Abstract
Objective
To investigate potential associations between dietary intake of polyunsaturated fatty acids (FAs) and pain patterns in early rheumatoid arthritis (RA) patients after 3 months of methotrexate (MTX) treatment.
Methods
We included 591 early RA patients with MTX monotherapy from a population‐based prospective case–control study, the Epidemiological Investigation of Rheumatoid Arthritis. Dietary data on polyunsaturated FAs (food frequency questionnaires) were linked with data on unacceptable pain (visual analog scale [VAS] >40 mm), noninflammatory/refractory pain (VAS >40 mm and C‐reactive protein [CRP] level <10 mg/liter), and inflammatory pain (VAS >40 mm and CRP level >10 mg/liter) after 3 months. Statistical analysis included logistic regression.
Results
After 3 months of MTX treatment, 125 patients (21.2%) had unacceptable pain, of which 92 patients had refractory pain, and 33 patients had inflammatory pain. Omega‐3 FA intake was inversely associated with unacceptable pain and refractory pain (odds ratio [OR] 0.57 [95% confidence interval (95% CI) 0.35–0.95] and OR 0.47 [95% CI 0.26–0.84], respectively). The omega‐6:omega‐3 FA ratio, but not omega‐6 FA alone, was directly associated with unacceptable pain and refractory pain (OR 1.70 [95% CI 1.03–2.82] and OR 2.33 [95% CI 1.28–4.24], respectively). Furthermore, polyunsaturated FAs were not associated with either inflammatory pain or CRP level and erythrocyte sedimentation rate at followup. Omega‐3 FA supplementation was not associated with any pain patterns.
Conclusion
Omega‐3 FA was inversely associated with, and the omega‐6:omega‐3 FA ratio was directly associated with, unacceptable and refractory pain, but not with inflammatory pain or systemic inflammation. The inverse association between omega‐3 FA and refractory pain may have a role in pain suppression in RA.
Introduction
Pain is a dominant and common feature of rheumatoid arthritis (RA) and a cause of both distress and decreased work capacity 1. In early disease, pain is usually related to inflammation and correlates well with disease activity 2. However, some patients continue to exhibit high levels of pain, even after adequate antirheumatic treatment. Thus, earlier data have shown that a subset of patients has significant pain persistence in low disease activity 3, 4. Moreover, remaining pain in early RA may also be common after good treatment responses 5, 6.
Significance & Innovations.
This study is the first, to our knowledge, to examine the association between dietary intake of omega‐3 fatty acid and omega‐6 fatty acid and noninflammatory pain in a large group of patients with early rheumatoid arthritis.
The results of this study suggest that dietary omega‐3 fatty acid may be associated with noninflammatory chronic pain in methotrexate‐treated patients with early rheumatoid arthritis.
Omega‐3 fatty acids (FAs) and omega‐6 FAs are polyunsaturated FAs that have been associated with inflammation. Earlier data suggest that omega‐3 FAs are antiinflammatory and can decrease disease activity in RA 7. Also, excessive intake of omega‐6 FA as well as increased omega‐6:omega‐3 FA ratio have been linked with proinflammatory properties in RA 8. Increased dietary intake of omega‐3 FA has been associated with decreased serum levels of tumor necrosis factor (TNF) and C‐reactive protein (CRP) in RA. Higher omega‐6 FA intake, on the other hand, has been associated with increased serum levels of interleukin 6 (IL‐6) and CRP in RA 9. Although evidence exists on reduced CRP level and erythrocyte sedimentation rate (ESR) with increased omega‐3 FA, there are still no clear results regarding the long‐term effect of omega‐3 FA on inflammatory parameters 10. Important derivatives of omega‐3 FA are resolvins, protectins, and lipoxins. These nonclassical eicosanoids have antiinflammatory properties, and resolvins have been linked with pain suppression in experimental models 11, 12. Based on these observations, we hypothesized that dietary intake of omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio may affect not only inflammatory mechanisms, but also pain in RA. Therefore, we sought to investigate potential associations between dietary intake of these FAs and different pain patterns after antirheumatic treatment in early RA.
Patients and methods
Study population
This study included primarily 1,296 newly diagnosed RA patients (disease duration ≤12 months) from a population‐based prospective case–control study called Epidemiological Investigation of Rheumatoid Arthritis (EIRA). Controls from the general population had been matched by age, sex, and area of residence (n = 2,632). EIRA began in May 1996, and the study design has previously been described 13. EIRA has been linked to the Swedish Rheumatology Quality register to provide clinical data. Patients who had completed food frequency questionnaires (FFQs) at EIRA inclusion/baseline, and who had been on methotrexate (MTX) monotherapy for at least the first 3 months from baseline, were included. The inclusion period for this study was from October 2005 to March 2012. Patients who did not fully complete the FFQ, had other disease‐modifying antirheumatic drugs than MTX during the first 3 months, or had missing data on a visual analog scale (VAS) for pain and CRP level after 3 months, were excluded. After patient exclusion, 591 patients remained. An overview of the patient exclusions is shown in Figure 1.
Figure 1.
