Abstract
Chronic wasting disease (CWD) is an emergent prion disease affecting cervid species in North America, Canada, South Korea, and recently, Norway. Detection of CWD has been advanced by techniques that rely on amplification of low levels of prion amyloid to a detectable level. However, the increased sensitivity of amplification assays is often compromised by inhibitors and/or activators in complex biologic samples including body fluids, excreta, or the environment. Here, we adapt real-time quaking-induced conversion conditions to specifically detect CWD prions in fecal samples from both experimentally infected deer and naturally infected elk and estimate environmental contamination. The results have application to detection, surveillance and management of CWD, and potentially to other protein-misfolding diseases.
Keywords: chronic wasting disease, RT-QuIC, TSE, Prion, amyloid amplification
Introduction
Chronic wasting disease (CWD) is a prion disease affecting cervid species primarily in the USA and Canada, but is now emerging worldwide (e.g. South Korea and Norway) [1, 2] (www.nwhc.usgs.gov) due to its facile spread in natural susceptible populations. Depopulation/repopulation studies and the detection of CWD prions in blood, saliva, feces and urine of infected cervids has led to the tenet that the efficient spread of CWD may in substantial part be due to environmental contamination [2–11].
Prions are misfolded conformations of the normal, alpha-helical, cellular protein, PrPC, classically designated as PrPSc [12–14]. Detection of PrPSc is hampered by difficulty in distinguishing the disease-specific conformation, PrPSc, from the cellular form of the PrPC protein. Traditionally, the distinction has been predicated on the relative protease resistance of the disease-specific form of PrPSc in Western blots or enzyme-linked immunosorbent assays (ELISA) [15]. A seminal advancement in the detection of prion diseases was the discovery that the addition of PrPSc to the proper PrPC substrate results in amyloid amplification under appropriate reaction conditions such as those provided in the serial protein-misfolding cyclic amplification assay (sPMCA) [16, 17].
The real-time quaking-induced conversion (RT-QuIC) assay employs a recombinant PrPC (rPrPC) substrate and provides increased throughput, simplified read-out and reduced cost [18–20]. Tuning of RT-QuIC conditions and enrichment methods makes it possible to detect prion-seeding activity in an array of biologic and environmental samples including tissues, biologic fluids and excreta [10, 11, 21–29]. However, we have observed the presence of inhibitors and activators of amyloid seeding in certain tissues and excreta, including lower intestine and feces. Recently, we have utilized iron-oxide bead magnetic extraction to increase sensitivity in dilute prion-containing samples such as saliva, urine and clarified fecal homogenates [30].
In the present study, we apply modifications of RT-QuIC to favor prion-seeded conversion and limit non-specific conversion caused by unidentified factors present in the feces of deer and elk. This innovation is of practical importance since the detection of prions in feces of free-ranging animals can be used to estimate the presence (or prevalence) of CWD in an environment or in a cervid population without individual animal capture. In addition to CWD status, the amount of CWD environmental contamination and potentially an estimation of infectious doses shed during the disease course could be determined by careful analysis of CWD-seeding activity in feces. Moreover, detection of prion-seeding activity in accessible body fluids and excreta may have non-invasive diagnostic application for other protein-misfolding disorders.
Results
Selecting for seeded conversion while maintaining sensitivity
In our initial investigation of RT-QuIC seeding activity in a large sample size of deer feces we observed an unacceptably high level of false-positive reactions. One method for decreasing false-positive reactions is to lower reaction temperature. With this in mind we set out to determine the level of sensitivity lost by lowering reaction temperature by investigating the effect of temperature on reaction kinetics with a CWD(+) brain sample. We found that decreasing temperature saw a corresponding decrease in the rate of seeded conversion but had little affect on reaction curves (Fig. 1a). Reaction rates decreased (the time required to cross the threshold increased) at all dilutions in response to lowering reaction temperatures (Fig. 1b). The best detection of a CWD(+) brain sample was observed at 45 °C (one more 10-fold dilution was detectable vs 37 or 42 °C) (Fig. 1b). Further reduction to 25 or 30 °C reduced prion seed detection by 10-fold (Fig. 1b). Most importantly, assay sensitivity (dilution at which detection was lost) was the same between 42 and 37 °C, though a slightly slower reaction rate was observed at 37 °C (Fig. 1a, b). A negative brain homogenate was also tested at each temperature with the same dilutions (24 replicates at each temperature). Only one replicate of the CWD(−) homogenate at 42 °C was positive under the same reaction conditions. Based on these results, we concluded that the reduced reaction temperature would not have a drastic effect on the overall sensitivity.
Fig. 1.
