized a similar mechanism against bacterial growth by directly damaging the bacterial surface. The activities of these drugs against negatively charged lipopolysaccharides on the cell surface will result in changes of the surface charge and hydrophobicity, which further affect membrane permeability. However, these changes do not damage intact cells or induce leakage of macromolecules, DNA or RNA. Moreover, treatment with TiO2 and Ag+ could lead to reduced soluble protein expression by suppressing the synthesis of nucleic acids, which further inhibited the growth of S. aureus. The result showed that TiO2 and Ag+ could cause slight lesion in the membrane to effect S. aureus membrane permeability, but not decomposition of membrane, and that TiO2 and Ag+ could inhibit S. aureus nucleic acid synthesis and reduce expression of soluble proteins against bacterial growth. Pleskova et al. studied bactericidal activity of TiO2 film and also found that S. aureus is swollen by TiO2 through damaging the cell membrane [27]. An et al. also showed that silver nanoparticles could cause DNA to concentrate, lead DNA to lose replication and degrade, and inhibit soluble protein synthesis. Silver nanoparticles could destroy the genetic material of bacteria, so that bacteria could not grow and reproduce [28]. Thus, TiO2 and Ag+ antibacterial action against S. aureus was probably through inhibiting the synthesis of nucleic acid, thereby reducing protein synthesis against bacterial growth.
It should be emphasized that the main aim of this study was to investigate the bactericidal activity of TiO2 and Ag+ and the underling mechanisms. Although TiO2 and Ag+ have been shown to exhibit strong bactericidal properties on S. aureus, it is important to emphasize that prior to their practical applications in medical instruments, efforts should be made to fully evaluate the potential carcinogenicity and in vivo toxicity of TiO2 and Ag+ against animal cells and tissues.
Funding information
This work was supported by Zhejiang Provincial Natural Science Foundation of China (ZJNSF LY14H150004).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: DAPI, 4',6-diamidino-2-phenylindole; TTC, 2,3,5-triphenyltetrazolium chloride; TTF, 2,3,5-triphenyltetrazolium formazan; VAP, ventilator-associated pneumonia.
References
- 1.Parida KM, Sahu N, Nruparaj S. Visible light induced photocatalytic activity of rare earth titania nanocomposites. J Mol Catal A Chem. 2008;287:151–158. doi: 10.1016/j.molcata.2008.02.028. [DOI] [Google Scholar]
- 2.Yu JC, Ho W, Yu J, Yip H, Wong PK, et al. Efficient visible-light-induced photocatalytic disinfection on sulfur-doped nanocrystalline titania. Environ Sci Technol. 2005;39:1175–1179. doi: 10.1021/es035374h. [DOI] [PubMed] [Google Scholar]
- 3.Zhang LS, Wong KH, Yip HY, Hu C, Yu JC, et al. Effective photocatalytic disinfection of E. coli K-12 using AgBr-Ag-Bi2WO6 nanojunction system irradiated by visible light: the role of diffusing hydroxyl radicals. Environ Sci Technol. 2010;44:1392–1398. doi: 10.1021/es903087w. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann J. Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal Today. 1999;53:115–129. doi: 10.1016/S0920-5861(99)00107-8. [DOI] [Google Scholar]
- 5.Li Q, Xie R, Mintz A, Shang J. Enhanced visible-light photocatalytic degradation of humic acid by palladium-modified nitrogen-doped titanium oxide. J Am Ceram Soc. 2007;92:3863–3868. [Google Scholar]
- 6.Kowalska E, Remita H, Colbeau-Justin C, Hupka J, Belloni J. Modification of titanium dioxide with platinum ions and clusters: application in photocatalysis. J Phys Chem C. 2008;112:1124–1131. doi: 10.1021/jp077466p. [DOI] [Google Scholar]
- 7.Bannat I, Wessels K, Oekermann T, Rathousky J, Bahnemann D, et al. Improving the photocatalytic performance of mesoporous titania films by modification with gold nanostructures. Chem Mater. 2009;21:1645–1653. doi: 10.1021/cm803455k. [DOI] [Google Scholar]
- 8.Guo L, Shan C, Liang J, Ni J, Tong M. Bactericidal mechanisms of Au@TNBs under visible light irradiation. Colloids Surf B Biointerfaces. 2015;128:211–218. doi: 10.1016/j.colsurfb.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Inoue Y, Uota M, Torikai T, Watari T, Noda I. Antibacterial properties of nanostructured silver titanate thin films formed on a titanium plate. J Biomed Mater Res A. 2009;92A:1171–1180. doi: 10.1002/jbm.a.32456. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Z, Ma J, Ng WJ, Waite TD, Zhao XS. Silver-modified mesoporous TiO2 photocatalyst for water purification. Water Res. 2011;45:2095–2103. doi: 10.1016/j.watres.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Wang X, Zhang H, Wu C, Shen Q, et al. Preparation and photocatalytic properties of silver loaded TiO2 mesoporous materials. Bull Chinese Ceram Soc. 2014;33:880–884. [Google Scholar]
