Abstract
Statement of the Problem:
A great challenge in periodontal therapy is the regeneration enhancement of osseous defects through applying osteoinductive materials. Demineralized freeze-dried bone allograft (DFDBA) has already been introduced as an allograft with osteoconductive and variable osteoinductive properties. Calcium hydroxide [Ca(OH)2] is an available well-known material in dentistry, which induces hard tissue formation.
Purpose:
This study evaluated the efficiency of combination of DFDBA and Ca(OH)2 in improving the quality of osteoinduction of DFDBA.
Materials and Method:
Human bone marrow-derived mesenchymal stem cells were taken from volunteers’ iliac crest. Cell proliferation was determined by MTT test at 18, 24 and 48 hours post-culture in 10 groups. The employed material were 0.5, 1.0 mg/ml Ca(OH)2 in two forms of suspension and pH-adjusted solution, 10mg/ml DFDBA per se and in combination with 0.5 and 1.0 mg/ml Ca(OH)2. Mineralization was assessed by Alizarin red staining in 10 mg/ml DFDBA, DFDBA+ 0.5 and 1 mg/ml Ca(OH)2 in solution and suspension forms. The data were statistically analyzed by using one-way ANOVA and Tukey’s post-hoc test (p< 0.05).
Results:
The pH-adjusted solutions exhibited better cell proliferation compared with the suspension groups. The combination of 0.5mg/ml Ca(OH)2 solution and DFDBA increased the cell proliferation and mineralization compared with DFDBA per se (p= 0.033).
Conclusion:
The combination of Ca(OH)2 with DFDBA improved the osteoinductivity of DFDBA.
Keywords: Calcium Hydroxide , Stem Cells , Allografts , Bone Regeneration
Introduction
Periodontitis is an inflammatory disease initiated by the presence of bacterial plaque, leading to the destruction of periodontium (periodontal ligament, alveolar bone and cementum) and eventually tooth loss.[1] Regenerating the periodontal defects as well as the osseous reconstruction of edentulous ridge to place the implants is a major challenge in modern dentistry and can have great influence on the patient’s aesthetics, chewing ability and quality of life. Several approaches have been introduced to improve the reconstruction of alveolar bone defects.[2-3]
The process of periodontal and bone regeneration is generally complicated by different types of present cells. Active regeneration is valuable through induction of purposed cell population to differentiate or migrate to the defect area, which is the migration of bone progenitor cells to the injured site and differentiation of stem cells to osteoblasts that leads to the regeneration of bone structure.[4] Also, in the reconstruction of large bony defects, there should be a graft material to provide both scaffold and active signaling to the bone marrow mesenchymal stem cells (MSCs) inducing them to migrate and differentiate.[4-5]
Among bone replacement grafts, autograft is considered the gold standard. [6] They are the only bone grafts that provide all the three properties of an ideal graft material including osteogenesis (to provide vital osteoblasts), osteoinduction (induction of osteoprogenitor cells to differentiate to osteoblasts) and osteoconduction (to provide scaffold).[6] However, owing to the low level of patient acceptance of autografts, allografts (grafts from the same species) have become the most popular bone grafts in oral surgery. Allografts are being processed as fresh-frozen or freeze-drying.[6]
Demineralized freeze-dried bone allograft (DFDBA) is a type of allograft claimed to be superior because of the osteoconductive and osteoinductive properties. The osteoinductivity is obtained through demineralization of the graft material and exposure of the bone morphogenetic proteins (BMPs). These are a group of growth factors inducing MSCs differentiation into bone-producing osteoblast cells in bone matrix.[7-8] Therefore, the demineralization is critical for osteoinductive properties of DFDBA, since it provides adequate levels of BMPs and does not allow the mineral content to drop to very low levels.