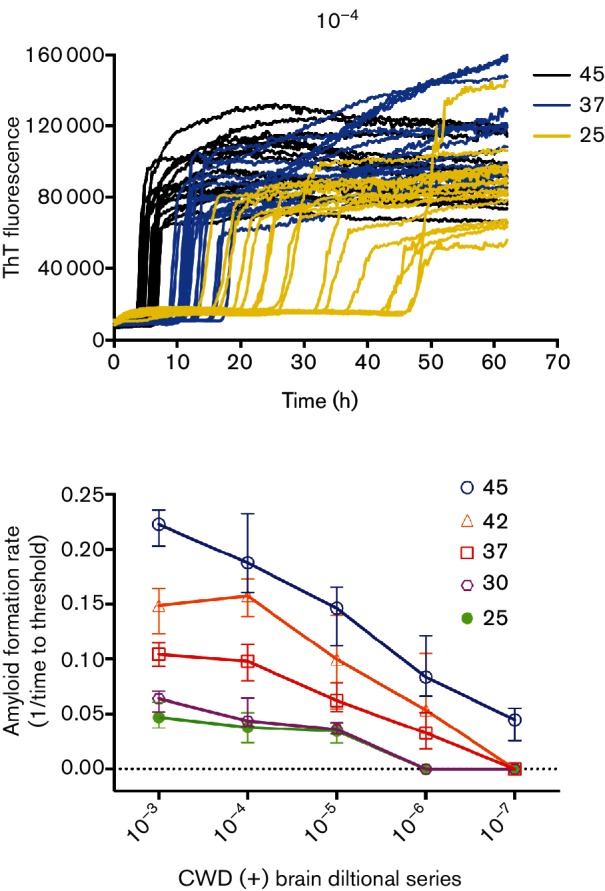
Seeded conversion is slower at lower temperatures, but the limit of detection is unchanged between 37 and 42 °C. The raw ThT fluorescence signal traces of a 10–4 dilution of a CWD positive brain homogenate is shown for three temperatures: 45 °C (black), 37 °C (blue) and 25 °C (yellow). Decreasing reaction temperature does not alter curve shape or smoothness. A serial dilution of CWD(+) (10−3 to 10−7) 10 % brain homogenate is analysed at 45 °C (blue circles), 42 °C (orange triangles), 37 °C (red squares), 30 °C (purple hexagons) and 25 °C (green solid circles) and reaction rate data (1 lag–1 phase) is plotted for each temperature and dilution. Temperature decreases reaction rate and reduces sensitivity below 37 °C. Each symbol represents the mean of eight replicates and error bars represent one standard deviation.
Detection of CWD prion seeding activity in deer feces
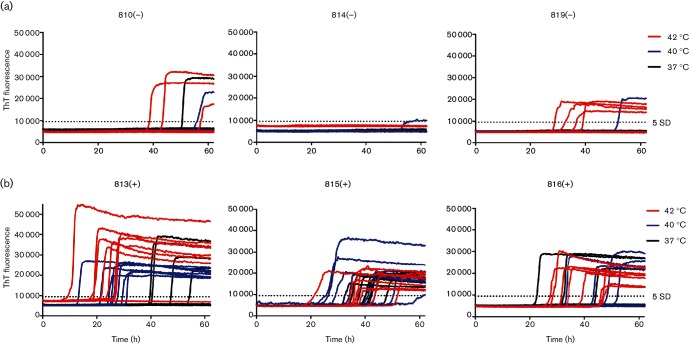
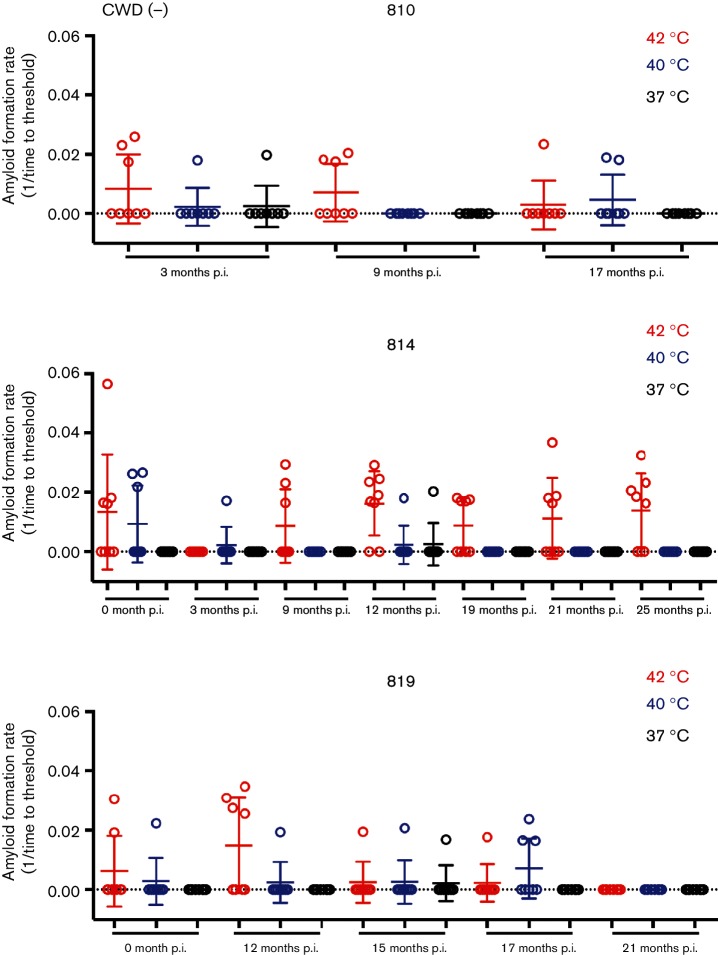
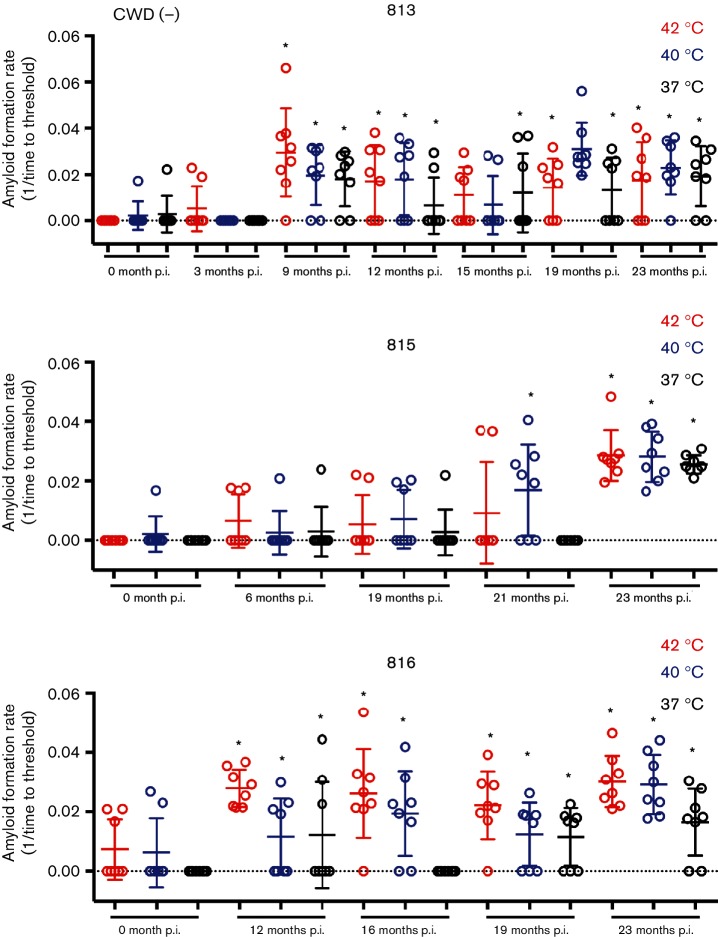
To expand our longitudinal analysis of prion shedding by cervids, we used iron-oxide particle magnetic extraction to enhance detection of CWD seeding activity in feces of CWD-exposed deer [11, 30]. To reduce the spontaneous amyloid formation that we have encountered with RT-QuIC of cervid fecal samples, we explored manipulation of reaction temperatures to select reaction conditions that favoured only prion-seeded vs non-specific amyloid conversion. We observed a temperature-dependent decrease in spontaneous/false-positive reactions in uninoculated, control deer with no significant differences in curve shape, which would hamper analysis using lag phase (Figs 2a and 3). The ThT traces provide a clear delineation between baseline fluorescence and reactions that crossed the reaction threshold in CWD(+) deer (Fig. 2). A positive reaction is defined as a single replicate that crosses a fluorescence value greater than five standard deviations of the average baseline fluorescence during the assay of 62.5 h. The percentage of false-positive reactions at 42 °C was 34.2 % and was decreased to 11.6 % at 40 °C, and to 2.5 % at 37 °C (n=120 replicates and n=15 fecal samples for each temperature) (Fig. 3 and Table 1). When we applied the same temperature manipulations to samples from CWD-exposed deer, we observed that some samples were only negligibly affected by decreasing temperature, which suggested that these were bona fide CWD-prion-seeded positive reactions (Fig. 4). Using a one-tailed Fisher’s exact test, we compared the number of false-positive reactions of pooled negative samples compared to each individual positive test at a given temperature. With this test we determined that samples assayed at 37 °C with ≥2/8 positive replicates and samples tested at 40 °C with ≥4/8 positive replicates were statistically different than negative samples at the same temperatures (P<0.05) (Fig. 4). Fecal samples tested at 42 °C having ≥5/8 positive replicates