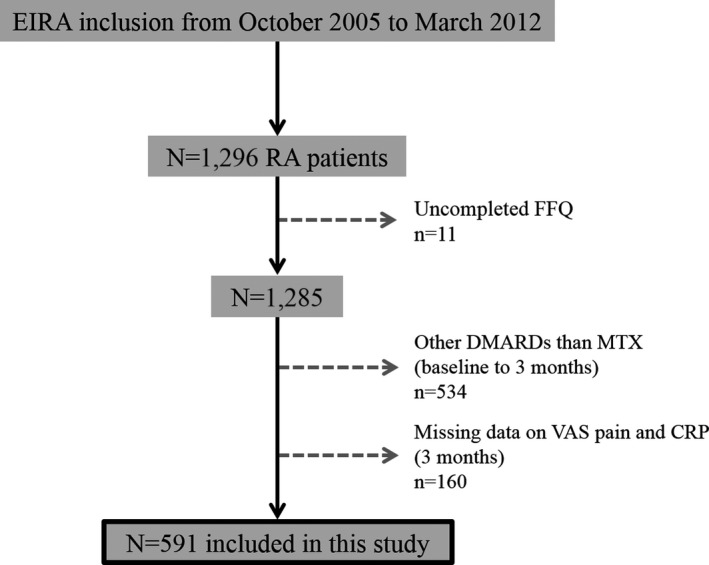
Overview of patient exclusions. EIRA = Epidemiological Investigation of Rheumatoid Arthritis; RA = rheumatoid arthritis; FFQ = food frequency questionnaire; DMARD = disease‐modifying antirheumatic drug; MTX = methotrexate; VAS = visual analog scale; CRP = C‐reactive protein.
Dietary assessment
Patients were asked to complete an FFQ at baseline regarding their food intake and habits. This specific self‐administered, semiquantitative FFQ included questions regarding patients’ frequency intake of 123 food items and beverages during the previous year from baseline. Frequency food intake ranged over 8 categories from never to ≥3 times per day. Furthermore, information on portion size (i.e., small, medium, large) or quantity (i.e., slice, cup, glass, deciliter) of frequently consumed food items in Sweden was obtained. Dietary nutrient intakes (grams/day) of omega‐3 FA (FA C18:3/10 + FA C20:5 + FA C22:5 + FA C22:6) 14, 15 and omega‐6 FA (FA C18:2 + FA C20:4) were calculated by multiplying the average frequency of consumption of each food item by the nutrient content, obtained from the Swedish National Food Administration Database 16. All dietary nutrients were energy adjusted using the residual method 17. This dietary assessment method and the validation of this FFQ have been described previously 18, 19. The FFQ also included questions regarding patients’ supplement use of omega‐3 FA/fish oil.
Definition of pain outcome
Pain in the previous week was assessed according to a VAS (range 0–100 mm) at baseline and at 3 months of treatment. Information on pain assessment was retrieved from the Swedish Rheumatology Quality register. Patient acceptable symptom state (PASS) is a validated measure that indicates the level of acceptable pain, among other clinical measures 20. PASS uses a threshold of VAS 40 mm, meaning that VAS >40 mm indicates unacceptable pain. Based on PASS, we defined a new outcome, refractory pain, which indicates pain in spite of inflammatory control. Refractory pain was defined as VAS pain >40 mm and CRP level <10 mg/liter 21 after antirheumatic treatment. In addition, inflammatory pain after treatment was defined as VAS pain >40 mm and CRP level >10 mg/liter. As a control group for refractory pain and inflammatory pain, we included patients with VAS pain <40 mm together with CRP level <10 mg/liter on the same visit as mentioned above. An overview of each defined pain group is presented in Figure 2.
Figure 2.
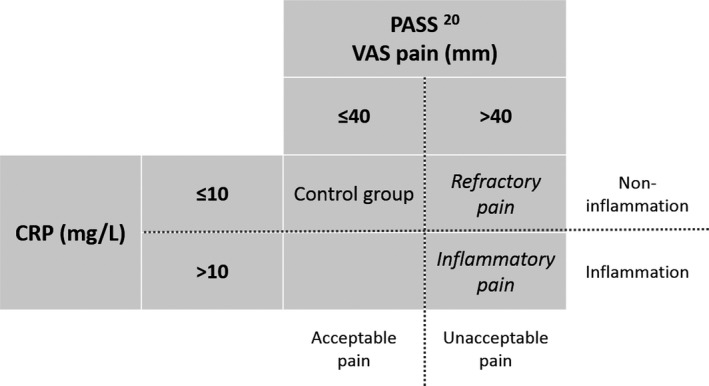
Definition of pain groups. PASS = patient acceptable symptom state; VAS = visual analog scale; CRP = C‐reactive protein.
Demographic and lifestyle variables
Data on age, smoking, weight and height, education level, and physical activity were obtained through the EIRA questionnaire. Patients were asked to report their smoking habit. Smoking status was categorized as current, occasional, former, and never smoker, and smoking intensity was based on pack‐years; 1 pack‐year was equal to 20 smoked cigarettes, cigars, and/or pipes per day during 1 year. Body mass index (BMI) was calculated based on weight (kg) and height (m) as kg/m2. Education level was categorized into high school degree and university degree. Patients were asked to report the level of physical activity that they performed during the previous year from baseline, based on 4 categories, ranging from low to high physical activity: sedentary physical activity, occasional moderate physical activity, regular moderate physical activity, and regular exercise. Disease activity levels were based on the 28‐joint Disease Activity Score (DAS28) and were classified according to the definition of the European League Against Rheumatism (EULAR) 22.
