Abstract
Animals in a natural environment confront many sensory cues. Some of these cues bias behavioral decisions independent of experience, and action selection can reveal a stimulus–response (S–R) connection. However, in a changing environment it would be a benefit for an animal to update behavioral action selection based on experience, and learning might modify even strong S–R relationships. How animals use learning to modify S–R relationships is a largely open question. Three sensory stimuli, air, light, and gravity sources were presented to individual Drosophila melanogaster in both naïve and place conditioning situations. Flies were tested for a potential modification of the S–R relationships of anemotaxis, phototaxis, and negative gravitaxis by a contingency that associated place with high temperature. With two stimuli, significant S–R relationships were abandoned when the cue was in conflict with the place learning contingency. The role of the dunce (dnc) cAMP-phosphodiesterase and the rutabaga (rut) adenylyl cyclase were examined in all conditions. Both dnc1 and rut2080 mutant flies failed to display significant S–R relationships with two attractive cues, and have characteristically lower conditioning scores under most conditions. Thus, learning can have profound effects on separate native S–R relationships in multiple contexts, and mutation of the dnc and rut genes reveal complex effects on behavior.
Learning in the environment requires a complex interplay between sensory information, ongoing behavior, and the consequences of a given behavior. When animals are confronted with a sensory cue, an evolutionarily conserved stimulus–response (S–R) relationship can be revealed through behavioral action selection (Heisenberg 2015). In a complex environment, however, learning through experience might provide a benefit to an animal by altering the selection of a particular S–R pathway. How native S–R-behavior interacts with associative processes is a largely open question. Indeed, tests of learning typically avoid biased environments that might test for interactions of this sort. For example, spatial learning in rodents and flies typically use visual stimuli in the environment which do not bias spatial preferences in search quadrants prior to conditioning (i.e., a weak or absent S–R environment) (Morris 1984; Foucaud et al. 2010; Ofstad et al. 2011). Moreover, classical olfactory conditioning in adult flies is usually done in dark conditions with balanced aversive odor cues to minimize potential interactions of intrinsic biases and memory formation (Tully and Quinn 1985; Zars et al. 2000a; McGuire et al. 2005; Kahsai and Zars 2011). However, classical olfactory conditioning with different odor concentrations in larval Drosophila animals suggests an interaction of learning with intrinsic chemotactic S–R behavior (Schleyer et al. 2015). Moreover, the ability of adult flies to suppress a light preference in the aversive phototaxic suppression (APS) paradigm when light is associated with quinine suggests an interaction of learning with this sort of S–R behavior (Seugnet et al. 2009; Dissel et al. 2015). To understand better how action selection occurs in a complex environment with potentially competing S–R behaviors and learning, what is needed is a robust conditioning paradigm that allows for exposure to different sensory stimuli with individual animals.
Drosophila exhibit multiple experience-independent innate S–R behaviors that lend themselves to examining interaction with learning. Three S–R relationships were examined here. If a fly is presented with a localized air source, at least in flight, flies will typically orient and move toward an air source, which is termed anemotaxis (Budick et al. 2007; van Breugel and Dickinson 2014). Flies also prefer to move toward a light source in fast phototaxis (Benzer 1967; Kain et al. 2012). The S–R behavior here is an attraction to lit versus dark targets (Le Bourg and Buecher 2002; Seugnet et al. 2008; Kain et al. 2012). Finally, flies move against gravitation. For example, if given sequential choices to go up or down in a repeated Y-maze, flies largely prefer the up-choice (McMillan and McGuire 1992; Armstrong et al. 2006).
A potential modification of S–R behavior was tested in an operant place learning paradigm in Drosophila using the heat-box. In this assay, a fly is typically placed in a dark long narrow chamber (1 × 2 × 34 mm). In the absence of conditioning flies walk back and forth between the chamber ends, presumably searching for an escape from this environment (Zars et al. 2000b). Addition of localized air, light, and gravitaxis cues might be used by flies as potential cues for an escape route, and biased searches toward these sensory cues could reveal S–R behaviors. Importantly, flies can be conditioned by associating movement to different parts of the chamber with rising high temperatures (Wustmann et al. 1996; Wustmann and Heisenberg 1997; Zars 2010; Ostrowski and Zars 2014). Flies can be quickly trained and show a persistent place preference in the chambers for several minutes (Zars et al. 2000b; LaFerriere et al. 2011; Ostrowski et al. 2015). Individual flies are conditioned, and positional information is recorded at high spatial (0.2 mm) and temporal (10 Hz) resolution (Sitaraman and Zars 2010).
Interaction of S–R behaviors with place learning and memory in the heat-box was tested. Naïve and potential interactions of preference behavior with learning were done with a localized air source, one lit chamber end, and on an incline (Fig. 1). That is, we tested whether S–R behaviors would be revealed in the heat-box with air source, light, and gravitational cues, with and without conditioning. Moreover, flies with mutations in the dunce (dnc) cAMP-phosphodiesterase and rutabaga (rut) adenylyl cyclase were examined in all conditions.
Figure 1.
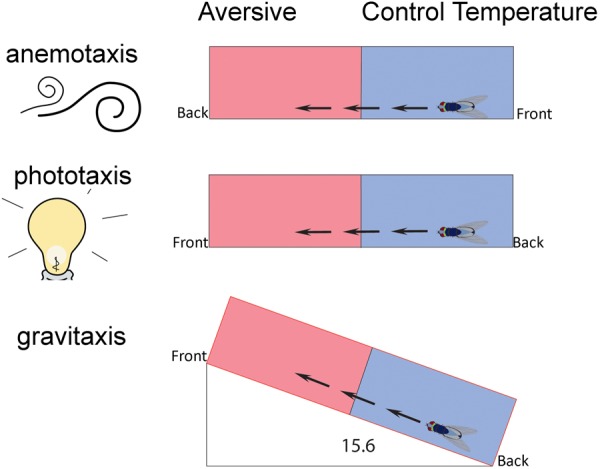
Flies were conditioned in the presence of stimuli that could act as potential attractive cues. In the anemotaxis experiments, an air source was provided at the back of the chambers. A fly with a potential preference for the air source is represented, with the arrows suggesting movement toward that source (front and back refer to either end of the chamber). Training associated the chamber end with the air source with 41°C. In the phototaxis experiments, a light source was provided at the front of the chambers. Training associated the lit end with the aversive temperature of 41°C. In the gravitaxis experiments, chambers were shifted at 7.8° and 15.6o from the horizon. Training associated the higher end with the aversive temperature of 41°C. Controls for each of these cues were without the potentially attractive cue. A second set of controls used the nonaversive temperature of 24°C as the “training” temperature. No conditioning was expected in these control conditions, and provided information about the attractiveness of the cues presented.
