Abstract
The partial success of the RV144 trial underscores the importance of envelope-specific antibody responses for an effective HIV-1 vaccine. Oligomeric HIV-1 envelope proteins delivered with a potent adjuvant are expected to elicit strong antibody responses with broad neutralization specificity. To test this hypothesis, two SIV envelope proteins were formulated with delta inulin-based adjuvant (Advax) and used to immunize nonhuman primates. Oligomeric gp140–gp145 from SIVmac251 and SIVsmE660 was purified to homogeneity. Oligomers showed high-affinity interaction with CD4 and were highly immunogenic in rabbits, inducing Tier 2 SIV-neutralizing antibodies. The immunogenicity of an oligomeric Env DNA prime and protein boost together with Advax was evaluated in Chinese rhesus macaques. DNA administration elicited antibodies to both envelopes, and titres were markedly enhanced following homologous protein boosts via intranasal and intramuscular routes. Strong antibody responses were detected against the V1 and V2 domains of gp120. During peak immune responses, a low to moderate level of neutralizing activity was detected against Tier 1A/1B SIV isolates, with a moderate level noted against a Tier 2 isolate. Increased serum antibody affinity to SIVmac251 gp140 and generation of Env-specific memory B cells were observed in the immunized macaques. Animals were subjected to low-dose intravaginal challenge with SIVmac251 one week after the last protein boost. One out of three immunized animals was protected from infection. Although performed with a small number of macaques, this study demonstrates the utility of oligomeric envelopes formulated with Advax in eliciting broad antibody responses with the potential to provide protection against SIV transmission.
Keywords: HIV, SIV, envelope, vaccine, Advax, BLI
Introduction
Despite an intensive global effort to control HIV-1 infection, nearly 36.7 million people are living with HIV and 2.1 million new infections occurred during 2015 alone [1, 2]. A prophylactic vaccine, effective in various regions of the world, is desperately needed to eradicate this pandemic. This is especially true in developing nations, where HIV-1 infection is still spreading at a higher rate than in more economically developed countries, and opportunities for drug interventions are limited. The development of such a broadly effective vaccine is challenging, since distinct subtypes of HIV-1 are prevalent in different regions of the world, and these subtypes are often divergent [3–5] from one another in their serotype. Despite these challenges, progress has been made toward the development of an HIV vaccine. Of the three major HIV vaccine efficacy human trials performed to date, the RV144 trial, which combined a prime vaccine called ALVAC‐HIV with a boost of the AIDSVAX gp120 vaccine formulated with alum, is the only successful intervention study, yielding 31 % protective efficacy in human volunteers [6]. Retrospective analysis of the HIV vaccine efficacy trials, including the RV144 trial, has yielded valuable information about immune correlates of protection in the vaccinated population. While 31 % protection was observed in the RV144 trial, protection did not correlate with a strong virus-neutralizing antibody titre or cytolytic CD8 T-cell response. Instead, protection correlated with plasma IgG titres against the V1/V2 loop region of gp120 and acquisition of infection correlated inversely with the IgA response to Env [7–9]. This suggests a possible role for anti-Env antibodies for protective efficacy against HIV-1. Therefore, a vaccine regimen consisting of natively constituted Env delivered with a more potent adjuvant than Alum may be capable of delivering improved vaccine protection.
Envelope protein is the primary target of the humoral immune response in HIV-infected individuals, which may induce antibodies with unique binding properties that have specific antiviral functions, including neutralization and the activation of effector cells. The role of neutralizing antibodies in the prevention of virus infection has been well documented in the non-human primate model, where neutralizing antibodies with various specificities conferred either complete or partial protection to macaques from pathogenic SHIV challenge following passive transfer [10–14]. It is therefore hypothesized that the Env immunogens can be used as a vaccine to elicit broad neutralizing antibody responses in immunized hosts. Both monomeric gp120 and trimeric HIV gp140 have been used as immunogens in preclinical and clinical trials. Earlier studies demonstrated that there were no significant differences in immunogenicity between monomeric and oligomeric Env [15–17]. However, trimeric HIV-1 Env immunogens were recently shown to expose several broadly neutralizing epitopes in stable conformation, and in some instances they elicited cross-clade neutralizing antibody responses with titres that were substantially higher than those observed with monomeric gp120 [18–20].
In protein vaccination regimens, adjuvants have the potential to modulate and enhance vaccine responses by activating different sets of signalling pathways. DNA vaccines that are delivered by electroporation have been shown to act like an adjuvant by enhancing the immunogenicity of antigens in both macaques and humans [21–24]. Priming with DNA in mice and macaques increases CD4 T-cell help, which improves subsequent Env protein boosting and increases the antibody response [25]. Recombinant antigens alone tend to be poorly immunogenic and may therefore benefit from formulation with a potent adjuvant. An ideal adjuvant should induce a strong immune response with long-lasting protection and low reactogenicity. Advax delta inulin is a polysaccharide adjuvant derived from plant polyfructofuranosyl-d-glucose. Advax alone or combined with the Toll-like receptor (TLR) 9 agonist, CpG oligonucleotide (ADAVAX-1), has been shown to enhance antibody and T-cell responses to a wide variety of viral vaccines, including seasonal influenza, avian influenza, Japanese encephalitis, West Nile virus, SARS coronavirus, RSV and HIV (in mice) [26–30]. Unlike other adjuvants, delta inulin stimulates adaptive immunity directly without activating typical innate immune inflammatory pathways, which explains its low reactogenicity.
In this pilot study, we report on the immunogenicity and efficacy of a DNA prime/protein boost vaccine using the trimeric SIV Env from two divergent isolates, SIVmac251 and SIVsmE660. This study demonstrates that the immunization of macaques using a mixture of plasmids encoding both forms of Env elicited high titre antibody responses that were significantly boosted following immunization with trimeric Env protein from both isolates. These immune responses showed protective efficacy against repeated low-dose intravaginal challenge with SIVmac251. Although performed with a small number of macaques, this pilot study demonstrates that a DNA prime/protein boost regimen encoding trimeric Env combined with appropriate adjuvant is capable of eliciting broad antibody responses with protective efficacy, and is suitable as a potential HIV-1 vaccine approach.
Results
Antigenic and immunogenic characterization of trimeric SIV Env proteins
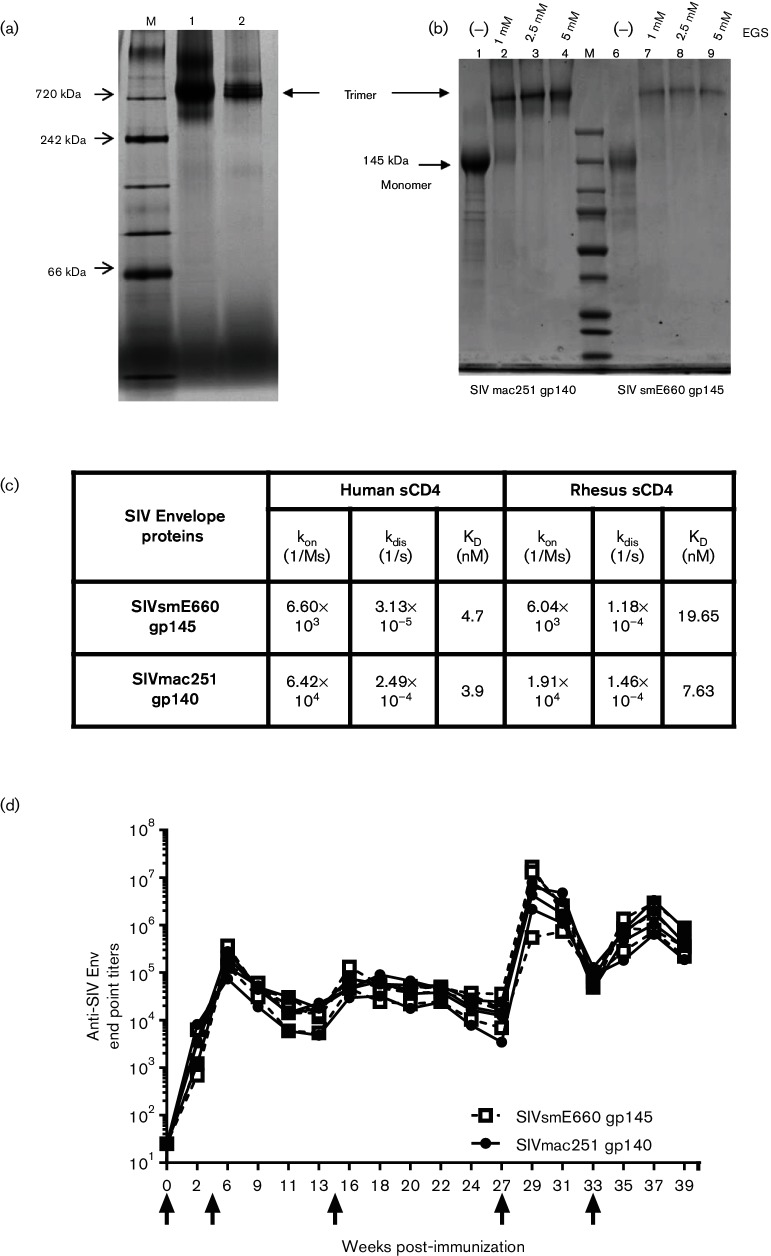
Codon-optimized genes of both SIVmac251 gp140 and SIVsmE660 gp145 were synthesized by abolishing the gp120–gp41 cleavage site and eliminating the transmembrane domain (TM) and cytoplasmic region of gp160, and then cloned into an expression vector under the control of the CMV promoter. Trimeric SIV envelope proteins were stably expressed following transfection of the Env expression vector DNAs into 293 cells and purified to homogeneity by lectin affinity, anion exchange and gel filtration chromatography [31]. As shown in Fig. 1(a), both proteins were shown to migrate predominantly as an oligomer with an approximate molecular weight of 720 kDa in blue native PAGE. To provide additional evidence that the SIVmac251 gp140 and SIVsmE660 gp145 immunogens had a trimeric structure, the purified proteins were treated with 1, 2.5 and 5 mM of ethylene glycol bis (succinimidyl succinate) (EGS), an amine-reactive crosslinking reagent, and analysed by SDS-PAGE under reducing conditions. As is evident in Fig. 1(b), in the absence of EGS, both proteins migrated in monomeric form with a molecular weight of 140 kDa, but following crosslinking with all three concentrations of EGS, both Env proteins migrated as trimers with a molecular weight of 720 kDa, thereby confirming their trimeric structure.
