Abstract
Background
Screening for abdominal aortic aneurysm (AAA) is known to reduce AAA-related mortality; however, the psychological impact of population AAA screening is unclear. The aim was to assess the impact of AAA diagnosis on quality of life (QoL) using data from an established AAA screening programme.
Methods
Mental and physical QoL scores for men diagnosed with AAA through participation in the English and Welsh AAA screening programmes were compared with no-AAA controls. Participants were identified through the United Kingdom Aneurysm Growth Study (UKAGS), a nationwide prospective cohort study of men with an AAA of less than 55 mm diagnosed through voluntary participation in screening. The UKAGS participants completed QoL questionnaires at the time of screening and annually thereafter.
Results
A transient reduction in mental QoL scores was observed following the diagnosis of AAA, returning to baseline levels after 12 months. Physical QoL remained consistently lower in the AAA cohort. Participants thought about their AAA and the AAA growth progressively less 12 months after the initial screening diagnosis. AAA growth rate had no influence over QoL parameters.
Discussion
This study suggests that screening for AAA does reduce mental QoL; however, this effect is transient (less than 12 months). Men diagnosed with AAA have a consistently worse physical QoL compared with controls.
Small initial reduction
Introduction
Abdominal aortic aneurysm (AAA) is defined as an abnormal dilatation of the abdominal aorta of 30 mm or more, and constitutes a significant health problem worldwide1. Each year in England and Wales, AAAs cause over 4000 deaths following aortic rupture2, with approximately 8000 patients a year undergoing surgery to prevent this3. In 2013, the National Health Service (NHS) AAA screening programme (NAAASP) was fully rolled out across England, based on evidence from several RCTs suggesting that AAA-related mortality was reduced through participation in AAA screening4. The NAAASP currently invites all men in their 65th year to receive a one-off non-invasive abdominal ultrasound scan. In England in 2015–2016, 227 543 men were screened and 2549 (1·1 per cent) were diagnosed with an AAA; however, only 723 men (0·3 per cent) had an AAA large enough (at least 55 mm) to require referral for consideration of surgery5. This highlights one of the major issues with screening for AAA in that, although it remains cost-effective, the majority of patients identified do not require immediate surgery and are subsequently entered into ongoing surveillance, either 6-monthly or annually. Most men with a screen-detected AAA will spend 3–5 years in surveillance before reaching the threshold for elective AAA repair, rising to over 7 years for men with a 30-mm AAA6. Currently 13 104 men in England are in AAA surveillance5.
This has led to questions being raised over the psychological impact of AAA screening. Some have even suggested that AAA screening may do more harm than good7. A small number of observational studies have investigated quality of life (QoL) in those who are identified at screening to have an AAA8–13, demonstrating varying results and conclusions when comparing screened and unscreened cohorts.
The United Kingdom Aneurysm Growth Study (UKAGS) is a prospective observational cohort study currently recruiting men with AAA identified through the English NHS AAA screening programme (NAAASP) and the Welsh AAA screening programme (WAAASP), with the aim of investigating the growth rates of small AAAs. All recruited men (those with an AAA and controls without) are sent an annual self-completed postal questionnaire to obtain longitudinal clinical and QoL data. This resource was used to assess the contemporary impact of screening for AAA on men who attend the NAAASP and WAAASP.
Methods
NAAASP and WAAASP invite all men in England and Wales during their 65th year of age to attend AAA screening14. Eligible men are sent an invitation letter to attend a local clinic for an ultrasound scan. A technician measures the maximal anteroposterior inner wall to inner wall diameter of the infrarenal aorta. Those with a diameter of less than 3 cm are discharged; those with a diameter between 3·0 and 5·4 cm are offered ultrasound surveillance every 6 or 12 months (based on AAA diameter); and men with an aortic diameter above 5·4 cm are directly referred for possible surgical repair.
UKAGS is a prospective observational cohort study that recruits men with an AAA, as well as individuals without AAA (controls) who have attended AAA screening, from NAAASP or WAAASP15. All recruited men are sent annual self-completion postal questionnaires (Appendix S1, supporting information) to obtain longitudinal clinical information and QoL information. Additionally, those with AAA on initial screening undergo annual ultrasound screening measurements through the standard surveillance procedures14. UKAGS is currently recruiting individuals from 14 units across England and Wales, and aims to recruit 20 000 men over 5 years. Ethical approval has been granted by an NHS research ethics committee, and men have provided their written informed consent upon recruitment for QoL data collection and analyses.