Results
Air source preference interaction with learning and memory
We first examined the potential influence of learning on anemotactic S–R behavior. The heat-box was fitted with a potential air source at the back of the chambers. A valve allowed us to provide a low level of airflow into the chamber. Flies were either exposed to an air speed of ∼0.7 m/sec or no-air flow in the chamber. In all conditioning experiments the back end of the chamber was associated with 41°C. The no-conditioning group similarly “associated” the back half of the chamber with the baseline temperature of 24°C. A performance index (PI) represents the proportion of time spent in the front half of the chamber. A PI of zero indicates an equal preference for the front and back half of the chamber. A PI of 1 would be a perfect preference for the front of the chamber and a negative PI indicates a preference for the end with the air source.
Wild-type flies show a strong effect of an air source on average spontaneous place preference, but learning completely suppresses this S–R behavior. Wild-type CS flies had an average place preference score of between −0.3 and −0.4 in the presence of the air source in the no-conditioning group (Fig. 2A). This shows that as a group flies preferred the end of the chamber with the air source. In the conditioning group, CS flies again showed a strong preference of the end of the chamber with the air source, but only in the pretest phase. This preference was completely suppressed during the training and post-test phases (Fig. 2A). The air-source preference is similar to the preference flies have to orient toward and approach an air source in flying flies (Budick et al. 2007; van Breugel and Dickinson 2014).
Figure 2.
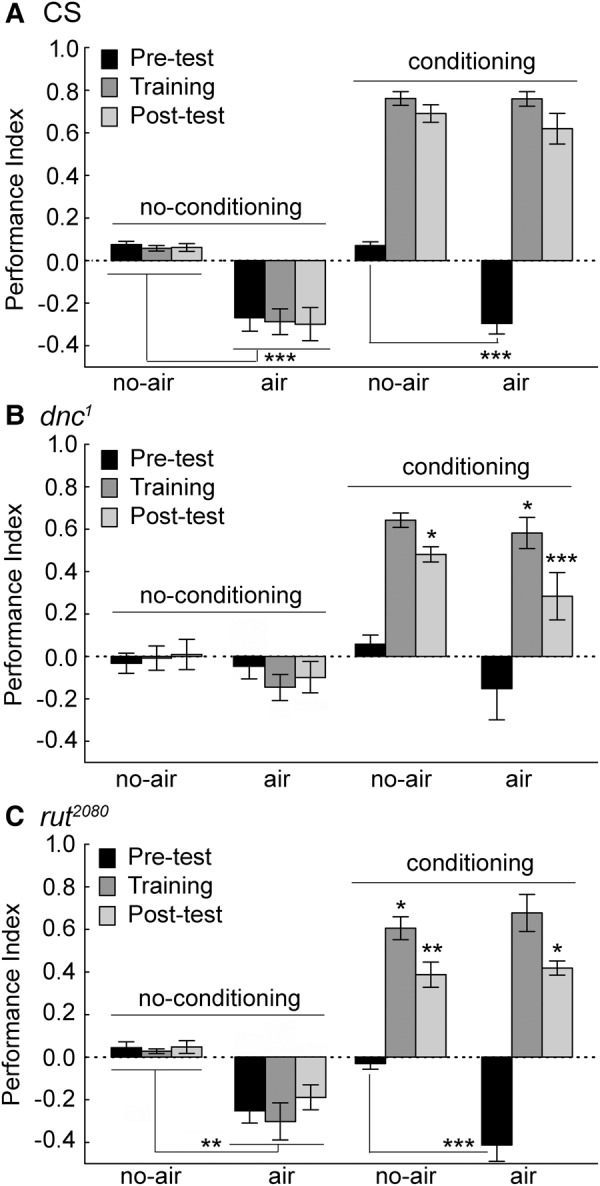
Flies were conditioned in the presence of an air source that could act as a potential attractive cue. (A) Wild-type flies have a preference for an air source. Under no-conditioning, wild-type CS flies show a preference for the side of the chamber with the air source compared to flies not exposed to an air source, evident in negative values (Wilks λ = 0.0378 F(33,313.0) = 20.3, P < 0.00001 for all groups and conditions. Duncan post hoc tests with significant differences are represented, (*) P < 0.05; (**) P < 0.01; (***) P < 0.001). Moreover, the pretest phase in a training experiment also shows a significant negative value compared to flies from the no-air group. In both training conditions, the Training and Post-test phases are strongly positive, but are not statistically distinguishable in the air and no-air groups. (B) There were no preferences for an air source in dnc1 flies compared to flies from the no-air group. Only the Post-test performance of dnc1 flies was significantly lower than that of CS flies in the absence of air. The dnc1 flies had a low Training and Post-test performance in the presence of air compared to CS flies. (C) The rut2080 flies had a significantly lower Training and Post-test performance in the absence of air compared to CS performance levels. Only the Post-test score in rut2080 flies was significantly lower than the CS flies levels in the air groups. The pretest preference was significantly lower in rut2080 flies in the air versus no-air groups. N = 16 trials for CS in each of the conditions; N’s = 8 trials for dnc1 and rut2080 in each of the conditions. Values are presented as means and error bars are SEMs.
We also tested dnc1 and rut2080 mutant flies. The dnc1 flies showed no average preference for the end of the chamber with the air source (Fig. 2B). In contrast, the rut2080 mutant flies showed a preference for the chamber end with the air source (Fig. 2C). The training scores gave mixed results for dnc1 and rut2080 mutant flies compared to CS, with dnc1 mutant flies having a significantly lower performance in the presence of airflow, but not in the control case (Fig. 2B). For rut2080 mutant flies, a lower training score was evident in only the no-air control condition (Fig. 2C). The post-test scores were significantly lower in dnc1 and rut2080 mutant flies compared to those of CS flies in all cases. The results from the control conditions are similar to previous findings, where dnc1 mutant flies have a relatively normal training performance but lower post-test score, and rut2080 flies have lower scores in both phases (Wustmann et al. 1996; Zars et al. 2000b).
