Abstract
Pyriculol was isolated from the rice blast fungus Magnaporthe oryzae and found to induce lesion formation on rice leaves. These findings suggest that it could be involved in virulence. The gene MoPKS19 was identified to encode a polyketide synthase essential for the production of the polyketide pyriculol in the rice blast fungus M. oryzae. The transcript abundance of MoPKS19 correlates with the biosynthesis rate of pyriculol in a time-dependent manner. Furthermore, gene inactivation of MoPKS19 resulted in a mutant unable to produce pyriculol, pyriculariol and their dihydro derivatives. Inactivation of a putative oxidase-encoding gene MoC19OXR1, which was found to be located in the genome close to MoPKS19, resulted in a mutant exclusively producing dihydropyriculol and dihydropyriculariol. By contrast, overexpression of MoC19OXR1 resulted in a mutant strain only producing pyriculol. The MoPKS19 cluster, furthermore, comprises two transcription factors MoC19TRF1 and MoC19TRF2, which were both found individually to act as negative regulators repressing gene expression of MoPKS19. Additionally, extracts of ΔMopks19 and ΔMoC19oxr1 made from axenic cultures failed to induce lesions on rice leaves compared to extracts of the wild-type strain. Consequently, pyriculol and its isomer pyriculariol appear to be the only lesion-inducing secondary metabolites produced by M. oryzae wild-type (MoWT) under these culture conditions. Interestingly, the mutants unable to produce pyriculol and pyriculariol were as pathogenic as MoWT, demonstrating that pyriculol is not required for infection.
Keywords: pyriculol, phytotoxin, Magnaporthe oryzae, secondary metabolite, PKS gene cluster, secondary metabolite biosynthesis, gene cluster regulation, virulence, polyketide synthases, plant–microbe interaction
Introduction
Fungal secondary metabolites play crucial roles in plant–pathogen interactions and it appears to be of particular interest to elucidate their function in respect of the biological activity and the organization of their biosynthetic pathways. Fungal pathogens, especially hemibiotrophic specimens, initially establish a biotrophic relationship with their host and and are supposed to produce toxic secondary metabolites mostly during late infection [1]. Magnaporthe oryzae causes rice blast disease and is one of the most significant plant pathogens worldwide [2]. This filamentous ascomycete has been studied intensively and has become an excellent model organism for studying the molecular mechanisms of plant–pathogen interaction. In contrast to infection-related morphogenesis ex planta [3, 4], which has been investigated intensively, little is known about invasive growth, metabolic adjustments and microbe–host interaction during the colonization in planta. One of the fungal secondary metabolites studied most intensively is the polyketide dihydroxynaphthalene (DHN) melanin. Melanin is a dark brown pigment accumulating in fungal cell walls, thereby protecting the mycelium and conidia against desiccation, reactive oxygen species and UV light [5]. DHN melanin was found to be essential in M. oryzae for appressorium function and, thereby, for pathogenic development. DHN-melanin is required for the generation of an enormous turgor within the appressorium needed for host penetration [6]. The biosynthetic pathway of DHN was investigated by using melanin-deficient mutant strains, and the main biosynthetic intermediates were uncovered [5]. Further secondary metabolites involved in the virulence of the rice blast fungus were believed to be produced by the polyketide synthase [PKS-nonribosomal peptide synthase (NRPS)] MoAce1p. The gene MoACE1 encodes an intracellular hybrid protein which was found to be involved in effects concerning the rice resistance gene Pi33 [7, 8]. Species of the rice blast fungus were found to produce a variety of phytotoxic secondary metabolites dependent on the way it was cultivated in vitro. For example, Magnaporthe grisea was found to produce a species-specific metabolite, pyrichalasin [9, 10]. The virulence of the Digitaria- pathogenic M. grisea strains putatively correlates with the quantity of pyrichalasin H because toxin-free mutants were non-pathogenic – but a comprehensive characterization of these mutants has not been not conducted to date [11]. Additional studies have identified other metabolites produced by Magnaporthe strains, i.e. tenuazonic acid and Magtoxin [10, 12–14]. Tenuazonic acid is a mycotoxin synthesized by various plant pathogenic fungi. The biosynthesis was hypothesized in M. oryzaefrom isoleucine and acetoacetyl-CoA by TeA synthetase 1 (TAS1) [14]. Furthermore, metabolome studies resulted in varieties of metabolites, i.e. siderophores found to be essential for host invasion. One of them is ferricrocin which was suggested to play a role in pathogenicity since mutants unable to produce ferricrocin were reduced in virulence [15]. Furthermore, it was hypothesized that the loss of an Abc3 pump leads to excessive accumulation of its physiological substrate, a digoxin-like endogenous steroidal glycoside, to likely inhibitory levels resulting in appressorial dysfunction [16]. The many common patterns of metabolomic re-programming during in planta growth was described in pre-symptomatic tissues, proliferation and hyphal growth of M. oryzae, indicating that that fungal pathogens deploy a common metabolic re-programming strategy in diverse host species to suppress plant defence and colonize plant tissue. Metabolization of monosaccharides into mannitol and glycerol for carbon sequestration and osmolyte production was suggested to drive hyphal growth in planta [17]. Furthermore, a series of salicylaldehyde-type phytotoxins, such as pyriculol and pyriculariol, were found and putative biosynthetic intermediates of these compounds were synthesized to elucidate the biosynthesis [18]. However, no further studies have been published to date concerning the M. oryzae PKS gene cluster with respect to the biosynthesis of pyriculol and pyriculariol. We identified the PKS19 gene cluster within this study and present the four genes MoPKS19, MoC19OXR1, MoC19TRF1 and MoC19TRF2 contributing to the regulation of the biosynthesis of pyriculol and pyriculariol. Furthermore, both compounds were found to be dispensable for pathogenicity in the rice blast fungus, since mutants unable to synthesize the heptaketides were found to be as virulent as the wild-type strain.
Methods
Strains, culture/growth conditions and oligonucleotides
All mutants described in this study were generated from M. oryzae (M. oryzae 70-15 strain: MoWT, Fungal Genetics Stock Centre, Kansas City, USA). The strains were grown at 26 °C on complete medium (CM) [19]. All oligonucleotides used in this study are listed in Table S1 (available in the online Supplementary Material) and were obtained from Eurofins MWG Operon. All chemicals used were of p.a. (pro analysis) quality unless otherwise stated.
Identification and sequence analysis of polyketide synthase genes in M. oryzae
Sequence analysis and comparison of the PKS-related genes in the M. oryzae genome [Magnaporthe Comparative Sequencing Project, Broad Institute of Harvard and MIT (www.broadinstitute.org/); MG8] were performed by the Basic Local Alignment Search Tool (blast; http://genome.jgi.doe.gov/pages/blast-query.jsf?db=Maggr1). Protein domains and further information were obtained from the InterProScan database (www.ebi.ac.uk/Tools/pfa/iprscan).
DNA manipulation/construction of gene deletion/expression vectors
The DNA of MoWT was isolated from mycelia of 3-day-old liquid cultures (grown in CM at 26 °C and 120 r.p.m.) using a DNeasy Plant Mini kit (QIAGEN) following the manufacturer’s instructions for purification of DNA from plants and filamentous fungi. Standard procedures were carried out for DNA manipulation [20].
Fungal transformations were conducted using Agrobacterium tumefaciens-mediated transformation, as described in previous studies [21, 22]. Mutant strains were generated using hygromycin resistance to interrupt and respectively replace parts of the coding sequences of the target genes. By contrast, the complementation of mutant strains was conducted using the ILV gene mediating resistance to chlorimuron ethyl [23]. See supplementary material S1 for detailed information on the deletion/expression strategies.