Data collection and quality-of-life assessments
Data collected at baseline included AAA diameter (inner wall to inner wall measurement), demographics, standard cardiovascular co-morbidities and QoL-related fields. This includes eight questions adapted from the Medical Outcomes Study Short Form 36 questionnaire (SF-8), a survey that has previously been recommended specifically for vascular disease-related QoL outcome analyses16 and has demonstrated high reliability and validity17. Several other QoL questionnaires are available, and have been used by other groups. The present questions were considered most suitable for the UKAGS.
The SF-8 questionnaire uses single-item scales addressing eight domains of general health, physical functioning, role limitations (due to physical health), bodily pain, vitality, social functioning, mental health and role limitations (due to emotional health). These parameters are then used to produce two outcome measures of QoL: the Physical Component Summary (PCS) and the Mental Component Summary (MCS). The scores range from 0 to 100, where zero indicates the lowest and 100 the highest level of health, calibrated so that the average score is 50, with a standard deviation of 1017. Scores were calculated using the QualityMetric Health Outcomes™ Scoring Software 4.5 (Optum®; QualityMetric, Lincoln, Rhode Island, USA). Additionally, using a Likert scale (1, not at all; 5, all the time), men with a known AAA were asked how often they thought about their aneurysm and how often they had thought about the potential for aneurysm growth in the preceding 4 weeks.
As recruitment occurred through the traditional screening pathway, participants were recruited into the study across an extended period (September 2011 to July 2015). Relative to each man's baseline recruitment date, QoL data collection follow-up was divided into four groups after initial screening: 0–12, 13–24, 25–36 and 37 or more months. The primary outcome measure was comparing PCS and MCS at each time point between men with a diagnosed AAA on initial screening and those without an AAA (control group).
Data collection was done at yearly follow-up intervals with recruitment from NAAASP and WAAASP into the study occurring continuously throughout the 4-year study. Thus, participants were in the study for varying lengths of time and had completed a varying number of questionnaires (Table 1).
Table 1.
Total number of questionnaire respondents for each time interval after initial screening
| Time from initial screening (months) | No aneurysm | Aneurysm | Total |
|---|---|---|---|
| 0–12 | 4807 | 174 | 4981 |
| 13–24 | 4232 | 238 | 4470 |
| 25–36 | 914 | 142 | 1056 |
| ≥ 37 | 52 | 93 | 145 |
Statistical analysis
The SF-8 QoL data collected were analysed using ANOVA, comparing the AAA group with the no-AAA group at each interval. Regression analyses accounted for potential confounding factors, including demographics and co-morbidities. Mean growth rate (cm/month) during surveillance was calculated and linear regression was used to identify any association of growth rate with the QoL parameters. ANOVA was used to assess changes in the frequencies over time with which respondents thought about their aneurysm and aneurysm growth; data for the intervals of 13–24, 25–36 and 37 or more months were compared with those for the 0–12-month interval, which acted as a baseline value. Continuous variables are presented as mean(s.d.) or mean(s.e.m.) values, as appropriate. A Pearson χ2 test was used to compare categorical variables and a paired t test to compare continuous data. Data were analysed using IBM SPSS® version 22.0 (IBM, Armonk, New York, USA). P < 0·050 was considered statistically significant.
Results
A total of 5011 men were recruited into the study, of whom 381 (7·6 per cent) had an AAA identified via screening. Overall, they were followed for a mean of 19·0(9·1) months from their initial screening appointment.
Men with an AAA were older (age 72·6 versus 69·8 years; P < 0·001), had a higher BMI (28·1 versus 27·0 kg/m2; P < 0·001) and were more likely to be a current smoker (15·1 versus 5·2 per cent; P < 0·001) than those in the control group (Table 2). When comparing co-morbidities to the control group, men with an AAA were more likely to have diabetes mellitus (18·8 versus 10·0 per cent), ischaemic heart disease (12·2 versus 4·4 per cent), high cholesterol (53·2 versus 30·8 per cent), previous stroke (6·1 versus 2·9 per cent) and a previous myocardial infarction (21·1 versus 5·8 per cent) (all P < 0·001).
Table 2.