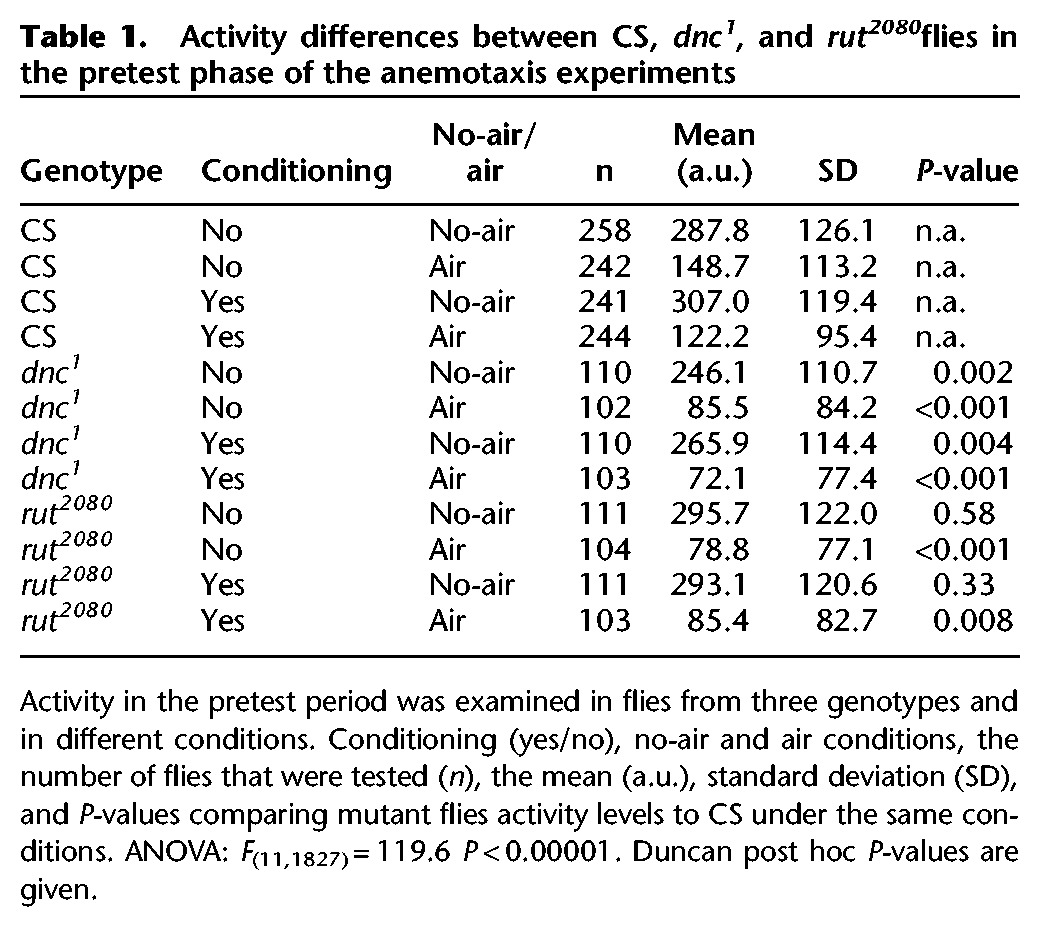
We also examined activity levels in the pretest phase for all genotypes and conditions. In this case, we identified a strong effect of the presence of air on locomotor activity across all genotypes (Table 1), consistent with previous observations (Yorozu et al. 2009). In these measures, we removed the requirement for an eventual experience with the high-temperature chamber half during the conditioning phase since these activity measures were made before there were any chances to experience a high-temperature exposure, and this reveals an effect of dnc1 and rut2080 mutation on behavior in the presence of an air source. In wild-type CS flies, activity levels in the presence of an air source were reduced more than 50% compared to the levels of flies in the absence of an air source. Both dnc1 and rut2080 flies have reduced activity levels in the absence of an air source, and an apparent strong sensitivity to an air source in that the activity levels are again significantly lower in mutant flies compared to CS flies (Table 1). Relative changes in activity were also examined by normalizing activity levels to those in the absence of an air source. CS activity levels in the presence of air were 0.50 and 0.41 in the no-conditioning and conditioning groups, respectively. Both dnc1 and rut2080 flies were still significantly lower in activity in the presence of air after normalization (dnc1 = 0.34 and 0.28 in no-conditioning and conditioning groups, P’s < 0.01 with Duncan post hoc tests; rut2080 = 0.27 and 0.29 in no-conditioning and conditioning groups, P’s < 0.01 with Duncan post hoc tests). The reductions in locomotor activity in the presence of air for dnc1 and rut2080 were consistent across all of the experiments, and suggest this as an effect of air exposure on the behavior of these mutant flies.
Table 1.
Activity differences between CS, dnc1, and rut2080flies in the pretest phase of the anemotaxis experiments
Light preference interaction with learning and memory
We next examined the potential interaction of learned behavior on a light-based S–R behavior. The opaque stoppers that are typically used to hold flies in individual chambers in the heat-box were replaced with translucent stoppers and the room remained lit, in contrast to conditions that were used in all other experiments. The effect was that flies had an approximate 200 lux light source at the front of the chamber. In all conditioning experiments the front, lit end, of the chamber was associated with 41°C. The no-conditioning group similarly “associated” the front half of the chamber with a baseline temperature of 24°C.