Fig. 1.
Vaccine immunogens. (a) Blue native PAGE analysis of SIVmac251 gp140 and SIVsmE660 gp145 envelope trimers. (b) SDS PAGE analysis of crosslinked SIVmac251 gp140 (lane 1) and SIVsmE660 gp145 (lane 2) under reducing conditions. (c) Binding affinity constants of SIVmac251 and SIVsmE660 gp140 trimers to human and rhesus sCD4 as measured by bio-layer interferometry (kon, on-rate constant; kdis, off-rate constant; KD, equilibrium dissociation constant). (d) Antibody response in rabbits immunized with SIVsmE660 gp145 and SIVmac251 gp140. Serum antibody binding titres in NZW rabbits against SIVmac251 gp145 and SIVsmE660 gp145, as measured by reciprocal endpoint ELISA. SIVsmE660 gp145 is represented as an open square (□) and SIVmac251 gp140 is represented as a closed circle (⚫). Black arrows indicate the time ofimmunization. On weeks 0, 4, and 14 animals were electroporated with DNA. On weeks 27 and 33 animals were immunized with envelope proteins.
The antigenicity of the trimeric envelopes was characterized by measuring their binding affinity to human and rhesus soluble CD4 by bio-layer interferometry (BLI). The on-rate constant (kon), dissociation constant (kdis) and equilibrium constant (KD) associated with the binding of SIVmac251 gp140 and SIVsmE660 gp145 to both human and rhesus sCD4 are shown in Fig. 1(c). The binding affinities of both envelopes to rhesus and human CD4 were found to be in the nanomolar range (3.9 to 19.7 nM), suggesting that Envs are properly folded with the CD4 binding site intact.
Prior to evaluating the immunogenicity of these trimeric Env proteins in macaques, it was of interest to demonstrate whether the proteins could elicit a neutralizing antibody response in small animals. To this end, the immunogenicity of the Env proteins was evaluated as a DNA prime/protein boost vaccine in rabbits. Four rabbits were immunized intramuscularly (IM) by electroporation three times with a mixture of DNA encoding SIVmac251 gp140 and SIVsmE660 gp145, followed by two IM boosts of a mixture of the trimeric homologous proteins formulated in Adjuplex adjuvant. Adjuplex adjuvant was selected for this evaluation as our earlier study has shown that this adjuvant was highly effective in eliciting a robust immune response in rabbits when used with HIV Env [32]. The antibody titres against the two homologous envelope proteins measured throughout the course of immunization are shown in Fig. 1(d). Clearly, DNA immunization (week 4) elicited a persistent level of anti-Env titres in all four animals, which were significantly enhanced following immunization with Env protein. Neutralization titres were also evaluated 2 weeks after the third DNA immunization (week 16) and each protein immunization (weeks 29 and 35) in TZM-bl cells against two Tier 1B viruses (SIVmac251/M766 and SIVsmE660-BR/CG7V.IR) and a Tier 2 virus (SIVmac251.30). In addition, post-protein boost sera were assayed against the Tier 1A isolates SIVmac251.6 and SIVsmE660-BR/CG7G.IR1. As shown in Table 1, a low to moderate level of neutralization was noted after the second protein boost against both Tier 1A/Tier 1B and Tier 2 isolates. However, the titres were markedly higher against Tier 1A isolates. Collectively, these data suggest that both SIVmac251 and SIVsmE660 Envs are immunogenic in rabbits and are capable of eliciting neutralizing antibodies against Tier 1 and, to a limited extent, Tier 2 isolates.
Table 1. Neutralizing antibody response in rabbits immunized with SIVsmE660 gp145 and SIVmac251 gp140 at various weeks after immunization.
Neutralization titres are expressed as the dilution of serum inhibiting infection of TZM-bl cells by 50 % (ID50) compared to the untreated control cells. nd, not determined.
| Animal ID | Bleed week | Neutralization titres ID50 | ||||
|---|---|---|---|---|---|---|
| Tier 1A | Tier 1B | Tier 2 | ||||
| SIVmac251.6 | SIVsmE660-BR/CG7G.IR1 | SIVmac251/M766 | SIVsmE660-BR/CG7V.IR | SIVmac 251.30 | ||
| C2471 | 0 | <20 | 63 | <20 | <20 | <20 |
| 16 | nd | nd | 29 | <20 | <20 | |
| 29 | nd | nd | 35 | <20 | 34 | |
| 35 | 12 882 | 77 916 | 146 | 27 | 57 | |
| C2472 | 0 | <20 | <20 | <20 | <20 | <20 |
| 16 | nd | nd | <20 | <20 | <20 | |
| 29 | nd | nd | 36 | <20 | 35 | |
| 35 | 81 901 | 133 790 | 69 | 26 | 98 | |
| C2473 | 0 | <20 | <20 | <20 | <20 | <20 |
| 16 | nd | nd | 44 | <20 | <20 | |
| 29 | nd | nd | 57 | 26 | 137 | |
| 35 | 31 204 | 94 478 | 133 | 41 | 79 | |
| C2474 | 0 | <20 | <20 | <20 | <20 | <20 |
| 16 | nd | nd | 39 | <20 | <20 | |
| 29 | nd | nd | nd | nd | nd | |
| 35 | 14 859 | 78 935 | 472 | 37 | 98 | |
Data are displayed in a heat map with shades of grey indicating higher nuetralizing antibody titre.
▪40–100, ▪ 100–1000, ▪ >1000.
Immunogenicity of trimeric envelope proteins formulated with Advax adjuvant in Chinese rhesus macaques
We next evaluated the immunogenicity of a prime-boost vaccination regimen in Chinese rhesus macaques. Only systemic humoral immune responses were evaluated in this study because of the small immunized animal group size. A combination of both intranasal (IN) and IM routes were selected for protein immunization with inulin adjuvants, as our earlier study had shown that this mode of vaccination elicited robust immune responses in mice when tested with HIV-1 Env [27]. To that end, animals were immunized with DNA vaccines twice by the IM route, followed by electroporation, and then subsequently with Env proteins once by the IN route and then three times by the IM route, as described in the Methods section.