Phylogenetic tree of MoPks19p and sequence analysis
Phylogenetic analysis was carried by using the program mega 5.2 [24]. Sequence alignments were performed using clustalw [25]. blosum was used as ‘cost matrix’ with ‘gap open cost’: 10 and ‘gap extend cost’: 0.1. Phylograms were made using the neighbour-joining algorithm with the Jones–Taylor–Thornton model [26]. The bootstrapping analysis involved 200 replicates. In order to visualize the data, the Geneious 6.1.7 software was applied.
RNA isolation, cDNA amplification and quantitative real-time PCR analysis
The RNA of the MoWT and the mutants was isolated using an RNeasy Plant Mini kit (QIAGEN) following the manufacturer’s instructions for purification of total RNA from plants and filamentous fungi. The cDNA amplification and quantitative real-time PCR (qPCR) were performed using the iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad Laboratories) following the manufacturer’s instructions. We used the constitutive expressed fungal elongation factor EF-1 alpha (MGG_03641) genes and actin (MGG_03982) as reference (housekeeping) genes for the relative quantification of the expression ratio. Calculations were based on the relative quantification calculation method of Pfaffl [27].
Fermentation and growth conditions for secondary metabolism and quantitative real-time PCR analysis
Three agar blocks of 10 mm diameter from 11-day-old M. oryzae cultures were aseptically transferred as inoculum to 200 ml liquid CM in 500 ml glass flasks with one baffle. These cultures were grown for 72 h at 26 °C and 120 r.p.m. The mycelium was washed with sterile water and was transferred to different liquid starvation media, and the fermentation was then continued at 26 °C and 120 r.p.m. in an orbital shaker. The media used in this study were minimal medium (MM) [19] and rice-extract medium (REM; 10 g rice leaves of 28-day-old rice cultivar CO39 were shredded and added to 1 l MM before sterilization).
Preparation of crude extracts from M. oryzae cultures
The mycelium was separated from the culture fluid by filtration. The culture fluid was extracted twice with ethyl acetate and equal amounts of the crude extracts obtained were then dried with Na2SO4. After evaporation of the organic solvent in vacuo at 40 °C to dryness, the residue was dissolved in methanol to give 10 mg ml−1. The mycelium was extracted for 60 min with a methanol/acetone mixture (1 : 1, v/v). The crude extract was filtered and evaporated in vacuo at 40 °C to dryness and dissolved in methanol to give 10 mg ml−1.
Isolation of the pyriculol derivatives from cultures of M. oryzae
A fermenter (20 l; B. Braun) with MM was inoculated with a 5-day-old M. oryzae 70-15 (MoWT) fluid culture (500 ml CM in a 1 l glass flask with one baffle). The culture conditions were as follows: 3 l air min−1; 28 °C; 120 r.p.m. Fermentation was stopped when pyriculol was produced in good yields (traced by HPLC). The culture fluid was separated from the mycelium by filtration and extracted with ethyl acetate (1 : 1, v/v) to yield 280 mg crude extract. Solid-phase extraction (Chromabond C182 g; Macherey-Nagel) of the crude extract with a H2O/acetonitrile gradient yielded 68 mg intermediate 1 [66 % (v/v) acetonitrile]. Intermediate 1 was subjected to a preparative separation [column: Waters Sunfire C18, 19×250 mm, 5 µm; solvents: H2O/acetonitrile; isocratic flow: 27 % (v/v) acetonitrile; flow: 15 ml min−1]. Pyriculol was isolated (28 mg) at retention time 6 min.
Furthermore, a fermenter (20 l) with BAF medium [ingredients per litre (H2O): 20 g maltose, 10 g glucose, 2 g soy peptone, 1 g yeast extract, 0.5 g KH2PO4, 1 g MgSO4.7H2O, 10 mg FeCl3, 1 mg ZnSO4.7H2O and 5 ml 0.1 M CaCl2] was inoculated with a 7-day-old MoWT fluid culture (500 ml BAF medium in a 1 l glass flask with one baffle). The culture conditions were as follows: 3 l air min−1; 28 °C; 130 r.p.m. Fermentation was stopped when the compounds desired were produced in good yields (traced by HPLC/MS; 7 days). The culture fluid was separated from the mycelium by filtration and extracted with ethyl acetate (1 : 1, v/v) to yield 806 mg crude extract. Solid-phase extraction (Chromabond C182 g; Macherey-Nagel) of the crude extract with a H2O/acetonitrile gradient yielded 365 mg intermediate 1 [10 % (v/v) acetonitrile], 195 mg intermediate 2 [35 % (v/v) acetonitrile, containing the pyriculol derivatives] and 24 mg intermediate 3 [100 % (v/v) acetonitrile]. Intermediate 2 was subjected to a preparative separation [column: Waters Sunfire C18, 19×250 mm, 5 µm; solvents: H2O/acetonitrile; gradient: 5–25 % (v/v) acetonitrile in 30 min; 25–32 % (v/v) acetonitrile in 60 min; flow: 13.6 ml min−1]. The following pyriculol derivatives were isolated: dihydropyriculariol (1.5 mg; retention time, 26.5 min), dihydropyriculol (3.4 mg; retention time, 27–27.5 min) and pyriculariol (7.3 mg; retention time, 48.5–49 min).
HPLC/MS method for analysis of secondary metabolites from M. oryzae
Crude extracts were analysed by means of HPLC (Agilent 1100 Series) equipped with a LiChrospher RP 18 (3×125 mm; 5 µm, Merck) and a diode array detector. In order to analyse the extracts, the temperature of the column was set to 40 °C and a flow rate of 1 ml min−1 was used with an elution gradient composed of H2O and acetonitrile.
The molecular weight of the peaks selected was determined using an HPLC/MS (Agilent 1260 Series LC and 6130 Series Quadrupole MS System). The mass spectra were recorded using atmospheric pressure chemical ionization with positive and negative polarization. A Superspher RP 18 (125×2 mm; 4 µm, Merck) column was used at 40 °C. For every run, 1 µl of a sample at a concentration of 1 mg ml−1 was injected. The elution was performed with a gradient of H2O and acetonitrile, and a flow rate of 0.45 ml min−1.
Identification of pyriculol, pyriculariol, dihydropyriculol and dihydropyriculariol
Isolated samples were analysed by 1H NMR (600 MHz, CDCl3, Bruker DRX-600 spectrometer). The NMR spectrum as well as the 1H NMR data is given in supplementary material S2.
Phytotoxicity assay with rice and plant infections/pathogenicity assays with rice
The phytotoxicity assays were carried out using leaf segments of 21-day-old plants of dwarf indica rice cultivar CO39. Plants were cultivated using a daily cycle of 16 h light followed by 8 h darkness [28 °C, 90 % (v/v) relative humidity]. Leaves were fixed on water agar and the extracts were applied in 10 µl droplets of H2O containing 0.2 % (w/v) gelatin to a final amount of 10 µg of each extract. Pure compounds were applied in 10 µl droplets with an additional control of the solvent used. The results were documented by photography 5 days post-application. The plant infection assays were carried out as described previously [19].