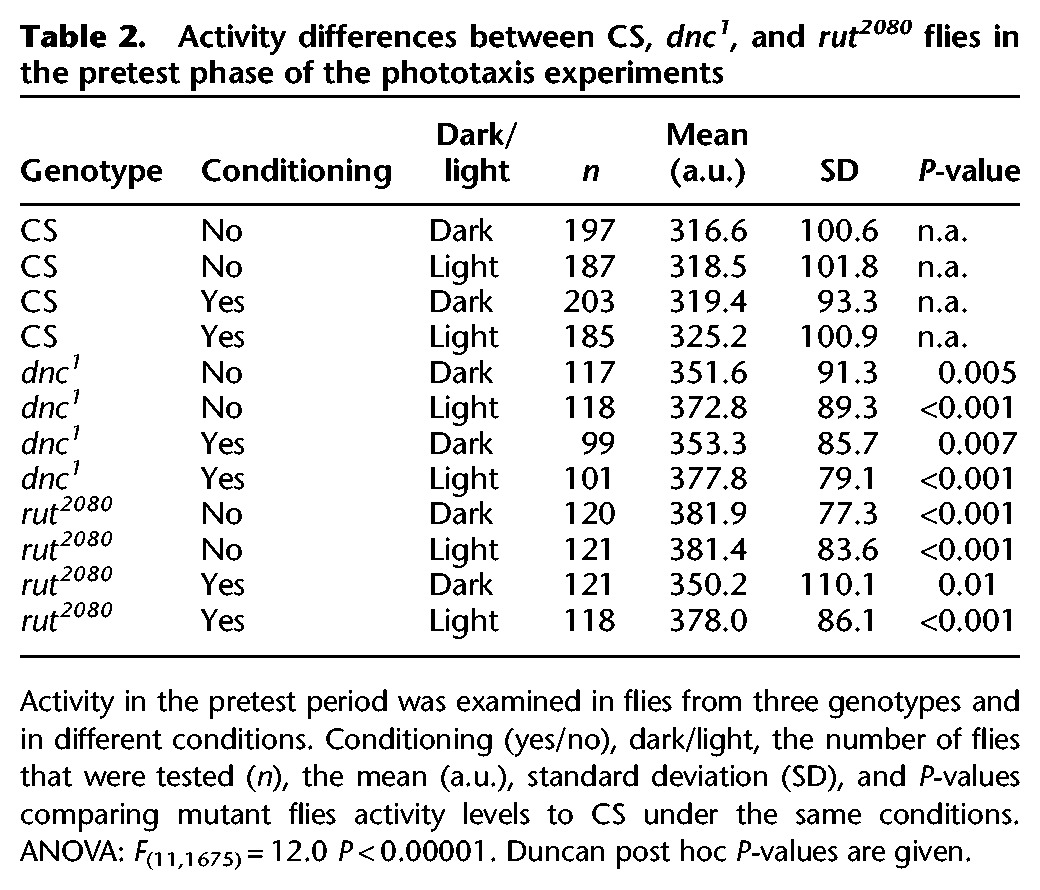
Flies show a preference for the lit end of the chamber, but conditioning completely suppressed this behavior. In the absence of conditioning, flies showed a significant stable light preference, evident in the negative PI for each of the phases of an experiment (Fig. 3A). This preference was also evident in the pretest phase of the conditioning experiment. In contrast, the training and post-test phases completely suppressed the light preference S–R behavior.
Figure 3.
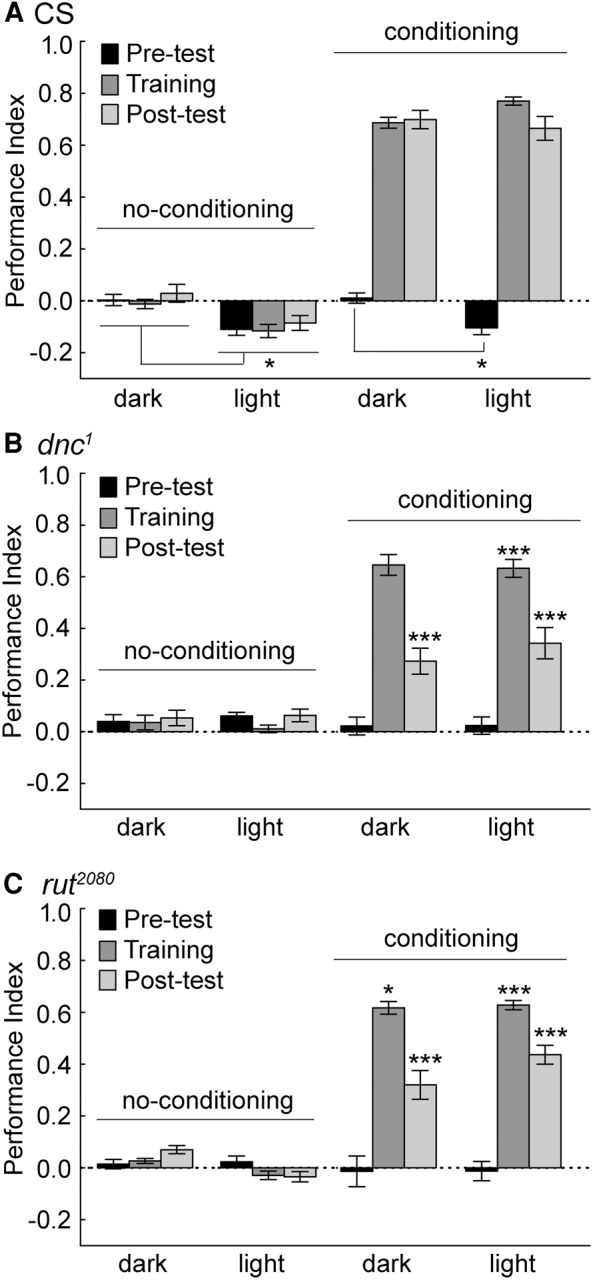
Flies were conditioned in the presence of a light stimulus that could act as a potential attractive cue. (A) In the phototaxis experiments, wild-type CS flies are attracted to the light cue in the absence of conditioning, evident in negative values (Wilks λ = 0.0122 F(33,357.2) = 37.5, P < 0.00001 for all groups and conditions. Duncan post hoc tests with significant differences are represented, (*) P < 0.05; (***) P < 0.001). This preference was also evident in the pretest phase of the conditioning experiment in the light compared to the dark. The learning and memory score during the training and post-test phases were statistically indistinguishable in the presence or absence of a lit chamber end. (B,C) Mutant dnc1 and rut2080 flies did not show a preference for the lit half of the chamber. Mutant dnc1 and rut2080 flies had statistically lower training and post-test performance compared to CS flies. The exception was the training performance of dnc1 flies in the dark. N = 16 trials for CS in each of the conditions; N’s = 8 trials for dnc1 and rut2080 in each of the conditions. Values are presented as means and error bars are SEMs.