were statistically significant compared to negative controls (P<0.05) (Fig. 4). Prion seeding activity was more commonly found later in the CWD disease course. The earliest positive sample was observed at 9 months post prion exposure (Fig. 4). At 12 months after CWD exposure, 9 of 11 (81.8 %) samples tested at 40 °C from three different deer were positive. The same samples tested at 37 or 42 °C saw a slightly lower detection level of 72.7 % (8 of 11) (Fig. 4). In spite of a slightly higher false-positive rate, a reaction temperature of 40 °C had the best detection of positive feces samples compared to 37 °C. With the hope of increasing detection we chose 40 °C as the reaction temperature for feces experiments for the remainder of this manuscript.
Fig. 2.
Reaction temperature does not alter ThT traces of fecal samples. Fecal samples from (a) CWD(−) and (b) CWD(+) deer were analysed via iron-oxide-mediated extraction (IOME) in two separate experiments with a total of eight replicates at 42 °C (red), 40 °C (blue) and 37 °C (black). Representative raw ThT traces are shown for six fecal samples. Readings were taken at 15 min intervals during the 62.5 h reaction times. Symbols for each time point were removed for clarity. Lowering reaction temperature does not alter curve shape, but reduces spontaneous reactions.
Fig. 3.
Lowering reaction temperature reduces RT-QuIC spontaneous amyloid formation. Fecal samples from CWD(−) deer were analysed via IOME in two separate experiments with a total of eight replicates at 42 °C (red), 40 °C (blue), and 37 °C (black). Time in months post inoculation (p.i.) is displayed below each sample. Circles represent the reaction rate of each replicate, the bar represents the mean of eight replicates and the error bars represent one standard deviation.
Table 1. False-positive replicates at each temperature.
| Animal no. | No. of replicates | False positive | % False positive |
|---|---|---|---|
| 810 | |||
| 42 °C | 24 | 7 | 16.6 |
| 40 °C | 24 | 1 | 4.16 |
| 37 °C | 24 | 1 | 4.16 |
| 814 | |||
| 42 °C | 40 | 22 | 55.0 |
| 40 °C | 40 | 1 | 2.5 |
| 37 °C | 40 | 1 | 2.5 |
| 819 | |||
| 42 °C | 40 | 8 | 20.0 |
| 40 °C | 40 | 6 | 15.0 |
| 37 °C | 40 | 1 | 2.5 |
| Total | |||
| 42 °C | 104 | 37 | 35.5 |
| 40 °C | 104 | 8 | 7.7 |
| 37 °C | 104 | 3 | 2.9 |
Fig. 4.
Lowering reaction temperature does not reduce seeding for all feces samples from CWD(+) deer. Feces samples from inoculated CWD(+) deer were analysed via IOME in two separate experiments with a total of eight replicates at 42 °C (red), 40 °C (blue) and 37 °C (black). Time in months p.i. is displayed below each sample. Many samples remained positive even as reaction temperature was lowered, suggesting they are bona fide positive samples. Circles represent the reaction rate of each replicate, the bar represents the mean of eight replicates and the error bars represent one standard deviation. Statistically significant (Fisher’s exact test P<0.05) samples are denoted with a star.