Results
Secondary metabolites produced by M. oryzae strain 70-15 in axenic cultures
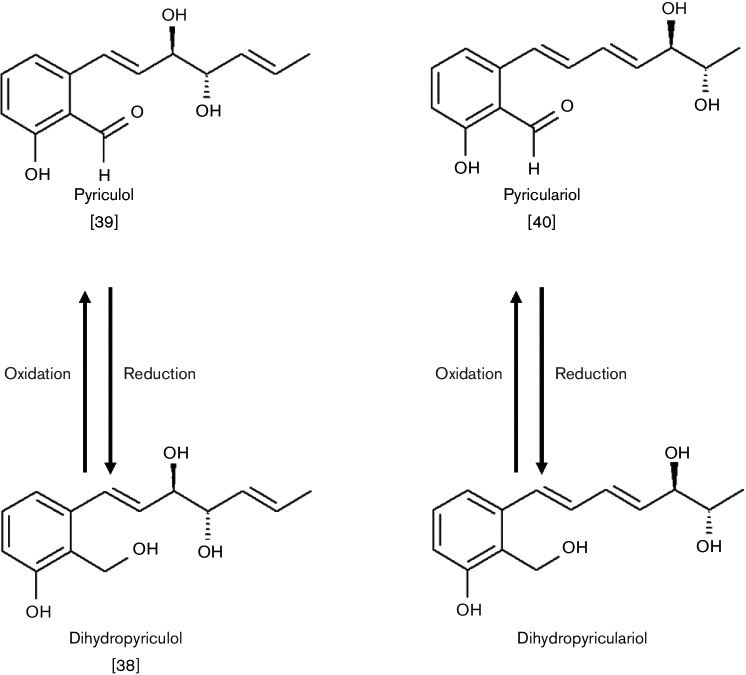
In order to investigate the secondary metabolism of M. oryzae 70-15 (MoWT), the fungal strain was grown under different culture conditions that affect secondary metabolite production. Crude extracts of the submerged cultures were analysed by HPLC/MS to identify secondary metabolites produced by the rice blast fungus. Levels of four polyketides were found to be boosted in axenic cultures in MM (synthetic medium) and in REM (rice leaf extract based medium). The respective metabolites produced under these conditions were identified as the heptaketides pyriculol [2-((1E,5E)-3,4-dihydroxy-1,5-heptadienyl)-6-hydroxybenzaldehyde], pyriculariol [2-((1E,3E)-5,6-dihydroxy-1,3-heptadienyl)-6-hydroxybenzaldehyde], dihydropyriculol [2-((1E,5E)-3,4-dihydroxy-1,5-heptadienyl)-2-hydroxymethylphenol] and dihydropyriculariol [3-((1E,3E)-5,6-dihydroxy-1,3-heptadienyl)-2-hydroxymethylphenol] (Fig. 1). The same compounds were found in the corresponding extracts of mycelia, but only in very low quantities. The highest total yield of secondary metabolites was observed in extracts of cultures grown in MM, and the best pyriculol production in REM (data not shown).
Fig. 1.
Secondary metabolites produced by M. oryzae 70-15 (MoWT). The structures of the major polyketides found in extracts of MoWT after fermentation in MM or in REM are shown.
Pyriculol is a heptaketide consisting of seven C2 groups, wherein of the seven C2 groups within the native polyketo compound, only one aldehyde group remains in the C1position (Fig. 1). The hydroxyl group at the C11 position of pyriculol can be obtained via a single reduction step and further reductions on the C5, C7, C9 and C13 positions may lead to the double bonds within the molecule. Furthermore, the C10 position of pyriculol contains a third hydroxyl moiety. Since pyriculol and its derivatives should be produced by a PKS, we searched for genes encoding such proteins in the M. oryzae genome. Several reduced moieties (-OH/-H) within the structure of pyriculol indicate that reductase and dehydratase domains are essential, whereas methyltransferase activity is apparently not required for the biosynthesis of heptaketides.
Sequence analysis and identification of polyketide synthase-related genes in M. oryzae
Sequence analysis and comparison of the predicted protein sequences of putative PKS-encoding genes revealed a set of 20 relevant ORFs within the M. oryzae genome (Table S2). Selected genes were found to contain the four PKS-characteristic protein domains [PF00109 (KS-N, N-terminal β-ketoacyl synthase), PF02801 (KS-C, C-terminal β-ketoacyl synthase), PF00698 (AT, acyltransferase) and PF00550 (PP, phosphopanthetin-binding sequence)]. In addition, the resulting sequences contained further significant domains: PF14765 (DH, dehydratase), PF08659 (KR, ketoreductase), PF08242 (MT, methyltransferase), PF08240/PF00107 (ADH/ADHZ, alcohol dehydrogenases) and PF00975 (TE, thioesterase). Furthermore, we also identified a set of 18 putative NRPS and PKS-NRPS hybrids (Table S3). These ORFs were found to contain additional NRPS-characteristic protein domains (PF00501: AMP, AMP-binding enzyme and PF00668: CD, condensation domain).
Transcript level of MoPKS19 correlates with the biosynthesis rate of pyriculol/pyriculariol
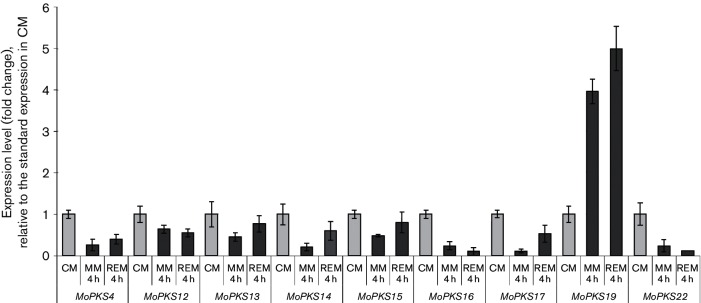
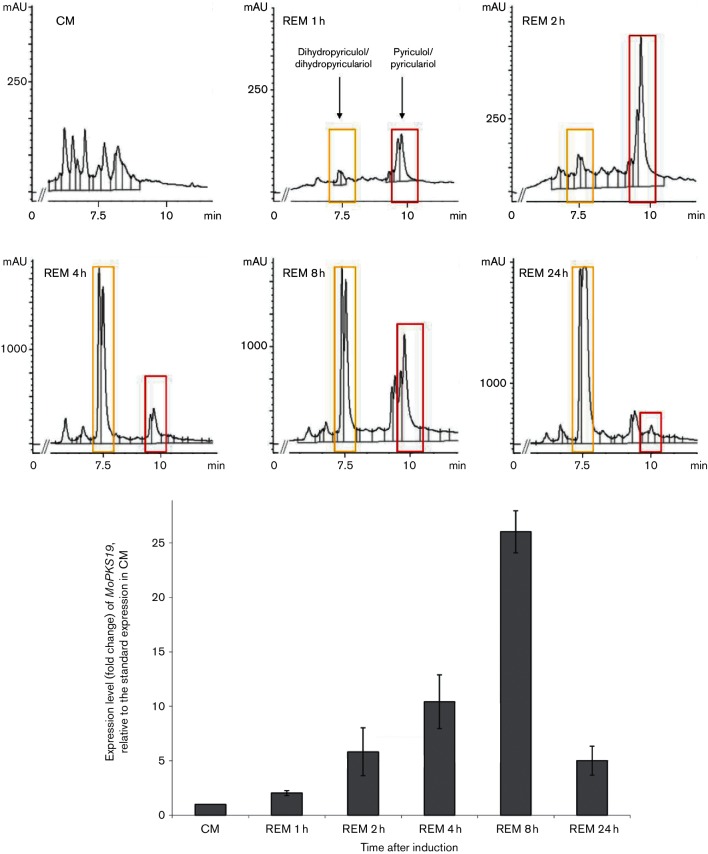
The PKS-encoding genes MoPKS4, MoPKS12-MoPKS17, MoPKS19 and MoPKS22 were assumed to be the most promising candidates for initial analysis. Their predicted protein sequences contain essential KR and DH domains, whereas MT domains were not found. The transcript abundance of MoPKS19 was the most significantly affected in the experiments. MoPKS19 transcript was increased by fivefold in REM compared to CM at 4 h after transfer to REM (Fig. 2). This increase in MoPKS19 expression is correlated with the production of pyriculol. Expression of other PKSs was either repressed in REM compared to CM (MoPKS4, MoPKS12, MoPKS14, MoPKS16, MoPKS17 and MoPKS22) or not upregulated (MoPKS13 and MoPKS15). REM was selected for a time course experiment to investigate the MoPKS19 transcript abundance in comparison to the pyriculol/pyriculariol production rate. The relative transcript abundance of MoPKS19 increased in a time-dependent manner, similar to increasing amounts of pyriculol in corresponding extracts of the culture filtrate samples (Fig. 3). The maximal pyriculol concentration and the highest MoPKS19 transcript abundance were observed 8 h after transfer from CM to REM. Overall, the expression pattern of MoPKS19 in REM was found to correlate with the kinetics of pyriculol production. Additionally, the MoPKS19 transcript abundance was analysed in planta during rice infection. The MoPKS19 transcript was found to be increased during invasive growth (Fig. S1).