Mutant dnc1 and rut2080 flies do not show a light preference, and have a deficit in training and post-test performance. In the no-conditioning experiments, dnc1 mutant flies show no preference for the lit end of the chamber (Fig. 3B). The dnc1 mutant flies only had a significantly reduced training score in the presence of light compared to those of wild-type flies, and strongly reduced post-test scores in both dark and light conditions. The rut2080 mutant flies also showed no preference for the lit end of the chamber (Fig. 3C). The rut2080 flies also showed a significantly lower training and post-test score compared to those of wild-type flies during the training and post-test phases of the conditioning experiments. Thus, at least under these conditions, dnc1 and rut2080 flies do not show an obvious light preference, and have a characteristic lower training and post-test score.
We examined activity levels in the pretest phase for all genotypes and conditions. In this case, there was some variation in activity between the different conditions for wild-type and mutant flies (Table 2). The presence or absence of a light cue did not consistently alter activity levels. The rut2080 mutant flies showed inconsistent differences with wild-type flies. In the dark, rut2080 mutant flies had significant higher activity level in the no-conditioning experiments, but not in the conditioning experiment. Similarly, in the light rut2080 mutant flies had a significantly higher activity level in the no-conditioning experiment but not in the conditioning experiment. Since the pretest activity levels in the conditioning and no-conditioning experiments are essentially identical with respect to the temperature and temperature change contingencies, the significant differences are likely without impact on interpretation of training, post-test, and spontaneous place preference behaviors in the presence and absence of a light cue. The dnc1 mutant flies’ activity levels were not significantly different from those of wild-type levels in any condition.
Table 2.
Activity differences between CS, dnc1, and rut2080 flies in the pretest phase of the phototaxis experiments
Potential gravitational preference interaction with learning and memory
Finally, we examined how a gravitation cue might influence native preferences and interact with conditioned behavior. The heat-box was either not tilted, or tilted at 7.8° and 15.6° from the horizon, positioning the front of the chamber higher than the back. In all conditioning experiments the front, elevated end, of the chamber was associated with 41°C. The no-conditioning group similarly “associated” the front half of the chamber with a baseline temperature of 24°C. In this control group, the chambers were treated in exactly the same fashion as the conditioning group, but the chamber temperature never changed.
Flies show no effect of an incline in the range tested on group average spontaneous preference, but show an interaction with place memory. Wild-type CS flies had an average place preference score of near zero in the three groups, 0°, 7.8°, and 15.6° in the “no-conditioning” group (Fig. 4A). This shows that as a group, flies preferred neither the higher nor the lower ends of the chamber. In the conditioning group, CS flies had similar training performance at all angles from the horizon, but in the post-test phase flies trained against the higher end showed a significantly higher post-test performance level. Both the dnc1 and rut2080 flies showed no average preference for the elevated chamber end, similar to wild-type flies (Fig. 4B,C). The training score was usually lower than the wild-type levels, the exception being dnc1 flies at 0 degrees from the horizon. In all cases, the post-test score of the mutant flies was significantly different from the post-test scores of CS flies. Remarkably, and in contrast to the CS flies, there was no post-test phase advantage of training with the incline. Thus, in addition to the typically reduced post-test score of dnc1 and rut2080 flies, these mutant flies also fail to take advantage of the gravity vector to improve post-test performance.
Figure 4.
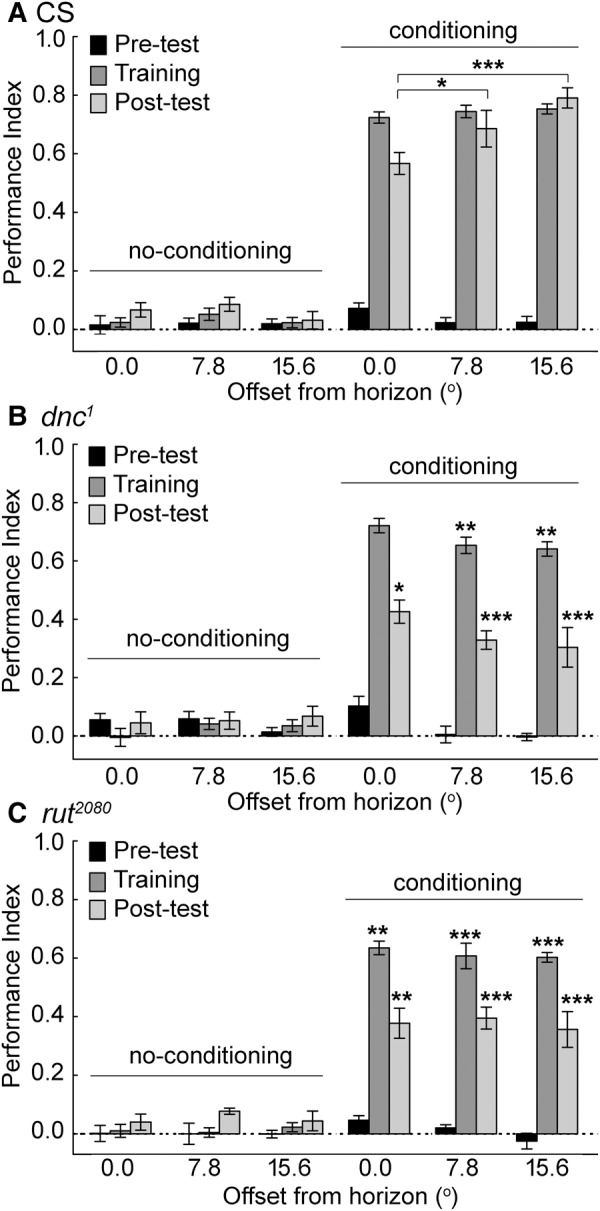
Wild-type CS, dnc1, and rut2080 flies were presented with gravitaxis cues and trained against a potential preference. (A) There were no obvious group average preferences for a chamber end that was raised up to 15.6°. Conditioning, however, led to high training and post-test scores compared to no-conditioning (Wilks λ = 0.0184 F(51,509.9) = 28.3, P < 0.00001 for all groups and conditions. Duncan post hoc tests with significant differences are represented, (*) P < 0.05; (**) P < 0.01; (***) P < 0.001). The post-test scores were higher with 15.6° compared to the 0.0°, and 7.8° compared to the 0.0°, conditions. (B) Mutant dnc1 flies showed no obvious preference for an elevated chamber end. The training and post-test performance was lower in dnc1 flies compared to CS flies, with the exception of the training score in control conditions. (C) Training and Post-test scores were lower in rut2080 flies compared to CS levels tested under the same conditions. N = 16 trials for CS in each of the conditions; N’s = 8 trials for dnc1 and rut2080 in each of the conditions. Values are presented as means and error bars are SEMs.