Detection of CWD prion seeding activity in feces of naturally exposed elk
The analysis of feces can potentially be affected by the foraging environment diet of cervids. The previously described studies in Fig. 3 employed samples from CWD-inoculated white-tailed deer housed indoors and fed commercial, pelleted diets supplemented with hay forage. To translate this work to a more natural context, we tested fecal samples from elk living in a large, fenced range in Colorado. None of the animals tested had overt clinical signs of any disease at the time of sampling. In this herd we determined the CWD status of 450 elk using rectal-anal mucosal-associated lymphoid tissue (RAMALT) biopsies screened by both RT-QuIC and immunohistochemistry (IHC). Testing determined that 27 of the individuals with RT-QuIC-positive RAMALT biopsies had fecal samples collected at the time of sampling and that 15 of those fecal samples came from elk that tested positive by both RT-QuIC and IHC in RAMALT biopsies (Haley and others, unpublished data). We went on to test each of these 27 fecal samples and 10 samples from elk that tested negative for CWD by both RT-QuIC and IHC in RAMALT biopsy (Fig. 5). Elk fecal samples with at least four positive replicates out of a total of 16 were statistically different from negative controls based on Fisher’s exact test as used above. We found a total of 12 positive fecal samples out of the 27 tested (44.4 %). Fecal samples from elk that tested positive by both IHC and RT-QuIC in RAMALT biopsies had a slightly higher detection rate 53.3 % (8 of 15) (Fig. 5).
Fig. 5.
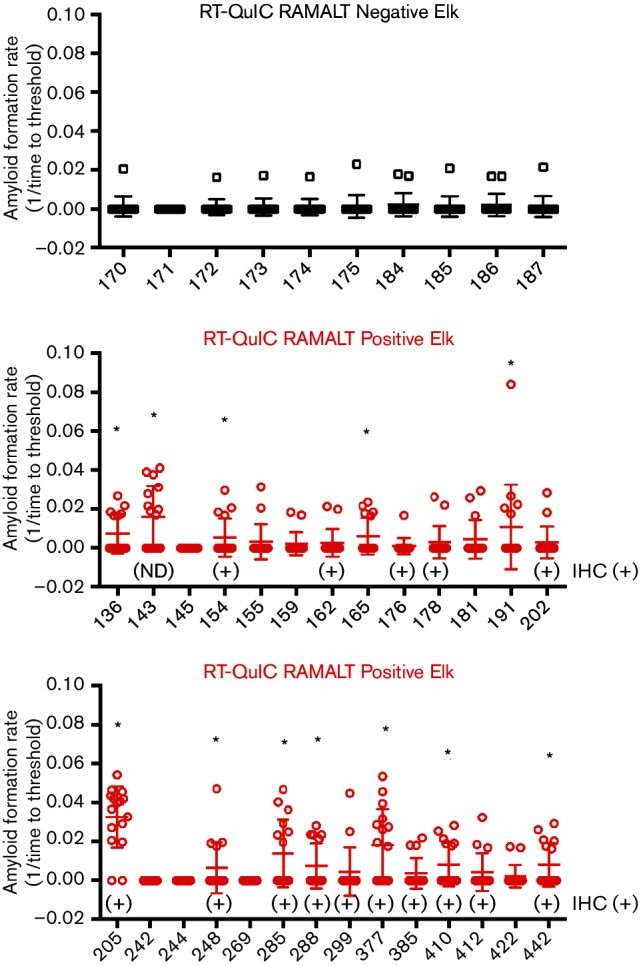
Detection of prion seeding activity in feces from free-ranging elk. Each feces sample was tested at 40 °C with IOME in four separate experiments for a total of 16 replicates. Red circles are from elk that tested CWD(+) by RT-QuIC analysis of a rectal biopsy. Elk with biopsy samples that were also CWD(+) by IHC are marked with a (+) sign. Black circles are from elk that tested CWD(−) by RT-QuIC and IHC analysis of rectal biopsy. Circles represent the reaction rate of each replicate, the bar represents the mean of 16 replicates and the error bars represent one standard deviation. Statistically significant (Fisher’s exact test, P<0.05) samples are denoted with a star.
Comparison of seeding activity in feces to a known CWD(+) reference
Understanding the levels of environmental contamination with prions during the CWD disease course would be useful for both management and for understanding the spread of CWD in natural populations. Previously, we estimated the CWD titre in saliva and urine samples from CWD-positive deer using transgenic mouse bioassay [11]. Here, we compared the CWD seeding activity in fecal samples to the seeding activity in a reference brain sample. Because we know the LD50 for the reference brain sample from end-point titration bioassay, we can extrapolate an LD50 for the fecal samples. We observed relatively consistent CWD seeding activity in fecal samples collected after 12 months p.i. in our experimentally inoculated deer (~80 % of the samples were positive). We calculated the average rate of amyloid formation for the post-12 months p.i. experimentally inoculated deer whose fecal samples were positive and extrapolated the estimated LD50 based on the reference CWD(+) brain sample (Fig. 6). The average feces rate of amyloid formation is equivalent to the rate of amyloid formation when 1.1×10−10 grams of CWD(+) brain is added to the reaction (Table 2).
Fig. 6.