Demographics of men included
| No AAA (n = 4630) | AAA (n = 381) | P † | |
|---|---|---|---|
| Age (years)* | 69·8(3·5) | 72·6(5·5) | < 0·001‡ |
| BMI (kg/m2)* | 27·0(4·4) | 28·1(4·3) | < 0·001‡ |
| AAA diameter at initial screening (mm)* | 17·8(0·2) | 36·1(0·7) | < 0·001‡ |
| Current smoker | 237 of 4596 (5·2) | 57 of 378 (15·1) | < 0·001 |
| Diabetes mellitus | 454 of 4536 (10·0) | 69 of 367 (18·8) | < 0·001 |
| IHD | 197 of 4507 (4·4) | 43 of 352 (12·2) | < 0·001 |
| High cholesterol | 1401 of 4542 (30·8) | 194 of 365 (53·2) | < 0·001 |
| Previous stroke | 134 of 4612 (2·9) | 23 of 376 (6·1) | < 0·001 |
| Previous MI | 269 of 4614 (5·8) | 80 of 379 (21·1) | < 0·001 |
Values in parentheses are percentages unless indicated otherwise;
values are mean(s.d.). AAA, abdominal aortic aneurysm; IHD, ischaemic heart disease; MI, myocardial infarction.
Pearson χ2 test, except
paired t test.
Quality of life
For the PCS, scores in the AAA group were significantly lower at 0–12, 13–24 and 25–36 months than those in the control group (P < 0·001, P = 0·028 and P < 0·001 respectively) (Table 3 and Fig. 1a). Over 37 months after screening, differences in PCS were non-significant. For the MCS, scores were significantly lower immediately after screening in men with an AAA versus the control group (P < 0·001) (Table 4 and Fig. 1b). However, after 12 months, MCS scores from the AAA cohort returned to baseline levels, equivalent to those of men with no AAA, and continued thus for the remainder of the follow-up.
Table 3.
SF-8 Physical Component Summary scores for men with and those without an abdominal aortic aneurysm for each time interval after initial screening
| Time from initial screening (months) | PCS score | P * | |
|---|---|---|---|
| No AAA | AAA | ||
| 0–12 | 51·4(7·9) | 47·6(8·9) | < 0·001 |
| 13–24 | 50·7(8·4) | 49·5(8·7) | 0·028 |
| 25–36 | 50·8(8·3) | 47·5(10·0) | < 0·001 |
| ≥ 37 | 51·3(8·8) | 48·5(9·4) | 0·077 |
The SF-8 questionnaire included eight questions adapted from the Medical Outcomes Study Short Form 36. Values are mean(s.d.). PCS, Physical Component Summary; AAA, abdominal aortic aneurysm.
ANOVA.
Fig. 1.
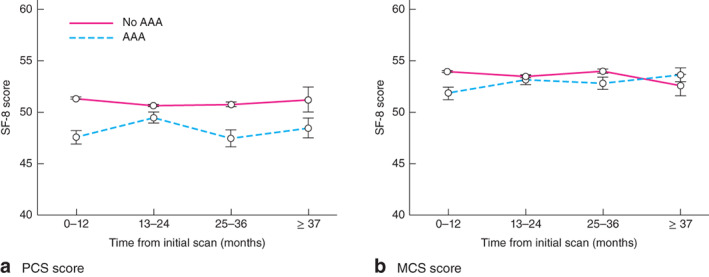
Changes in SF-8 scores for a Physical Component Summary (PCS) score and b Mental Component Summary (MCS) score after initial screening. Values are mean(s.e.m.). The SF-8 questionnaire included eight questions adapted from the Medical Outcomes Study Short Form 36
Table 4.
SF-8 Mental Component Summary scores for men with and those without an abdominal aortic aneurysm for each time interval after initial screening
| Time from initial screening (months) | MCS score | P * | |
|---|---|---|---|
| No AAA | AAA | ||
| 0–12 | 54·0(7·0) | 51·9(8·3) | < 0·001 |
| 13–24 | 53·5(7·5) | 53·2(7·4) | 0·610 |
| 25–36 | 54·0(7·1) | 52·9(7·1) | 0·884 |
| ≥ 37 | 52·7(7·6) | 53·7(6·7) | 0·408 |
The SF-8 questionnaire included eight questions adapted from the Medical Outcomes Study Short Form 36. Values are mean(s.d.). MCS, Mental Component Summary; AAA, abdominal aortic aneurysm.
ANOVA.
Regression analysis was done, adjusting for the additional co-variables collected, for both PCS and MCS (Tables S1 and S2, supporting information). The lower PCS scores remained significant across all AAA groups after screening (P < 0·001), whereas the MCS scores overall showed no differences between the AAA and control group (P = 0·443).