Fig. 2.
qRT-PCR analysis of the expression level from genes encoding reducing non-methylating PKS in MoWT. The M. oryzae cultures were grown in CM for 72 h at 26 °C and 120 r.p.m. The mycelium was then transferred to MM, REM or CM for further submersed cultivation at 26 °C and 120 r.p.m. Samples were taken after 2 and 4 h and RNA was isolated from the mycelium samples for qRT-PCR analysis. The results of transcript abundance in MM or REM are given relative to quantification in CM. The experiments were conducted in triplicate. CM, complete medium; MM, minimal medium; REM, rice-extract medium.
Fig. 3.
Time-dependent HPLC analysis of culture filtrate extracts from MoWT in correlation to qRT-PCR analysis of the expression level from the MoPKS19 gene. The M. oryzae cultures were grown as described in Methods. Samples were taken 1, 2, 4, 8 and 24 h after transfer to REM. The HPLC analysis was conducted using extracts of the culture broth, and RNA was isolated from the mycelium samples. The results of transcript abundance in REM are given relative to quantification in CM. The experiments were conducted in triplicate. CM, complete medium; REM, rice-extract medium; mAU, milli-absorption units. Bars represent (+/−) sem.
Analysis of MoPKS19 and identification of MoPKS19-clustered genes
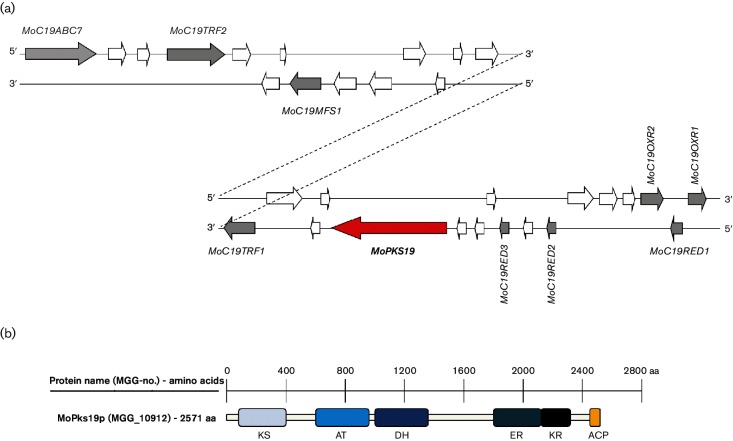
The gene sequence, including the flanking areas, was analysed by genome analysis in order to get more detailed information concerning MoPKS19. Therefore, sequence analysis of MoPKS19 was conducted by means of the InterProScan database and conserved domains are presented in a scheme of the protein sequence (Fig. 4b, Table 1). In addition, phylogenetic analysis of amino acid sequences of MoPks19 was conducted and is provided in Fig. S2.
Fig. 4.
Schematic presentation of the MoPKS19 gene cluster in the genome of MoWT. (a) A scheme of 30 ORFs neighbouring the MoPKS19 gene is presented. The genes are shown with their position either on the ‘sense (5′–3′)’ or on the ‘antisense (3′–5′)’ strand. White arrows imply genes with no obvious functions with significant use in this study (e.g. hypothetical proteins without predicted protein domains). (b) Schematic presentation of protein domains of MoPks19p. KS, β-Ketoacyl synthase; AT, acyltransferase; DH, dehydratase; ER, enoyl reductase; KR, ketoreductase; ACP, ACP domain.
Table 1. Neighbouring genes to MoPKS19 within the MoWT genome with putative function of the corresponding proteins.
The MGG numbers of the genes neighbouring MoPKS19 (30 ORFs) of the eighth annotation of the M. oryzae genome are listed. The InterPro search of conserved protein domains resulted in information concerning the putative protein function.
| MGG–no. | Gene name | Protein size (aa) | Protein domains (InterPro) | Putative function |
|---|---|---|---|---|
| MGG_04855 | MoC19ABC7 | 1683 | ABC_membrane (PF00664) ABC_trans (PF00005) |
Membrane transport |
| MGG_04854 | Hyp. prot | 394 | Adaptin-binding (PF10199) | – |
| MGG_16817 | Hyp. prot | 81 | – | – |
| MGG_04853 | MoC19TRF2 | 625 | Homeobox (PF00046) | Transcription factor |
| MGG_04852 | – | 1279 | E1-E2_ATPase (PF00122) HAD (PF12710) |
Membrane ATPase |
| MGG_04851 | Hyp. prot | 392 | – | – |
| MGG_16816 | Hyp. prot | 57 | – | – |
| MGG_04850 | MoC19MFS1 | 616 | MFS_1 (PF07690) | Membrane transport |
| MGG_12978 | – | 505 | FAD_binding_3 (PF01494) | Monooxygenase |
| MGG_12979 | Hyp. prot | 545 | – | – |
| MGG_04847 | – | 449 | Peptidase_M14 (PF00246) | Carboxypeptidase |
| MGG_04846 | Hyp. prot | 198 | – | – |
| MGG_04845 | – | 246 | – | – |
| MGG_04844 | Hyp. prot | 512 | – | – |
| MGG_04843 | MoC19TRF1 | 610 | Fungal_trans (PF04082) | Transcription factor |
| MGG_04842 | – | 776 | Aconitase (PF00330) | Isomerization reaction |
| MGG_04841 | Hyp. prot | 241 | – | |
| MGG_16815 | Hyp. prot | 210 | – | – |
| MGG_10912 | MoPKS19 | 2571 | KS (PF00109) AT (PF00698) DH (PF14765) ER (IPR020843) KR (PF08659) ACP (IPR009081) |
Polyketide synthase |
| MGG_10911 | – | 198 | Cupin_2(PF07883) | Storage protein |
| MGG_15115 | Hyp. prot | 104 | – | – |
| MGG_16814 | Hyp. prot | 81 | – | – |
| MGG_10910 | MoC19RED3 | 250 | adh_short (PF00106) | Dehydrogenase/reductase |
| MGG_12981 | – | 205 | Cupin_2 (PF07883) | Storage protein |
| MGG_12982 | MoC19RED2 | 278 | adh_short_C2 (PF13561) | Dehydrogenase/reductase |
| MGG_15114 | Hyp. prot | 458 | FAD_binding_3 (PF01494) | Monooxygenase |
| MGG_16813 | Hyp. prot | 363 | Aldo_ket_red (PF00248) | – |
| MGG_16812 | MoC19OXR2 | 520 | FAD_binding_4 (PF01565) BBE (PF08031) |
Oxidase |
| MGG_12983 | MoC19RED1 | 284 | adh_short (PF00106) | Dehydrogenase/reductase |
| MGG_10961 | MoC19OXR1 | 507 | FAD_binding_4 (PF01565) BBE (PF08031) |
Oxidase |
Since multiple genes may participate in one biosynthetic gene cluster close together in filamentous fungi, we also analysed the genes neighbouring MoPKS19. A total of 30 ORFs next to MoPKS19 were examined and their putative function with respect to secondary metabolism and the biosynthesis of pyriculol was assessed (Table 1, Fig. 4a).