We also examined activity levels in the pretest phase for all genotypes and conditions. In this case, there was some variation in activity between the different conditions, but no significant differences were identified between flies of the different genotypes when compared within specific conditions (Table 3).
Table 3.
Activity differences between CS, dnc1, and rut2080 flies in the pretest phase of the gravitaxis experiments
Discussion
A conceptual model explains the interaction of S–R behaviors with place learning and memory. We posit that a learning module provides inhibitory input to S–R based modules such that learning over-rides experience-independent behaviors. That is, cellular and circuit mechanisms of learning impinge on and suppress the systems that are critical for expression of stereotyped behavior. The stereotyped behaviors that were examined here are shifts in group average place preference with air and light. Moreover tests with a gravitaxis sensory modality suggests that cues that provide stereotyped behaviors can provide a positive input to a learning module to enhance memory performance.
In Drosophila place conditioning, learning has a remarkably strong influence on air and light-induced place preferences. Both light and air cues induced a significant group-average preference for the source of these cues, evident in place preferences that were significantly biased toward the chamber ends with the source of these stimuli (Figs. 2, 3). However, when conditioned to avoid this chamber end, learning during the training phase and memory in the post-test were statistically indistinguishable in flies with and without these cues. Thus, a learning module completely suppresses the native S–R behavior in two different cases. The sole interaction was with a gravitaxis cue and an increase in memory levels as the incline increased (Fig. 4), even though this cue did not induce a spontaneous average preference for this chamber end. This memory performance increase was not a result of increased training, since learning performance levels were similar across the different inclines. The increased memory performance was in the direction opposite of a potential native preference of moving up the chamber incline. The gravitaxis experiments suggest that nonobvious conditions can provide input to a learning module to positively influence place memory levels.
What influences the increased memory performance of flies in the presence of a gravitaxis cue, but completely suppresses light or air cue induced preferences? Even though light and air sources provide attractive cues to wild-type flies, this preference was completely abolished by learning conditions. Thus, it appears that the high-temperature reinforced place memory mechanisms can readily over-ride mechanisms for these attractions. Perhaps it is important that the gravitaxis cue was also the only cue that did not influence a spontaneous preference. It seems that in place conditioning with obvious cues that the suppression of the behavioral action of phototaxis and anemotaxis by conditioning is of paramount importance. In the presence of a subtle cue, flies take advantage of all information, and use the gravitational vector as an orientation aid. Place conditioning with aversive temperatures could be using very different mechanisms that impinge on the experience-independent visual- and air-dependent circuits. The gravitaxis circuits could be more integrated in the neural circuits that influence place conditioning (Zars et al. 2000b; Armstrong et al. 2006; Kamikouchi et al. 2009; Zars 2010). The caveat here is that the stimulus intensities for the three cues have not been examined extensively. It may be that light brightness and air speed levels could be found that provide little or no spontaneous preference but have an impact on memory levels. Future experiments will determine if this is the case.
We find that mutation of the dnc cAMP-phosphodiesterase gene (Nighorn et al. 1991) has complex effects on learned and innate S–R behaviors. In the absence of overt sensory cues, dnc1 mutant flies never had a significant reduction in training performance, but had a significant reduction in memory performance in all cases (Figs. 2–4). In the presence of sensory cues, both the training and memory scores were significantly lower in all cases. The variable effect on place learning but overall reduction in memory performance is consistent with previous findings (Wustmann et al. 1996). Moreover, in all cases dnc1 mutant flies did not display an average preference for any discrete cue, including light and air source experiments. The lack of a phototactic response in dnc1 mutant flies in the current conditions, with fairly low-light levels and in a narrow chamber, are in contrast to other results in which these flies have a normal attraction to light but a specific deficit in learning (e.g., Dissel et al. 2015). The specific low-light conditions in the current experiments are likely responsible for these differences. The dnc1 mutant flies also did not show an advantage in the memory score in the presence of potential gravitational cues. In our simple model, a learning module negatively influences nonconditioned behaviors. In the case of dnc1 mutant flies, the learning module is altered such that the input on naïve behaviors is less influential.
Mutation of the rut adenylyl cyclase (Levin et al. 1992) is largely restricted to learning and memory phenotypes. In the absence of overt sensory cues, rut2080 mutant flies have significantly lower learning and memory scores in all cases (Figs. 2–4). Moreover, in the presence of overt cues, learning and memory scores are significantly lower in nearly all of the cases, the exception being in the presence of an air cue. The rut2080 mutant flies only showed a consistent preference for an air source, and there was no effect of a potential gravitational cue on memory performance. As with dnc1 flies, rut2080 flies showed an enhanced reaction to the air stimulus in terms of locomotor activity reduction (Table 1). Thus, although spontaneous preferences were not readily detected in most cases by rut2080 flies, it seems likely from the results that the primary deficit in these flies is in the associative process, the learning module, which is consistent with previous views for the function of this gene (Dudai 1985; Levin et al. 1992; Zars et al. 2000a; Schwaerzel et al. 2002; Gervasi et al. 2010).
The interaction of gravitaxis cues with place conditioning and the suppression of light and air source preferences add to a few examples of integration across different sensory modalities in Drosophila (Guo and Guo 2005; Chow and Frye 2008; Seugnet et al. 2008; Gaudry et al. 2012; Zhang et al. 2013; van Breugel and Dickinson 2014; Wasserman et al. 2015). Flies can use visual feedback in a flight simulator to better orient toward a virtually local attractive odor source (Chow and Frye 2008; Wasserman et al. 2015). Moreover, in cross-modal and sensory preconditioning learning experiments with visual and odor cues, a clear interaction of visual and olfactory information can influence learned behaviors (Guo and Guo 2005; Zhang et al. 2013). That is, flies both show an enhanced conditioned avoidance of a visual or olfactory cue when the two cues that were conditioned were suboptimal and that flies can be trained to avoid one cue if it has been preassociated with a second cue. These studies suggest that the visual and olfactory neural systems converge at some level to influence action selection and ongoing behavior. We see a similar interaction with gravitaxis and place memory (Fig. 4). Moreover, examination of chemotactic behavior in Drosophila larvae with paired and unpaired conditioning experiments suggests that learning can influence native S–R behaviors within a sensory modality (Schleyer et al. 2015). In adult flies examined with a quinine conditioned suppression of light preference (the APS paradigm), a few paired experiences with the aversive gustatory cue is enough to suppress the light preference in a light/dark choice point. The effect of this conditioning is short-lived, with flies going back to a light preference quickly after the quinine contingency is removed (Seugnet et al. 2008, 2009). We see a similar profile with the suppression of light and air source cues (Figs. 2, 3). The difference here is that the suppression is longer lived, lasting at least 3 min.