The effect of growth rate on QoL was analysed by comparing QoL to the mean growth rate (cm/month) recorded for all patients with AAA. QoL for both MCS and PCS showed no relationship with growth rate (Figs S1 and S2, supporting information).
Impact of AAA and AAA growth
ANOVA demonstrated a progressive reduction in the frequency with which men with an AAA had thought about their aneurysm in the preceding 4 weeks, at 13–24 months (P = 0·025), 25–36 months (P = 0·040) and 37 months or more (P = 0·005), all showing a significant reduction relative to baseline values at 0–12 months (Fig. 2a; Table S3, supporting information). When men with a small AAA were asked how often they had thought about aneurysm growth in the preceding 4 weeks, there was also a significant reduction in frequency at 25–36 months (P = 0·004) and 37 months or more (P = 0·006) (Fig. 2b; Table S4, supporting information).
Fig. 2.
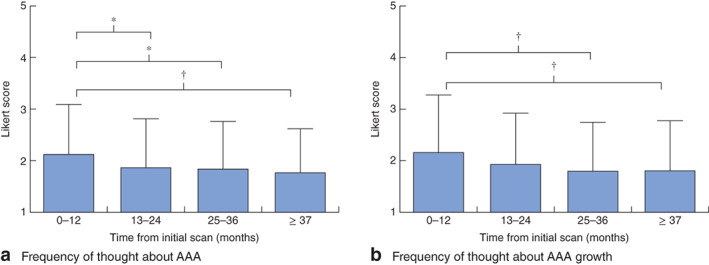
Likert scores of the frequency with which men had thought about a their abdominal aortic aneurysm (AAA) and b AAA growth in the preceding 4 weeks. Values are mean(s.d.). *P < 0·050, †P ≤ 0·010 (ANOVA)
Discussion
This analysis demonstrates that men diagnosed with an AAA through screening have a transient reduction in mental QoL during the first year, but this then returns to normal. Furthermore, with time, a man diagnosed with an AAA is likely to think progressively less about it and its growth. This study also demonstrates that men with an AAA have a consistently lower physical QoL than men without an AAA, even when adjusted for co-variables.
This is not the first time that a transient impact of mental QoL has been demonstrated in screening programmes: colorectal, prostate and breast cancer programmes have all been shown to have small effects on QoL that tend to diminish with long-term follow-up18–20. Faecal occult blood testing for colorectal cancer had no impact on mental QoL18, prostate cancer screening showed no influence on physical, psychological or social functioning19, and in women recalled after breast cancer screening, the initial anxiety and depression associated with the appointment had decreased significantly, even after a few days20. However, contemporaneous data on QoL in men screened for AAA were lacking.
For AAA screening, the majority of the QoL evidence on this topic has come from the Multi-Aneurysm Screening Study (MASS)9. Like the present study, the longer-term mental impact appeared to be negligible in MASS. Using similar methodology, the MASS data set demonstrated a significant reduction in mental component scores for QoL 6 weeks after initial AAA diagnosis, yet, as in the present study, an improvement was seen 12 months after diagnosis, back to near-baseline values.
Smaller historical studies have shown similar findings. Lucarotti and colleagues11 showed that initial screening investigations caused mild anxiety that did not persist following AAA diagnosis. Wanhainen and co-workers8 found that only individuals with a low QoL score before screening were susceptible to potential negative effects. If such transient negative effects on mental QoL are seen in men after screening, more work is warranted to evaluate the potential benefit that could occur from introducing counselling, with discussion surrounding the presence and growth of the AAA, and how this may impact on the man with the disease.
The finding that PCS scores were lower in men with an AAA compared with controls, even after adjustment for confounders, probably reflects the co-morbid nature of many men with AAA. This observation was also seen in MASS9, where men with an AAA had lower physical QoL scores, from both the Short Form 36 (QualityMetric, Lincoln, Rhode Island, USA) and EuroQol – 5D (EuroQol Group, Rotterdam, The Netherlands) questionnaires. AAA is an independent marker of cardiovascular risk, with a documented 3·0 per cent per year risk of cardiovascular death in patients with a small AAA21. AAA and cardiovascular disease have been shown to share risk factors22,23.
It might be assumed that AAA growth would have a negative effect on the MCS score. Here, no association between these parameters was seen. Indeed, the longer a man had been involved in the surveillance programme, the less he reported thinking about AAA growth. These findings all support the suggestion that AAA screening does not have a significant or long-term effect on QoL. These findings were echoed by Dahlberg and colleagues24, who demonstrated that the reassurance a patient received from increased AAA surveillance and the positive reinforcement of screening programmes eventually outweighed the potential negative effects that might be anticipated about worsening health.