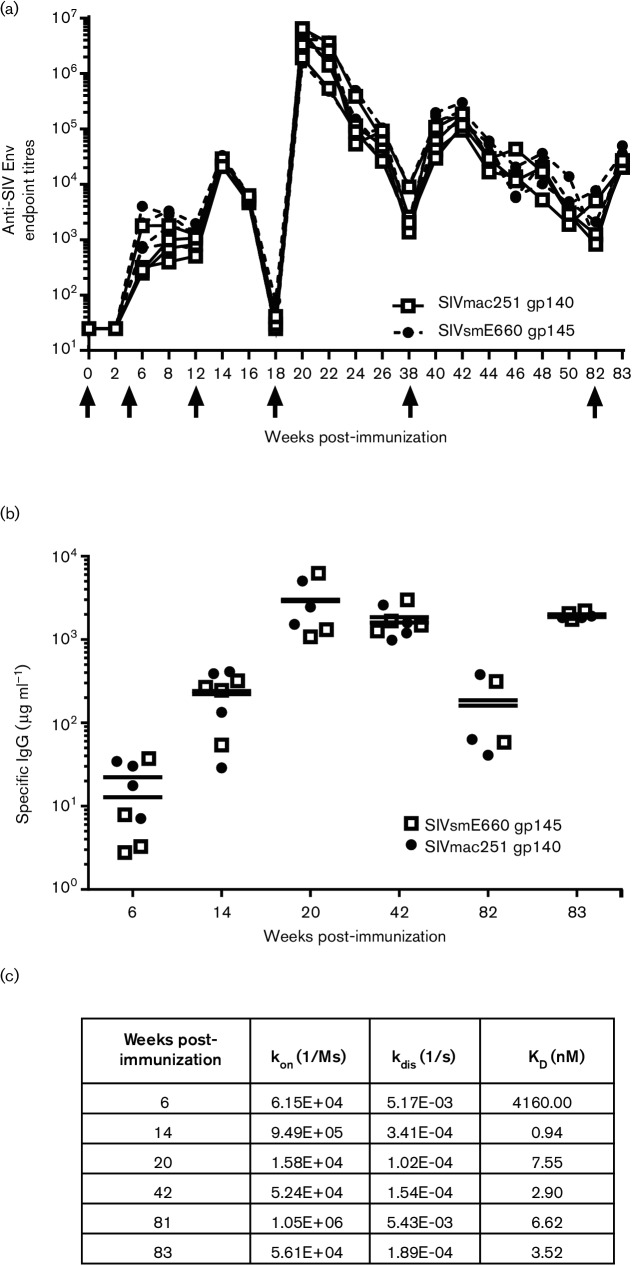
Serum was collected at regular intervals after every immunization and anti-Env titres were measured. As shown in Fig. 2(a), the anti-envelope antibody titres against both SIVmac251 gp140 and SIVsmE660 gp145 were comparable in all of the immunized animals. DNA priming elicited an endpoint titre of almost 3.5 log units, which increased to approximately 4.5 log units after the envelope protein boost via the intranasal route; however, this response decayed quickly. Two subsequent boosts of envelope proteins via IM routes enhanced the antibody titres markedly, and this persisted for 44 weeks. At the time of vaginal challenge, the antibody titres were in the range of 4.5 logs following a third immunization of the envelope(week 83) via the IM route.
Fig. 2.
Antibody response in macaques primed with DNA encoding SIVmac251 gp140 and SIVsmE660 gp145 and boosted with autologous protein. (a) Serum antibody-binding titres in immunized rhesus macaques against SIVmac251 gp140 and SIVsmE660 gp145, expressed as reciprocal endpoint ELISA titres. The results are presented as individual values for all four animals. The black arrows indicate the time of immunization. On weeks 0 and 4, animals were electroporated with DNA. On week 12, animals were boosted IN with envelope proteins and boosted again on weeks 18, 38 and 82 with envelope proteins by IM. (b) Quantitation of serum IgG antibodies specific for SIVmac251 and SIVsmE660 proteins in rhesus immune sera at indicated times post-immunization. In both panels, SIVmac251 gp140 is represented as an open square (□) and SIVsmE660 gp145 is represented as a closed circle (⚫). (c) Relative binding affinity of the immune sera at different times post-immunization to SIVmac251 gp140 as measured by bio-layer interferometry (kon, on-rate constant; kdis, off-rate constant; KD, equilibrium dissociation constant).
To further characterize the humoral immune response elicited by the vaccine regimen, we measured the envelope-specific IgG concentration in the sera by enzyme-linked immunosorbent assay (ELISA). Consistent with the endpoint titres, a low level of envelope-specific IgG was detected after the DNA immunizations (12.85 and 22.35 µg ml−1 for SIVsmE660 anti-gp145 and anti-SIVmac251 gp140, respectively). The levels of specific IgGincreased significantly after the IM protein boost to 3005.33 and 2005.00 µg ml−1 for the anti-SIVsmE660 gp145 and anti-SIVmac251 gp140 antibodies, respectively, which persisted at a high level until the day of challenge at week 83 (Fig. 2b). Next, we evaluated the relative affinity of serum antibodies for binding to Env. The term ‘relative affinity’ is used because the polyclonality of serum antibodies does not permit a true affinity to be determined between one antibody and one immunogen. Since the antibody titres and concentrations were similar for both proteins in the immune sera, we only measured the binding to SIVmac251 gp140 at selected time points using BLI. High-affinity/-avidity antibodies were elicited following the protein boost via the IN route (KD of 0.94 nM) and a similar high-affinity antibody persisted until the day of challenge, with a KD of 3.52 nM (Fig. 2c).
Since the RV144 trials showed that low levels of envelope-specific IgA and increased antibody titres against the V1 and V2 domains of the envelope correlated with decreased risk of infection [8], both of these responses were evaluated. No antigen-specific IgA response was detected after the DNA immunizations, however a low level of IgA against both SIVsmE660 gp145 and SIVmac251 gp140 was observed following the IN boost (week 14), and this level persisted until the day of challenge (Fig. 3a). Since the overall antibody titres were comparable for both Env proteins, we only determined the anti-V1/V2 responses against SIVmac251 gp140. Strong antibody reactivity to the V1 and V2 domains was observed at 14 weeks post-IN immunization, and this level persisted until the day of challenge (Fig. 3b). The antibody titres for the V1 and V2 regions were comparable at all the measured time points, except at week 20, when the response to the V1 region was slightly stronger (Fig. 3b).
Fig. 3.
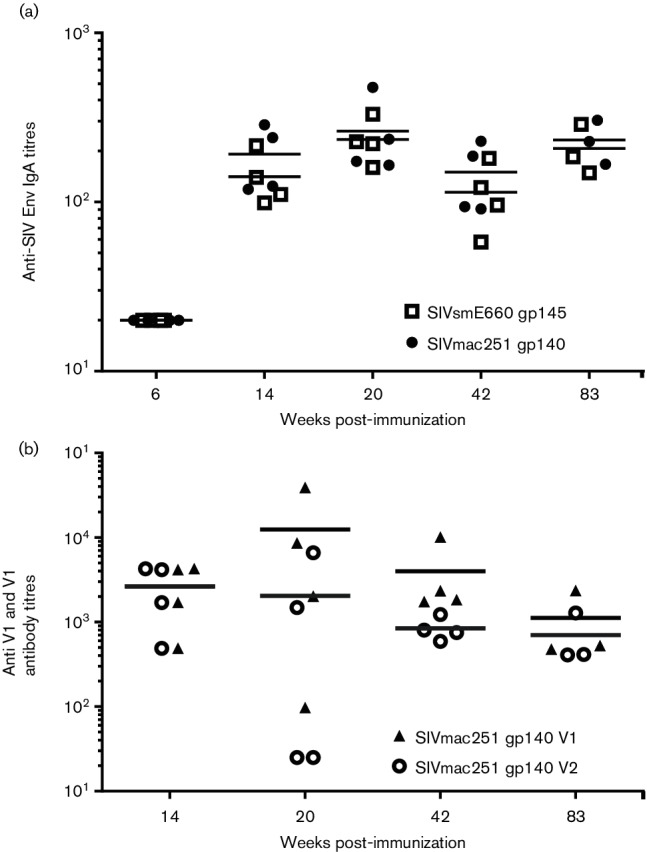
Anti-IgA and anti-V1 and V2 responses in the immunized animals measured at indicated time points post-immunization. (a) Serum IgA titres were measured against both SIVmac251 gp140 and SIVsmE660 gp145 by ELISA. SIVmac251 gp140 is represented as an open square (□) and SIVsmE660 gp145 is represented as a closed circle (⚫). (b) Antibody titres against V1 and V2 linear peptides of SIVmac251 gp140 were assayed by ELISA. SIVmac251 gp140 V1 is represented as a closed triangle (▲) and SIVmac251 gp140 V2 is represented as an open circle (○).