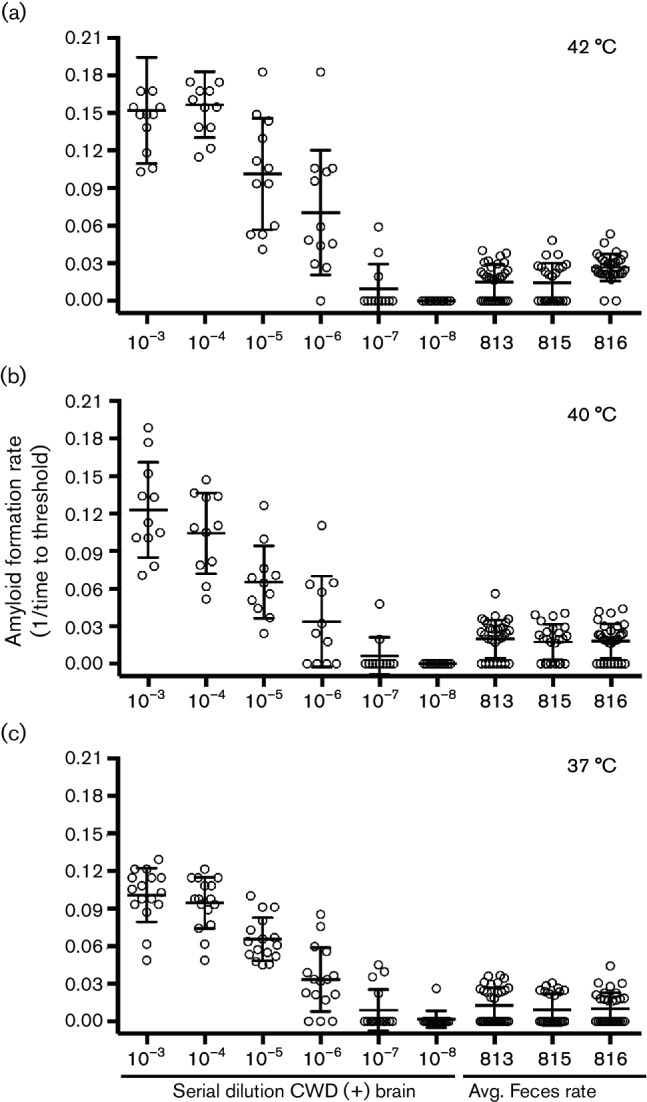
Reaction rates for fecal samples after 12 months p.i. fall between 10−6 and 10−7 dilution of a 10 % homogenate of a CWD positive brain. Serial dilutions of a reference 10 % CWD(+) brain homogenate are assayed by RT-QuIC and the reaction rate data are plotted for each temperature: (a) 42, (b) 40 and (c) 37 °C. Fecal samples from three CWD positive deer (813, 815 and 816) taken after 12 months p.i. were analysed by RT-QuIC and all replicates analysed were pooled for each animal. The reaction rate for each replicate is plotted for each deer at (a) 42, (b) 40 and (c) 37 °C. The mean and standard deviation are shown for each dilution and deer at every temperature.
Table 2. Brain equivalents in grams are multiplied by the LD50 doses per gram to obtain LD50 values for each brain equivalent.
| Brain equivalents (grams) | TG5037 mouse LD50 | ||
|---|---|---|---|
| 125 µl (amount assayed in one well) | 1.1×10−10 | 0.00037 | |
| 500 µl | 4.40×10−10 | 0.00147 | |
| 1 ml | 8.8×10−10 | 0.00293 | |
| 1 pellet=1 g=10 ml of 10 % homogenate | 8.8×10−9 | 0.02930 | LD50 doses in one pellet |
| 90 pellets pile–1 | 0.000000792 | 2.63736 | LD50 doses in one pile |
| 33 piles day–1 | 0.000026136 | 87.03 | LD50 doses per day |
| 365 days year–1 | 0.00953964 | 31767.00 | LD50 doses per year |
| CWD(+) brain TG5037 mouse LD50 doses gram–1 | 3.33×106 |
We use iron-oxide beads to concentrate the prions from 500 µl of a 10 % homogenate of feces (equivalent to 50 mg feces), and then distribute the beads among four wells, effectively adding 125 l (12.5 mg) feces to each well. Therefore, if one well is equivalent to 1.1×10−10 g brain, four wells (500 µl) are equivalent to ~4.4×10−10 grams of CWD(+) brain (Table 2). Therefore, one pellet of feces (estimated at a weight of 1 gram) is equivalent to approximately 8.8×10−9 grams of brain. The LD50 of our reference CWD(+) brain sample is 3.33×106 LD50 gram–1, so there are 0.029 LD50 (TgMo5037) in one pellet (Table 2) [31]. Deer defecate approximately 3000 pellets each day [32], which suggests that deer shed up to ~31700 LD50 (TgMo5037) units in 1year (Table 2).
Discussion
In this study, we have optimized RT-QuIC conditions to reduce the false-positive reactions inherent in complex samples like fecal homogenates. In deer fecal samples, simply reducing reaction temperature from 42 to 37 °C reduced spontaneous reactions from 34.2 to 2.5 %. Using these modifications, we found that most (81.8 %) fecal samples from deer infected with CWD for 1 year or longer contained prion seeding activity (Fig. 4). Applying this approach to elk living in a natural environment, we demonstrated that up to 40 % of asymptomatic, CWD(+) animals could be identified by RT-QuIC analysis of fecal samples.
It is clear that cervids infected with CWD shed infectious prions in saliva, nasal secretions, urine and feces [5–9, 33]. Pulford and colleagues have shown that sPMCA may detect CWD prions in feces; however large-scale assays of feces by sPMCA have not been undertaken [34]. To characterize environmental contamination with fecal prions, we have simplified an RT-QuIC protocol to detect CWD prion seeding activity in feces of deer and elk. Decreasing RT-QuIC reaction temperature significantly reduced non-specific or spontaneous amyloid formation associated with some fecal samples without decreasing sensitivity (Figs 3 and 4). Due to the inherently low levels of prions in excreta, increased experimental replicates and a temperature of 40 °C is likely to yield a more robust picture of fecal CWD shedding (Fig. 5).