Statistical analysis
Statistical analysis was performed with SPSS statistics software, version 23. Baseline characteristics and dietary intake between subgroups were compared with the Mann‐Whitney U test for continuous variables (mean ± SD) as well as Pearson's chi‐square test for proportions (%). Associations between dietary intake of omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio and unacceptable pain, refractory pain, and inflammatory pain were analyzed with logistic regression. In addition, associations between dietary omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio and inflammatory parameters, such as CRP level, ESR, swollen joint count (SJC), and DAS28 after 3 months, were studied in sensitivity analyses. All analyses were adjusted for potential confounders such as age, sex, smoking intensity, total energy intake, omega‐3 FA/fish oil supplementation, BMI, education, and physical activity. Dietary intake of omega‐3 FA and omega‐6 FA, and the omega‐6:omega‐3 FA ratio, were divided into tertiles based on the intake of the total EIRA sample, including matched controls; the first tertile was defined as referent group. Demographics and lifestyle variables were categorized as: age into 11 age groups with a 5‐year range for each, BMI into <25 and >25 kg/m², education into high school degree and university degree, physical activity level into either sedentary to occasional moderate physical activity, or regular moderate physical activity to regular exercise, total energy intake into tertiles, and supplementation of omega‐3 FA/fish oil into yes and no. Smoking adjustment was based on pack‐years. All levels of significance were set at 0.05.
Results
Patient characteristics
This study included 591 RA patients. Baseline characteristics are presented in Table 1. Overall, the disease activity of the participants was high at baseline, with a mean DAS28 of 5.2. Of the total study sample, 30.8% were smokers, 24.0% had obtained a university degree, and 31.6% performed regular physical activity or regular exercise.
Table 1.
Baseline characteristics (n = 591)a
| Characteristics | Total |
|---|---|
| Female, % | 70.6 |
| Age, years | 52.8 ± 13.0 |
| Body mass index, kg/m² | 25.8 ± 4.7 |
| Smoking status: never/former/current, % | 33.0/35.9/30.8 |
| Symptom duration, days | 289.7 ± 390.6 |
| Rheumatoid factor positive, % | 65.3 |
| Anti–citrullinated protein antibody positive, % | 67.9 |
| Disease Activity Score in 28‐joints | 5.2 ± 1.3 |
| C‐reactive protein, mg/liter | 22.4 ± 27.2 |
| Erythrocyte sedimentation rate (0–100 mm) | 32.0 ± 21.7 |
| HAQ visual analog scale (0–100 mm) | 1.1 ± 0.6 |
| Pain visual analog scale (0–100 mm) | 53.9 ± 24.7 |
| Physician's global assessment (5‐point scale) | 2.2 ± 0.7 |
| Patients’ global assessment (0–100 mm) | 50.5 ± 24.4 |
| SJC, mean ± SD / median (IQR) | 9.3 ± 5.5 / 9 (5–12) |
| TJC, mean ± SD / median (IQR) | 8.2 ± 5.9 / 7 (4–12) |
| Methotrexate, % | 100.0 |
| Glucocorticoids, % | 59.1 |
| Cyclooxygenase‐1 inhibitor, % | 52.6 |
| Cyclooxygenase‐2 inhibitor, % | 1.7 |
| Omega‐3 fatty acid/fish oil supplementation, % | 19.5 |
| Omega‐3 fatty acid intake, grams/day | 0.7 ± 0.4 |
| Omega‐6 fatty acid intake, grams/day | 7.6 ± 2.3 |
| Omega‐6:omega‐3 fatty acid ratio | 13.4 ± 7.2 |
Values are the mean ± SD unless indicated otherwise. HAQ = Health Assessment Questionnaire; SJC = swollen joint count; IQR = interquartile range; TJC = tender joint count.
After 3 months of MTX treatment, 125 patients (21.2%) had unacceptable pain, of which 92 patients (15.6%) had refractory pain, and 33 patients (5.6%) had inflammatory pain. When comparing baseline characteristics between subgroups, patients with refractory pain, compared to the control group (n = 420), had significantly higher BMI (mean ± SD 26.5 ± 4.5 versus 25.3 ± 4.3; P = 0.013), DAS28 (mean ± SD 5.4 ± 1.4 versus 5.0 ± 1.3; P = 0.017), Health Assessment Questionnaire (HAQ) score (mean ± SD 1.3 ± 0.6 versus 1.0 ± 0.6; P < 0.001), VAS pain (mean ± SD 61 ± 23 versus 51 ± 25; P < 0.001), patient's global assessment score (mean ± SD 58 ± 21 versus 48 ± 25; P < 0.001), and tender joint count (TJC) (mean ± SD 10 ± 6 versus 8 ± 6; P = 0.003) at baseline. Patients with refractory pain also had a significantly higher proportion of current smokers compared to the control group (42.4% and 30.9%, respectively; P = 0.009).