It was suggested that oxidation steps or reduction steps of the heptaketides are required in order to obtain the oxidized forms pyriculol and pyriculariol, respectively the reduced compounds dihydropyriculol and dihydropyriculariol (Fig. 1). In addition to the coding sequence of the MoPKS19 gene, two genes were identified containing conserved domains for FAD_binding (PF01565). This domain has been found to be characteristic for oxidase activity [28]. The two genes were named MoC19OXR1 and MoC19OXR2 (Table 1). Furthermore, three genes probably encoding reductases were located close to the MoPKS19 gene. The three ORFs possess sequences coding for short-chain dehydrogenase/reductase domains (PF00106/PF13561; Table 1).Due to the putative role of the corresponding proteins as reductases, the encoding genes were named MoC19RED1, MoC19RED2 and MoC19RED3. Since the transcriptional activation of the genes is likely to be needed for regulation of the MoPKS19 gene, two ORFs including sequences for a homeobox domain (PF00046) and a fungal-specific transcription domain (PF04082) were analysed in detail. The genes were named MoC19TRF1 and MoC19TRF2 (Table 1). Gene clusters often comprise genes coding for transporters in order to export secondary metabolites. Two genes with adequate domains were identified as MoC19ABC7 and MoC19MFS1. MoABC7 has already been published in a comprehensive study of ABC transporters in M. oryzae, where its function was not linked to secondary metabolism [29]. Function and position of genes identified near MoPKS19 suggest the presence of a putative cluster of secondary metabolite genes (Fig. 4a). Further studies (co-expression, deletion mutants) are needed to demonstrate the role of this putative secondary metabolite cluster in pyriculol biosynthesis.
Inactivation of MoPKS19 resulted in mutants deficient in heptaketide production
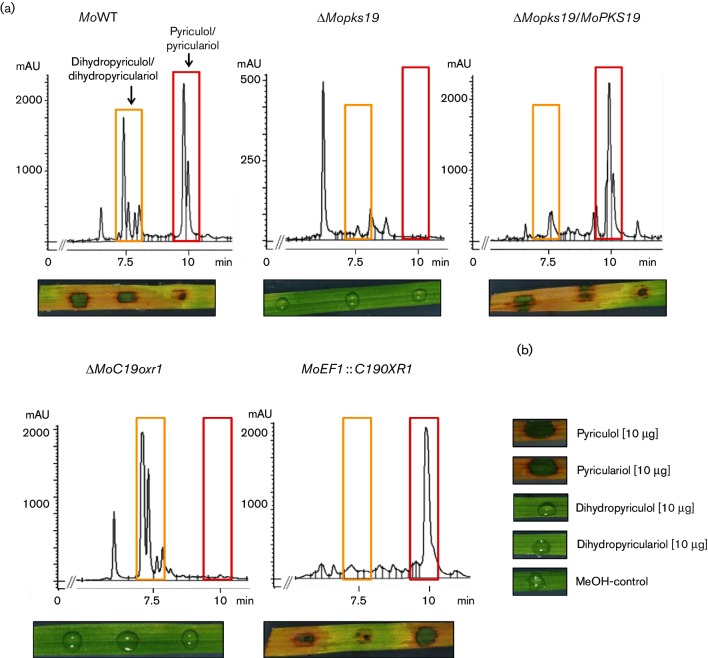
In order to prove that the gene product is the key enzyme in pyriculol biosynthesis, the MoPKS19 gene was inactivated, resulting in the mutant strain ΔMopks19. The HPLC chromatogram of the culture filtrate extract from MoWT in comparison to extracts of the ‘loss of function’ mutant ΔMopks19 and the complemented strain ΔMopks19/MoPKS19 showed complete absence of the heptaketides in extracts of ΔMopks19. Neither pyriculol nor pyriculariol nor the dihydro derivatives were detected in extracts of ΔMopks19, whereas reintegration of the intact gene MoPKS19 into the genome of ΔMopks19 rescued the phenotype in terms of the heptaketide biosynthesis (Fig. 5a). Thus, the secondary metabolite analysis revealed that the mutant strain ΔMopks19 was unable to produce pyriculol and pyriculariol, further underlining the essential role of MoPKS19 in the biosynthesis.
Fig. 5.
Analysis of polyketide in culture filtrate extracts from M. oryzae and phytotoxic activity of extracts and pure compounds. (a) HPLC chromatograms of extracts from MoWT, ΔMopks19, ΔMopks19/MoPKS19, ΔMoC19oxr1 and the overexpression mutant MoEF1 :: C19OXR1. The M. oryzae cultures were grown as described in Methods. Samples were taken 8 h after transfer to REM. The HPLC analysis was conducted using extracts of the culture broth (210 nm wavelength). mAU, Milli-absorption units. Phytotoxic activity of the extracts towards rice leaves is shown below each chromatogram. The assays were conducted as described in Methods. (b) Phytotoxicity was monitored for the pure compounds under equal conditions.
Since two putative oxidase-encoding genes (MoC19OXR1 and MoC19OXR2) and three reductase-encoding genes (MoC19RED1, MoC19RED2 and MoC19RED3) were identified within the putative MoPKS19 gene cluster (Table 1, Fig. 4a), these enzymes were of particular interest concerning their function in pyriculol biosynthesis. However, we were unable to generate inactivation mutants of MoC19OXR2, MoC19RED1, MoC19RED2 and MoC19RED3. Gene inactivation, loss of function andoverexpression mutants of the MoC19OXR1 gene, could be generated. The analysis of the HPLC chromatograms of extracts frosm ΔMoC19oxr1 and MoEF1 :: C19OXR1 validated the putative function in pyriculol biosynthesis because the reduced dihydro compounds were detectable exclusively in the extracts of the inactivation mutant ΔMoC19oxr1, whereas the oxidized forms were found exclusively in the extracts of the overexpression mutant MoEF1 :: C19OXR1 (Fig. 5a). Reintegration of the intact gene MoC19OXR1 into the genome of ΔMoC19oxr1 restored the phenotype of MoWT (data not shown). As a conclusion, MoC19Oxr1p appears to be responsible for conversion of dihydropyriculol and dihydropyriculariol into pyriculol and pyriculariol, respectively.