Immune sera collected at weeks 0, 20, 42 and 83 (day of first challenge) were screened for neutralizing activity against various SIVmac251 and SIVsmE660 isolates with varying levels of neutralization sensitivity. In addition, sera collected on the day of the second and third challenges (weeks 84 and 85) of the protected animals were similarly assayed. The neutralization titres were expressed as the dilution of sera inhibiting SIV infection of TZM-bl cells by 50 % (ID50) and 80 % (ID80) compared to the virus-only control, and these results are shown in Table 2. The neutralization of the Tier 1A viruses (SIVmac251.6 and SIVsmE660 BR/CG7G.IR1), expressed as ID50, was strong and consistent throughout the course of immunization. Relatively high-titre neutralization of the Tier 1B viruses (SIVmac251/M766 and SIVsmE660-BR/CG7V.IR) was also observed in a few serum samples and neutralization of the Tier 2 SIVmac251.30 was detectable in the week 83 serum. However, the titres noted above for the Tier 1B and Tier 2 isolates were no longer evident when these were analysed using the ID80 cut-off (Table 2).
Table 2. Neutralizing antibody response elicited in Chinese rhesus macaques immunized with SIVsmE660 gp145 and SIVmac251 gp140 at various weeks after immunization.
Neutralization titres are expressed as the dilution of serum inhibiting infection of TZM-bl cells by 50 % (ID50) or 80 % (ID80) compared to the untreated control cells. nd, not determined.
| Animal ID | Bleed week | Neutralization titres ID50 (ID80) | ||||
|---|---|---|---|---|---|---|
| Tier 1A | Tier 1B | Tier 2 | ||||
| SIVmac251.6 | SIVsmE660-BR/CG7G.IR1 | SIVmac251/M766 | SIVsmE660-BR/CG7V.IR | SIVmac251.30 | ||
| M907* | 0 | <20 | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) |
| 20 | 5428 (<20) | 29 170 (6735) | <20 (<20) | <20 (<20) | <20 (<20) | |
| 42 | 9354 (<20) | >43 740 (12 545) | <20 (<20) | 2672 (<20) | <20 (<20) | |
| M908 | 0 | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) |
| 20 | 2726 (<20) | >43 740 (26 104) | 41 (<20) | 7259 (<20) | <20 (<20) | |
| 42 | 3572 (<20) | >43 740 (12 300) | 26 (<20) | 5570 (<20) | <20 (<20) | |
| 83 (DOC) | 26 129 (49) | >43 740 (26 294) | 3664 (24) | 5374 (<20) | 118 (<20) | |
| 84 | 15 236 (<20) | >43 740 (24 171) | <20 (<20) | 7082 (<20) | <20 (<20) | |
| 85 | 8794 (<20) | >43 740 (15 850) | <20 (<20) | 3862 (<20) | <20 (<20) | |
| M909 | 0 | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) |
| 20 | 2542 (<20) | 8125 (1927) | <20 (<20) | <20 (<20) | <20 (<20) | |
| 42 | 4437 (28) | 9189 (2476) | <20 (<20) | <20 (<20) | <20 (<20) | |
| 83 (DOC) | 22 974 (122) | >43 740 (12 651) | 4672 (23) | 1203 (<20) | 144 (<20) | |
| 84 | 16 409 (<20) | 29 822 (5179) | <20 (<20) | <20 (<20) | <20 (<20) | |
| M910 | 0 | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) | <20 (<20) |
| 20 | 5377 (<20) | 34 428 (5758) | <20 (<20) | 2431 (<20) | <20 (<20) | |
| 42 | 5626 (<20) | 34 792 (5935) | <20 (<20) | <20 (<20) | <20 (<20) | |
| 83 (DOC) | 8478 (105) | >43 740 (15 459) | 44 (25) | 1881 (<20) | 56 (<20) | |
| 84 | 7556 (<20) | >43 740 (18 489) | <20 (<20) | <20 (<20) | <20 (<20) | |
Data are displayed in a heat map with shades of grey indicating higher nuetralizing antibody titre.
▪ 40–100, ▪ 100–1000, ▪ >1000.
*Animal was euthanized before challenge due to non-vaccine related issues.
To evaluate the neutralizing antibody response against the challenge virus, we determined the inhibition of infection of the challenge stock (SIVmac251-2010 day 8) in TZM-bl cells using a 1 : 10 dilution of immune sera harvested on week 83 (day of first challenge) compared to the pre-immune serum. Clearly, sera from all three animals inhibited in vitro infection of the challenge virus (Table 3). However, this inhibitory activity was short-lived and could not be detected in subsequent weeks, even in macaque M908, which resisted challenge (data not shown).
Table 3. Percentage inhibition of infection elicited against the challenge virus in Chinese rhesus macaques immunized with SIVsmE660 gp145 and SIVmac251 gp140 on the day of challenge.
Sera were measured against the SIVmac251-2010 day 8 virus used for vaginal challenge. Percentage inhibition of infection was calculated based on the level of infection noted in TZM-bl cells in the presence of 1 : 10 dilution of immune serum compared to that noted with similar dilution of pre-immune serum.
| Animal ID | Week | % Inhibition of infection |
|---|---|---|
| M908 | 83 | 89 |
| M909 | 83 | 83 |
| M910 | 83 | 70 |
Antigen-specific B- and T-cell responses following envelope immunization with Advax adjuvants
We evaluated the presence of memory B-cells in the three immunized macaques, which were challenged on week 83. Peripheral blood mononuclear cells (PBMC) collected on week 6 (two weeks after the second DNA immunization) as well as on week 83 (day of first challenge) were evaluated by a B-cell ELISpot assay. As shown in Fig. 4(a), the number of B-cells secreting total IgG was in the range of 927 to 2873 spots/106 PBMC at both measured time points. In contrast, the frequency of B-cells secreting IgG specific to each envelope was initially low after DNA immunization, but markedly higher on week 83 in two out of three immunized animals. We also evaluated T-cell responses in PBMC from immunized animals by IFN-γ ELISpot assay after stimulation with envelope peptide pools from SIVmac251 gp120. The frequency of envelope-specific interferon-gamma (IFN-γ) secreting T-cells was modest after immunizations with DNA on week 6, and a similarly modest level persisted until week 83 (Fig. 4b).
Fig. 4.
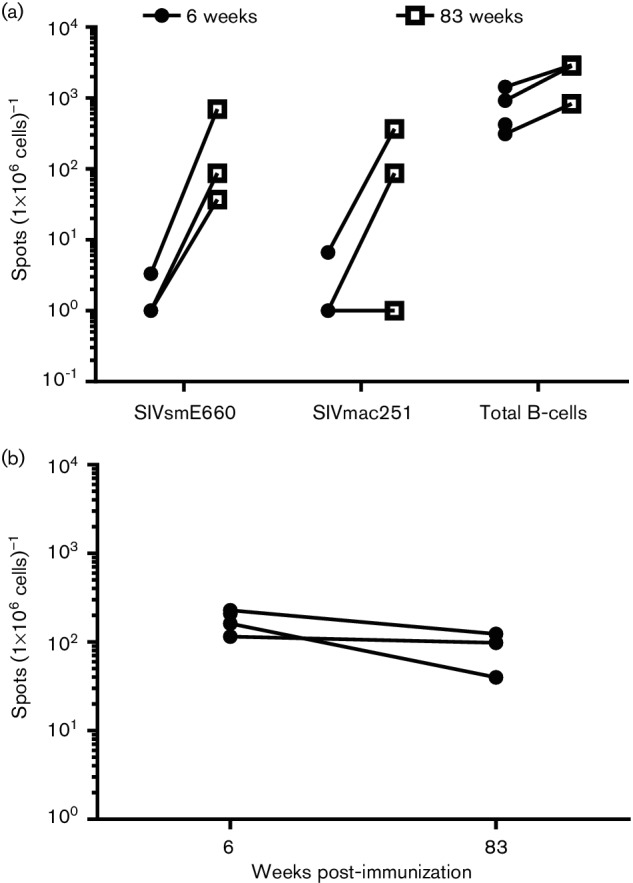
Generation of B- and T-cell responses during immunization. (a) Env-specific IgG-secreting B-cells in PBMC; (b) Env-specific T-cells in PBMC. Both B- and T-cell responses were evaluated by ELISpot assays as described in the Methods section. Data are shown for three macaques, which were vaginally challenged with SIVmac251 on week 83.