The mean ± SD energy intake of the total study sample was 1,956.6 ± 685.8 kcal/day and was normally distributed. The intake of omega‐3 FA and omega‐6 FA, and the omega‐6:omega‐3 FA ratio, for the whole study group was mean ± SD 0.7 ± 0.5 grams/day, 7.6 ± 2.3 grams/day, and 13.5 ± 7.2, respectively. Omega‐3 FA/fish oil supplementation was used by 19.5% of the patients. Omega‐3 FA intake was found to be lower in patients with refractory pain compared to those without refractory pain (mean ± SD 0.6 ± 0.3 versus 0.7 ± 0.4 grams/day; P = 0.006).
Association between dietary intake of polyunsaturated FAs and pain
Omega‐3 FA was inversely associated with, and the omega‐6:omega‐3 FA ratio was directly associated with, unacceptable pain, after adjustment for age, sex, smoking, total energy intake, and omega‐3 FA/fish oil supplementation (odds ratio [OR] 0.57 [95% confidence interval (95% CI) 0.35–0.95] and OR 1.70 [95% CI 1.03–2.82], respectively). These associations were based on comparison between the 3rd and the 1st tertile. Additional adjustments for anti–citrullinated protein antibody, BMI, education, and physical activity did not change the ORs remarkably. Omega‐6 FA alone did not significantly associate with unacceptable pain (OR 1.27 [95% CI 0.73–2.20]). Similar results were seen when analyzing the association between omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio and refractory pain (OR 0.47 [95% CI 0.26–0.84], OR 1.57 [95% CI 0.82–3.03], and OR 2.33 [95% CI 1.28–4.24], respectively). In contrast, omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio did not associate with inflammatory pain (OR 1.07 [95% CI 0.45–2.56], OR 0.81 [95% CI 0.32–2.07], and OR 0.65 [95% CI 0.26–1.62], respectively) (Table 2). Furthermore, the prevalence of omega‐3 FA/fish oil supplementation did not differ significantly between patients with and without unacceptable pain, and no statistically significant association was observed between omega‐3 FA/fish oil supplementation and unacceptable pain, after adjustment for age, sex, smoking, total energy intake, and dietary intake of omega‐3 FA (OR 0.97 [95% CI 0.58–1.64]).
Table 2.
Association between dietary intakes of omega‐3, omega‐6, and omega‐6:omega‐3 FA ratio and pain after 3 months of MTX treatment (n = 591)a
| Polyunsaturated FA intake (grams/day) | No. | Unacceptable pain | Refractory pain | Inflammatory pain |
|---|---|---|---|---|
| Omega‐3 FA, tertiles | ||||
| 1st ≤0.48 | 206 | 1.00 | 1.00 | 1.00 |
| 2nd 0.49–0.77 | 186 | 0.67 (0.41–1.09) | 0.65 (0.38–1.13) | 0.78 (0.30–1.97) |
| 3rd ≥0.78 | 199 | 0.57 (0.35–0.95)b | 0.47 (0.26–0.84)b | 1.07 (0.45–2.56) |
| P c | – | 0.029 | 0.011 | 0.873 |
| Omega‐6 FA, tertiles | ||||
| 1st ≤6.00 | 192 | 1.00 | 1.00 | 1.00 |
| 2nd 6.01–8.19 | 196 | 1.43 (0.85–2.38) | 1.89 (1.03–3.45)b | 0.79 (0.32–1.98) |
| 3rd ≥8.20 | 203 | 1.27 (0.73–2.20) | 1.57 (0.82–3.03) | 0.81 (0.32–2.07) |
| P c | – | 0.395 | 0.175 | 0.665 |
| Omega‐6:omega‐3 FA, tertiles | ||||
| 1st ≤9.74 | 202 | 1.00 | 1.00 | 1.00 |
| 2nd 9.75–14.19 | 191 | 1.07 (0.63–1.83) | 1.31 (0.68–2.49) | 0.63 (0.26–1.53) |
| 3rd ≥14.20 | 198 | 1.70 (1.03–2.82)b | 2.33 (1.28–4.24)b | 0.65 (0.26–1.62) |
| P c | – | 0.039 | 0.006 | 0.353 |
Values are the odds ratio (95% confidence interval) unless indicated otherwise, with adjustments for age, sex, smoking pack‐years, total energy intake, and omega‐3 FA/fish oil supplementation. Unacceptable pain: visual analog (VAS) pain >40 mm. Refractory pain: VAS pain >40 mm and C‐reactive protein (CRP) level <10 mg/liter. Inflammatory pain: VAS pain >40 mm and CRP level >10 mg/liter. FA = fatty acids; MTX = methotrexate.
Statistically significant.
Comparison between 3rd and 1st tertiles.
To test whether the low inflammatory state contributed in a major way to these results, we performed sensitivity analyses using inflammatory parameters. These analyses showed that dietary omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio did not significantly associate with either CRP level, ESR, SJC, or DAS28 after 3 months (Table 3). In addition, there was no significant association observed between use of omega‐3/fish oil supplements and any of the inflammatory markers, after adjustment for age, sex, smoking, total energy intake, and dietary intake of omega‐3 FA (OR 1.04 [95% CI 0.56–1.94] for CRP level, OR 1.15 [95% CI 0.75–1.76] for ESR, OR 1.20 [95% CI 0.82–1.94] for SJC, and OR 1.02 [95% CI 0.67–1.56] for DAS28).