Regulators of pyriculol biosynthesis within the putative PKS19 gene cluster
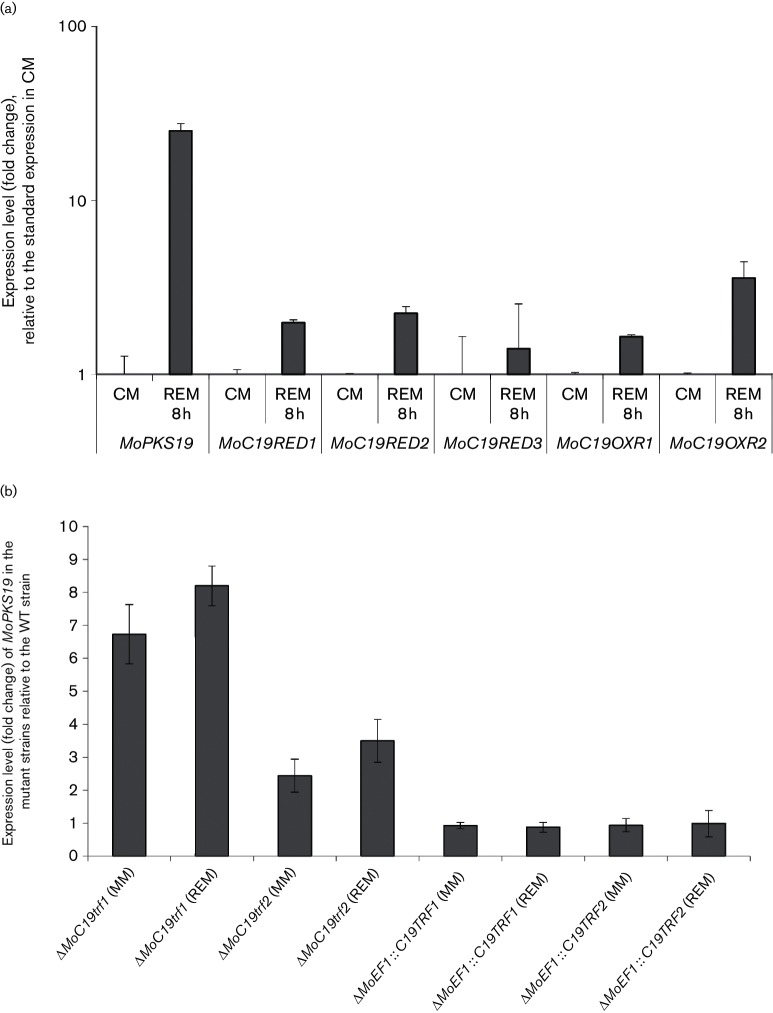
Since we were not able to inactivate the genes MoC19RED1, MoC19RED2, MoC19RED3 and MoC19OXR2, it was impossible to assess the function of these genes in secondary metabolite production. We conducted quantitative PCR analysis of these oxidase/reductase genes identified within the putative MoPKS19 gene cluster to identify enzymes responsible for the reduction of pyriculol and pyriculariol (Fig. 6a). Transcript abundance of the selected genes MoC19RED1, MoC19RED2, MoC19RED3, MoC19OXR1 and MoC19OXR2 during the growth in axenic culture in REM was found to be upregulated relative to the growth in CM (Fig. 6a). Furthermore, we checked the transcript abundance of the selected genes relative to elongation factor EF-1 alpha, which is known to have stable and high transcript levels. Solely MoRED3 transcript abundance was found to be even higher than MoEF1 abundance in CM, MM and REM (Fig. S3). All other genes showed lower transcript levels compared to MoEF1.
Fig. 6.
(a) qRT-PCR analysis of the expression level from the oxidase/reductase-encoding genes in the putative MoPKS19 gene cluster. The M. oryzae cultures were grown for 72 h in CM at 26 °C and 120 r.p.m. The mycelium was transferred for further submersed cultivation to REM at 26 °C and 120 r.p.m. Samples were taken before (CM control) and 8 h after the transfer to REM. The results of transcript abundance are given relative to quantification of the MoEF1 gene in the MoWT. Three replicates were made of each. (b) qRT-PCR analysis of the expression level from the MoPKS19 gene in the mutant strains. The M. oryzae cultures were grown for 72 h in CM at 26 °C and 120 r.p.m. The mycelium was transferred for further submersed cultivation to MM or REM at 26 °C and 120 r.p.m. Samples were taken after 8 h. The RNA was isolated from the mycelium samples and the results of transcript abundance in REM are given relative to quantification in the MoWT. Three replicates were made of each. Bars represent (+/−) sem.
The regulation dynamics within the putative PKS19 gene cluster and the contribution of the two transcription factors MoC19TRF1 (MGG_04843) and MoC19TRF2 (MGG_04853) to pyriculol production were also investigated. Therefore, the transcript abundance of the MoPKS19 gene was determined in the inactivation mutants ΔMoC19TRF1 and ΔMoC19TRF2 and in the expression mutants MoEF1 :: C19TRF1 and MoEF1 :: C19TRF2. Naturally, we initially checked the transcription of the target genes MoC19TRF1 and MoC19TRF2 in the generated mutant strains and found complete transcript absence in ΔMoC19TRF1 and ΔMoC19TRF2 whereas we observed a strong increase of transcript abundance in the overexpression strains (data not shown). The relative transcript level of MoPKS19 was found to be increased in the inactivation mutants, either in high pyriculol producing or in low producing conditions (Figs 6b and S1). By contrast, transcript abundance of MoPKS19 was found to be constant, at the mostslightly decreased in the overexpression mutants compared to MoWT (Figs 6b and S1). These results suggest that these two transcription factors act as negative regulators of MoPKS19.
Pyriculol and pyriculariol appear to induce lesion formation upon application on rice leaves but are dispensable for pathogenicity of M. oryzae 70-15
The crude extract of MoWT containing the heptaketides induced lesion or ‘green isle’ formation on host plant leaves, whereas no disease symptoms were observed after exposure of leaf segments to extracts of ΔMopks19 and ΔMoC19oxr1 containing no pyriculol, oronly the reduced dihydro derivatives (Fig. 5a). Pyriculol and pyriculariol appear to be essential for lesion-inducing effects on rice plants, while the dihydro derivatives appear to be inactive in that respect. Furthermore, qRT-PCR results showed a slightly increase of MoPKS19 transcript abundance in planta during infection (Fig. S1). Complementation experiments with reintegration of the intact gene MoPKS19 into the genome of ΔMopks19 and MoC19OXR1 into the genome of ΔMoC19oxr1 rescued the phenotype in terms of phytotoxicity and the results were comparable to wild-type extracts. The assays were conducted with the pure compounds isolated from the extracts of M. oryzae cultures in order to provide evidence for pyriculol and pyriculariol being responsible for the phytotoxic effects. Pyriculol and pyriculariol induced the ‘green isles’, whereas the dihydro compounds were not found to be phytotoxic (Fig. 5b). Furthermore, the same assays were carried out on leaf segments of wheat and barley and revealed comparable results (data not shown).
Rice plants were infected with conidia of MoWT and the mutant strains ΔMopks19, ΔMoC19oxr1, MoEF1 :: C19OXR1, ΔMoC19TRF1, ΔMoC19TRF2, MoEF1 :: C19TRF1 and MoEF1 :: C19TRF2 in order to further assess whether pyriculol or pyriculariol is of relevance for pathogenicity or virulence in the rice blast fungus. The numbers of lesions found on plants treated with conidia of the MoWT and the respective mutant strains were equal (Fig. S4). Thus, loss of pyriculol biosynthesis did not affect the pathogenicity in M. oryzae.