Understanding environmental contamination by prions and the exposure risk for other cervids is important for the management of CWD. Our estimate of fecal environmental contamination is based on the bioassay of CWD(+) brain in transgenic mice and extrapolation of RT-QuIC reaction rates for feces to reaction rates of brain. The use of transgenic mouse bioassay to estimate a dose at which 50 % of the mice die (LD50) is a well-established method but likely fails to predict the infectious doses for cervids exposed by a natural route of infection. Tamguney and colleagues found that 30 µl of 10 % irradiated homogenate of deer feces administered to transgenic mice by intracerebral injection produced fatal prion infection in 30 % of mice [8]. Our estimates of prion dose in feces are significantly lower and likely under-stated due to reaction inhibitors in feces as well as our ability to separate prions from the fecal milieu, using iron-oxide particle magnetic extraction. Further studies are needed to determine the relationship of RT-QuIC seeding activity and infectivity in cervids, and to determine the effects of time and exposure on the stability of prions in feces [35].
Cheng and colleagues [36] have recently reported the use of RT-QuIC to detect CWD prions in elk fecal samples using a similar protocol, but differing in the use of a full-length mouse rPrPC substrate and phosphotungstic acid precipitation (PTA) as an enrichment step. We have also previously tested excreta samples (saliva and urine) using PTA precipitation with success [11]. In the course of our fecal experiments we did not utilize PTA precipitation for fecal analysis but in our comparison of PTA and bead extraction in saliva and urine we observed comparable results in RT-QuIC for both methods. The results we report reinforce the findings by Cheng and colleagues and extend them to include another cervid species. Moreover, employing iron-oxide magnetic extraction may allow for the analysis of a larger volume of feces and enhance the potential for sensitivity.
Based on our preliminary analysis of elk on open ranch land, we propose that RT-QuIC analysis of feces has the potential to determine whether a population may harbour CWD-positive animals. Moreover, it may be possible to estimate the prevalence of CWD in a given area based on the relationship between positive fecal samples and IHC-positive rectal biopsies (40 % based on the elk in this study). Analysis of feces has been used to monitor bacterial pathogens in deer as well as other wild animals, including monitoring Ebola virus and SIV in non-human primates [37, 38], illustrating the utility of fecal analyses as a mechanism for disease surveillance.
Methods
Deer inoculation, sample collection and sample preparation
All animal studies in this manuscript received prior approval by the Institutional Animal Care and Use Committee at Colorado State University. White-tailed deer from a CWD-free region were provided by the Warnell School of Forestry and Natural Resources, University of Georgia and were transferred to the indoor CWD research facility at Colorado State University. Deer were inoculated, as previously stated, via aerosolization receiving two 1.0 ml doses of a 5 % CWD(+) brain homogenate [39]. Feces was collected with a fresh glove at each time point and stored at −80 °C until testing. Elk were from a ranch in Colorado with an estimated CWD prevalence of ~10–15 % were also collected with clean gloves (below). Fecal pellets or portions thereof were weighed and phosphate-buffered saline (PBS) pH 7.4 was added to make a 10 % homogenate. Fecal samples were vortexed and homogenized by disruption with a P1000 then centrifuged at 3000 g to pellet insoluble debris.
Naturally exposed elk samples
Fecal samples were collected rectally from elk (Cervus elaphus elaphus) involved in an ongoing study in a CWD endemic area. The animals range in a fenced area of approximately 3000 acres consisting of habitat typical of that of free-ranging elk. The animals were handled in a modern, conventional animal handling facility as part of a yearly inventory. With minimal restraint, each sample was collected cleanly prior to any additional sample collection, and placed in a sterile whirlpak bag and chilled. Fecal samples were then stored at −80 °C after shipment on wet ice. Assay conditions for RT-QuIC of RAMALT biopsies were performed as previously described and as developed in our laboratory [27, 28]. Reactions were carried out with 4 µl of a 10−2 dilution of a 10 % rectal biopsy sample in 0.05 % SDS in 1× PBS. Reaction conditions were the same as below except that the experiment was ended after 24 h. RT-QuIC positive biopsies were deemed positive only if all three replicates tested crossed the predetermined threshold (five standard deviations above baseline) during the 24 h reaction.
Iron-oxide bead extraction for RT-QuIC
500 µl of fecal homogenates and 2 µl of iron-oxide super-paramagnetic beads (~9 µm; Bangs Laboratories, IN, USA) were added to 1.7 ml microcentrifuge tubes and rotated at room temperature for 60 min. Beads were then subjected to a magnetic particle separator and fecal homogenate supernatant was removed. Beads were washed once with PBS (pH7.4) and then placed back into the magnetic particle separator to enable removal of the PBS wash. Beads were immediately resuspended in 10 µl of PBS with 0.1 % sodium dodecyl sulphate (SDS). 2 µl of resuspended beads were added to each RT-QuIC well. The feces from free-range elk was significantly more heterogeneous and required an extra wash to remove traces of fecal homogenate from the iron-oxide particles. For elk feces, each sample was assayed at 40 °C after washing the beads one additional time for a total of two washes.
RT-QuIC protocol and protein preparation
The PrPC substrate was the Syrian hamster recombinant PrP, amino acids 90–231, (SH-rPrP(90-231)) and was prepared as described in [10]. Briefly, protein expression was carried out in 1 litre cultures induced by Over Night Express (EMD-Millipore) auto induction media. Inclusion bodies were harvested according to the manufacturer’s protocol with Lysonase (EMD-Millipore). Inclusion bodies were solubilized in 8.0 M guanidine hydrochloride (GuHCl) with 100 mM NaPO4 rotating at room temperature. The solubilized SH-rPrP(90-231) was batch-bound to superflow Ni resin (Qiagen) and refolded with a 180 ml linear gradient of 6.0 GuHCl, 100 mM NaPO4, 10 mM Tris pH 8.0 to the same buffer without the GuHCl, flowing at 0.75 ml min–1. SH-rPrP(90-231) was eluted with a linear gradient of 100 mM NaPO4, 10 mM Tris pH 8.0 to 0.5 M imidazole in 100 mM NaPO4, 10 mM Tris pH 5.5 at 2.0 ml min–1. Eluted protein was dialysed overnight in two changes of 3.5 l 20 mM NaPO4 at pH 5.5. The concentration of SH-rPrP(90-231) was determined as previously described by A280 and stored at 4 °C for up to 1 month.