Table 3.
Dietary intake of omega‐3, omega‐6, and omega‐6:omega‐3 FA ratio and their association with inflammatory parameters (CRP, ESR, SJC, and DAS28) after 3 months of MTX treatmenta
| Polyunsaturated FA intake (grams/day) | No. | OR (95 % CI) | P |
|---|---|---|---|
| CRP >12.44 mg/liter (median) | |||
| Omega‐3 FA | 581 | 1.10 (0.73–1.65) | 0.665 |
| Omega‐6 FA | 581 | 1.07 (0.68–1.66) | 0.772 |
| Omega‐6:omega‐3 FA | 581 | 0.95 (0.68–1.43) | 0.800 |
| ESR >13.00 mm (median) | |||
| Omega‐3 FA | 561 | 0.97 (0.64–1.49) | 0.904 |
| Omega‐6 FA | 561 | 0.94 (0.60–1.48) | 0.798 |
| Omega‐6:omega‐3 FA | 561 | 0.85 (0.56–1.29) | 0.446 |
| SJC >1 swollen joint (median) | |||
| Omega‐3 FA | 579 | 0.92 (0.60–1.41) | 0.694 |
| Omega‐6 FA | 579 | 0.86 (0.56–1.42) | 0.640 |
| Omega‐6:omega‐3 FA | 579 | 0.93 (0.61–1.42) | 0.743 |
| DAS28 >3.2 | |||
| Omega‐3 FA | 559 | 0.70 (0.46–1.07) | 0.096 |
| Omega‐6 FA | 559 | 1.11 (0.71–1.72) | 0.654 |
| Omega‐6:omega‐3 FA | 559 | 1.24 (0.82–1.87) | 0.317 |
odds ratio (OR) adjusted for age, sex, smoking pack‐years, total energy intake, and omega‐3 fatty acid (FA)/fish oil supplementation. Comparison between 3rd and 1st tertiles. CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; SJC = swollen joint count; DAS28 = 28‐joint Disease Activity Score; MTX = methotrexate; 95% CI = 95% confidence interval.
Discussion
This study is to our knowledge the first to examine the association between dietary intake of polyunsaturated FAs and pain patterns in early RA patients. We found that higher intake of omega‐3 FA was inversely associated with both unacceptable pain and refractory pain. A higher omega‐6:omega‐3 FA ratio was directly associated with unacceptable pain and refractory pain. In contrast, neither omega‐3 FA, omega‐6 FA, nor the omega‐6:omega‐3 FA ratio were significantly associated with inflammatory pain or key inflammatory parameters at 3 months. These data suggest inflammatory independent associations between omega‐3 FA and the omega‐6:omega‐3 FA ratio and pain in early RA.
Pain is the most important symptom in RA and often brings the patient to health care for the first time. In earlier literature, there is a discrepancy between pain and inflammation in the course of RA. Lee et al 3 showed in 2012 that DAS28 remission does not exclude the persistence of significant pain. Moreover, we have earlier shown that remaining pain is common in early RA despite antirheumatic treatment 6. In the present study, we sought to investigate the prevalence not only of inflammatory‐related pain, but also pain in spite of inflammatory control. Although pain reduction is expected after MTX initiation in a majority of early RA patients, our data showed that 15.6% of all the patients still experienced pain after 3 months, in spite of low inflammation (refractory pain). A high frequency of significant pain after antirheumatic treatment is supported by other reports in the literature. For example, Andersson et al 23 reported chronic widespread pain in over one‐third of patients with established RA. Moreover, there are several reports of higher prevalence of fibromyalgia in patients with RA compared to the general population 24. Widespread pain is common in RA and has been associated with high levels of pain, fatigue, and sleep problems 25, especially during the first year after RA diagnosis 26. Diagnosis of fibromyalgia was not in the scope of the present report, but the comparably high prevalence of refractory pain after 3 months is indicative that pain in spite of inflammation is a significant problem in early RA, thus, immunosuppressive therapy is not likely to have further major effects on decreasing pain. Patients with refractory pain had higher BMI, DAS28, HAQ score, VAS pain, patient's global assessment score, and TJC at baseline compared to the control group, and these results are in line with previous findings 6, 27. HAQ scores have been observed to be higher in RA patients with fibromyalgia 28. The patient's global assessment and TJC have also shown to strongly correlate with pain and DAS28 in RA patients 29, 30.
In this study, we used the well‐defined PASS for pain assessment, which was described earlier and validated as the level of definite unacceptable pain, the result of the question “If you were to remain for the rest of your life as you were during the last 48 hours, would that be acceptable or unacceptable for you?” However, PASS alone is a subjective measure, and therefore we considered an additional objective measure that could differentiate noninflammatory pain from inflammatory pain. Low inflammation was defined as a CRP level <10 mg/liter. According to the American College of Rheumatology/EULAR definition of remission in RA, a CRP level of ≤10 mg/liter has been associated with low inflammatory RA core set measures, such as ESR, SJC, and DAS28, within the remission interval 21. Of note, there are also other assessments for noninflammatory pain described earlier in the literature. For instance, DAS28‐P, a modified DAS28 based on the patient's global assessment and TJC, has been used to measure noninflammatory pain in early RA 31. However, we did not consider DAS28‐P as appropriate for the current analysis, since we instead needed a dichotomous cutoff mirroring pain that the patient would find acceptable or unacceptable. Therefore, we instead used PASS in combination with low inflammation as stated above.