The results here represent the first attempts to examine potential interactions of overt sensory cues and place learning and memory in behavioral action selection. Potential for interaction was tested with three sensory stimuli and place learning and memory using high-temperature aversive conditioning. Wild-type CS flies show an unexpected enhancement of memory in the presence of a gravitaxis cue, even though these flies do not show an influence of this cue in average preferences. Wild-type flies completely suppress light and air source preferences in place learning and memory. Mutation of the dnc1 and rut2080 flies show complex phenotypes, with the rut2080 mutant flies consistently showing learning and memory deficits.
Materials and Methods
Drosophila treatment
Drosophila melanogaster were raised on cornmeal-based fly food media and maintained on a 12 h/12 h day/night cycle at 24°C and 60% relative humidity. For behavioral experiments wild-type Canton S (CS) flies at the age of 2–5 d were used. Prior to the behavioral experiments flies were provided 16–24 h on new fly food. The Canton S flies stem from the Martin Heisenberg laboratory stocks. The rutabaga adenylyl cyclase (rut2080) has a P-element insertion near the gene and has been described as a null allele. The dunce cAMP phosphodiesterase (dnc1) was isolated from an EMS mutagenesis and is thought to be a hypomorphic allele. Both are in a Canton S background (Nighorn et al. 1991; Levin et al. 1992; Crittenden et al. 1998).
Behavioral experiments
Place learning and memory was tested using the heat-box apparatus. The heat-box consists of multiple rectangular chambers in which single flies are allowed to walk freely back and forth (Zars 2009, 2010; Ostrowski and Zars 2014). The position of a single fly within each chamber is recorded throughout an experiment at 10 Hz and a resolution of 0.2 mm. Fast temperature changes within the chambers are provided by Peltier-elements on top and bottom. A computer coordinates rising temperatures with position of the fly. Before each training session flies are provided a pretest phase (60 sec) at constant 24°C to determine any potential spontaneous side preference. During conditioning (the training phases) one chamber half is defined as the side associated with high temperature and the other as not. Every time the fly enters the high temperature associated side the whole chamber heats up to an aversive temperature (41°C). The return of the fly to the other side quickly cools down the chamber to a nonaversive temperature (24°C) (Sayeed and Benzer 1996; Zars 2001). The training phase was for 10 min. The following 3-min post-test measures place preference while the chamber is kept at the same nonaversive temperature. A PI is calculated by the difference in time a fly spent in either chamber half (unpunished side versus punished side) divided by the total time within a pretest, training, or post-test session. The PI can vary from 1.0 to −1.0. Zero indicates that on average the flies spent equal time on both sides of the chamber, whereas 1.0 shows a perfect side preference of the fly for the unpunished chamber half.
Flies were exposed to one of three stimuli that could act as attractive cues. In the first case, a localized air source was used. In this case, the air supply that is traditionally used to expel the flies was slightly opened to allow an airflow of ∼0.7 m/sec during the experiment. Conditioning associated the back, air-source containing chamber end with 41°C during training. The second case used a light source. The traditional opaque stoppers were replaced with translucent stoppers and the fluorescent room light was left on, providing approximately 200 lux light. Conditioning associated the front, brighter chamber end with 41°C during training. Finally, the chambers were put on an incline of 7.8 or 15.6° from the horizon. The control situation was no incline. Conditioning associated the front, higher chamber end with 41°C during training.
Data analysis
Position of flies within the chambers is recorded by a custom-made program and spatial preference (PI) of individual flies during all phases of an experiment are automatically calculated. Flies that were inactive during pretest or did not experience heat during training were automatically discarded in all experiments. Average PIs were calculated for each of the 8 to 16 experiments per genotype and experiment type. Data from two sets of eight experiments were combined for the wild-types flies since results were consistent between the experiments. The mean values were used in parametric ANOVA and Duncan's post hoc tests. Inspection of residual versus expected normal values indicate that the data are normally distributed, as is typical for this type of analysis (Tully and Quinn 1985; Zars et al. 2000a). Data are shown as mean ± SEM. For statistical analysis Statistica 8 (StatSoft) was used.
Acknowledgments
Research in the laboratory of T.Z. is supported by NIH grant NS076980 and NSF grants 1535790 and 1654866. Helpful comments and discussion with Martin Heisenberg, Reinhard Wolf, and Bertram Gerber are gratefully acknowledged.
Author contributions: Experimental design by V.B., A.M., P.S., and T.Z. V.B., A.M., A.L.K., and A.O.R. performed experiments. Data collection and analysis by V.B., A.M., A.L.K., A.O.R., and T.Z. Writing of manuscript by V.B., A.M., A.L.K., A.O.R., P.S., and T.Z.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.046136.117.
