Abstract
Background
Reduced dopamine D2 receptor (D2R) ligand binding has repeatedly been demonstrated in the striatum of humans with alcohol use disorder (AUD). The attenuated D2R binding has been suggested to reflect a reduced D2R density, which in turn has been proposed to drive craving and relapse. However, results from rodent studies addressing the effects of alcohol drinking on D2R density have been inconsistent.
Methods
A validated alcohol drinking model (intermittent access to 20% alcohol) in Wistar rats was used to study the effects of voluntary alcohol drinking (at least 12 weeks) on the D2R in the striatum compared to age‐matched alcohol‐naïve control rats. Reverse transcriptase quantitative PCR was used to quantify isoform‐specific Drd2 gene expression levels. Using bisulfite pyrosequencing, DNA methylation levels of a regulatory region of the Drd2 gene were determined. In situ proximity ligation assay was used to measure densities of D2R receptor complexes: D2R‐D2R, adenosine A2A receptor (A2AR)‐D2R, and sigma1 receptor (sigma1R)‐D2R.
Results
Long‐term voluntary alcohol drinking significantly reduced mRNA levels of the long D2R isoform in the nucleus accumbens (NAc) but did not alter CpG methylation levels in the analyzed sequence of the Drd2 gene. Alcohol drinking also reduced the striatal density of D2R‐D2R homoreceptor complexes, increased the density of A2AR‐D2R heteroreceptor complexes in the NAc shell and the dorsal striatum, and decreased the density of sigma1R‐D2R heteroreceptor complexes in the dorsal striatum.
Conclusions
The present results on long‐term alcohol drinking might reflect reduced D2R levels through reductions in D2R‐D2R homoreceptor complexes and gene expression. Furthermore, based on antagonistic interactions between A2AR and D2R, an increased density of A2AR‐D2R heteroreceptor complexes might indicate a reduced affinity and signaling of the D2R population within the complex. Hence, both reduced striatal D2R levels and reduced D2R protomer affinity within the striatal A2AR‐D2R complex might underlie reduced D2R radioligand binding in humans with AUD. This supports the hypothesis of a hypodopaminergic system in AUD and suggests the A2AR‐D2R heteroreceptor complex as a potential novel treatment target.
Keywords: Receptor Dimers, Heteroreceptors and Homoreceptors, Epigenetics, DNA Methylation, Alcohol Dependence
The mesolimbic dopamine system plays a crucial role in alcohol's reinforcing effects, as well as in the development and maintenance of alcohol use disorder (AUD) (Jayaram‐Lindström et al., 2016). In particular, alcohol activates the projections from the ventral tegmental area to the nucleus accumbens (NAc; i.e., the ventral striatum) and induces striatal dopamine release in rats (Ericson et al., 1998; Weiss et al., 1996) and humans (Boileau et al., 2003; Urban et al., 2010). Furthermore, dopamine D2 receptors (D2Rs) in the NAc are crucial for alcohol reinforcement (Hodge et al., 1997; Jayaram‐Lindström et al., 2016). Moreover, D2Rs in the dorsolateral striatum are involved in alcohol seeking that after an extended period has become habitual, that is, is independent of alcohol reinforcement (Corbit et al., 2014), and is mainly influenced by alcohol‐associated cues (Corbit and Janak, 2016).
During protracted abstinence in humans, reduced central‐stimulant induced striatal dopamine release (Martinez et al., 2005; Volkow et al., 2007) and D2R radioligand binding (Hietala et al., 1994; Volkow et al., 1996) have been found. In addition, reduced striatal D2R binding and low dopamine levels have been correlated with craving (Heinz et al., 2004) and relapse (Guardia et al., 2000; Heinz et al., 1995), respectively. When evaluating neurochemical changes related to AUD in animal models, the importance of paradigms with face validity and in particular long‐term voluntary alcohol intake should not be underestimated. However, short‐term (Reggiani et al., 1980; Rommelspacher et al., 1992) and/or forced alcohol exposure (Hamdi and Prasad, 1992; Lograno et al., 1993; Syvalahti et al., 1988) have been frequently used. Thus, the limited face validity of the traditionally used alcohol intake models could potentially underlie the inconsistent results in rodent autoradiography studies with increased, decreased, or unchanged striatal D2R binding (e.g., see table 1 in Sari et al., 2006). To increase the arsenal of validated rodent AUD models, the 2‐bottle‐choice intermittent access to 20% ethanol (EtOH) (IA20E) model from Wise (1973) was reintroduced in 2008 (Simms et al., 2008). The IA20E model mimics several hallmarks of an AUD profile such as escalation of voluntary drinking, cycles of excessive drinking and abstinence, pharmacologically relevant blood alcohol concentrations (Simms et al., 2008), and compulsive drinking (Hopf et al., 2010). In addition, the IA20E model has shown predictive (Litten et al., 2013; Simms et al., 2008; Steensland et al., 2007) and constructive validity (Carnicella et al., 2014). Indeed, several months of voluntary drinking in the IA20E model induces a dopamine deficiency in the NAc during alcohol withdrawal in both Long‐Evans (Barak et al., 2011) and Wistar rats (Feltmann et al., 2016). The behavioral relevance of a dopamine deficiency is highlighted by a study showing that rats undergoing withdrawal and displaying a reduced NAc dopamine output induced by several weeks of alcohol liquid diet self‐administered just enough alcohol to restore the dopamine baseline (Weiss et al., 1996). Taken together, preclinical and clinical findings support the dopamine deficiency hypothesis, suggested to be responsible for driving craving and compulsive drinking, as well as contributing to relapse even after a period of protracted abstinence (Ravan et al., 2014).
The finding that the D2R is expressed in different isoforms, as well as assembles in homo‐ and heteroreceptor complexes, could be of relevance for the understanding of AUD and the development of potential treatment targets. Through alternative splicing, the D2R is expressed in a short and a long isoform (dal Toso et al., 1989), associated with different cellular signaling (Lindgren et al., 2003; Senogles, 1994). However, to the best of our knowledge, only 1 previous study has investigated alcohol‐induced effects on isoform‐specific D2R expression, although only in the pituitary gland (Oomizu et al., 2003). The dopamine D2R can form D2R‐D2R homoreceptor, as well as heteroreceptor complexes with a variety of receptors, for example, the adenosine A2A receptor (A2AR) (Borroto‐Escuela et al., 2013; Fuxe et al., 2014a; Trifilieff et al., 2011) and the sigma1 receptor (sigma1R) (Navarro et al., 2013), which have been suggested to be involved in alcohol reward (Blasio et al., 2015; Filip et al., 2012; Nam et al., 2013). A2AR and D2R are both expressed on striato‐pallidal GABAergic neurons, where they form A2AR‐D2R heteroreceptor complexes (Nam et al., 2013). Within the A2AR‐D2R heteroreceptor complex, antagonistic allosteric receptor–receptor interactions (Fuxe et al., 2014a) operate at the receptor recognition, signaling and trafficking level, probably underlying antagonistic A2AR‐D2R interactions displayed at the level of brain circuits and behavior (Filip et al., 2012; Fuxe et al., 2014b). These antagonistic interactions may in part underlie findings in alcohol‐preferring rats showing that A2AR agonists reduce, whereas antagonists increase, alcohol intake (Micioni Di Bonaventura et al., 2012), and findings in mice showing that dampening A2AR signaling in the dorsomedial striatum enhances alcohol‐seeking behavior (Nam et al., 2013).
In the present study, the effect of long‐term drinking on the Drd2 gene expression and the density of several D2R receptor complexes were evaluated in the ventral (NAc) and dorsal striatum of Wistar rats using the validated IA20E drinking model (Simms et al., 2008) compared to age‐matched alcohol‐naïve controls. First, using reverse transcriptase quantitative PCR (qPCR) with specific primers detecting exon–exon junctions, expression levels of D2R short and long isoform and the A2AR were measured. Second, to test whether a potential reduction in D2 expression in the NAc could be related to epigenetic changes, bisulfite pyrosequencing was used to determine DNA methylation levels of a regulatory sequence within the Drd2 gene. Finally, in situ proximity ligation assay (PLA) was used to evaluate the effects of long‐term drinking on D2R‐D2R homo, as well as A2AR‐D2R and sigma1R‐D2R heteroreceptor complexes (Borroto‐Escuela et al., 2013).