One of the key limitations of this work is the fact that QoL scores were not available before screening. As the scores of the no-AAA participants acted as a control group, and remained stable throughout the study, it can be assumed that QoL would be similar before screening. There was a discrepancy in the longer-term follow-up rate between men with an AAA and no-AAA controls, where reduced compliance may reflect decreasing engagement in those without an AAA.
The recruitment method employed meant that some men were recruited at the time of the first screening scan and some during AAA surveillance. To allow for this, the time of recruitment was recorded and used to adjust analyses of AAA growth accordingly. This is reflected in the difference in the mean age of men with and those without an AAA at baseline. It was not possible to conduct a direct regression analysis of the SF-8 data set owing to the clustered annual follow-up of the men, yet ANOVA was able to provide a more suitable and relevant alternative.
The conclusions drawn from this work are applicable only to men screened as positive for AAA. The overall effects of screening on the larger number of men with negative scans are not yet determined.
Collaborators
Other UK Aneurysm Growth Study investigators include: R. Pathak, M. Brooks, P. Hayes, C. Imray, J. Quarmby, S. Choksy, J. J. Earnshaw, C. P. Shearman, E. Grocott, T. Rix, I. Chetter, W. Tennant, G. Libertiny, T. Sykes, M. Dayer, L. Pike, A. Pherwani, C. Nice, N. Browning, C. McCollum, S. Yusuf, M. Gannon, J. Barwell, S. Baker, S. R. Vallabhaneni, A. Davies.
Supplementary Material
Appendix S1. Questionnaire sent to all recruited men for self-completion
Table S1 Regression analysis for Physical Component Summary score
Table S2 Regression analysis for Mental Component Summary score
Table S3 Likert scores of the frequency with which men had thought about their AAA in the preceding 4 weeks
Table S4 Likert scores of the frequency with which men had thought about their AAA growth in the preceding 4 weeks
Fig. S1 Linear regression for aneurysm growth rate and Physical Component Summary score
Fig. S2 Linear regression for aneurysm growth rate and Mental Component Summary score
Acknowledgements
A.S. and D.S. are funded by the National Institute for Health Research, and A.S. is also funded by the Academy of Medical Sciences (grant number SGCL13). The United Kingdom Aneurysm Growth Study is funded by the British Heart Foundation (grant number CS/14/2/30841).
Disclosure: The authors declare no conflict of interest.
References
- 1. Sidloff D, Stather P, Dattani N, Bown M, Thompson J, Sayers Ret al. . Aneurysm global epidemiology study: public health measures can further reduce abdominal aortic aneurysm mortality. Circulation 2013; 129: 747–753. [DOI] [PubMed] [Google Scholar]
- 2. Office for National Statistics . The 21st Century Mortality Files: 2001 to 2015. https://www.ons.gov.uk/releases/the21stcenturymortalityfiles2001to2015 [accessed 1 March 2017].
- 3. Vascular Services Quality Improvement Programme (VSQIP) . Elective AAA Repair; 2017. https://www.vsqip.org.uk/surgeon-outcomes/elective-aaa-repair/ [accessed 1 March 2017]. [Google Scholar]
- 4. Guirguis-Blake J, Beil T, Senger C. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the US preventive services task force. J Vasc Surg 2014; 60: 264–265. [DOI] [PubMed] [Google Scholar]
- 5. GOV.UK . Abdominal Aortic Aneurysm Screening: 2015 to 2016 Data; 2016. https://www.gov.uk/government/publications/abdominal-aortic-aneurysm-screening-2015-to-2016-data [accessed 1 March 2017]. [Google Scholar]
- 6. The RESCAN Collaborators . Surveillance intervals for small abdominal aortic aneurysms. JAMA 2013; 309: 806. [DOI] [PubMed] [Google Scholar]
- 7. Johansson M, Hansson A, Brodersen J. Estimating overdiagnosis in screening for abdominal aortic aneurysm: could a change in smoking habits and lowered aortic diameter tip the balance of screening towards harm? BMJ 2015; 350: h825. [DOI] [PubMed] [Google Scholar]
- 8. Wanhainen A, Rosén C, Rutegård J, Bergqvist D, Björck M. Low quality of life prior to screening for abdominal aortic aneurysm: a possible risk factor for negative mental effects. Ann Vasc Surg 2004; 18: 287–293. [DOI] [PubMed] [Google Scholar]