We investigated the dietary intake of polyunsaturated FAs in RA patients and its association with pain and inflammation. Omega‐3 FA and omega‐6 FA are essential nutrients and need to be obtained through diet or supplementation. These 2 FAs are distinguished based on the location of the first double bond, counting from the methyl end of the FA chain. As mentioned earlier, omega‐3 FA has been found to have antiinflammatory properties 7, 32, and omega‐6 FA has been linked to proinflammatory actions in RA 8. A diet rich in omega‐3 FA may have beneficial antiinflammatory effects for chronic diseases such as RA 32, but omega‐3 FA was not associated with high inflammation. This fact was illustrated by a trend towards negative association with DAS28 (OR 0.70; P = 0.096), which is in line with earlier data 7, 32. Earlier studies on clinical effects of omega‐3 FA supplementation in RA found primarily reduction of TJC and morning stiffness, and not peripheral inflammation to the same extent 7.
The results of this study may be interpreted as omega‐3 FA also having inflammation‐independent actions on pain. This interpretation was also supported by the lack of associations between omega‐3 FA and inflammatory parameters at the 3‐month followup, including inflammatory pain. In addition, omega‐3 FA has earlier been linked to the production of mediators involved in pain suppression. The latter include resolvins, protectins, and lipoxins, which are omega‐3 FA–derived nonclassical eicosanoids with antiinflammatory properties. Interestingly, resolvins (e.g., RvE1, RvD1) have been shown to directly suppress pain in experimental models 11, 12. These effects are mediated through inhibition of the actions of transient receptor potential cation (TRP) channels, such as TRPV1 and TRPA1 16, both known to be strongly implicated in nociceptive mechanisms 33, 34. Based on the direct effects of RvD on nociceptive mechanisms, we might hypothesize that higher levels and activity of these mediators, related to an increased intake of omega‐3 FA, may result in direct effects on pain perception, although the exact clinical mechanisms need to be further explored. Potential neuroprotective actions of omega‐3 FA are further supported by earlier reports that omega‐3 FA can decrease neuroinflammation in animal models 35. Notably, RvD1 has also been found to have pro‐resolving and cartilage protective actions in inflammatory arthritis 36, and resolvins may also inhibit the production of proinflammatory omega‐6 FA–derived eicosanoids 37, leading to inflammation resolution. Another potential explanation for omega‐3 FA effects on pain in RA may be the interaction with microbiota in the gut. Omega‐3 FA supplementation was earlier shown to affect the gut microbiome in rodents 38, and intervention with gut microbiome regulation has been shown to affect neurodevelopment, also with potential effects on nociceptive mechanisms 39.
Earlier literature shows that a low ratio between omega‐6 FA and omega‐3 FA (recommended ratio range 1:1–4:1) needs to be maintained in order to avoid trigger of inflammation 40. In the present study, omega‐6 FA alone was not associated with unacceptable pain or refractory pain, but omega‐6:omega‐3 FA ratio was significantly associated with increased risk of unacceptable pain and refractory pain. A high omega‐6:omega‐3 FA ratio increases the risk for obesity 41, and high BMI has been associated both with high disease severity and pain in RA 42. Thus, obese RA patients were shown to have increased pain levels. In our study, associations between BMI, DAS28, and ESR and unacceptable pain and refractory pain were also confirmed (results not shown). Therefore, we adjusted the results for BMI, but with remaining associations between FA and refractory pain. Thus, the association between these polyunsaturated FAs and refractory pain seems to be independent of the BMI of the early RA patients in this context.
There are contradictory results regarding the benefits of omega‐3 FA supplementation. Several studies have shown that omega‐3 FA supplementation lowers inflammatory markers such as CRP, IL‐6, and TNF levels 43. In addition, cod liver oil supplements containing omega‐3 FA can be used as a sparing agent of nonsteroidal antiinflammatory drugs in RA patients 44, 45. Other results have not shown a superior clinical effect of omega‐3 FA supplementation 46. In our study, we found no association between use of omega‐3 FA/fish oil supplementation and refractory pain or inflammatory parameters. Thus, our data on potential protective effects of omega‐3 FA on pain should not be interpreted as showing that supplementation can be used therapeutically to reduce pain.
Regular physical activity before the onset of RA has been associated with reduced disease severity and pain 47. Moreover, patients with higher physical activity may have a different diet than other patients, and this diet could be composed of higher content of omega‐3 FA. However, when we stratified for physical activity in the present study, our results showed that physical activity levels did not differ between patients with refractory pain and the control group. In addition, further adjustment for physical activity in our main analyses did not change the ORs markedly.