Reduction of viral acquisition in the immunized rhesus macaques following vaginal challenge
Although four animals were initially enrolled in the study, macaque M907 was euthanized on week 45 due to clinical conditions of the urethra, not induced by the vaccine or adjuvant. On week 82, the three remaining animals were boosted via the IM route with the mixture of SIVmac251 gp140 and SIVsmE660 gp145 proteins in ADVAX-1 adjuvant, and after 7 days subjected to weekly low-dose vaginal challenge with SIVmac251 isolate (2000 TCID50). We selected primary SIVmac251 for challenge, as this virus is pathogenic in macaques and difficult to neutralize. Moreover, this stock was thoroughly characterized for low-dose vaginal transmission by titration studies in Chinese rhesus macaques. Five unvaccinated macaques of Chinese origin served as controls in the study and were challenged with the same dose of virus. While all of the control animals were infected by the second exposure tovirus, one out of the three immunized animals remained protected. even after four vaginal challenges (Fig. 5a). The plasma viral loads measured at 4 additional weeks post-infection to confirm virus acquisition are shown in Fig. 5(b). Although the viral load was slightly lower at peak infection in the two infected immunized animals compared to the controls, this effect was not statistically significant. Macaque M908, which resisted challenge following each virus exposure, had a plasma viral RNA load below the detection limit of the assay (<50 copies; data not shown). These results demonstrate, albeit in a small number of animals, that DNA priming followed by protein boosting with SIV envelopes in Advax adjuvant elicited protective immune responses against vaginal transmission of SIV.
Fig. 5.
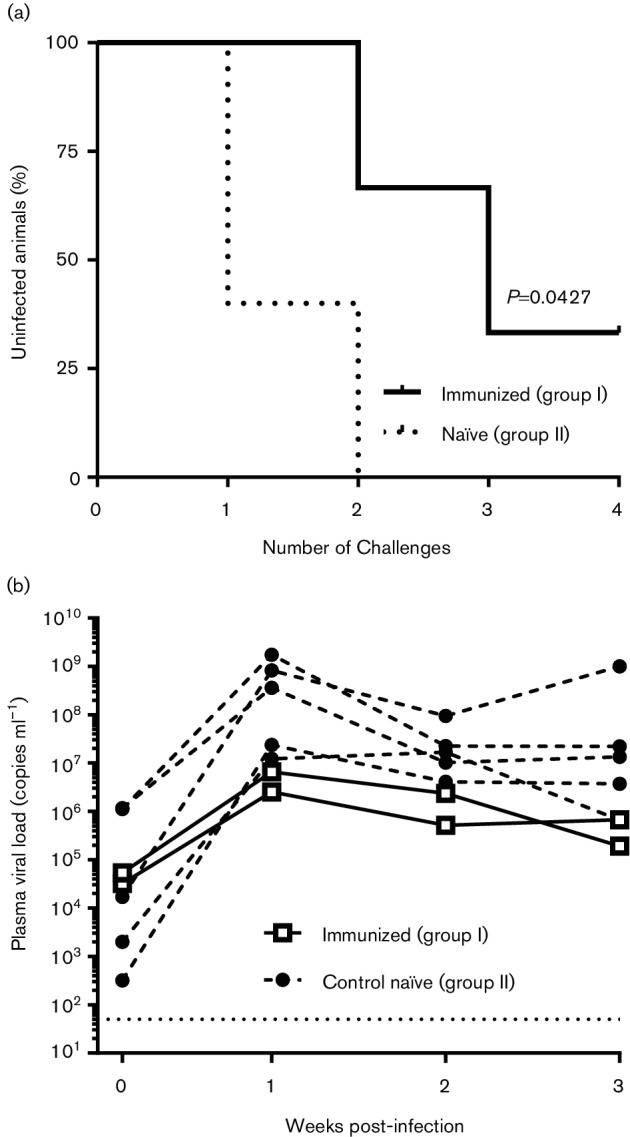
Virus acquisition and plasma viremia in the immunized and control animals following vaginal challenge with SIVmac251. (a) Post-challenge survival in vaccinated and control animals following repeated low-dose challenges with SIVmac251. (b) Plasma viral RNA loads in the infected macaques. RNA loads were plotted as a function of time after virus acquisition for all infected animals measured over a 4-week period (week 0 represents the first day when the animal was shown to be infected). The immunized (group I) animals are represented as open squares (□) and the control naïve (group II) animals are represented as closed circles (⚫). The lower limit of detection for the viral RNA assay (50 copies) is shown as a dotted line. The viral loads of only two infected immunized animals are shown, along with those for five control animals, as one immunized macaque (M908) resisted infection and had an undetectable viral load (<50 copies).
Discussion
The partial protection noted in the RV144 trial would suggest that a better selection of HIV-1 Env delivered with an adjuvant capable of eliciting a persistent antibody response in the immunized host will confer enhanced protective efficacy. In a previous study, we evaluated a DNA envelope prime–gp120 protein boost approach in which sequential nasal and parenteral protein administrations were performed with two delta inulin-based adjuvants, ADVAX-M and ADVAX-P. Mice primed with HIV-1 DNA followed by subsequent nasal and parenteral HIV-1 gp120 protein boost with the respective Advax adjuvants generated strong mucosal and systemic immunity [27]. Here we extended those earlier findings to the SIV rhesus macaque model using a similar design incorporating an oligomeric form of the virus envelope from two distinct SIV isolates. Animals were primed with DNA encoding SIV envelopes and boosted intranasally and intramuscularly with purified SIVmac251 gp140 and SIVsmE660 gp145 envelope oligomeric immunogens formulated with Advax adjuvants. We observed low to moderate levels of neutralizing antibodies against Tier 1B and Tier 2 SIV isolates and specific antibodies against the V1 and V2 regions. Neutralization of the challenge virus was noted with immune sera collected on the day of challenge. Additionally, one out of three animals resisted infection following four low-dose intravaginal challenges, while all of the control animals were infected after the second challenge.
In this study, we chose to immunize with two distinct SIV envelopes from SIVmac251 and SIVsmE660 to broaden the anti-Env antibody response. Vaccination with a mixture of either monomeric or trimeric Envs has been shown to be an effective strategy to enhance HIV-1 vaccine-elicited neutralizing antibody responses [33] and a number of oligomeric Env structures have been designed and tested in preclinical studies [15, 20, 34–36]. We selected the uncleaved gp140 trimeric protein design in which the Env gp120–gp41 cleavage sites in both proteins were abolished by amino acid substitution and two stop codons were introduced before the gp41 TM and after the consensus YIQ (‘YIK’ HIV nomenclature), resulting in uncleaved soluble gp140/gp145 proteins. The uncleaved proteins formed oligomeric species that included higher order oligomers, trimers and dimers. The trimers were purified to homogeneity by lectin-affinity, size-exclusion and anion-exchange chromatography. This envelope design has proven to be quite effective in eliciting functional and protective B-cell and antibody responses in animal models [32, 34, 37–39]. Our results showed that uncleaved envelopes had strong affinity interactions with rhesus and human CD4 proteins, and clearly formed trimers, as was evident from the EGS crosslinking and subsequent SDS-PAGE analysis. Administration of the two antigens elicited a robust antibody response to both envelopes, with comparable titres at all time points, suggesting that several immunogenic epitopes were equally exposed in all the antigens. Additional characterization of these antigens for the potential exposure of broadly neutralizing epitopes will be probed using recently described anti-SIV Env monoclonal antibodies [40].
The combination of the SIVmac251 and SIVsmE660 envelopes administered in Advax adjuvants led to the generation of memory B- and T-cell responses against both antigens, as was evident from the ELISpot assays. This vaccine regimen elicited a broadly neutralizing antibody response (ID50) against Tier 1A/1B isolates, and a limited response against a Tier 2 isolate. However, this breadth of neutralizing activity was not observed when the titres were calculated as ID80. This type of incomplete neutralization has been noted with both monoclonal antibodies and immune sera, and can be explained at least in part by the variability in the glycosylation of the viral Env protein, which is also the current hypothesis for incomplete virus neutralization by polyclonal sera [41–43].