Materials and Methods
Animals and Drinking Model
Male Rcc Wistar Han rats were voluntarily drinking alcohol in the IA20E drinking model (Simms et al., 2008; Wise, 1973) under conditions described previously (Steensland et al., 2012). In brief, rats were housed individually, under a reversed light/dark 12‐hour cycle and had ad libitum access to food and water throughout the study. They had access to 1 bottle of 20% EtOH and 1 bottle of water for 24 hours on Monday, Wednesday, and Friday and 2 bottles of water on the remaining days of the week. The positions of the alcohol and water bottles were switched for every alcohol session. Alcohol presentation started approximately 10 minutes after initiation of the dark phase. Individual fluid intake was measured every 4 and 24 hours. Rats were weighted daily to calculate the grams of alcohol intake per kilogram body weight (g/kg). A description of the escalation in drinking following IA20E can be found in Fig. S1. An alcohol‐naïve, age‐matched group of control rats were housed and handled under identical conditions with the only exception that they had continuous access to 2 water bottles rather than intermittent access to an alcohol bottle. The study was approved by the Swedish Ethical Committee on Animal Research in Stockholm (Dnr N163/14) and carried out in accordance with the European Directive 2010/63/EU.
Extraction of DNA and RNA
Following 5 months of drinking in the IA20E model, rats were sacrificed by decapitation after approximately 24 hours of alcohol withdrawal. Brain regions were dissected, immediately frozen on dry ice, and stored at −80°C until further processing. DNA and RNA were isolated from NAc using the AllPrep DNA/RNA/Protein Minikit according to manufacturer instructions (Qiagen, Sollentuna, Sweden). Briefly, frozen tissues were lysed in 600 μl RLT buffer using 5‐mm stainless steel beads and the TissueLyser LT (Qiagen) at 20 Hz for 2 minutes twice. First, after centrifugation to separate insoluble components, the supernatant of the lysate was passed through a DNA‐binding column, whereafter the DNA‐column flow‐through was passed through another column binding RNA. Following several washing steps and centrifugations of the columns, RNA and DNA were eluted in 40 μl RNAse‐free water and 100 μl EB buffer (70°C), respectively. Nucleic acid concentration was measured using a Nanodrop (Infinite 200 NanoQuant; Tecan, Männedorf, Switzerland). Nucleic acids were stored at −80°C.
Gene Expression Analysis: qPCR
To eliminate any remaining genomic DNA, DNAse digestion was performed on RNA samples. RNA (200 ng) was diluted with water to 12 μl; 1.5 μl of DNAse I and 1.5 μl of DNAse buffer (New England Biolabs, Hitchin, UK) were added; and the samples were incubated at 37°C for 30 minutes. Then, 1.5 μl of EDTA was added to each sample followed by incubation for 10 minutes at 65°C.
DNAse‐treated RNA was converted into complementary DNA using the iScript cDNA synthesis kit (Bio‐Rad, Solna, Sweden) according to the manufacturer's protocol. In brief, reverse transcriptase and reaction mix containing random primers were added to DNAse‐treated RNA and exposed to the following protocol: annealing at 25°C for 5 minutes, transcription at 42°C for 30 minutes, and termination at 85°C for 5 minutes. cDNA samples were stored at −20°C. As a control for successful removal of genomic DNA, 1 sample was exposed to the same treatment except that the reverse transcriptase was not added (RT control).
Gene expression was assessed using qPCR. Reactions were performed in triplicates using SYBR green (Qiagen) and the Rotor‐Gene Q (Qiagen) machine under the following condition: 95°C for 5 minutes followed by 40 cycles of 95°C for 5 seconds, 60°C for 10 seconds. Primers were designed to detect exon–exon junctions (for primer sequences, see Table S1) and were tested for specific target amplification in the sample compared to the RT control using melt curve analysis (from 60 to 95°C) and agarose gel electrophoresis. Moreover, reaction efficiencies of each primer pair were assessed with cDNA serial dilution of 1:5. Due to too low RNA concentration, 1 sample was not converted into cDNA (alcohol‐naïve rat).
DNA Methylation Analysis: Bisulfite Pyrosequencing
Using bisulfite pyrosequencing, the methylation of the following DNA sequence within exon 1 of the Drd2 gene was analyzed: TTCCCGACGCCCGAGGCGCAATCTGCCCGTCGGA. This region was chosen for analysis based on a previous study showing correlation of methylation levels of a sequence spanning exon 1 and part of intron 1 and Drd2 gene expression (Rodrigues et al., 2012).
First, 500 ng of genomic DNA was bisulfite‐converted using the EZ DNA Methylation‐Gold™ Kit (ZYMO Research, Irvine, CA), following the manufacturer's instructions. Briefly, 130 μl of C to T conversion reagent was added to 20 μl of DNA sample and incubated at 98°C for 10 minutes and at 64°C for 2.5 hours. The sample was loaded onto a Zymo‐Spin™ IC column that had been equilibrated with 600 μl of M‐Binding buffer. Following a 30 seconds centrifugation at 10,000×g, the column was washed with 100 μl of M‐Wash buffer and centrifuged again. Thereafter, 200 μl of M‐desulphonation buffer was added to the column and incubated for 20 minutes followed by centrifugation. The converted DNA was eluted in 10 μl M‐Elution buffer and stored at −80°C until use.
The bisulfite‐converted DNA was amplified with PCR using biotinylated primers and reagents from a Custom Assay (Qiagen) under the following conditions: 95°C for 15 minutes followed by 45 cycles of 94°C for 30 seconds (denaturation), 58°C for 30 seconds (annealing), 72°C for 30 seconds (extension), and a final extension at 72°C for 10 minutes. PCR products were incubated with Sepharose beads (Sarstedt, Germany) and binding buffer (Qiagen) under shaking (14,000 rpm) for 10 minutes. Using vacuum suction and filter probes, bead‐bound PCR products were taken from solution and washed with 70% EtOH for 5 seconds, denaturation buffer (Qiagen) for 10 seconds and wash buffer for 10 seconds. Vacuum was turned off, and filter probes were lowered into the sequencing PCR plate containing sequencing primers (Custom Assay) and annealing buffer to release PCR products. Samples were heated at 80°C for 2 minutes and cooled down to room temperature. Thereafter, pyrosequencing was performed using the PyroMark Q96 ID (Qiagen) instrument and percent methylation at 6 CpG positions was calculated with PyroMark Q96 software (Qiagen).
Receptor Complex Formation Analysis In Situ: PLA
PLA is a sensitive technique, allowing specific identification of receptor complexes, for example, in situ at the subcellular level. In brief, primary antibodies binding to the receptors of interest are detected by secondary antibodies labeled with short nucleotides. When the receptors are in close proximity (up to 10 to 20 nm), these nucleotides form a ring, which can be amplified and visualized with fluorescent probes.
After the 3 months of IA20E (or water‐only access), rats were transcardially perfused under deep pentobarbital anesthesia with phosphate‐buffered saline (PBS) followed by 4% paraformaldehyde after approximately 24 hours of alcohol withdrawal. Brains were incubated in 10% sucrose for 24 hours and 30% sucrose for 1 week until cryostat sectioning. In situ PLA was performed as described previously (Borroto‐Escuela et al., 2013) and according to the manufacturer's instructions (Duolink in situ PLA detection kit; Olink, Uppsala, Sweden). In brief, frozen floating frontal forebrain sections (+1.0 mm from Bregma) were washed with PBS, quenched with 10 mM glycine buffer for 20 minutes, permeabilized with 10% fetal bovine serum, and 0.5% Triton X‐100 for 30 minutes and blocked with 0.2% bovine serum albumin (BSA) solution for 30 minutes. Sections were washed with PBS thoroughly between incubation steps (performed at room temperature) described above. Sections were incubated with the primary antibodies for 1 to 2 hours at 37°C or at 4°C overnight. Incubation steps described in the following were conducted in humidity chambers at 37°C unless stated otherwise. Sections were washed with PBS twice, incubated with the proximity probe mixture for 1 hour. Unbound probes were washed away with blocking solution at room temperature. Sections were incubated with the hybridization–ligation solution (250 g/ml BSA, 0.05 U/μl T4 DNA ligase, 0.05% Tween‐20, 250 mM NaCl, 1 mM ATP, 125 to 250 nM circularization, or connector oligonucleotides) for 30 minutes. Excess connector oligonucleotides were removed with the washing buffer A (Olink; Duolink kit, at room temperature). Then, sections were incubated with the rolling circle amplification mixture for 100 minutes and the detection solution for 30 minutes and washed twice for 10 minutes in the dark with the washing buffer B (Duolink kit, at room temperature). Finally, sections were mounted on microscope slides with VectaShield (Vector Laboratories, Burlingame, CA), covered, sealed, and stored at −20°C (protected from light). PLA signal was visualized and quantified from 20 z‐layers (40 × 40 μm) using a confocal microscope Leica TCS‐SL confocal microscope (Leica, Weizlar, Germany) and the Duolink Image Tool software (Olink). Illustration of the sampling fields in NAc shell and core from microphotographs of frontal sections of the rat ventral striatum (Bregma level: 1.00 mm) is indicated in Fig. S2. The Bregma level was reached with the help of the Paxinos and Watson Atlas. The first frontal section was taken at the very anterior beginning of the striatum. Then, the number of required sections to reach Bregma 1.00 mm level was calculated (Paxinos and Watson, 2007). The location was validated by the appearance of a single myelinated bundle of the anterior limb of the anterior commissure because prior to this Bregma level, 2 myelinated bundles exist of the anterior limb. The analysis was made by scientists blind to the treatment conditions.