Dietary data from FFQs were based on estimated dietary consumption. Recall bias as well as under‐ and overreporting may have occurred when completing the FFQ. Dietary patterns were assumed to be unchanged during the first 3 months from baseline. Clinical manifestations, treatment history, adherence, doses, and side effects of MTX from diagnosis were not considered in this study. Moreover, we had no data on depression or psychological factors, which are known to associate with self‐reported pain in RA 48. However, due to the registry‐based approach, we believe that the impact of depression on the current results should be limited. The VAS indicated overall pain and not disease‐related pain. In this study, refractory pain was defined as noninflammatory pain, but a CRP level <10 mg/liter may still indicate some active inflammation.
In conclusion, omega‐3 FA was inversely, and the omega‐6:omega‐3 FA ratio was directly, associated with both unacceptable pain and refractory pain after 3 months of MTX treatment. These associations seemed to be independent of inflammation. A higher intake of omega‐3 FA may have reduced the central sensitization in patients in the control group. In addition, omega‐3 FA, omega‐6 FA, and the omega‐6:omega‐3 FA ratio did not associate with inflammatory pain nor with key inflammatory parameters after 3 months. Our data suggest that dietary omega‐3 FA may associate with noninflammatory chronic pain in MTX‐treated patients with early RA.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Lourdudoss had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Lourdudoss, Di Giuseppe, van Vollenhoven, Lampa.
Acquisition of data
Alfredsson.
Analysis and interpretation of data
Lourdudoss, Di Giuseppe, Wolk, Westerlind, Klareskog, van Vollenhoven, Lampa.
References
- 1. De Croon EM, Sluiter JK, Nijssen TF, Dijkmans BA, Lankhorst GJ, Frings‐Dresen MH. Predictive factors of work disability in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2004;63:1362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odegard S, Finset A, Mowinckel P, Kvien TK, Uhlig T. Pain and psychological health status over a 10‐year period in patients with recent onset rheumatoid arthritis. Ann Rheum Dis 2007;66:1195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 2011;13:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koop SM, ten Klooster PM, Vonkeman HE, Steunebrink LM, van de Laar MA. Neuropathic‐like pain features and cross‐sectional associations in rheumatoid arthritis. Arthritis Res Ther 2015;17:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti‐tumor necrosis factor therapy. J Rheumatol 2007;34:1674–83. [PubMed] [Google Scholar]
- 6. Altawil R, Saevarsdottir S, Wedren S, Alfredsson L, Klareskog L, Lampa J. Remaining pain is common in early RA patients treated with methotrexate: results from the EIRA cohort and the Swedish Rheumatology Quality Register [abstract]. Arthritis Rheum 2013;65:S340. [Google Scholar]
- 7. Ariza‐Ariza R, Mestanza‐Peralta M, Cardiel MH. Omega‐3 fatty acids in rheumatoid arthritis: an overview. Semin Arthritis Rheum 1998;27:366–70. [DOI] [PubMed] [Google Scholar]
- 8. James MJ, Cleland LG. Dietary n‐3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum 1997;27:85–97. [DOI] [PubMed] [Google Scholar]
- 9. Sundrarjun T, Komindr S, Archararit N, Dahlan W, Puchaiwatananon O, Angthararak S, et al. Effects of n‐3 fatty acids on serum interleukin‐6, tumour necrosis factor‐alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. J Int Med Res 2004;32:443–54. [DOI] [PubMed] [Google Scholar]
- 10. Ruggiero C, Lattanzio F, Lauretani F, Gasperini B, Andres‐Lacueva C, Cherubini A. Omega‐3 polyunsaturated fatty acids and immune‐mediated diseases: inflammatory bowel disease and rheumatoid arthritis. Curr Pharm Des 2009;15:4135–48. [DOI] [PubMed] [Google Scholar]
- 11. Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 2010;16:592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bang S, Yoo S, Yang TJ, Cho H, Kim YG, Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti‐nociception. Br J Pharmacol 2010;161:707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case‐control study, using incident cases. Ann Rheum Dis 2003;62:835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerster H. Can adults adequately convert alpha‐linolenic acid (18:3n‐3) to eicosapentaenoic acid (20:5n‐3) and docosahexaenoic acid (22:6n‐3)? Int J Vitamin Nutr Res 1998;68:159–73. [PubMed] [Google Scholar]
- 15. Davis BC, Kris‐Etherton PM. Achieving optimal essential fatty acid status in vegetarians: current knowledge and practical implications. Am J Clin Nutr 2003;78:640s–6s. [DOI] [PubMed] [Google Scholar]
- 16. Bergström LK, Hagman U, Eriksson HB, Bruce Å. Livsmedelsdatasystemet KOST: livsmedelsverkets system för information om näringsvärden i livsmedel. Vår Föda 1991;43:439–47. [Google Scholar]
- 17. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65 Suppl:1220S–8S. [DOI] [PubMed] [Google Scholar]
- 18. Messerer M, Johansson SE, Wolk A. The validity of questionnaire‐based micronutrient intake estimates is increased by including dietary supplement use in Swedish men. J Nutr 2004;134:1800–5. [DOI] [PubMed] [Google Scholar]
- 19. Larsson SC, Hakansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst 2006;98:407–13. [DOI] [PubMed] [Google Scholar]
- 20. Pham T, Tubach F. Patient acceptable symptomatic state (PASS). Joint Bone Spine 2009;76:321–3. [DOI] [PubMed] [Google Scholar]