- 9. Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RAet al. ; Multicentre Aneurysm Screening Study Group . The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002; 360: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 10. Spencer C, Norman P, Jamrozik K, Tuohy R, Lawrence-Brown M. Is screening for abdominal aortic aneurysm bad for your health and well-being? ANZ J Surg 2004; 74: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 11. Lucarotti M, Heather B, Shaw E, Poskitt K. Psychological morbidity associated with abdominal aortic aneurysm screening. Eur J Vasc Endovasc Surg 1997; 14: 499–501. [DOI] [PubMed] [Google Scholar]
- 12. Lesjak M, Boreland F, Lyle D, Sidford J, Flecknoe-Brown S, Fletcher J. Screening for abdominal aortic aneurysm: does it affect men's quality of life? Aust J Prim Health 2012; 18: 284–288. [DOI] [PubMed] [Google Scholar]
- 13. Scalone L, Cortesi P, Spinella G, Pane B, Cesana G, Mantovani Let al. . Use of health-related quality-of-life measurements to estimate individuals' health on screening. Acta Chir Belg 2013; 113: 406–414. [PubMed] [Google Scholar]
- 14. Public Health England . NHS Abdominal Aortic Aneurysm (AAA) Screening Programme: Essential Elements in Providing an AAA Screening and Surveillance Programme; 2017. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/598365/AAA_Screening_Standard_Operating_Procedures_March_2017.pdf [accessed 1 May 2017]. [Google Scholar]
- 15. University of Leicester . The United Kingdom Aneurysm Growth Study (UKAGS) – Study Information. http://www2.le.ac.uk/projects/ukags [accessed 1 May 2017].
- 16. Beattie D, Golledge J, Greenhalgh R, Davies A. Quality of life assessment in vascular disease: towards a consensus. Eur J Vasc Endovasc Surg 1997; 13: 9–13. [DOI] [PubMed] [Google Scholar]
- 17. Ware J How to Score and Interpret Single-Item Health Status Measures (1st edn). QualityMetric: Lincoln, 2001. [Google Scholar]
- 18. Mahabaleshwarkar R, Khanna R, West-Strum D, Yang Y. Association between health-related quality of life and colorectal cancer screening. Popul Health Manag 2013; 16: 178–189. [DOI] [PubMed] [Google Scholar]
- 19. Essink-Bot M, de Koning H, Nijs H, Kirkels W, van der Maas P, Schroder F. Short-term effects of population-based screening for prostate cancer on health-related quality of life. J Natl Cancer Inst 1998; 90: 925–931. [DOI] [PubMed] [Google Scholar]
- 20. Lampic C, Thurfjell E, Bergh J, Sjödén P. Short- and long-term anxiety and depression in women recalled after breast cancer screening. Eur J Cancer 2001; 37: 463–469. [DOI] [PubMed] [Google Scholar]
- 21. Bath M, Gokani V, Sidloff D, Jones L, Choke E, Sayers Ret al. . Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br J Surg 2015; 102: 866–872. [DOI] [PubMed] [Google Scholar]
- 22. Alcorn H, Wolfson S, Sutton-Tyrrell K, Kuller L, O'Leary D. Risk factors for abdominal aortic aneurysms in older adults enrolled in the cardiovascular health study. Arterioscler Thromb Vasc Biol 1996; 16: 963–970. [DOI] [PubMed] [Google Scholar]
- 23. Saratzis A, Bown M. The genetic basis for aortic aneurysmal disease. Heart 2014; 100: 916–922. [DOI] [PubMed] [Google Scholar]
- 24. Dahlberg M, Mani K, Öhman M, Wanhainen A. Health Information and Well-Being: Evidence from an Asymptomatic Disease (Department of Economics, Uppsala University, Working paper 2016: 2.) http://uu.diva-portal.org/smash/get/diva2:896885/FULLTEXT01.pdf [accessed 1 March 2017]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Questionnaire sent to all recruited men for self-completion
Table S1 Regression analysis for Physical Component Summary score
Table S2 Regression analysis for Mental Component Summary score
Table S3 Likert scores of the frequency with which men had thought about their AAA in the preceding 4 weeks
Table S4 Likert scores of the frequency with which men had thought about their AAA growth in the preceding 4 weeks
Fig. S1 Linear regression for aneurysm growth rate and Physical Component Summary score
Fig. S2 Linear regression for aneurysm growth rate and Mental Component Summary score