The reaction mixtures for the RT-QuIC reaction were prepared as before: 20 mM NaPO4 pH 7.4, 1 mM ethylenediaminetetraacetic acid (EDTA), 320 mM sodium chloride (NaCl), 0.1 mg ml−1 SH-rPrP(90-231) and 10 µM thioflavin T (Sigma Cat# T3516) [10]. Shaking and reading settings were as previously described and a BMG fluostar was used for all experiments [10]. Temperature changes were made by editing the script used for the RT-QuIC assay developed by BMG.
Reactions in RT-QuIC were deemed positive if the fluorescence in a given well surpassed five standard deviations over the mean baseline fluorescence of all 96 wells. The time until the threshold was crossed was calculated using the time-to-threshold calculator in the BMG Mars software. Rates were calculated as previously described [24] by dividing one by the lag phase. The log-linear regressions were fitted and plotted in GraphPad Prism.
We compared the total number of positive replicates from the negative feces samples to the number of positive replicates for each unknown sample with Fisher’s exact test.
Calculation of feces infectivity
The calculation of feces infectivity is based on RT-QuIC reaction rates reflecting the amount of initial seed added to a reaction. Our previous results have indicated that there is a linear relationship between the reaction rate and the amount of seed added [11, 24]. In order to estimate feces infectivity we converted the reaction rates of 12 month or later fecal samples tested by RT-QuIC to brain equivalents. The reaction rates from an end-point-diluted CWD(+) brain sample were plotted and it was determined that the fecal sample reaction rates fell between a 10−6 and 10−7 dilution of the reference brain homogenate at each temperature tested (Fig. 6). Since the value between these dilutions differed slightly between reaction temperatures we chose to simplify the estimation by using a value of brain equivalent that fell half way between a 10−6 and 10−7 dilution which was 1.1×10−10 grams of CWD(+) brain. This value represents the 125 µl volume of assayed 10 % fecal homogenate in each well extracted by the iron-oxide beads. The total volume assayed for each fecal sample was 500 µl and the beads from each 500 µl sample were separated into four wells to achieve four technical replicates per 500 µl assayed. Therefore, the total brain equivalents for each fecal sample tested was 1.1×10−10×4 or 4.4×10−10. To calculate the amount of brain equivalent seeding activity per gram of feces we multiplied the amount in 500 µl tested by 2 to represent the seeding activity in 1 ml of a 10 % homogenate and then by 10 to represent the amount in 1 gram of solid feces which is 8.8×10−9. In a earlier study we determined the LD50 of a CWD(+) brain pool using end-point dilution bioassay in TG5037 mice which are engineered to express the cervid PrPC in substitute of the mouse PrPC [31]. We found that there were 3.3×106 LD50 doses per gram of brain and 3.3×105 LD50 doses per ml of a 10 % brain homogenate [31]. The brain equivalent per gram of feces was calculated and extrapolated to defecation rates in the literature. Values for defecation were sourced from Rogers and seasonal totals were averaged for simplicity [32]. Pellets were estimated to weigh 1 gram. Average pellets per pile were 90 and the number of seasonally averaged pellet piles per day was 33 [32]. The volume of feces analysed was used to extrapolate the amount of brain equivalent doses per gram of feces. Brain equivalents of the volumes analysed and relevant fecal amounts are given in Table 2. TG5037 LD50 doses were calculated by multiplying the brain equivalents in grams by the LD50 doses per gram of the reference end-point bioassayed CWD(+) brain homogenate (Table 2).
Funding information
This work was supported by grant NIH-1-R01-NS061902.
Acknowledgement
We thank Kristen Davenport for the critical review of this manuscript.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Ethical statement
All appropriate institutional protocols for animal handling and treatment were properly followed and approved by the Colorado State University Research Integrity and Compliance Review and the Institutional Animal Care and Use Committee (IUCAC).
Footnotes
Abbreviations: CWD, Chronic wasting disease; EDTA, ethylenediaminetetraacetic acid; ELISA, enzyme-linked immunosorbent assays; GuHCl, guanidine hydrochloride; IHC, immunohistochemistry; LD50, Lethal dose for 50 % of inoculated animals; p.i., post inoculation; PrPC, cellular prion protein; PrPSc, Prion form of PrP; RAMALT, rectal-anal mucosal-associated lymphoid tissue; RT-QuIC, real-time quaking-induced conversion; SH-rPrP(90-231), Syrian hamster recombinant PrP, amino acids 90–231; sPMCA, serial protein-misfolding cyclic amplification assay.