Although the challenge study was limited to three immunized macaques, the combination vaccine regimen showed partial efficacy against vaginal transmission of SIVmac251. While all of the control animals were infected, one out of three experimental animals showed no sign of infection after four challenges. The nature of the immune correlates responsible for this partial protection is not clearly understood. Although neutralization of the challenge virus, as well as neutralization of other SIV isolates, was noted with sera collected from the day of the first challenge, this activity did not persist for long, even in the animal that resisted subsequent challenges. Therefore, it is likely that while the neutralizing antibody may have played a partial role in the observed protection, other immune correlates, including antibody-dependent cell-mediated cytotoxicity and phagocytosis within vaginal tissues, may have contributed to protection. Planned future studies with more animals will evaluate the immune responses elicited by this vaccine formulation extensively in both systemic and mucosal compartments. Given the small number of animals in this study, and the lack of a comparative adjuvant, it is not possible to say how great a role the inclusion of Advax adjuvant in the protein boosts played in the robust Tier 1 antibody responses and partial efficacy noted here. However, recent human vaccine studies have started to tease out how Advax adjuvant broadens cross-protective B-cell responses, a feature first identified when it was found that an inactivated Japanese encephalitis virus antigen formulated with Advax adjuvant could cross-protect against a lethal West Nile virus challenge [44]. Additionally, studies showed that subjects immunized with influenza vaccine formulated with Advax adjuvant not only had higher serum neutralizing antibodies, but also had a higher frequency of day 7 plasmablasts in their peripheral blood. These plasmablasts exhibited significantly higher variability of amino acids in the CDR3 region of their B-cell receptors, which was associated with increased expression of activation-induced cytidine deamidase (AID) in the responding plasmablasts, which is consistent with Advax inducing a higher degree of B-cell receptor avidity maturation [45]. Thus, it will be useful in the future to look specifically at whether Advax adjuvant can similarly induce a broader B-cell repertoire against HIV envelope antigens.
Since mucosal transmission represents a major route of HIV-1 infection, we used a combination of mucosal and systemic immunization routes to elicit antibody responses both systemically and at the site of virus transmission. Earlier studies demonstrated that a combination of parental and mucosal protein immunization routes elicited robust mucosal antibody and T–cell responses in mice and macaques [25, 46–48]. Our earlier observation in mice demonstrated that immunization via the IN route followed by immunization via the IM route with Advax adjuvant elicited a robust immune response in both the mucosal and cellular compartments. Similarly, a combined-route immunization strategy consisting of HIV-1 envelope formulated in the adjuvant, MF59, was effective at eliciting robust antibody responses both systemically and in mucosal compartments in macaques. This vaccine had protective efficacy in 12 out of 12 vaccinated rhesus macaques after vaginal challenge with SHIV [46]. Furthermore, several studies have shown that DNA priming enhances cellular T-cell responses and antigen presentation to promote functional, long-lasting systemic and mucosal antigen-specific antibodies that can be masked in the protein immunogen [49, 50]. Based on these observations, we chose to prime with DNA by electroporation followed by a single IN protein boost and three subsequent IM protein boosts. DNA electroporation induced low to moderate envelope binding antibody titres, which were later enhanced by the subsequent IN and IM protein boosts. Future studies may also consider pulmonary delivery of DNA, which has been shown to enhance mucosal and systemic antibody responses in a noninvasive way [51, 52]. However, further studies are needed first to test whether the IN immunization contributed to the observed protection.
In future studies we also plan to modify our envelope design to improve antigen immunogenicity and the functionality of the induced antibody responses. Studies have suggested that envelopes with a closed conformation that is resistant to CD4-induced changes may be better antigens. The recently described SOSIP and NFL trimers have made great strides towards this effort, as they form soluble well-ordered native-like trimers [20, 53, 54]. These proteins have improved antigenicity over uncleaved gp140 envelopes, bind fewer non-neutralizing antibodies and can elicit potent Tier 2 neutralizing antibodies [55, 56]. Another approach that has been used to stabilize the envelope structure and increase envelope thermostability is glutaraldehyde fixation. This method conformationally fixes the envelope into a locked position to prevent ‘breathing’, going from an open to a closed conformation, or even falling apart. Increased thermostability improves the B-cell response by allowing the immunogen to persist within the B-cell follicle for longer periods and engage more cells [55].
In summary, we have shown that an oligomeric envelope vaccine formulated with Advax adjuvant and given as a booster after DNA vaccine priming is capable of inducing a strong humoral response in rhesus macaques and may be a useful framework for the development of HIV vaccines.
Methods
Immunogens and Adjuvants
DNA
Codon-optimized env genes for SIVmac251 gp140 and SIVsmE660 gp145, lacking the native signal peptide, were cloned in frame with the tissue plasminogen activator signal peptide in a mammalian expression vector, pSWTK. Truncations were generated by site-directed mutagenesis using the QuickChange site-directed mutagenesis kit (Stratagene). The gp120–gp41 cleavage sites were abolished by the substitution of an arginine for a serine residue at R527S and potential secondary sites R516S in SIVmac251 and R533S in SIVsmE660. Two stop codons were introduced before the gp41 TM after the consensus YIQ (‘YIK’ HIV nomenclature). The DNA used for immunization was prepared using an EndoFree plasmid giga kit (Qiagen) according to the manufacturer’s protocol and was shown to contain >90 % supercoil structure with low endotoxin levels (<20 EU).
Envelope proteins
Gibco 293 H cells (Thermo Fisher Scientific) were transfected with the above DNAs using Transfectamine 2000 (Invitrogen) as performed previously [32]. Envelope proteins were purified from conditioned media in a three-step purification process: affinity chromatography using Galanthus nivalis lectin (GNL) agarose beads (Vector Laboratories), anion-exchange chromatography using mono-Q sepharose beads (GE Healthcare) and a gel filtration step to selectively isolate trimeric protein. Proteins were concentrated and buffer exchanged into 1× PBS. Protein estimations were performed using a Bradford protein assay kit (Pierce, Rockford). Purified proteins were analysed by SDS-PAGE and blue native PAGE. To demonstrate the trimeric nature of the preparations, envelope proteins were crosslinked with EGS crosslinker (Pierce) according to the manufacturer’s protocol.
Adjuvants
ADVAX-1 (Vaxine Pty Ltd) was provided as a 50 mg ml−1 aseptic suspension of delta inulin. The adjuvant was simply mixed with the envelope protein immediately prior to immunization. ADVAX-M (Vaxine) is a proprietary glycolipid adjuvant based on alpha galactosyl ceramide, and was similarly mixed with antigen immediately prior to immunization. Adjuplex adjuvant was obtained from Sigma as a liquid concentrate.
Immunizations
Rabbits
Four New Zealand White (NZW) rabbits were housed at the animal facility of Advanced BioScience Laboratories under protocols approved by the IACUC. Animals were immunized with 250 µg of each plasmid DNA encoding the envelope gp145 of SIVmac251 and SIVE660 intramuscularly followed by electroporation using a BTX AgilePulse device (BTX Harvard Apparatus) on weeks 0, 4 and 14. SIV envelope proteins (50 µg/animal) were formulated with Adjuplex adjuvant (Sigma) by mixing prior to immunization and were injected simultaneously via intramuscular, intradermal and subcutaneous routes as per the manufacturer’s protocol on weeks 27 and 33. Serial bleeds were collected 2 weeks post each immunization.
Rhesus macaques
Four female Chinese rhesus macaques (M907, M908, M909 and M910), weighing 4–5 kg and virologically and immunologically negative for type D retroviruses, SIV and simian T-lymphotropic virus (STLV), were immunized in this study.Five similar unvaccinated macaques were used as controls. Animals in the immunization group were vaccinated intramuscularly with 500 µg of each plasmid DNA encoding the envelope of SIVmac251 and SIVE660, followed by electroporation using a BTX AgilePulse device (BTX Harvard Apparatus) on weeks 0 and 4. Recombinant envelope gp140/gp145 proteins were mixed and formulated with ADVAX-M or ADVAX-1 adjuvants provided by Vaxine (Australia) by mixing prior to immunization. The animals were boosted with a mixture of SIVmac251 gp140 and SIVsmE660 gp145 proteins (200 µg each protein) intranasally on week 12 with ADVAX-M (25 µg/animal) adjuvant. Subsequently macaques were immunized intramuscularly with the same envelope proteins on weeks 18, 38 and 82 with ADVAX-1 (5 mg/animal). Macaque M907 was euthanized on week 45 due to clinical conditions that were affecting the animal’s ability to urinate. These symptoms developed after an episode of self-inflicted trauma near the base of the tail and were not related to either the vaccine or the adjuvant. One week after the last protein immunization (week 83), the three remaining macaques and the controls were challenged intravaginally with SIVmac251 (2000 TCID50) and weekly thereafter for three additional exposures until the naïve controls were infected. This challenge stock was generated in the laboratory of Dr Desrosiers (University of Miami) by in vitro infection of naïve rhesus PBMC. It contained 10.8 ng ml−1 of SIV p27 protein.