For D2R‐D2R homoreceptor complex detection, mouse monoclonal anti‐D2R (MABN53, 1:600; Merck Millipore, Burlington, MA) and rabbit monoclonal anti‐D2R (HPA015691, 1 μg/ml; Human Atlas Project, Stockholm, Sweden) were used. For A2AR‐D2R and sigma1R‐D2R heteroreceptor complex detection, mouse monoclonal anti‐D2R (MABN53, 1:600; Merck Millipore) and rabbit monoclonal anti‐A2A (AB1559F, 1:250; Merck Millipore) and rabbit monoclonal anti‐sigma1R (ab53852, 1:500; Abcam, Cambridge, UK) were used. The quality and specificity of the antibodies used were validated by single immunohistochemistry experiments in frontal sections of the rat ventral striatum (Bregma level: 1.00 mm) and in HEK cells expressing exogenously only dopamine D1 to D5 receptor subtypes. Dopamine D2R antibodies were also validated by the Human Atlas Project. As proximity probes (Duolink kit), oligonucleotide‐labeled secondary antibodies (anti‐mouse and anti‐rabbit) were used.
Statistical Analysis
For the gene expression experiments, target gene expression was quantified relative to the internal reference gene PPIA (ΔCT = CTtarget − CTreference). The 2ΔCT values were analyzed using 2‐way ANOVA with brain region (NAc/dorsal striatum) and drinking condition (alcohol naïve/alcohol drinking) as factors followed by Bonferroni post hoc. In addition, correlation between individual alcohol intake (daily alcohol intake in g/kg/24 h for each individual obtained from mean of the last 7 weeks of alcohol drinking or 0 g/kg/24 h for alcohol‐naïve rats) and 2ΔCT values were analyzed with Pearson correlation analysis. For the epigenetic experiments, DNA methylation levels between the alcohol drinking (only rats with a voluntary alcohol intake above 2 g/kg/24 h, n = 7) and the alcohol‐naïve group were compared, using unpaired Student's t‐test. Values for densities of receptor complexes were obtained as mean of 3 pictures per brain region for each rat and were analyzed by 2‐way ANOVA with brain region (NAc shell/NAc core/dorsal striatum) and drinking condition (alcohol naïve/alcohol drinking) as factors followed by Bonferroni post hoc. Analyses were performed with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). Values are presented as mean ± SEM, and the significant level was α = 0.05.
Results
Long‐Term Alcohol Drinking Reduces Expression of the D2R in the NAc
Effects of long‐term alcohol drinking on expression of dopamine D2R (mRNA levels of long and short isoform) and the adenosine A2AR in the NAc and dorsal striatum were measured using qPCR. Expression of each gene of interest was measured relative to the reference gene PPIA (2deltaCT) and was analyzed for the factors brain region and drinking condition. Drinking condition compared rats that had been drinking alcohol in the IA20E model for 5 months (n = 14) with age‐matched alcohol‐naïve controls (n = 8). Values of individual alcohol intake (g/kg/24 h) were obtained as mean of the last 7 weeks. In the NAc, 2 RNA samples in the alcohol‐drinking rats and 1 in the alcohol‐naïve rats could not be successfully converted into cDNA (after 2 attempts), because gene expression of the reference gene could not be detected.
For the dopamine D2 long isoform, there was no overall main effect of drinking condition, F(1, 37) = 0.3, nonsignificant (ns), or brain region, F(1, 37) = 1.8, ns, but a significant interaction between these 2 factors, F(1, 37) = 12.4, p < 0.01. Post hoc analysis revealed a significantly lower expression (Fig. 1 A) in the NAc in alcohol‐drinking rats compared to alcohol‐naïve rats, but no significant effect of alcohol drinking in the dorsal striatum (Fig. 1 C). Furthermore, there was a significant correlation between individual alcohol intake and expression levels in the NAc (Fig. 1 B, r = −0.77, p < 0.001, R 2 = 0.58) but not in the dorsal striatum (Fig. 1 D, r = 0.23, R 2 = 0.05, ns).
Figure 1.
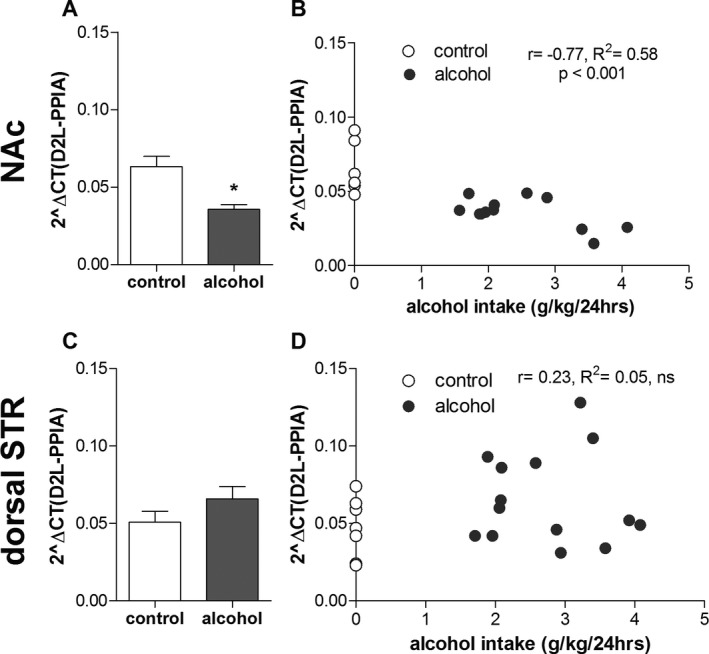
Effects of long‐term voluntary alcohol drinking on striatal gene expression of the long isoform of the dopamine D2 receptor. Wistar rats were exposed to intermittent access to 20% ethanol (alcohol, n = 14) or 2 bottles of water (age‐matched alcohol‐naïve control, n = 8) for 5 months. Individual alcohol intake was determined as mean g/kg/24 h alcohol intake over the last 7 weeks. Effects of alcohol drinking on gene expression of the dopamine D2 receptor long isoform (D2L) in the nucleus accumbens (NAc) and dorsal striatum (STR) were quantified using reverse transcriptase quantitative PCR. Specific primers were used detecting exon–exon junctions of the D2L. Expression levels are quantified relative to the reference gene peptidylprolyl isomerase A (PPIA). In the NAc, 3 RNA samples (n = 2 alcohol, n = 1 control) could not be successfully converted into cDNA. Alcohol drinking significantly decreased D2L gene expression in the NAc (A, B), but not the dorsal STR (C, D) (*p < 0.05 compared to alcohol‐naïve controls, 2‐way ANOVA with Bonferroni post hoc (A, C); Pearson's correlation (B, D)).
Regarding the dopamine D2 short isoform (Fig. 2 A,C), there was an overall main effect of brain region, F(1, 38) = 5.5, p < 0.05, but not of drinking condition, F(1, 38) = 0.34, ns, and no significant interaction, F(1, 38) = 1.9, ns, between these 2 factors. Hence, no post hoc tests were performed. However, there was a significant correlation between individual alcohol intake and expression levels in the NAc (Fig. 2 B, r = −0.61, p < 0.01, R 2 = 0.37). No such correlation was found in the dorsal striatum (Fig. 2 D, r = 0.08, R 2 = 0.01, ns).
Figure 2.
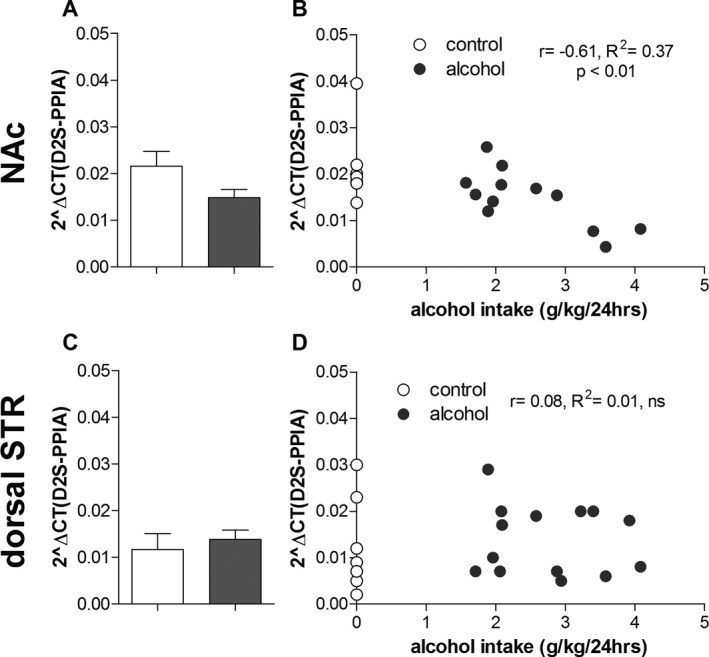
Effects of long‐term voluntary alcohol drinking on the short isoform of the dopamine D2 receptor (D2S). Wistar rats were exposed to intermittent access to 20% ethanol (alcohol, n = 14) or 2 bottles of water (age‐matched alcohol‐naïve control, n = 8) for 5 months. Individual alcohol intake was determined as mean g/kg/24 h alcohol intake over the last 7 weeks. Effects of alcohol drinking on gene expression of the D2S in the nucleus accumbens (NAc) and dorsal striatum (STR) were quantified using reverse transcriptase quantitative PCR. Specific primers were used detecting exon–exon junctions of the D2S. Expression levels for each gene are quantified relative to the reference gene peptidylprolyl isomerase A (PPIA). In the NAc, 3 RNA samples (n = 2 alcohol, n = 1 control) could not be successfully converted into cDNA. There were no significant overall effects of drinking condition or interaction of drinking condition*brain region (NAc: A; dorsal STR: C) (2‐way ANOVA) on D2S gene expression. There was a significant correlation between individual alcohol intake and D2S expression levels in the NAc (B), but not the dorsal STR (D) (Pearson's correlation).