References
- Armstrong JD, Texada MJ, Munjaal R, Baker DA, Beckingham KM. 2006. Gravitaxis in Drosophila melanogaster: a forward genetic screen. Genes Brain Behav 5: 222–239. [DOI] [PubMed] [Google Scholar]
- Benzer S. 1967. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci 58: 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budick SA, Reiser MB, Dickinson MH. 2007. The role of visual and mechanosensory cues in structuring forward flight in Drosophila melanogaster. J Exp Biol 210: 4092–4103. [DOI] [PubMed] [Google Scholar]
- Chow DM, Frye MA. 2008. Context-dependent olfactory enhancement of optomotor flight control in Drosophila. J Exp Biol 211: 2478–2485. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. 1998. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem 5: 38–51. [PMC free article] [PubMed] [Google Scholar]
- Dissel S, Angadi V, Kirszenblat L, Suzuki Y, Donlea J, Klose M, Koch Z, English D, Winsky-Sommerer R, van Swinderen B, et al. 2015. Sleep restores behavioral plasticity to Drosophila mutants. Curr Biol 25: 1270–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. 1985. Some properties of adenylate cyclase which might be important for memory formation. FEBS letters 191: 165–170. [DOI] [PubMed] [Google Scholar]
- Foucaud J, Burns JG, Mery F. 2010. Use of spatial information and search strategies in a water maze analog in Drosophila melanogaster. PLoS One 5: e15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudry Q, Nagel KI, Wilson RI. 2012. Smelling on the fly: sensory cues and strategies for olfactory navigation in Drosophila. Curr Opin Neurobiol 22: 216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi N, Tchénio P, Preat T. 2010. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron 65: 516–529. [DOI] [PubMed] [Google Scholar]
- Guo J, Guo A. 2005. Crossmodal interactions between olfactory and visual learning in Drosophila. Science 309: 307–310. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. 2015. Outcome learning, outcome expectations, and intentionality in Drosophila. Learn Mem 22: 294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahsai L, Zars T. 2011. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol 99: 139–167. [DOI] [PubMed] [Google Scholar]
- Kain JS, Stokes C, de Bivort BL. 2012. Phototactic personality in fruit flies and its suppression by serotonin and white. Proc Natl Acad Sci 109: 19834–19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, Ito K. 2009. The neural basis of Drosophila gravity-sensing and hearing. Nature 458: 165–171. [DOI] [PubMed] [Google Scholar]
- LaFerriere H, Speichinger K, Stromhaug A, Zars T. 2011. The radish gene reveals a memory component with variable temporal properties. PLoS One 6: e24557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourg E, Buecher C. 2002. Learned suppression of photopositive tendencies in Drosophila melanogaster. Anim Learn Behav 30: 330–341. [DOI] [PubMed] [Google Scholar]
- Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. 1992. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell 68: 479–489. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Deshazer M, Davis RL. 2005. Thirty years of olfactory learning and memory research in Drosophila melanogaster. Prog Neurobiol 76: 328–347. [DOI] [PubMed] [Google Scholar]
- McMillan PA, McGuire TR. 1992. The homeotic gene spineless-aristapedia affects geotaxis in Drosophila melanogaster. Behav Genet 22: 557–573. [DOI] [PubMed] [Google Scholar]
- Morris RG. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Meth 11: 47–60. [DOI] [PubMed] [Google Scholar]
- Nighorn A, Healy MJ, Davis RL. 1991. The cyclic AMP phosphodiesterase encoded by the Drosophila dunce gene is concentrated in the mushroom body neuropil. Neuron 6: 455–467. [DOI] [PubMed] [Google Scholar]
- Ofstad TA, Zuker CS, Reiser MB. 2011. Visual place learning in Drosophila melanogaster. Nature 474: 204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski D, Kahsai L, Kramer EF, Knutson P, Zars T. 2015. Place memory retention in Drosophila. Neurobiol Learn Mem 123: 217–224. [DOI] [PubMed] [Google Scholar]
- Ostrowski D, Zars T. 2014. Place Memory. In Handbook of behavior genetics of Drosophila melanogaster: behavioral phenotypes and models of neurobehavioral disorders (ed. Dubnau J), pp. 125–134. Cambridge University Press, Cambridge. [Google Scholar]
- Sayeed O, Benzer S. 1996. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci 93: 6079–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M, Reid SF, Pamir E, Saumweber T, Paisios E, Davies A, Gerber B, Louis M. 2015. The impact of odor-reward memory on chemotaxis in larval Drosophila. Learn Mem 22: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M, Heisenberg M, Zars T. 2002. Extinction antagonizes olfactory memory at the subcellular level. Neuron 35: 951–960. [DOI] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Stidd R, Shaw PJ. 2009. Aversive phototaxic suppression: evaluation of a short-term memory assay in Drosophila melanogaster. Genes Brain Behav 8: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. 2008. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol 18: 1110–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Zars T. 2010. Lack of prediction for high-temperature exposures enhances Drosophila place learning. J Exp Biol 213: 4018–4022. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. 1985. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A 157: 263–277. [DOI] [PubMed] [Google Scholar]
- van Breugel F, Dickinson MH. 2014. Plume-tracking behavior of flying Drosophila emerges from a set of distinct sensory-motor reflexes. Curr Biol 24: 274–286. [DOI] [PubMed] [Google Scholar]
- Wasserman SM, Aptekar JW, Lu P, Nguyen J, Wang AL, Keles MF, Grygoruk A, Krantz DE, Larsen C, Frye MA. 2015. Olfactory neuromodulation of motion vision circuitry in Drosophila. Curr Biol 25: 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wustmann G, Heisenberg M. 1997. Behavioral manipulation of retrieval in a spatial memory task for Drosophila melanogaster. Learn Mem 4: 328–336. [DOI] [PubMed] [Google Scholar]
- Wustmann G, Rein K, Wolf R, Heisenberg M. 1996. A new paradigm for operant conditioning of Drosophila melanogaster. J Comp Physiol A 179: 429–436. [DOI] [PubMed] [Google Scholar]
- Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, Ito K, Anderson DJ. 2009. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature 458: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T. 2001. Two thermosensors in Drosophila have different behavioral functions. J Comp Physiol A 187: 235–242. [DOI] [PubMed] [Google Scholar]
- Zars T. 2009. Spatial orientation in Drosophila. J Neurogenet 23: 104–110. [DOI] [PubMed] [Google Scholar]
- Zars T. 2010. Short-term memories in Drosophila are governed by general and specific genetic systems. Learn Mem 17: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zars T, Fischer M, Schulz R, Heisenberg M. 2000a. Localization of a short-term memory in Drosophila. Science 288: 672–675. [DOI] [PubMed] [Google Scholar]
- Zars T, Wolf R, Davis R, Heisenberg M. 2000b. Tissue-specific expression of a type I adenylyl cyclase rescues the rutabaga mutant memory defect: In search of the engram. Learn Mem 7: 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ren Q, Guo A. 2013. Parallel pathways for cross-modal memory retrieval in Drosophila. J Neurosci 33: 8784–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]