References
- 1.Sigurdson CJ. A prion disease of cervids: chronic wasting disease. Vet Res. 2008;39:41. doi: 10.1051/vetres:2008018. [DOI] [PubMed] [Google Scholar]
- 2.Williams ES. Chronic wasting disease. Vet Pathol. 2005;42:530–549. doi: 10.1354/vp.42-5-530. [DOI] [PubMed] [Google Scholar]
- 3.Williams ES, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerg Infect Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 6.Safar JG, Lessard P, Tamgüney G, Freyman Y, Deering C, et al. Transmission and detection of prions in feces. J Infect Dis. 2008;198:81–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamgüney G, Miller MW, Wolfe LL, Sirochman TM, Glidden DV, et al. Asymptomatic deer excrete infectious prions in faeces. Nature. 2009;461:529–532. doi: 10.1038/nature08289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, et al. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol. 2011;85:6309–6318. doi: 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, et al. Rapid antemortem detection of CWD prions in deer saliva. PLoS One. 2013;8:e74377. doi: 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, et al. Longitudinal detection of prion shedding in Saliva and urine by chronic wasting disease-infected deer by real-time quaking-Induced conversion. J Virol. 2015;89:9338–9347. doi: 10.1128/JVI.01118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prusiner SB, Groth DF, Bolton DC, Kent SB, Hood LE. Purification and structural studies of a major scrapie prion protein. Cell. 1984;38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- 13.Telling GC, Parchi P, Dearmond SJ, Cortelli P, Montagna P, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 14.Colby DW, Prusiner SB. Prions. Cold Spring Harb Perspect Biol. 2011;3:a006833. doi: 10.1101/cshperspect.a006833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabizon R, Telling G, Meiner Z, Halimi M, Kahana I, et al. Insoluble wild-type and protease-resistant mutant prion protein in brains of patients with inherited prion disease. Nat Med. 1996;2:59–64. doi: 10.1038/nm0196-59. [DOI] [PubMed] [Google Scholar]
- 16.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 18.Atarashi R, Sano K, Satoh K, Nishida N. Real-time quaking-induced conversion: a highly sensitive assay for prion detection. Prion. 2011;5:150–153. doi: 10.4161/pri.5.3.16893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilham JM, Orrú CD, Bessen RA, Atarashi R, Sano K, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS Pathog. 2010;6:e1001217. doi: 10.1371/journal.ppat.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colby DW, Zhang Q, Wang S, Groth D, Legname G, et al. Prion detection by an amyloid seeding assay. Proc Natl Acad Sci USA. 2007;104:20914–20919. doi: 10.1073/pnas.0710152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atarashi R, Satoh K, Sano K, Fuse T, Yamaguchi N, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med. 2011;17:175–178. doi: 10.1038/nm.2294. [DOI] [PubMed] [Google Scholar]
- 22.Mcguire LI, Peden AH, Orrú CD, Wilham JM, Appleford NE, et al. Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol. 2012;72:278–285. doi: 10.1002/ana.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orrú CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, et al. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. MBio. 2011;2:e00078-11. doi: 10.1128/mBio.00078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, et al. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol. 2015;96:210–219. doi: 10.1099/vir.0.069906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanusso G, Monaco S, Pocchiari M, Caughey B. Advanced tests for early and accurate diagnosis of Creutzfeldt-Jakob disease. Nat Rev Neurol. 2016;12:325–333. doi: 10.1038/nrneurol.2016.65. [DOI] [PubMed] [Google Scholar]
- 26.Mcguire LI, Poleggi A, Poggiolini I, Suardi S, Grznarova K, et al. Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: an international study. Ann Neurol. 2016;80:160–165. doi: 10.1002/ana.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haley NJ, Siepker C, Walter WD, Thomsen BV, Greenlee JJ, et al. Antemortem detection of chronic wasting disease prions in nasal brush collections and rectal biopsy specimens from White-Tailed deer by real-time quaking-induced conversion. J Clin Microbiol. 2016;54:1108–1116. doi: 10.1128/JCM.02699-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haley NJ, Siepker C, Hoon-Hanks LL, Mitchell G, Walter WD, et al. Seeded amplification of chronic wasting disease prions in nasal brushings and recto-anal mucosa-associated lymphoid tissues from elk by real-time quaking-induced conversion. J Clin Microbiol. 2016;54:1117–1126. doi: 10.1128/JCM.02700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, et al. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. MBio. 2015;6:e02451-14. doi: 10.1128/mBio.02451-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denkers ND, Henderson DM, Mathiason CK, Hoover EA. Enhanced prion detection in biological samples by magnetic particle extraction and real-time quaking-induced conversion. J Gen Virol. 2016;97:2023–2029. doi: 10.1099/jgv.0.000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoover CE, Davenport KA, Henderson DM, Pulscher LA, Mathiason CK, et al. Detection and quantification of CWD Prions in fixed Paraffin embedded tissues by real-time auaking-induced conversion. Sci Rep. 2016;6:25098. doi: 10.1038/srep25098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers LL. Seasonal changes in defecation rates of free-ranging white-tailed deer. J Wildl Manage. 1987;51:330–333. doi: 10.2307/3801011. [DOI] [Google Scholar]
- 33.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One. 2009;4:e7990. doi: 10.1371/journal.pone.0007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulford B, Spraker TR, Wyckoff AC, Meyerett C, Bender H, et al. Detection of PrPCWD in feces from naturally exposed Rocky Mountain elk (Cervus elaphus nelsoni) using protein misfolding cyclic amplification. J Wildl Dis. 2012;48:425–434. doi: 10.7589/0090-3558-48.2.425. [DOI] [PubMed] [Google Scholar]
- 35.Saunders SE, Yuan Q, Bartz JC, Bartelt-Hunt S. Effects of solution chemistry and aging time on prion protein adsorption and replication of soil-bound prions. PLoS One. 2011;6:e18752. doi: 10.1371/journal.pone.0018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, et al. Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS One. 2016;11:e0166187. doi: 10.1371/journal.pone.0166187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renter DG, Gnad DP, Sargeant JM, Hygnstrom SE. Prevalence and serovars of Salmonella in the feces of free-ranging white-tailed deer (Odocoileus virginianus) in Nebraska. J Wildl Dis. 2006;42:699–703. doi: 10.7589/0090-3558-42.3.699. [DOI] [PubMed] [Google Scholar]
- 38.Reed PE, Mulangu S, Cameron KN, Ondzie AU, Joly D, et al. A new approach for monitoring ebolavirus in wild great apes. PLoS Negl Trop Dis. 2014;8:e3143. doi: 10.1371/journal.pntd.0003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, et al. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol. 2013;87:1890–1892. doi: 10.1128/JVI.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]