Regarding the adenosine A2A expression (Fig. 3 A,C), there was no overall main effect of drinking condition, F(1, 37) = 2.5, ns, brain region, F(1, 37) = 3.1, ns, or condition*brain region interaction, F(1, 37) = 0.8, ns. Hence, no post hoc tests were conducted. Furthermore, there was no significant correlation between individual alcohol intake and expression levels in the NAc (Fig. 3 B, r = −0.37, R 2 = 0.14, ns, or in the dorsal striatum (Fig. 3 D, r = −0.15, R 2 = 0.02, ns).
Figure 3.
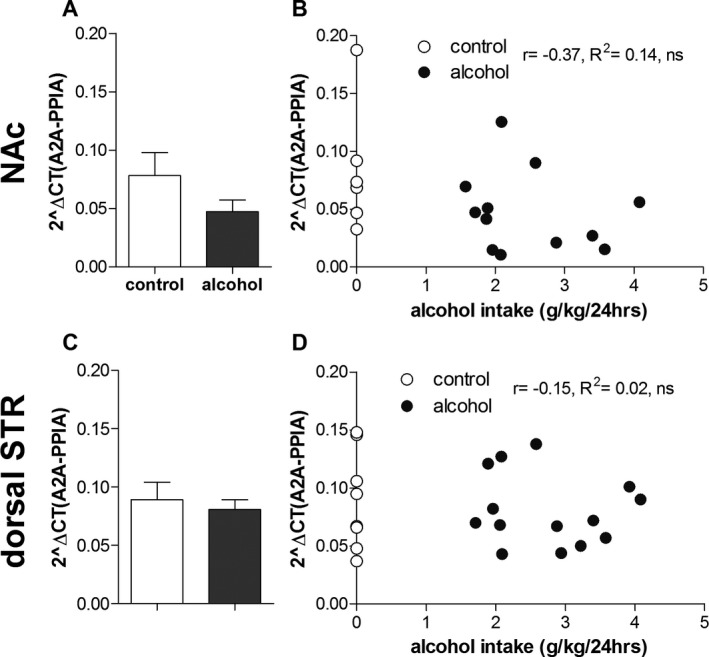
Effects of long‐term voluntary alcohol drinking on striatal gene expression of the adenosine A2A receptor. Wistar rats were exposed to intermittent access to 20% ethanol (alcohol, n = 14) or 2 bottles of water (age‐matched alcohol‐naïve control, n = 8) for 5 months. Individual alcohol intake was determined as mean g/kg/24 h alcohol intake over the last 7 weeks. Effects of alcohol drinking on adenosine A2A receptor gene expression in the nucleus accumbens (NAc) and dorsal striatum (STR) were quantified using reverse transcriptase quantitative PCR. A2A expression levels were quantified relative to the reference gene peptidylprolyl isomerase A (PPIA). In the NAc, 3 RNA samples (n = 2 alcohol, n = 1 control) could not be successfully converted into cDNA. Alcohol drinking did not affect Adora2a gene expression in the NAc (A, B) or the dorsal STR (C, D) (2‐way ANOVA with Bonferroni post hoc (A, C); Pearson's correlation (B, D)).
No Evidence for Drinking‐Induced Changes in DNA Methylation of the Drd2 Gene in the NAc
To investigate whether the above‐mentioned significant decrease in D2R expression was due to alcohol‐induced epigenetic changes, the percentage of DNA methylation of 6 CpGs of a short sequence within exon 1 of the Drd2 gene was measured in the NAc and compared between the same alcohol‐drinking rats (n = 12) and alcohol‐naïve rats (n = 7) used in the expression analysis. This region was chosen for analysis based on a previous study showing correlation of methylation levels of a sequence spanning exon 1 and part of intron 1 and Drd2 gene expression (Rodrigues et al., 2012).
Long‐term voluntary alcohol drinking did not have any significant effect on methylation levels of any CpG analyzed within the Drd2 gene (Fig. 4, CpG1: t 17 = 1.0, CpG2: t 17 = 0.8, CpG3: t 17 = 0.3, CpG4: t 17 = 0.5, CpG5: t 17 = 0.5, CpG6: t 17 = 0.4). Furthermore, no significant correlation was found between methylation levels and gene expression levels of the long isoform at any CpG position (data not shown; CpG1: r = −0.40, R 2 = 0.16, CpG2: r = −0.10, R 2 = 0.01, CpG3: r = 0.05, R 2 = 0.00, CpG4: r = −0.20, R 2 = 0.04, CpG5: r = −0.27, R 2 = 0.07, CpG6: r = −0.21, R 2 = 0.05; CpG1‐6: ns).
Figure 4.
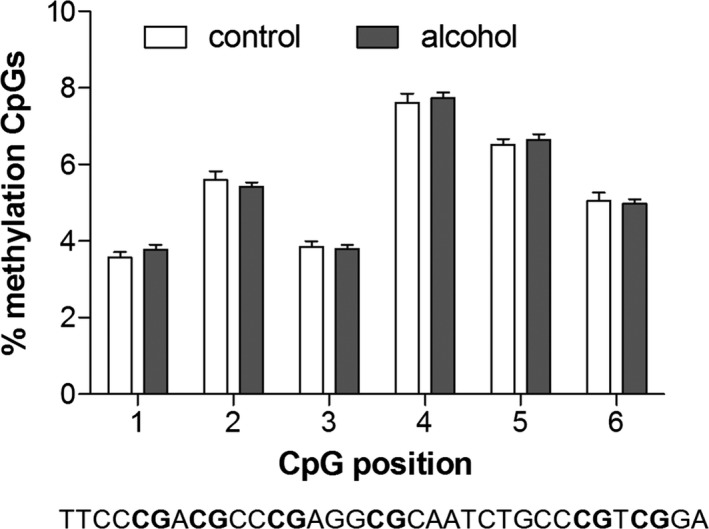
Effects of long‐term voluntary alcohol drinking on dopamine Drd2 gene methylation in the nucleus accumbens. Wistar rats were exposed to intermittent access to 20% ethanol (alcohol) or 2 bottles of water (age‐matched alcohol‐naïve control) for 5 months. Individual alcohol intake was determined as mean g/kg/24 h alcohol intake over the last 7 weeks. Individuals included in the CpG analysis are the same as the ones included in the expression analysis (n = 12 alcohol‐drinking, n = 7 alcohol‐naïve rats). Effects of alcohol drinking on methylation levels of a sequence within a CpG island in exon 1 of the Drd2 gene (see sequence above) in the NAc were quantified using bisulfite pyrosequencing. Alcohol‐drinking rats showed no changes in methylation levels of any CpG analyzed compared to alcohol‐naïve controls (unpaired Student's t‐test).
Alcohol Drinking Affects D2R Receptor Complex Formations in NAc Shell and Dorsal Striatum
PLA was used to investigate effects of voluntary long‐term alcohol drinking on densities of D2R‐D2R homo‐, A2AR‐D2R, and sigma1R‐D2R heteroreceptor complexes in the NAc shell, NAc core, and dorsal striatum by comparing the number of positive PLA clusters/nucleus/field (Fig. 5) between age‐matched alcohol‐naïve rats (n = 5), and rats that had IA20E for 3 months (4.7 ± 0.4 g alcohol/kg/24 h, n = 5). The PLA‐positive clusters were found to be associated with soma and neuropil. Both, the soma and the neuropil locations of the adenosine A2AR and the dopamine D2R are further demonstrated in single Z scan immunohistochemistry images (see supplementary information, Figs S3–S6).
Figure 5.