- 21. Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 23. Andersson ML, Svensson B, Bergman S. Chronic widespread pain in patients with rheumatoid arthritis and the relation between pain and disease activity measures over the first 5 years. J Rheumatol 2013;40:1977–85. [DOI] [PubMed] [Google Scholar]
- 24. Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia. I: examination of rates and predictors in patients with rheumatoid arthritis (RA). Pain 2011;152:291–9. [DOI] [PubMed] [Google Scholar]
- 25. Ulus Y, Akyol Y, Tander B, Durmus D, Bilgici A, Kuru O. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol 2011;29:S92–6. [PubMed] [Google Scholar]
- 26. Lee YC, Lu B, Boire G, Haraoui BP, Hitchon CA, Pope JE, et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis 2013;72:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kronisch C, McLernon DJ, Dale J, Paterson C, Ralston SH, Reid DM, et al. Predicting functional disability: one‐year results from the Scottish Early Rheumatoid Arthritis Inception Cohort. Arthritis Rheumatol 2016;68:1596–602. [DOI] [PubMed] [Google Scholar]
- 28. Ranzolin A, Tavares Brenol JC, Bredemeier M, Guarienti J, Rizzatti M, Feldman D, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and Short Form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum 2009;61:794–800. [DOI] [PubMed] [Google Scholar]
- 29. Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken) 2012;64:206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 2010;49:924–8. [DOI] [PubMed] [Google Scholar]
- 31. Joharatnam N, McWilliams DF, Wilson D, Wheeler M, Pande I, Walsh DA. A cross‐sectional study of pain sensitivity, disease‐activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther 2015;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti‐inflammatory potential of long‐chain omega‐3 fatty acids. Nutr Rev 2010;68:280–9. [DOI] [PubMed] [Google Scholar]
- 33. Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002;36:57–68. [DOI] [PubMed] [Google Scholar]
- 34. Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, et al. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 2007;117:1979–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trepanier MO, Hopperton KE, Orr SK, Bazinet RP. N‐3 polyunsaturated fatty acids in animal models with neuroinflammation: an update. Eur J Pharmacol 2016;785:187–206. [DOI] [PubMed] [Google Scholar]
- 36. Norling LV, Headland SE, Dalli J, Arnardottir HH, Haworth O, Jones HR, et al. Proresolving and cartilage‐protective actions of resolvin D1 in inflammatory arthritis. JCI Insight 2016;1:e85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie 2009;91:791–5. [DOI] [PubMed] [Google Scholar]
- 38. Pusceddu MM, El Aidy S, Crispie F, O'Sullivan O, Cotter P, Stanton C, et al. N‐3 Polyunsaturated fatty acids (PUFAs) reverse the impact of early‐life stress on the gut microbiota. PloS One 2015;10:e0139721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. O'Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, et al. Disturbance of the gut microbiota in early‐life selectively affects visceral pain in adulthood without impacting cognitive or anxiety‐related behaviors in male rats. Neuroscience 2014;277:885–901. [DOI] [PubMed] [Google Scholar]
- 40. Simopoulos AP. The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomed Pharmacother 2002;56:365–79. [DOI] [PubMed] [Google Scholar]
- 41. Simopoulos AP. An increase in the omega‐6/omega‐3 fatty acid ratio increases the risk for obesity. Nutrients 2016;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ajeganova S, Andersson ML, Hafstrom I. Association of obesity with worse disease severity in rheumatoid arthritis as well as with comorbidities: a long‐term followup from disease onset. Arthritis Care Res (Hoboken) 2013;65:78–87. [DOI] [PubMed] [Google Scholar]
- 43. Li KL, Huang T, Zheng JS, Wu KJ, Li D. Effect of marine‐derived n‐3 polyunsaturated fatty acids on C‐reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta‐analysis. PloS One 2014;9:e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galarraga B, Ho M, Youssef HM, Hill A, McMahon H, Hall C, et al. Cod liver oil (n‐3 fatty acids) as a non‐steroidal anti‐inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:665–9. [DOI] [PubMed] [Google Scholar]
- 45. Kremer JM, Lawrence DA, Petrillo GF, Litts LL, Mullaly PM, Rynes RI, et al. Effects of high‐dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs: clinical and immune correlates. Arthritis Rheum 1995;38:1107–14. [DOI] [PubMed] [Google Scholar]
- 46. Remans PH, Sont JK, Wagenaar LW, Wouters‐Wesseling W, Zuijderduin WM, Jongma A, et al. Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: clinical and biochemical effects. Eur J Clin Nutr 2004;58:839–45. [DOI] [PubMed] [Google Scholar]
- 47. Sandberg ME, Wedren S, Klareskog L, Lundberg IE, Opava CH, Alfredsson L, et al. Patients with regular physical activity before onset of rheumatoid arthritis present with milder disease. Ann Rheum Dis 2014;73:1541–4. [DOI] [PubMed] [Google Scholar]
- 48. Hewlett S, Sanderson T, May J, Alten R, Bingham CO III, Cross M, et al. “I'm hurting, I want to kill myself”: rheumatoid arthritis flare is more than a high joint count: an international patient perspective on flare where medical help is sought. Rheumatology (Oxford) 2012;51:69–76. [DOI] [PubMed] [Google Scholar]