Discussion
Toxins produced by the pathogen were found to be important virulence factors in many fungal plant diseases [30]. The secondary metabolite spectrum of the hemibiotrophic fungus MoWT includes among others the polyketide pyriculol, its isomer pyriculariol and reduced dihydro derivatives of both compounds (Fig. 1). Further secondary metabolites previously described to be produced by the rice blast fungus, for example, pyrichalasin H [31], pyriculol [32] or tenuazonic acid [12–14], were not found in this study. Based on considerations concerning the chemical structure of pyriculol, we searched for PKS-encoding genes in the genome of M. oryzae. The pyriculol molecule contains several hydroxyl moieties and double bonds, so it can be assumed that reduction steps occurred during biosynthesis. This reaction could be executed by a PKS itself or partly by tailoring enzymes [33]. The variety of PKS-related genes was, consequently, restricted to genes containing a conserved ketoreductase domain (PF08659). Moreover, pyriculol is composed of a linear polyketide chain of seven C2 units without any branch. Subsequent methylation could not been hypothesized since the biosynthetic process of polyketide methylation followed by demethylation has never been observed in fungi [34] and, subsequently, the number of candidate genes involved in pyriculol biosynthesis was further reduced to those without a methyltransferase domain (PF08242). The qRT-PCR analysis of the remaining nine putative PKS-encoding genes resulted in a high transcript abundance of MoPKS19 (Fig. 2). The detailed sequence analysis of MoPKS19 resulted in significantly conserved protein domains (Fig. 4b). With these conserved domains, MoPks19p should be able to synthesize a polyketide such as pyriculol and implement the two reduction steps to the double bonds. Nine genes likely involved in secondary metabolism were identified near MoPKS19 (Fig. 4a). Three reductases, two oxidases, two putative transcription factors and two membrane transporters were located close to MoPKS19 in the genome (Table 1; Fig. 4a). We identified that MoPKS19 is involved in pyriculol synthesis and MoC19OXR1, as an oxidase gene, is involved in the biosynthetic pathway. The two transcription factors MoC19TRF1 and MoC19TRF2 appear to act as negative regulators of MoPKS19, since the transcript level of MoPKS19 in the loss of function mutants was found to be increased (Fig. 6b). There was no need to compare the expression pattern of MoC19OXR1, MoC19TRF1 and MoC19TRF2 to MoPKS19 in this study because transcript abundance must not correlate with protein function. The extracts of the mutant strain ΔMopks19 did not contain heptaketides, the oxidized forms pyriculol/pyriculariol or the reduced dihydro derivatives (Fig. 5a). qRT-PCR analysis showed a strong correlation of MoPKS19 transcript abundance with pyriculol production (Fig. 3) suggesting the essential role of MoPKS19 in pyriculol/pyriculariol production. Modifications of the heptaketide-like conversions from the oxidized molecules into the reduced dihydro derivatives and vice versa can be accomplished from the remaining enzymes coded in the putative MoPKS19 cluster (Table 1; Fig. 4a). In this context, we identified MoC19OXR1 as essential for pyriculol synthesis, since the mutant strain ΔMoC19oxr1 was not able to produce the oxidized molecules pyriculol and pyriculariol, but rather the reduced dihydro compounds. By contrast, overexpression of MoC19OXR1 resulted in a mutant strain producing the oxidized molecules pyriculol/pyriculariol exclusively (Fig. 5a). Since fungal PKSs are known to use C2, C3 or C4 starter molecules [35], and heptaketides, such as rubrofusarin from Fusarium graminearum [36] or naphthopyran and isocoumarin from Aspergillus nidulans [37], were built from acetyl-CoA and malonyl-CoA, the biosynthetic pathway of pyriculol may proceed in an equivalent manner. The recently proposed biosynthesis process describes pyriculol as the intermediate product and the dihydro derivatives need the pyriculol molecule as a precursor [18]. That hypothesis could be underpinned with the results in our study concerning the correlation of heptaketide synthesis and MoPKS19 gene expression in axenic cultures of M. oryzae. Analysis of pyriculol production kinetics in REM showed that it is formed before its dihydro derivative (Fig. 3). In addition, we showed that only dihydro derivatives were found in extracts of ΔMoC19oxr1 (Fig. 5a). That could be furthermore underpinned with the results of the extremely high transcript levels of MoC19RED3 even in CM and still higher in REM (Figs 6a and S3). The pyriculol precursor molecule may be reduced to the dihydro form immediately after production. In contrast to that hypothesis, it was found that neither pyriculol nor pyriculariol but rather the dihydro forms were found in extracts of the mutant strain ΔMoC19oxr1, whereas pyriculol/pyriculariol could be identified exclusively in extracts of the overexpression mutant MoEF1 :: C19OXR1 (Fig. 5a). That indicates that the dihydro derivatives may be precursors and, finally, might be oxidized by MoC19Oxr1p to become pyriculol/pyriculariol. Overexpression of MoC19OXR1 resulted in a mutant strain in which maybe the reductase activity of MoC19Red3p is dominated by the oxidase activity of MoC19Oxr1p. Consequently, only oxidized heptaketides were found (Fig. 5a). The dihydro derivatives of the heptaketides have been described previously as biologically inactive and not phytotoxic [38]. These findings were confirmed in our study because extracts in which only the dihydro derivatives and the pure dihydro compounds were detected failed to induce lesion formation on rice leaves (Fig. 5a, b). By contrast, extracts containing pyriculol and pyriculariol induced lesion and ‘green isles’ formation. Since the extracts of the ΔMopks19 and ΔMoC19oxr1 mutant strains failed to induce phytotoxic lesions on rice leaves, the heptaketides can be assumed to be the sole lesion-inducing compound produced by MoWT under the culture conditions applied within the present study. As a consequence, pyriculol and pyriculariol do not appear to be virulence factors in M. oryzae, since the mutant strains ΔMopks19 and ΔMoC19oxr1 were found to be as virulent as the MoWT strain, whereas these mutant strains have lost the capacity to make the lesion-inducing constituents (Figs 5a, b and S4). This conclusion gives rise to the question why a pathogen wastes energy to produce a lesion-inducing secondary metabolite if the phytotoxin is not required for disease development? Since we found increased MoPKS19 expression levels in planta during invasive growth (Fig. S1), it might be possible that pyriculol can be detoxified by the plant in lower concentrations. The concentrations of the heptaketide in the extracts applied in the phytotoxicity assays resulted in phytotoxic effects maybe because of excessive compound concentrations. We have not yet been able to detect either pyriculol or the derivatives in infected rice plants and the mechanisms of induction of pyriculol synthesis are still unknown. However, the results obtained in this study concerning the molecular basis and the dynamics of regulation of the putative MoPKS19 gene cluster give insights into the biosynthesis of the phytotoxic secondary metabolites pyriculol and pyriculariol. The identification of the genes MoPKS19, MoC19OXR1, MoC19TRF1 and MoC19TRF2 and their contribution to heptaketide production are reported for the first time to our knowledge and will promote further investigations concerning secondary metabolism in the rice blast fungus.
Funding information
Funding was supported by DFG SFB1212: ‘Mikrobielle Umprogrammierung der Pflanzenzell-Entwicklung’ and by ‘Ministerium für Bildung, Wissenschaft, Weiterbildung und Kultur RLP’.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Data
Footnotes
Abbreviations: DHN, dihydroxynaphthalene; PKS, polyketide synthase; qRT-PCR, quantitative real-time PCR.