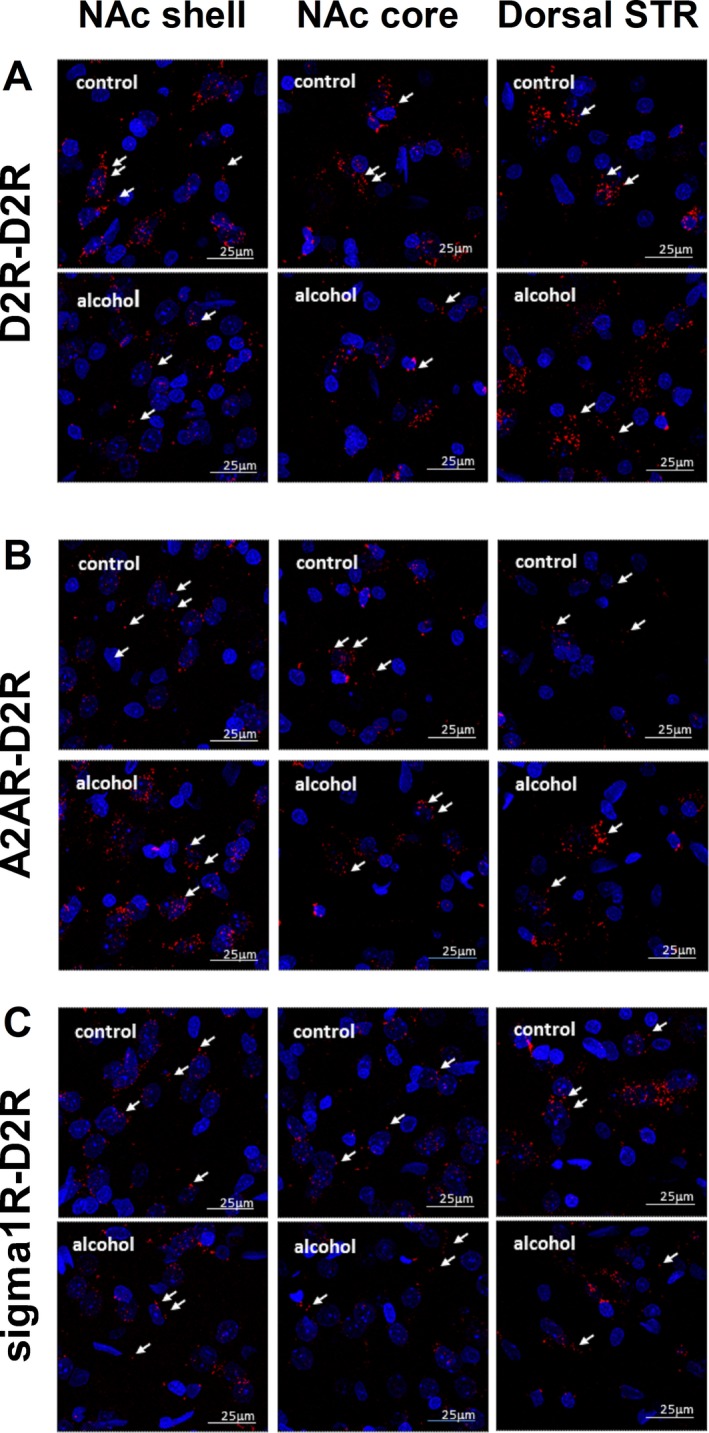
D2R‐D2R homoreceptor and A2AR‐D2R and sigma1R‐D2R heteroreceptor complexes in the striatum of alcohol‐naïve and alcohol‐drinking rats. These panels provide representative examples of the changes in the density of D2R‐D2R homoreceptor (A), the A2AR‐D2R (B), and sigma1R‐D2R (C) heteroreceptors complexes in the nucleus accumbens (NAc) shell (left panels), core (center panels), and the dorsal striatum (right panels) after at least 12 weeks of voluntary alcohol drinking in the intermittent access to 20% ethanol paradigm (lower panels) compared to control (upper panels). Using the in situ proximity ligation assay (in situ PLA) technique (Borroto‐Escuela et al., 2013; Fuxe et al., 2014a), together with confocal laser microscopy, receptor complexes were identified and visualized as red blobs (clusters, white arrows). PLA blobs were found in high densities per cell in a large number of nerve cells (picture: 25 × 25 μm, nuclei shown in blue by DAPI, 20 z‐layers) in frontal sections of the rat ventral and dorsal striatum (Bregma level: 1.00 mm). No specific PLA blobs were found in the anterior commissure and corpus callosum regions for either complex.
The analysis of the density of the D2R‐D2R homoreceptor complexes (Fig. 6 A) showed an overall main effect of drinking condition, F(1, 15) = 4.5, p < 0.05, and brain region, F(2, 15) = 6.3, p < 0.05, but no significant drinking condition*region interaction, F(2, 15) = 0.7, ns. Hence, no post hoc tests were performed.
Figure 6.
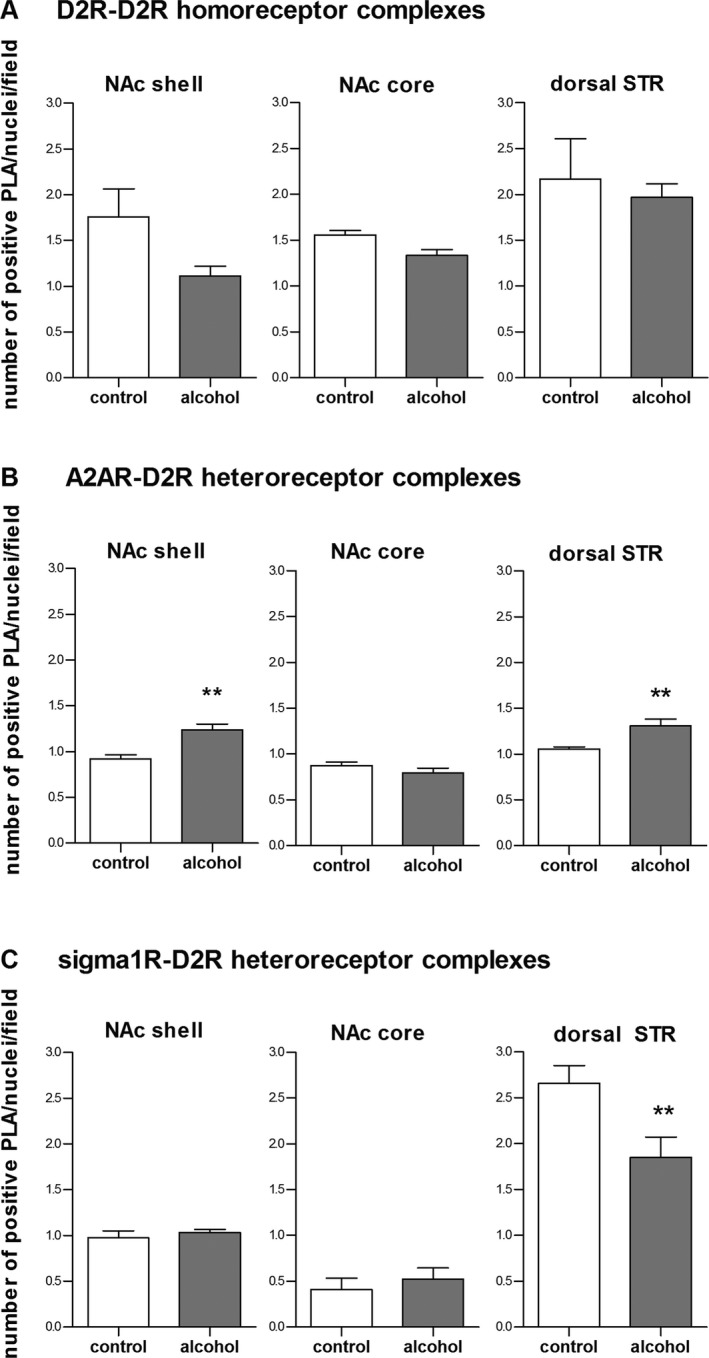
Effects of voluntary alcohol drinking on striatal density of the D2R‐D2R homoreceptor, A2AR‐D2R, and sigma1R‐ D2R heteroreceptor complexes. Rats were exposed to intermittent access to 20% ethanol (alcohol, 4.7 ± 0.4 g/kg/24 h) or 2 bottles of water (age‐matched alcohol‐naïve control), respectively, for at least 12 weeks. Effects of alcohol drinking on the density of D2R complexes in the nucleus accumbens (NAc) core and shell and the dorsal striatum (STR) were quantified using in situ proximity ligation assay (PLA). Data were analyzed using 2‐way ANOVA followed by Bonferroni post hoc with brain region and drinking condition as factors (n = 3 to 4, per group). There was a significant effect of alcohol drinking on D2R‐D2R homoreceptor complexes, but no drinking condition*brain region interaction (A). Alcohol drinking significantly increased the density of A2AR‐D2R heteroreceptor complexes in the NAc shell and the dorsal striatum, but not in the NAc core (B). Alcohol drinking significantly decreased the density of sigma1R‐D2R heteroreceptor complexes in the dorsal STR, but not in the NAc core or shell (C). Values represent group mean±SEM number of positive PLA dots per nuclei/sample field, for each rat given as mean values of 3 pictures (40 × 40 μm). **p < 0.01 compared to corresponding alcohol‐naïve controls.