ELISA and specific IgG quantitation
Sera collected at different time points during the course of the study were assayed for anti-gp145-specific IgG antibodies using an ELISA as previously described [57]. Binding titres were determined as the highest dilution of immune serum producing ELISA values (A450 nm) greater than or equal to two times the signal detected with a corresponding dilution of pre-immune serum. The reactivity of the immune sera to the peptides representing the V1 and V2 domains was also assessed by ELISA as described above. The assay was performed using the peptides for V1-NKSETDRWGLTKSSTTTTTAAPTSAPVSEKTDMVNETSSC and V2-IAQNNCTGLEQEQMISCKFTMTGLKRDKTKEYNETWYSTDLVCEQGNSTDNESRCYMNHC.
Antibody affinity measurements
The binding kinetics of the immunoglobulins or CD4 to SIVmac251 gp140 and SIVsmE660 gp145 was measured by BLI using an OctetRED-96 platform (ForteBio) with biotinylated envelope proteins as described elsewhere [32]. The data were analysed using the latest version of Data Analysis 9.0 evaluation software (ForteBio) to measure the response curves and calculate the kinetic parameters (kon, kdis, KD) using a global fit 1 : 1 model. Pre-immune sera were used a controls in the affinity measurements to establish baseline responses.
Neutralization assays
The neutralizing antibody titres for the rabbit and macaque sera were evaluated in the TZM-bl Luc assay using a panel of SIVmac251 and SIVsmE660 isolates with different neutralization sensitivities (SIVmac251.30, SIVmac251/M766, SIVsmE660-BR/CG7V.IR, SIVmac251.6 and SIVsmE660-BR/CG7G.IR1) as previously described [58]. The titres were defined as the reciprocal serum dilution at which there was a 50 or 80 % reduction in the relative luminescence units (RLUs) compared to virus control wells with no test sample. Inhibition of the infectivity of the challenge virus was performed in a similar manner with fixed (1 : 10) dilution of the immune sera.
B- and T-cell ELISpot assays
Total IgG and envelope-specific IgG B cells were quantitated by ELISpot using the Human IgG ELISpot basic kit (Mabtech) according to the manufacturer’s instructions. For both total and envelope-specific IgG, the mean B-cell count was recorded from triplicate wells. T-cell ELISpot assays for IFN-γ release from PBMC stimulated with SIVmac251 envelope peptide pools were performed as previously described [23, 27].
Quantitation of plasma viremia
Plasma viral RNA load in challenged macaques was quantitated by nucleic acid sequence-based amplification (NASBA) as previously described [59, 60].
Statistical analyses
Student’s t-test was used to compare the immunological outcomes for the vaccinated groups and controls. Statistical analyses were computed using the statistical software, GraphPad Prism 6.0 (GraphPad Software, Inc.). Differences between groups were considered to be significant at a P value of <0.05.
Funding information
Work performed at Duke University was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272201100016C.
Acknowledgements
We would like to thank Dr Deborah Weiss and Mr Jim Treece for their veterinary services, and Ms Sharon Orndorff for her coordination of the animal studies and for editing the manuscript. We also thank Drs Ronald Desrosiers and Nancy Miller for providing the SIVmac251 challenge stock.
Conflicts of interest
Nikolai Petrovsky is an employee of Vaxine Pty Ltd, which has interests in Advax adjuvant. This does not alter the authors’ adherence to all of Journal of General Virology’s policies on sharing data and materials.
Ethical statement
All animal studies were performed in compliance with NIH guidelines for housing and care of laboratory animals. Performed procedures were under anaesthesia that adhere to protocols approved by the Institutional Animal Care and Use Committee (IACUC; protocols AUP500 and AUP558) at Advanced BioScience Laboratories in Rockville, MD, USA and comply with the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) guidelines.
Footnotes
Abbreviations: BLI, bio-layer interferometry; CMV, cytomegalovirus; Env, Envelope; gp, glycoprotein; HIV, human immunodeficiency virus; IFN-γ, interferon-gamma; IM, intramuscular; IN, intranasal; KD, equilibrium constant; Kdis, off-rate constant; Kon, on-rate constant; RSV, respiratory syncytial virus; SARS, severe acute respiratory syndrome; SIV, simian immunodeficiency virus; SHIV, simian-human immunodeficiency virus; TM, transmembrane domain.
References
- 1.HIV/AIDS, J. U. N. P. o . According to the UNAIDS’estimate the Number of New Infections in the Region Increased From 21, 22,000-047,000. Geneva: UNAIDS; 2015. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. [Google Scholar]
- 2.HIV/AIDS, J. U. N. P. o . Global AIDS Update. Geneva: UNAIDS: 2016. [Google Scholar]
- 3.Letvin NL, Barouch DH, Montefiori DC. Prospects for vaccine protection against HIV-1 infection and AIDS. Annu Rev Immunol. 2002;20:73–99. doi: 10.1146/annurev.immunol.20.081501.094854. [DOI] [PubMed] [Google Scholar]
- 4.Nabel GJ. Challenges and opportunities for development of an AIDS vaccine. Nature. 2001;410:1002–1007. doi: 10.1038/35073500. [DOI] [PubMed] [Google Scholar]
- 5.Nabel GJ. HIV vaccine strategies. Vaccine. 2002;20:1945–1947. doi: 10.1016/S0264-410X(02)00074-9. [DOI] [PubMed] [Google Scholar]
- 6.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 7.de Souza MS, Ratto-Kim S, Chuenarom W, Schuetz A, Chantakulkij S, et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol. 2012;188:5166–5176. doi: 10.4049/jimmunol.1102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes BF, Gilbert PB, Mcelrath MJ, Zolla-Pazner S, Tomaras GD, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, et al. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci USA. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudicell RS, Kwon YD, Ko SY, Pegu A, Louder MK, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88:12669–12682. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, et al. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beddows S, Franti M, Dey AK, Kirschner M, Iyer SP, et al. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology. 2007;360:329–340. doi: 10.1016/j.virol.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 16.Grundner C, Li Y, Louder M, Mascola J, Yang X, et al. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology. 2005;331:33–46. doi: 10.1016/j.virol.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Qiao ZS, Montefiori DC, Haynes BF, Reinherz EL, et al. Comparison of HIV type 1 ADA gp120 monomers versus gp140 trimers as immunogens for the induction of neutralizing antibodies. AIDS Res Hum Retroviruses. 2005;21:58–67. doi: 10.1089/aid.2005.21.58. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs JM, Nkolola JP, Peng H, Cheung A, Perry J, et al. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc Natl Acad Sci USA. 2012;109:12111–12116. doi: 10.1073/pnas.1204533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nkolola JP, Peng H, Settembre EC, Freeman M, Grandpre LE, et al. Breadth of neutralizing antibodies elicited by stable, homogeneous clade A and clade C HIV-1 gp140 envelope trimers in guinea pigs. J Virol. 2010;84:3270–3279. doi: 10.1128/JVI.02252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders RW, Derking R, Cupo A, Julien JP, Yasmeen A, et al. A next-generation cleaved, soluble HIV-1 Env trimer, BG505 SOSIP.664 gp140, expresses multiple epitopes for broadly neutralizing but not non-neutralizing antibodies. PLoS Pathog. 2013;9:e1003618. doi: 10.1371/journal.ppat.1003618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cristillo AD, Wang S, Caskey MS, Unangst T, Hocker L, et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology. 2006;346:151–168. doi: 10.1016/j.virol.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Pal R, Kalyanaraman VS, Nair BC, Whitney S, Keen T, et al. Immunization of rhesus macaques with a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine elicits protective antibody response against simian human immunodeficiency virus of R5 phenotype. Virology. 2006;348:341–353. doi: 10.1016/j.virol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Pal R, Wang S, Kalyanaraman VS, Nair BC, Whitney S, et al. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J Med Primatol. 2005;34:226–236. doi: 10.1111/j.1600-0684.2005.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Kennedy JS, West K, Montefiori DC, Coley S, et al. Cross-subtype antibody and cellular immune responses induced by a polyvalent DNA prime-protein boost HIV-1 vaccine in healthy human volunteers. Vaccine. 2008;26:3947–3957. doi: 10.1016/j.vaccine.2007.