It is believed that a certain concentration of Ca2+ ions is needed for the osteoinductive properties of DFDBA; therefore, addition of Ca2+ ions might provide the necessary Ca2+ ions for the optimum function of these allografts.[6,9] Moreover, other factors such as the donor’s age make the osteoinductivity of DFDBA variable and unpredictable.[10] Therefore, the recent studies aimed at improving the active bone and periodontal regeneration investigated the addition of different growth factors such as emdogain or rh-PDGF to DFDBA. It was noted that although such additives improved the regeneration, these growth factors are expensive and not readily accessible.[11-12]
In contrast, calcium hydroxide, [Ca(OH)2] with hard tissue formation and antimicrobial properties, is a readily accessible material and quite well known in dentistry, particularly in endodontics, for pulp capping or apexification. Furthermore, Ca(OH)2 is a strong base with low solubility in water that forms suspension in aqueous environment and releases hydroxyl and calcium ions over time. Most properties of the Ca(OH)2 come from this ionic dissociation.[13]
Based on in vitro studies, Ca(OH)2 increases the recruitment, migration, and proliferation of periodontal ligament (PDL) stem cells and promotes the mineralization and cementogenesis. However, scholars do not have consensus over its efficiency mainly because it is dose-dependent.[14-15] Paula-Silva et al.[15] in their in vitro study found that low dosage of Ca(OH)2 did not influence the induction of mineralization and high dosage was cytotoxic to cells.[15] Bone marrow-derived MSCs are a group of multipotent stem cells that are sensitive to their local environment and differentiate into different types of cells including periodontal ligament-like or alveolar bone cell types.[16]
The present study evaluated the effect of adding different doses of Ca(OH)2 in both solution and suspension forms to DFDBA on viability, proliferation and differentiation of human bone marrow-derived mesenchymal stem cells (hBM-MSCs) into osteoblasts. The significance of this study is that Ca(OH)2 is inexpensive, abundant and easy to obtain compared with other materials. To the best of the authors’ knowledge, this is the first study carried out on this issue. Moreover, the controversies about the osteoinductivity of DFDBA encouraged the evaluation of in vitro behavior of hBM-MSCs in the presence of DFDBA (Cenobone, Demineralized Cortical Cancellous Powder; Tissue Regeneration Corporation Co., Kish, Iran) as a popular allograft material routinely used in periodontal and osseous reconstructive surgeries.
Materials and Method
Isolation and culture of human MSCs
Human MSCs were obtained from 5 ml bone marrow aspirated from iliac crest of normal donors within the age range of 19-45 years. They were donors of bone marrow to relatives after obtaining approval of Ethic Committee. Written informed consent was also taken to permit the analysis of the clinical data. Bone marrow-derived MSCs were isolated from mononuclear cell (MNC) layer using our previously method[17] which is briefly mentioned.
Each aspirated sample was diluted (1:1) with Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen; Merelbeke, Belgium) containing 10% fetal bovine serum (FBS), 1% penicillin, 1% streptomycin, and 2 mM glutamine as the basal DMEM media. The cells were layered over about 5 ml of Ficoll (Lymphoprep; Oslo, Norway), then centrifuged at 338 g for 15 min to obtain MNC layer. The MNC layer was seeded on culture flasks, and maintained at 37°C in 5% CO2 atmosphere. In order to obtain the MSCs cells, the adhered monolayer cells was detached and expanded for successive passages, and characterized.
Characterization of human MSCs
The cells viability was analyzed by using trypan blue staining. Flow cytometric analysis for detection of MSC-morphologic markers was also achieved following the method used by Ayatollahi et al.[18]
Proliferation assay
The two types of employed Ca(OH)2 were suspension (non-pH-adjusted) and solution (pH-adjusted). For the suspension groups, 50 and 100mg Ca(OH)2 (Merck; Germany) were added to 10 ml normal saline, yielding 5- and 10-mg/ml concentration of Ca(OH)2. Then, they were diluted in the culture medium in a 1:10 ratio to obtain 0.5- and 1-mg/ml doses of Ca(OH)2. In the solution group, the powder was first dissolved in HCl, then, NaOH was added and the pH was adjusted to 7.4 via a pH meter.
Accordingly, 10 designed study groups were as follows; group 1: control group (MSCs in culture medium with no treatment), group 2: 0.5mg/ml Ca(OH)2 solution, group 3: 0.5mg/ml Ca(OH)2 suspension, group 4: 1mg/ml Ca(OH)2 solution, group 5: 1mg/ml Ca(OH)2 suspension, group 6: 10mg/ml DFDBA, group 7: 10mg/ ml DFDBA+ 0.5mg/ml Ca(OH)2 solution, group 8: 10 mg/ml DFDBA+ 0.5mg/ml Ca(OH)2 suspension, group 9: 10mg/ml DFDBA+ 1mg/ml Ca(OH)2 solution and group 10: 10mg/ml DFDBA+ 1mg/ml Ca(OH)2 suspension. Each experiment was repeated 4 times.