Regarding the density of the A2AR‐D2R heteroreceptor complexes (Fig. 6 B), there was an overall main effect of drinking condition, F(1, 18) = 15.0, p < 0.01, brain region, F(2, 18) = 24.0, p < 0.001, and an interaction for drinking condition*brain region, F(2, 18) = 8.5, p < 0.01. Post hoc analysis revealed that the density of A2AR‐D2R heteroreceptor complexes was significantly higher in alcohol‐drinking, compared to alcohol‐naïve rats, in the NAc shell and in the dorsal striatum, but not in the NAc core.
Regarding the density of the sigma1R‐D2R heteroreceptor complexes (Fig. 6 C), there was an overall main effect of brain region, F(1, 5) = 28.0, p < 0.01, no significant overall main effect of drinking condition, F(1, 18) = 3.3, ns, but a significant drinking condition*brain region interaction, F(2, 18) = 6.6, p < 0.01. Post hoc analysis revealed a significant reduction in these complexes in the dorsal striatum of the alcohol drinking compared to the alcohol naïve rats. No significant effects were found in the NAc core or shell.
Discussion
The main findings in the present study were that long‐term voluntary alcohol drinking in the IA20E model, induced changes in Drd2 gene expression and D2R complex formations in the striatum, compared to age‐matched alcohol‐naïve control rats. Specifically, alcohol drinking significantly reduced mRNA levels of the D2R long isoform in the NAc and decreased the density of D2R‐D2R homoreceptor complexes in the striatum, as well as sigma1R‐D2R heteroreceptor complexes in the dorsal striatum. In addition, alcohol‐drinking rats displayed a significantly increased density of A2AR‐D2R heteroreceptor complexes in the NAc shell and the dorsal striatum, compared to alcohol‐naïve rats. Collectively, these findings support the dopamine deficiency hypothesis in AUD.
Reduced striatal D2R radioligand binding in alcohol‐dependent patients compared to healthy controls as consistently found in human imaging studies (Hietala et al., 1994; Volkow et al., 1996) could reflect several neurobiological differences. First, D2R levels could be reduced. This suggestion is supported by the present findings of a reduced striatal density of D2R‐D2R homoreceptor complexes, as well as a reduced Drd2 gene expression in the long‐term drinking rats. Second, the D2R affinity might be reduced. An increase in the density of A2AR‐D2R heteroreceptor complexes found in the present study could potentially result in a reduced D2R affinity due to reciprocal allosteric antagonism between A2AR and D2R (Fernandez‐Duenas et al., 2012; Fuxe et al., 2014b). Although this suggestion remains to be demonstrated, this hypothesis is supported by studies showing that an A2AR agonist can reduce the affinity of D2R for dopamine in the striatum (Fuxe et al., 2014b) and that reinstatement of cocaine seeking induced by the D2R agonist quinpirole can be blocked by systemic A2AR agonist treatment (Wydra et al., 2015) as well as intra‐accumbal A2AR agonist infusions (O'Neill et al., 2012). Finally, increased endogenous dopamine levels could compete with the radioligand at the D2R and thereby reduce D2R radioligand binding. However, our previous rodent study showing decreased striatal dopamine output following long‐term alcohol drinking (Feltmann et al., 2016), as well as a reduced central‐stimulant induced striatal dopamine release in alcohol‐dependent patients (Martinez et al., 2005; Volkow et al., 2007) makes this possibility less likely.
The mRNA levels of the long, but not the short, D2R isoform was significantly lower in the NAc alcohol‐drinking rats compared to age‐matched alcohol‐naïve controls. However, the expression levels of both isoforms were negatively correlated with individual alcohol intake, indicating that the alcohol‐induced Drd2 gene expression changes might not be isoform specific. It has been suggested that the long and short D2R isoforms are mainly expressed post‐ and presynaptically, respectively (Khan et al., 1998; Usiello et al., 2000). However, by isolating RNA from the NAc, the obtained expression levels do not represent receptor levels on presynaptic dopamine terminals, which originate from projections from the ventral tegmental area. Thus, the present changes in D2R expression levels in the NAc most likely represent changes on postsynaptic membranes. However, the reduced receptor availability indicated by the density of D2R‐D2R homoreceptor complexes could be induced by many other processes than gene expression, such as posttranslational modifications of proteins and receptor trafficking. The effects of long‐term voluntary alcohol consumption on D2R function, that is affinity and signaling, deserve to be further investigated.
The present result, showing that long‐term voluntary alcohol intake (IA20E) decreases expression of the long isoform of the dopamine D2R in the NAc, supports previous findings that long‐term IA20E drinking reduces dopamine output in the NAc of standard laboratory rats (Barak et al., 2011; Feltmann et al., 2016). Moreover, a rat study with 8 months of voluntary, albeit continuous, alcohol intake inducing similar levels of alcohol intake as in the present study, has shown a variety of changes in dopamine‐regulating proteins relevant for AUD (Kashem et al., 2012). Together, these findings highlight the importance of voluntary alcohol intake over several weeks or months to induce a dopamine deficiency. As mentioned in the introduction, rodent studies with short‐term and or forced alcohol administration show inconsistent results with regard to effects on the dopamine system, with down‐regulated (Rommelspacher et al., 1992), unchanged (Reggiani et al., 1980), or increased (Kim et al., 1997) D2R densities in the striatum. Nevertheless, studies with forced alcohol intake (using alcohol as the only available fluid or food) during 8 (Syvalahti et al., 1988) and 12 months (Rothblat et al., 2001) have been shown to down‐regulate the dopamine system. These studies combined indicate that oral administration together with the length of the alcohol exposure, rather than the voluntary aspect, is crucial for inducing a dopamine deficiency. However, it should be noted that 14 weeks of voluntary alcohol intake through a 2‐bottle‐choice paradigm, with and without repeated cycles of withdrawal, increased D2R binding density in the NAc shell and core and dorsal striatum in rats selectively bred for alcohol preference (Sari et al., 2006). Nevertheless, based on the present findings and previous studies, we hypothesize that the dopamine deficiency consistently found in AUD patients (Ravan et al., 2014) might be induced by chronic excessive alcohol drinking rather than reflecting an underlying vulnerability factor contributing to the development of AUD. This suggestion is supported by the fact that the outbred rats in these studies were randomized to alcohol drinking or alcohol‐naïve control groups and thus, most likely, lacked prevailing differences in D2R expression at the start of the experiment.
The present results showing no effect of alcohol drinking on DNA methylation of any CpG within a short sequence of the exon 1 of the Drd2 gene indicate that the epigenetic mechanism of DNA methylation seems not to be responsible for the found alcohol‐induced reduction on Drd2 gene expression at least for the sequence analyzed. Exon 1 was chosen for analysis based on a previous study showing that methylation levels of a large sequence spanning between exon 1 and part of intron 1 correlated with mRNA levels (Rodrigues et al., 2012). However, in the present study, none of the CpG positions analyzed correlated with mRNA expression levels. Therefore, the observed effects on gene expression could be caused by changes in methylation of CpGs outside the sequence analyzed or through other mechanisms regulating gene expression, for example, histone modifications or miRNAs.
In the dorsal striatum, there was no significant difference in the mRNA levels of the long or short isoform of the D2R between long‐term drinking and alcohol‐naïve rats. Furthermore, the gene expression of the long or short isoform of the D2R did not correlate with individual alcohol intake levels. These results indicate that alcohol drinking does not affect Drd2 gene expression in the dorsal striatum. However, the present findings that A2AR‐D2R and sigma1R‐D2R heteroreceptor complexes were changed by alcohol drinking in the dorsal striatum indicate that alcohol‐induced dopaminergic receptor changes do occur and might contribute to a hypodopaminergic state. In fact, it has recently been shown that cycles of excessive alcohol consumption and withdrawal in mice induce changes in synaptic strength serving to inhibit the D2‐positive dorsal striato‐pallidal GABA neurons and that inhibition and excitation of these cells, promotes, and reduces alcohol consumption, respectively (Cheng et al., 2017).