Four supplementary figures and three supplementary tables are available with the online Supplementary Material
Edited by: A. Alastruey-Izquierdo and V. J. Cid
References
- 1.Horbach R, Navarro-Quesada AR, Knogge W, Deising HB. When and how to kill a plant cell: infection strategies of plant pathogenic fungi. J Plant Physiol. 2011;168:51–62. doi: 10.1016/j.jplph.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Dean R, van Kan JA, Pretorius ZA, Hammond-Kosack KE, di Pietro A, et al. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan X, Talbot NJ. Investigating the cell biology of plant infection by the rice blast fungus Magnaporthe oryzae. Curr Opin Microbiol. 2016;34:147–153. doi: 10.1016/j.mib.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Thines E. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell. 2000;12:1703–1718. doi: 10.1105/tpc.12.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langfelder K, Streibel M, Jahn B, Haase G, Brakhage AA. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet Biol. 2003;38:143–158. doi: 10.1016/S1087-1845(02)00526-1. [DOI] [PubMed] [Google Scholar]
- 6.Howard RJ, Ferrari MA. Role of melanin in appressorium function. Exp Mycol. 1989;13:403–418. doi: 10.1016/0147-5975(89)90036-4. [DOI] [Google Scholar]
- 7.Collemare J, Billard A, Böhnert HU, Lebrun MH. Biosynthesis of secondary metabolites in the rice blast fungus Magnaporthe grisea: the role of hybrid PKS-NRPS in pathogenicity. Mycol Res. 2008;112:207–215. doi: 10.1016/j.mycres.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Song Z, Bakeer W, Marshall JW, Yakasai AA, Khalid RM, et al. Heterologous expression of the avirulence gene ACE1 from the fungal rice pathogen Magnaporthe oryzae. Chem Sci. 2015;6:4837–4845. doi: 10.1039/C4SC03707C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nukina M. The blast disease fungi and their metabolic products. J Pestic Sci. 1999;24:293–298. doi: 10.1584/jpestics.24.293. [DOI] [Google Scholar]
- 10.Tsurushima T, Minami Y, Miyagawa H, Nakayashiki H, Tosa Y, et al. Induction of chlorosis, ROS generation and cell death by a toxin isolated from Pyricularia oryzae. Biosci Biotechnol Biochem. 2010;74:2220–2225. doi: 10.1271/bbb.100404. [DOI] [PubMed] [Google Scholar]
- 11.Tsurushima T, don LD, Kawashima K, Murakami J, Nakayashiki H, et al. Pyrichalasin H production and pathogenicity of Digitaria-specific isolates of Pyricularia grisea. Mol Plant Pathol. 2005;6:605–613. doi: 10.1111/j.1364-3703.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- 12.Umetsu N, Kaji J, Tamari K. Investigation on the toxin production by several blast fungus strains and isolation of tenuazonic acid as a novel toxin. Agric Biol Chem. 1972;36:859–866. doi: 10.1080/00021369.1972.10860315. [DOI] [Google Scholar]
- 13.Lebrun MH, Dutfoy F, Gaudemer F, Kunesch G, Gaudemer A. Detection and quantification of the fungal phytotoxin tenuazonic acid produced by Pyricularia oryzae. Phytochemistry. 1990;29:3777–3783. doi: 10.1016/0031-9422(90)85330-I. [DOI] [Google Scholar]
- 14.Yun CS, Motoyama T, Osada H. Biosynthesis of the mycotoxin tenuazonic acid by a fungal NRPS-PKS hybrid enzyme. Nat Commun. 2015;6:8758. doi: 10.1038/ncomms9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hof C, Eisfeld K, Welzel K, Antelo L, Foster AJ, et al. Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity in rice. Mol Plant Pathol. 2007;8:163–172. doi: 10.1111/j.1364-3703.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Patkar RN, Xue YK, Shui G, Wenk MR, Naqvi NI. Abc3-mediated efflux of an endogenous digoxin-like steroidal glycoside by Magnaporthe oryzae is necessary for host invasion during blast disease. PLoS Pathog. 2012;8:e1002888. doi: 10.1371/journal.ppat.1002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker D, Beckmann M, Zubair H, Enot DP, Caracuel-Rios Z, et al. Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J. 2009;59:723–737. doi: 10.1111/j.1365-313X.2009.03912.x. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Sasaki A, Cao H-Q, Yamada T, Igarashi M, et al. Synthesis and biotransformation of plausible biosynthetic intermediates of salicylaldehyde-type phytotoxins of rice blast fungus, Magnaporthe grisea. European J Org Chem. 2011;2011:6276–6280. doi: 10.1002/ejoc.201100771. [DOI] [Google Scholar]
- 19.Jacob S, Foster AJ, Yemelin A, Thines E. Histidine kinases mediate differentiation, stress response, and pathogenicity in Magnaporthe oryzae. MicrobiologyOpen. 2014;3:668–687. doi: 10.1002/mbo3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green MR, Sambrook J. Molecular cloning: a laboratory manual. 4th ed. Cold Spring Harbor, NY: Cold Spring Harbor laboratory; 2012. [Google Scholar]
- 21.Odenbach D, Breth B, Thines E, Weber RW, Anke H, et al. The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol Microbiol. 2007;64:293–307. doi: 10.1111/j.1365-2958.2007.05643.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacob S, Foster AJ, Yemelin A, Thines E. High osmolarity glycerol (HOG) signalling in Magnaporthe oryzae: identification of MoYPD1 and its role in osmoregulation, fungicide action, and pathogenicity. Fungal Biol. 2015;119:580–594. doi: 10.1016/j.funbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Kramer B, Thines E, Foster AJ. MAP kinase signalling pathway components and targets conserved between the distantly related plant pathogenic fungi Mycosphaerella graminicola and Magnaporthe grisea. Fungal Genet Biol. 2009;46:667–681. doi: 10.1016/j.fgb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 26.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattevi A, Fraaije MW, Mozzarelli A, Olivi L, Coda A, et al. Crystal structures and inhibitor binding in the octameric flavoenzyme vanillyl-alcohol oxidase: the shape of the active-site cavity controls substrate specificity. Structure. 1997;5:907–920. doi: 10.1016/S0969-2126(97)00245-1. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Park SY, Kim D, Choi J, Lee YH, et al. Genome-scale analysis of ABC transporter genes and characterization of the ABCC type transporter genes in Magnaporthe oryzae. Genomics. 2013;101:354–361. doi: 10.1016/j.ygeno.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Markham JE, Hille J. Host-selective toxins as agents of cell death in plant–fungus interactions. Mol Plant Pathol. 2001;2:229–239. doi: 10.1046/j.1464-6722.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 31.Nukina M, Namai T. Productivity of pyrichalasin H, a phytotoxic metabolite, from different isolates of Pyricularia grisea and from other isolates of Pyricularia spp. Agric Biol Chem. 1991;55:1899–1900. doi: 10.1271/bbb1961.55.1899. [DOI] [Google Scholar]
- 32.Kim JC, Min JY, Kim HT, Cho KY, Yu SH. Pyricuol, a new phytotoxin from Magnaporthe grisea. Biosci Biotechnol Biochem. 1998;62:173–174. doi: 10.1271/bbb.62.173. [DOI] [PubMed] [Google Scholar]
- 33.Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106:3468–3496. doi: 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 34.Baker SE, Kroken S, Inderbitzin P, Asvarak T, Li B-Y, et al. Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol Plant Microbe Interact. 2006;19:139–149. doi: 10.1094/MPMI-19-0139. [DOI] [PubMed] [Google Scholar]
- 35.Khosla C, Gokhale RS, Jacobsen JR, Cane DE. Tolerance and specificity of polyketide synthases. Annu Rev Biochem. 1999;68:219–253. doi: 10.1146/annurev.biochem.68.1.219. [DOI] [PubMed] [Google Scholar]
- 36.Frandsen RJ, Nielsen NJ, Maolanon N, Sørensen JC, Olsson S, et al. The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between the naphthoquinones and naphthopyrones. Mol Microbiol. 2006;61:1069–1080. doi: 10.1111/j.1365-2958.2006.05295.x. [DOI] [PubMed] [Google Scholar]
- 37.Fujii I, Watanabe A, Sankawa U, Ebizuka Y. Identification of Claisen cyclase domain in fungal polyketide synthase WA, a naphthopyrone synthase of Aspergillus nidulans. Chem Biol. 2001;8:189–197. doi: 10.1016/S1074-5521(00)90068-1. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki S, Muro H, Sasaki K, Nozoe S, Okuda S, et al. Isolations of phytotoxic substances produced by Pyricularia oryzae Cavara. Tetrahedron Lett. 1973;14:3537–3542. doi: 10.1016/S0040-4039(01)86964-1. [DOI] [Google Scholar]
- 39.Manabu N, Takeshi S, Michimasa I, Takeshi U, Hisao T. Pyriculariol, a new phytotoxic metabolite of Pyricularia oryzae cavara. Agric Biol Chem. 1981;45:2161–2162. doi: 10.1271/bbb1961.45.2161. [DOI] [Google Scholar]
- 40.Iwasaki S, Nozoe S, Okuda S, Sato Z, Kozaka T. Isolation and structural elucidation of a phytotoxic substance produced by Pyricularia oryzae Cavara. Tetrahedron Lett. 1969;10:3977–3980. doi: 10.1016/S0040-4039(01)88591-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.