Cell proliferation in each of the above groups was assessed by using Thiazolyl Blue Tetrazolium bromide (MTT) (Sigma-Aldrich Co.; St. Louis, MO, USA) assay. The hBM-MSCs (5.8×10³) were cultured with 200µL of DMEM containing 10% FBS. After 3 days of culture, treatment in the mentioned groups was done for 18, 24 and 48 hours. Finally, MTT assay was conducted via 0.5 mg/ml 3-((4,5-dimethyl thiazole-2-yl)-2,5-diphe-nyl tetrazolium) bromide for 3 hours. Following the formation of formazan solution in DMSO (dimethyl sulfoxide), the absorbance of the solution was measured by an absorbance microplate reader at 570 and 630nm as the reference wavelength (BioTek Instruments Inc.;USA).
Osteogenic differentiation assay
The potential of human MSCs to differentiate into osteogenic lineages was examined in the presence of osteogenic media in four groups by using Alizarin red staining (Sigma-Aldrich Co., USA) within 5, 12 and 14 days after staining. The groups were group 1: MSCs in osteogenic media containing DFDBA (10mg/ml), group 2: MSCs in osteogenic media containing 0.5mg/ml Ca(OH)2 (solution)+ DFDBA 10mg/ml, group 3: MSCs in osteogenic media containing 0.5mg/ml Ca(OH)2 (suspension)+ DFDBA 10mg/ml, and group 4: MSCs in osteogenic media (positive control).
The osteogenic media consisted of basal DMEM media supplemented with 10 M/L dexamethasone, 10 mM/L glycerol phosphate, 3.7 g/L sodium bicarbonate and 0.05 g/L ascorbic acid (all from Sigma-Aldrich; St. Louis, USA). The osteogenesity was assessed by Alizarin red staining. The morphologic differentiation of cells and red mineralized nodule formation were observed by using microscope (Nikon) under 10×40 magnification.
Statistical analysis
The triplicate samples obtained from different cases were analyzed in duplicate. The statistical analyses were performed by using SPSS software, version 16 (IBM; USA). One-way ANOVA was used to analyze the data. Tukey’s post-hoc test was used for pair-wise comparisons between the study groups. p< 0.05 was considered to be statistically significant. Alizarin red staining results were presented qualitatively.
Results
Characterization of human MSCs
The rapidly grown fibroblast-like cells, which exhibited homogeneous morphology, were selected for culture expansion after inverted microscopic evaluation (Figure 1a). The viability assessed by trypan blue staining analysis showed 98-100% viability in human MSCs. The flow cytometry determined the surface phenotype to be positive for CD90 and CD73. Additionally, no cell expressed the hematopoietic markers CD45 and CD34 (Figure 1).
Figure1.

Characterization of human mesenchymal stem cells. Relatively homogenous fibroblast-like cells were obtained within 9-10 days after cell culture [A]. The surface phenotypic markers were positive for CD90, and CD73. Additionally, no cells expressed the hematopoietic markers CD45, CD34. The shaded area shows the profile of the negative control.
Cell proliferation analysis
There was a significant interaction effect between the time and group, i.e., the culture duration and material concentrations significantly affected the cellular proliferation (p< 0.001). The cell proliferation was compared among the groups at each time point of 18, 24 and 48 hours as demonstrated in Table 1.
Table 1.
Bone morrow-derived mesenchymal stem cells viability in different study groups (significant differences have been marked)
| Group | Time (hr) | ||
|---|---|---|---|
| 18h | 24h | 48h | |
| 1 | 0.98 ± 0.10a | 0.96 ± 0.07a,b | 0.96 ± 0.05a,b,c |
| 2 | 0.8 ± 0.06a | 0.91 ± 0.06a,b | 0.93 ± 0.04a,b,c |
| 3 | 0.88 ± 0.07a | 0.74 ± 0.13b | 0.76 ±0.05b,d,f |
| 4 | 0.78 ± 0.02a | 0.90 ± 0.05a,b | 0.93 ± 0.01a,b,c |
| 5 | 0.91 ± 0.05a | 0.17 ± 0.02a | 0.14 ± .03a,c |
| 6 | 0.86 ± 0.08a | 0.92 ± 0.02a,b | 0.90 ± 0.04a,b,d |
| 7 | 0.84 ± 0.17a | 0.86 ± 0.13a,b | 1.04 ± 0.05a,b,c,e |
| 8 | 0.78 ± 0.04a | 0.73 ± 0.22b | 0.82 ± 0.06a,b,c,d,f |
| 9 | 0.86 ± 0.09a | 0.98 ± 0.04a,b | 1.00 ± 0.10a,b,c,e |
| 10 | 0.39 ± 0.08 | 0.53 ± 0.16b | 0.66 ± 0.05b,c,d,e,f |
p< 0.05 as compared with group 10, 5, 3, 7, 8 and 9 respectively
After 18 hours treatment
After 18 hours of treatment, the cell proliferation was significantly lower in the group 10 compared with the other groups, which were not significantly different from the control group.