- 12.Simchi A, Tamjid E, Pishbin F, Boccaccini A. Recent progress in inorganic and composite coatings with bactericidal capability for orthopaedic applications. Nanomed Nanotech Biol Med. 2011;7:22–39. doi: 10.1016/j.nano.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Lv B, Shen Q, Wang X. Preparation of silicon-modified antimicrobial polyethylene endotracheal tubes. J Biomed Mater Res B Appl Biomater. 2017;105:91–98. doi: 10.1002/jbm.b.33530. [DOI] [PubMed] [Google Scholar]
- 14.Zhou W, Liu H, Wang J, Liu D, du G, et al. Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. Am Chem Soc Appl Mater Interfaces. 2010;2:2385–2392. doi: 10.1021/am100394x. [DOI] [PubMed] [Google Scholar]
- 15.Chen F, Yang X, Xu F, Wu Q, Zhang Y. Correlation of photocatalytic bactericidal effect and organic matter degradation of TiO2. part I: observation of phenomena. Environ Sci Technol. 2009;43:1180–1184. doi: 10.1021/es802499t. [DOI] [PubMed] [Google Scholar]
- 16.Heidenau F, Mittelmeier W, Detsch R, Haenle M, Stenzel F, et al. A novel antibacterial titania coating: metal ion toxicity and in vitro surface colonization. J Mater Sci Mater Med. 2005;16:883–888. doi: 10.1007/s10856-005-4422-3. [DOI] [PubMed] [Google Scholar]
- 17.Baldi C, Minoia C, di Nucci A, Capodaglio E, Manzo L. Effects of silver in isolated rat hepatocytes. Toxicol Lett. 1988;41:261–268. doi: 10.1016/0378-4274(88)90063-X. [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Dai Z, Liu F, Kim H, Tong M, et al. Bactericidal mechanisms of Ag2O/TNBs under both dark and light conditions. Water Res. 2013;47:1837–1847. doi: 10.1016/j.watres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Tan Y, Tan G, Ning C, Rong X, Zhang Y, et al. Bioinspired polydopamine functionalization of titanium surface for silver nanoparticles immobilization with antibacterial property. J Inorg Mater. 2014;29:1320–1326. [Google Scholar]
- 20.Shi Z, Xue Q, Guo S. Preparation, photocatalytic property and antibacterial property of Ag@TiO2@SiO2 composite nanomaterials. J Inorg Mater. 2016;31:465–472. [Google Scholar]
- 21.Jin J-C, Xu Z-Q, Dong P, Lai L, Lan J-Y, et al. One-step synthesis of silver nanoparticles using carbon dots as reducing and stabilizing agents and their antibacterial mechanisms. Carbon N Y. 2015;94:129–141. doi: 10.1016/j.carbon.2015.05.084. [DOI] [Google Scholar]
- 22.Fang M, Gao J, Shen Q, Wang P, Yang H. Fine structure of TiO2 sol and its photocatalytic activity. Bull Chinese Ceram Soc. 2006;34:438–442. [Google Scholar]
- 23.Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23:3359–3368. doi: 10.1016/S0142-9612(02)00036-4. [DOI] [PubMed] [Google Scholar]
- 24.Pablos C, van Grieken R, Marugán J, Muñoz A. Simultaneous photocatalytic oxidation of pharmaceuticals and inactivation of Escherichia coli in wastewater treatment plant effluents with suspended and immobilised TiO2. Water Sci Technol. 2012;65:2016–2023. doi: 10.2166/wst.2012.868. [DOI] [PubMed] [Google Scholar]
- 25.Jiang X, Wang Y, Hua J, Lyu B. Study on antibacterial property of silver loaded titanium dioxide antibacterial coated endotracheal intubation tube [Chinese] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2014;26:343–346. doi: 10.3760/cma.j.issn.2095-4352.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Xu J, Cao J, Jiang X. A experiment on cytotoxicity test in vitro of antibacterial coating endotracheal tube with silver loaded titanium dioxide. Chinese J Integr Trad Western Med Intensive Crit Care. 2016;23:920–922. [Google Scholar]
- 27.Pleskova SN, Golubeva IS, Verevkin YK. Bactericidal activity of titanium dioxide ultraviolet-induced films. Mater Sci Eng C Mater Biol Appl. 2016;59:807–817. doi: 10.1016/j.msec.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 28.An J, Zhang H, Zhang J, Zhao Y, Yuan X. Preparation and antibacterial activity of electrospun chitosan/poly(ethylene oxide) membranes containing silver nanoparticles. Colloid Polym Sci. 2009;287:1425–1434. doi: 10.1007/s00396-009-2108-y. [DOI] [Google Scholar]