The present results suggest, to our knowledge for the first time, that a reorganization of the D2R homo‐ and A2AR‐D2R heteroreceptor complexes could take place following long‐term alcohol drinking and potentially contribute to the results in studies showing a reduction in D2R radioligand binding in AUD (Ravan et al., 2014). Thus, targeting specific receptor complexes might provide a novel treatment strategy in AUD. In fact, it has previously been suggested that specific blockage of the A2AR protomer within the A2AR‐D2R heteroreceptor complex could increase D2R function and thereby compensate for a dysfunctioning dopamine system during dependence disorders (Filip et al., 2012; Kravitz et al., 2015). Furthermore, it was recently shown that chronic cocaine self‐administration, similar to the present results in long‐term drinking rats, increased the density of A2AR‐D2R heteroreceptor complexes in the NAc shell, decreased the density of sigma1R‐D2R heteroreceptor complexes in the dorsal striatum, and had no effect on the density of either receptor complex in the NAc core. In contrast to the present results, cocaine self‐administration increased the density of sigma1R‐D2R heteroreceptor complexes in the NAc shell and caused a nonsignificant reduction in the density of the A2AR‐D2R heteroreceptor complexes in the dorsal striatum (Borroto‐Escuela et al., 2017).
The finding that the density of A2AR‐D2R heteroreceptor complexes was increased in the NAc shell, but not in the NAc core, indicates that long‐term alcohol consumption induces different effects in these subregions. This suggestion is in line with studies showing that drugs of abuse preferentially induce dopamine release in the NAc shell compared to the core (Pontieri et al., 1995) and that alcohol is self‐administered into the NAc shell, but not NAc core (Engleman et al., 2009). Furthermore, the finding that A2AR‐D2R heteroreceptor complexes were increased, whereas D2R‐D2R homoreceptor complexes were decreased could indicate that the A2AR‐D2R increase is driven by changes in the A2AR. However, our present results, showing no effect on Adora2a gene expression, indicate that the observed changes are related to a reorganization of the heteroreceptor complexes studied. However, subregion‐specific changes of Adora2a gene expression might have been missed, because it is not possible to differentiate between NAc subregions using brain dissection. Nevertheless, to the best of our knowledge, no differences between NAc core and shell have been reported for A2AR levels.
The significantly increased density of A2AR‐D2R heteroreceptor complexes in the NAc shell and dorsal striatum of the long‐term alcohol drinking rats in the present study could possibly indicate that alcohol indirectly may have induced conformational changes in the A2AR and/or D2R, enhancing the affinity between these 2 receptors. Such changes could lead to increased antagonistic allosteric A2AR‐D2R receptor–receptor interactions (Borroto‐Escuela et al., 2017). Due to the role of the sigma1R as an adaptor protein in receptor complexes (Navarro et al., 2013), the sigma1R could be part of the A2AR‐D2R heteroreceptor complex (Borroto‐Escuela et al., 2016). The formation of higher order complexes (A2AR‐D2R‐sigma1R) contributes to conformational changes, as well as to an altered balance and expression level of multiple complexes. Thus, the higher density of the D2‐sigma1 heteroreceptor complexes in the NAc shell and dorsal striatum, compared to the NAc core (Fig. 6), could potentially contribute to the present region‐specific alcohol‐induced increase in the A2AR‐D2R heteroreceptor complexes via the formation of the A2AR‐D2R‐sigma1R complex.
It could be argued that the stronger staining in the cortex in relation to the striatum (Fig. S5) could be due to unspecific binding of the A2A antibody. Nevertheless, the present level of A2AR immunoreactivity distribution in the striatum is in line with previous publications on striatal A2AR immunoreactivity (Durieux et al., 2009; Trifilieff et al., 2011). It should, however, be considered that cortical, but not striatal, A2AR immunoreactivity could potentially be higher in rat than that observed in previous work in mice (Durieux et al., 2009; Trifilieff et al., 2011). An unspecific immunoreactivity with the commercial A2AR antibodies used is therefore possible. However, such an unspecific protein staining, if at all present in the striatum, will most likely not physically interact with striatal D2R and form a heteromer, which is visualized with PLA, as is the case for the A2AR. Thus, PLA clusters will not appear between an unspecifically stained protein and D2Rs because they probably do not physically interact with each other.
In conclusion, the present study demonstrates that long‐term voluntary alcohol consumption induces changes of the D2R in the striatum, including gene expression and receptor complexes in Wistar rats. Together with our previous microdialysis study (Feltmann et al., 2016), these findings are in line with the dopamine deficiency hypothesis in AUD (Ravan et al., 2014) and might contribute to novel treatment strategies in dependence disorders (Filip et al., 2012; Kravitz et al., 2015).
Funding
This work was supported by grants from the Swedish Research Council (04X‐715 and VR‐link) to KFu and (2015‐03525) to PS, the Swedish Research Council for Health, Working Life and Welfare (2013‐1781) to PS, the Swedish Brain Foundation: (FO2014‐0137) to PS; (FO2016‐0302) to DOB‐E; (FO2014‐0223 and F02016‐0231) to TJE, AFA Insurance (130328) to KFu and DOB‐E, as well as Psykiatrifonden to KFe, and the Swedish Research Council Formas (210‐2012‐1502) to JR. DOB‐E belongs to Academia de Biólogos.
Declaration of Interest
The authors declare no competing financial interest.
Supporting information
Fig. S1. Escalation of voluntary alcohol intake in the intermittent‐access to 20% ethanol paradigm.
Fig. S2. Illustration of the sampling fields used in the proximity ligation assay.
Fig. S3. Illustrations of the dopamine D2 receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Fig. S4. Illustrations of the dopamine D2 receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Fig. S5. Illustrations of the adenosine A2A receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Fig. S6. Illustrations of the sigma1 receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Table S1. Primer sequences used in qPCR and pyrosequencing.
Acknowledgments
We thank the technicians Malin Wirf, Linnea Tankred, and Monica Aronson and undergraduate student Maria Östman for excellent assistance with the IA20E model and Beth Hagman for assistance with perfusions and sectioning of the brains in the PLA experiment. We thank undergraduate students Radwa Almamoun and Sabina Risén Rimfors for teaching Kristin Feltmann the qPCR and pyrosequencing methods and Dr. Vicente Martínez Redondo for assistance in designing the qPCR primers.
References
- Barak S, Carnicella S, Yowell QV, Ron D (2011) Glial cell line‐derived neurotrophic factor reverses alcohol‐induced allostasis of the mesolimbic dopaminergic system: implications for alcohol reward and seeking. J Neurosci 31:9885–9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Valenza M, Iyer MR, Rice KC, Steardo L, Hayashi T, Cottone P, Sabino V (2015) Sigma‐1 receptor mediates acquisition of alcohol drinking and seeking behavior in alcohol‐preferring rats. Behav Brain Res 287:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A (2003) Alcohol promotes dopamine release in the human nucleus accumbens. Synapse 49:226–231. [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela DO, Narvaez M, Wydra K, Pintsuk J, Pinton L, Jimenez‐Beristain A, di Palma M, Jastrzebska J, Filip M, Fuxe K (2017) Cocaine self‐administration specifically increases A2AR‐D2R and D2R‐sigma1R heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder. Pharmacol Biochem Behav 155:24–31. [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela DO, Romero‐Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO, Palkovits M, Agnati LF, Fuxe K (2013) G protein‐coupled receptor heterodimerization in the brain. Methods Enzymol 521:281–294. [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela DO, Wydra K, Pintsuk J, Narvaez M, Corrales F, Zaniewska M, Agnati LF, Franco R, Tanganelli S, Ferraro L, Filip M, Fuxe K (2016) Understanding the functional plasticity in neural networks of the basal ganglia in cocaine use disorder: a role for allosteric receptor‐receptor interactions in A2A‐D2 heteroreceptor complexes. Neural Plast 2016:4827268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D, Barak S (2014) Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 48:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang CCY, Ma T, Wei X, Wang X, Lu J, Wang J (2017) Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biol Psychiatry 81:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Janak PH (2016) Changes in the influence of alcohol‐paired stimuli on alcohol seeking across extended training. Front Psychiatry 7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH (2014) Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front Behav Neurosci 8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, De Kerchove D'exaerde A (2009) D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci 12:393–395. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA (2009) Ethanol is self‐administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res 33:2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358:189–196. [DOI] [PubMed] [Google Scholar]