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Valentin A, Kulkarni V, Rosati M, Beach RK, et al. HIV/SIV DNA vaccine combined with protein in a co-immunization protocol elicits highest humoral responses to envelope in mice and macaques. Vaccine. 2013;31:3747–3755. doi: 10.1016/j.vaccine.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielefeldt-Ohmann H, Prow NA, Wang W, Tan CS, Coyle M, et al. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Vet Res. 2014;45:130. doi: 10.1186/s13567-014-0130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, et al. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol. 2011;92:128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honda-Okubo Y, Kolpe A, Li L, Petrovsky N. A single immunization with inactivated H1N1 influenza vaccine formulated with delta inulin adjuvant (Advax™) overcomes pregnancy-associated immune suppression and enhances passive neonatal protection. Vaccine. 2014;32:4651–4659. doi: 10.1016/j.vaccine.2014.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larena M, Prow NA, Hall RA, Petrovsky N, Lobigs M. JE-ADVAX vaccine protection against Japanese encephalitis virus mediated by memory B cells in the absence of CD8(+) T cells and pre-exposure neutralizing antibody. J Virol. 2013;87:4395–4402. doi: 10.1128/JVI.03144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Layton RC, Petrovsky N, Gigliotti AP, Pollock Z, Knight J, et al. Delta inulin polysaccharide adjuvant enhances the ability of split-virion H5N1 vaccine to protect against lethal challenge in ferrets. Vaccine. 2011;29:6242–6251. doi: 10.1016/j.vaccine.2011.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangasamy SP, Menon V, Dhopeshwarkar P, Pal R, Vaniambadi KS, et al. Membrane bound Indian clade C HIV-1 envelope antigen induces antibodies to diverse and conserved epitopes upon DNA prime/protein boost in rabbits. Vaccine. 2016;34:2444–2452. doi: 10.1016/j.vaccine.2016.03.062. [DOI] [PubMed] [Google Scholar]
- 32.Sneha Priya R, Veena M, Kalisz I, Whitney S, Priyanka D, et al. Antigenicity and immunogenicity of a trimeric envelope protein from an Indian clade C HIV-1 isolate. J Biol Chem. 2015;290:9195–9208. doi: 10.1074/jbc.M114.621185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bricault CA, Kovacs JM, Nkolola JP, Yusim K, Giorgi EE, et al. A multivalent clade C HIV-1 Env trimer cocktail elicits a higher magnitude of neutralizing antibodies than any individual component. J Virol. 2015;89:2507–2519. doi: 10.1128/JVI.03331-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Earl PL, Sugiura W, Montefiori DC, Broder CC, Lee SA, et al. Immunogenicity and protective efficacy of oligomeric human immunodeficiency virus type 1 gp140. J Virol. 2001;75:645–653. doi: 10.1128/JVI.75.2.645-653.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma SK, de Val N, Bale S, Guenaga J, Tran K, et al. Cleavage-independent HIV-1 Env trimers engineered as soluble native spike mimetics for vaccine design. Cell Rep. 2015;11:539–550. doi: 10.1016/j.celrep.2015.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, et al. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol. 2002;76:4634–4642. doi: 10.1128/JVI.76.9.4634-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Hao Y, Luo Z, Huang Y, Hu X, et al. Broad HIV-1 neutralizing antibody response induced by heterologous gp140/gp145 DNA prime-vaccinia boost immunization. Vaccine. 2012;30:4135–4143. doi: 10.1016/j.vaccine.2012.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundling C, Forsell MN, O'Dell S, Feng Y, Chakrabarti B, et al. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J Exp Med. 2010;207:2003–2017. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieczorek L, Krebs SJ, Kalyanaraman V, Whitney S, Tovanabutra S, et al. Comparable antigenicity and immunogenicity of oligomeric forms of a novel, acute HIV-1 subtype C gp145 envelope for use in preclinical and clinical vaccine research. J Virol. 2015;89:7478–7493. doi: 10.1128/JVI.00412-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason RD, Welles HC, Adams C, Chakrabarti BK, Gorman J, et al. Targeted isolation of antibodies directed against major sites of SIV Env vulnerability. PLoS Pathog. 2016;12:e1005537. doi: 10.1371/journal.ppat.1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doores KJ, Burton DR. Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16. J Virol. 2010;84:10510–10521. doi: 10.1128/JVI.00552-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim AS, Leaman DP, Zwick MB. Antibody to gp41 MPER alters functional properties of HIV-1 Env without complete neutralization. PLoS Pathog. 2014;10:e1004271. doi: 10.1371/journal.ppat.1004271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovsky N, Larena M, Siddharthan V, Prow NA, Hall RA, et al. An inactivated cell culture Japanese encephalitis vaccine (JE-ADVAX) formulated with delta inulin adjuvant provides robust heterologous protection against West Nile encephalitis via cross-protective memory B cells and neutralizing antibody. J Virol. 2013;87:10324–10333. doi: 10.1128/JVI.00480-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Honda-Okubo Y, Li C, Sajkov D, Petrovsky N. Delta inulin adjuvant enhances plasmablast generation, expression of activation-induced cytidine deaminase and B-cell affinity maturation in human subjects receiving seasonal influenza vaccine. PLoS One. 2015;10:e0132003. doi: 10.1371/journal.pone.0132003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS. 2008;22:339–348. doi: 10.1097/QAD.0b013e3282f3ca57. [DOI] [PubMed] [Google Scholar]
- 47.Jalah R, Kulkarni V, Patel V, Rosati M, Alicea C, et al. DNA and protein co-immunization improves the magnitude and longevity of humoral immune responses in macaques. PLoS One. 2014;9:e91550. doi: 10.1371/journal.pone.0091550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vargas-Inchaustegui DA, Tuero I, Mohanram V, Musich T, Pegu P, et al. Humoral immunity induced by mucosal and/or systemic SIV-specific vaccine platforms suggests novel combinatorial approaches for enhancing responses. Clin Immunol. 2014;153:308–322. doi: 10.1016/j.clim.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valentin A, von Gegerfelt A, Rosati M, Miteloudis G, Alicea C, et al. Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit. Vaccine. 2010;28:1962–1974. doi: 10.1016/j.vaccine.2009.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Gegerfelt AS, Rosati M, Alicea C, Valentin A, Roth P, et al. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency virus SIVmac251 after therapeutic DNA immunization. J Virol. 2007;81:1972–1979. doi: 10.1128/JVI.01990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murugappan S, Frijlink HW, Petrovsky N, Hinrichs WL. Enhanced pulmonary immunization with aerosolized inactivated influenza vaccine containing delta inulin adjuvant. Eur J Pharm Sci. 2015;66:118–122. doi: 10.1016/j.ejps.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Rajapaksa AE, Ho JJ, Qi A, Bischof R, Nguyen TH, et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization. Respir Res. 2014;15:60. doi: 10.1186/1465-9921-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guenaga J, de Val N, Tran K, Feng Y, Satchwell K, et al. Well-ordered trimeric HIV-1 subtype B and C soluble spike mimetics generated by negative selection display native-like properties. PLoS Pathog. 2015;11:e1004570. doi: 10.1371/journal.ppat.1004570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, et al. Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science. 2013;342:1477–1483. doi: 10.1126/science.1245625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng Y, Tran K, Bale S, Kumar S, Guenaga J, et al. Thermostability of Well-Ordered HIV spikes correlates with the elicitation of autologous tier 2 neutralizing antibodies. PLoS Pathog. 2016;12:e1005767. doi: 10.1371/journal.ppat.1005767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, et al. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pal R, Venzon D, Letvin NL, Santra S, Montefiori DC, et al. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J Virol. 2002;76:292–302. doi: 10.1128/JVI.76.1.292-302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005;Chapter 12:12.11. 11-12.11. 17. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 59.Lee EM, Chung HK, Livesay J, Suschak J, Finke L, et al. Molecular methods for evaluation of virological status of nonhuman primates challenged with simian immunodeficiency or simian-human immunodeficiency viruses. J Virol Methods. 2010;163:287–294. doi: 10.1016/j.jviromet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Romano JW, Shurtliff RN, Dobratz E, Gibson A, Hickman K, et al. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J Virol Methods. 2000;86:61–70. doi: 10.1016/S0166-0934(99)00184-6. [DOI] [PubMed] [Google Scholar]