- Feltmann K, Fredriksson I, Wirf M, Schilstrom B, Steensland P (2016) The monoamine stabilizer (‐)‐OSU6162 counteracts downregulated dopamine output in the nucleus accumbens of long‐term drinking Wistar rats. Addict Biol 21:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Duenas V, Gomez‐Soler M, Jacobson KA, Kumar ST, Fuxe K, Borroto‐Escuela DO, Ciruela F (2012) Molecular determinants of A2AR‐D2R allosterism: role of the intracellular loop 3 of the D2R. J Neurochem 123:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K (2012) The importance of the adenosine A(2A) receptor‐dopamine D(2) receptor interaction in drug addiction. Curr Med Chem 19:317–355. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Borroto‐Escuela DO, Romero‐Fernandez W, Palkovits M, Tarakanov AO, Ciruela F, Agnati LF (2014a) Moonlighting proteins and protein‐protein interactions as neurotherapeutic targets in the G protein‐coupled receptor field. Neuropsychopharmacology 39:131–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Tarakanov A, Romero Fernandez W, Ferraro L, Tanganelli S, Filip M, Agnati LF, Garriga P, Diaz‐Cabiale Z, Borroto‐Escuela DO (2014b) Diversity and bias through receptor‐receptor interactions in GPCR heteroreceptor complexes. Focus on examples from dopamine D2 receptor heteromerization. Front Endocrinol (Lausanne) 5:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia J, Catafau AM, Batlle F, Martin JC, Segura L, Gonzalvo B, Prat G, Carrio I, Casas M (2000) Striatal dopaminergic D(2) receptor density measured by [(123)I]iodobenzamide SPECT in the prediction of treatment outcome of alcohol‐dependent patients. Am J Psychiatry 157:127–129. [DOI] [PubMed] [Google Scholar]
- Hamdi A, Prasad C (1992) Bidirectional changes in striatal D2‐dopamine receptor density during chronic ethanol intake. Alcohol 9:133–137. [DOI] [PubMed] [Google Scholar]
- Heinz A, Dettling M, Kuhn S, Dufeu P, Graf KJ, Kurten I, Rommelspacher H, Schmidt IG (1995) Blunted growth hormone response is associated with early relapse in alcohol‐dependent patients. Alcohol Clin Exp Res 19:62–65. [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P (2004) Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161:1783–1789. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U (1994) Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology 116:285–290. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM (1997) Alcohol self‐administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res 21:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A (2010) Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self‐administration. Alcohol Clin Exp Res 34:1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram‐Lindström N, Ericson M, Steensland P, Jerlhag E (2016) Dopamine and alcohol dependence: from bench to clinic, in Recent Advances in Drug Addiction Research and Clinical Applications (Meil DW. ed), pp 81–114. InTech doi: 10.5772/63144. Available at: https://www.intechopen.com/books/recent-advances-in-drug-addiction-research-and-clinical-applications/dopamine-and-alcohol-dependence-from-bench-to-clinic. Accessed December 10, 2017. [Google Scholar]
- Kashem MA, Ahmed S, Sarker R, Ahmed EU, Hargreaves GA, McGregor IS (2012) Long‐term daily access to alcohol alters dopamine‐related synthesis and signaling proteins in the rat striatum. Neurochem Int 61:1280–1288. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman‐Rakic PS (1998) Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A 95:7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MO, Lee YK, Choi WS, Kim JH, Hwang SK, Lee BJ, Kang SG, Kim K, Baik SH (1997) Prolonged ethanol intake increases D2 dopamine receptor expression in the rat brain. Mol Cells 7:682–687. [PubMed] [Google Scholar]
- Kravitz AV, Tomasi D, Leblanc KH, Baler R, Volkow ND, Bonci A, Ferre S (2015) Cortico‐striatal circuits: novel therapeutic targets for substance use disorders. Brain Res 1628:186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G (2003) Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci U S A 100:4305–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE, Green AI, Pettinati HM, Ciraulo DA, Sarid‐Segal O, Kampman K, Brunette MF, Strain EC, Tiouririne NA, Ransom J, Scott C, Stout R, NCIG (National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group) Study Group (2013) A double‐blind, placebo‐controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 7:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lograno DE, Matteo F, Trabucchi M, Govoni S, Cagiano R, Lacomba C, Cuomo V (1993) Effects of chronic ethanol intake at a low dose on the rat brain dopaminergic system. Alcohol 10:45–49. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi‐Dargham A (2005) Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58:779–786. [DOI] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Cifani C, Lambertucci C, Volpini R, Cristalli G, Froldi R, Massi M (2012) Effects of A(2)A adenosine receptor blockade or stimulation on alcohol intake in alcohol‐preferring rats. Psychopharmacology 219:945–957. [DOI] [PubMed] [Google Scholar]
- Nam HW, Bruner RC, Choi DS (2013) Adenosine signaling in striatal circuits and alcohol use disorders. Mol Cells 36:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G, Moreno E, Bonaventura J, Brugarolas M, Farre D, Aguinaga D, Mallol J, Cortes A, Casado V, Lluis C, Ferre S, Franco R, Canela E, McCormick PJ (2013) Cocaine inhibits dopamine D2 receptor signaling via sigma‐1‐D2 receptor heteromers. PLoS One 8:e61245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill CE, Letendre ML, Bachtell RK (2012) Adenosine A2A receptors in the nucleus accumbens bi‐directionally alter cocaine seeking in rats. Neuropsychopharmacology 37:1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomizu S, Boyadjieva N, Sarkar DK (2003) Ethanol and estradiol modulate alternative splicing of dopamine D2 receptor messenger RNA and abolish the inhibitory action of bromocriptine on prolactin release from the pituitary gland. Alcohol Clin Exp Res 27:975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The Rat Brain in Stereotaxic Coordinates Academic Press/Elsevier, Amsterdam, Boston. [Google Scholar]
- Pontieri FE, Tanda G, di Chiara G (1995) Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A 92:12304–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravan S, Martinez D, Slifstein M, Abi‐Dargham A (2014) Molecular imaging in alcohol dependence. Handb Clin Neurol 125:293–311. [DOI] [PubMed] [Google Scholar]
- Reggiani A, Barbaccia ML, Spano PF, Trabucchi M (1980) Dopamine metabolism and receptor function after acute and chronic ethanol. J Neurochem 35:34–37. [DOI] [PubMed] [Google Scholar]
- Rodrigues AJ, Leao P, Pego JM, Cardona D, Carvalho MM, Oliveira M, Costa BM, Carvalho AF, Morgado P, Araujo D, Palha JA, Almeida OF, Sousa N (2012) Mechanisms of initiation and reversal of drug‐seeking behavior induced by prenatal exposure to glucocorticoids. Mol Psychiatry 17:1295–1305. [DOI] [PubMed] [Google Scholar]
- Rommelspacher H, Raeder C, Kaulen P, Bruning G (1992) Adaptive changes of dopamine‐D2 receptors in rat brain following ethanol withdrawal: a quantitative autoradiographic investigation. Alcohol 9:355–362. [DOI] [PubMed] [Google Scholar]
- Rothblat DS, Rubin E, Schneider JS (2001) Effects of chronic alcohol ingestion on the mesostriatal dopamine system in the rat. Neurosci Lett 300:63–66. [DOI] [PubMed] [Google Scholar]
- Sari Y, Bell RL, Zhou FC (2006) Effects of chronic alcohol and repeated deprivations on dopamine D1 and D2 receptor levels in the extended amygdala of inbred alcohol‐preferring rats. Alcohol Clin Exp Res 30:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senogles SE (1994) The D2 dopamine receptor isoforms signal through distinct Gi alpha proteins to inhibit adenylyl cyclase. A study with site‐directed mutant Gi alpha proteins. J Biol Chem 269:23120–23127. [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE (2008) Intermittent access to 20% ethanol induces high ethanol consumption in Long‐Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Fredriksson I, Holst S, Feltmann K, Franck J, Schilstrom B, Carlsson A (2012) The monoamine stabilizer (‐)‐OSU6162 attenuates voluntary ethanol intake and ethanol‐induced dopamine output in nucleus accumbens. Biol Psychiatry 72:823–831. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518–12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syvalahti EK, Hietala J, Roytta M, Gronroos J (1988) Decrease in the number of rat brain dopamine and muscarinic receptors after chronic alcohol intake. Pharmacol Toxicol 62:210–212. [DOI] [PubMed] [Google Scholar]
- dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH (1989) The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J 8:4025–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J, Schmauss C, Slattman M, Gullberg M, Javitch JA (2011) Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2‐adenosine A2A receptor complexes in the striatum. Biotechniques 51:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O'Malley SS, Krystal JH, Abi‐Dargham A (2010) Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry 68:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge‐Pont F, Picetti R, Dierich A, Lemeur M, Piazza PV, Borrelli E (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408:199–203. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K (1996) Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 20:1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C (2007) Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 27:12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF (1996) Ethanol self‐administration restores withdrawal‐associated deficiencies in accumbal dopamine and 5‐hydroxytryptamine release in dependent rats. J Neurosci 16:3474–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1973) Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29:203–210. [DOI] [PubMed] [Google Scholar]
- Wydra K, Suder A, Borroto‐Escuela DO, Filip M, Fuxe K (2015) On the role of A(2)A and D(2) receptors in control of cocaine and food‐seeking behaviors in rats. Psychopharmacology 232:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Escalation of voluntary alcohol intake in the intermittent‐access to 20% ethanol paradigm.
Fig. S2. Illustration of the sampling fields used in the proximity ligation assay.
Fig. S3. Illustrations of the dopamine D2 receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Fig. S4. Illustrations of the dopamine D2 receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Fig. S5. Illustrations of the adenosine A2A receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Fig. S6. Illustrations of the sigma1 receptor single immunolabelling studies in the dorsal and ventral striatum of rat brain.
Table S1. Primer sequences used in qPCR and pyrosequencing.