After 24 hours treatment
The cell proliferation in groups 5 and 10 was significantly lower than the control group. 1-mg/ml Ca(OH)2 solution yielded significantly higher cell proliferation compared with the suspension form. Furthermore, cell proliferation was significantly lower in 1 mg/ml Ca(OH)2 suspension than in 0.5 mg/ml Ca(OH)2 suspension. Compared with 1 mg/ml Ca(OH)2 suspension per se, the combination of 1 mg/ml Ca(OH)2 suspension and DFDBA significantly increased the cell proliferation. However, the cell proliferation in DFDBA was higher than its combination with 1 mg/ml Ca(OH)2 suspension.
After 48 hours treatment
The above-mentioned differences were still present among the groups. Both 0.5 and 1 mg/ml Ca(OH)2 suspensions and DFDBA+ 1mg/ml Ca(OH)2 suspension exhibited significantly lower proliferation in comparison with the control group. Both 0.5 and 1mg/ml Ca(OH)2 solutions exhibited significantly higher cell proliferation than the same doses in suspension form. Furthermore, the combination of DFDBA with 0.5 mg/ml, Ca(OH)2 solution significantly increased the cell proliferation in comparison with DFDBA per se. The cell proliferation in groups 7 and 9 showed an ascending trend from 18 to 48 hours to reach to a level higher than the control group but the difference was not statistically significant (Figure 2 and Figure 3).
Figure2.
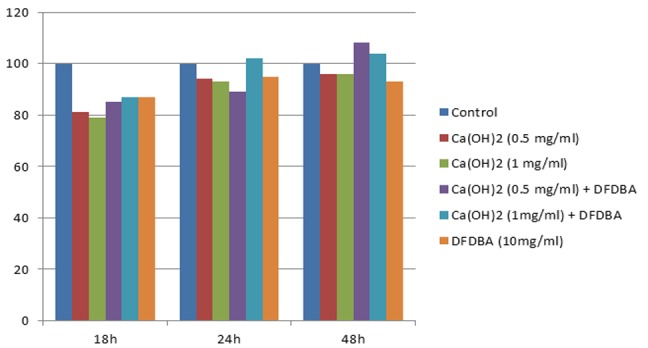
percentage change of proliferation and viability in different groups as compared with the control in different time points. DFDBA per se, Ca(OH)2 solution per se, and in combination with DFDBA
Figure3.
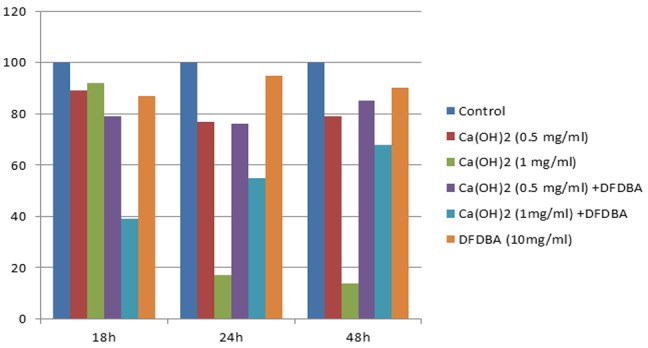
percentage of cell proliferation and viability in different groups in comparison with the control in different time points. DFDBA per se, Ca(OH)2 suspension per se and in combination with DFDBA
Osteogenic differentiation assay
Qualitatively, Alizarin red staining results showed osteogenic differentiation in BM-MSCs in four designed groups. The morphologic differentiation of cells and red mineralized nodule formation were observed by microscope. It was noted that only MSCs in osteogenic media containing Ca(OH)2 in the solution group qualitatively exhibited mineralization in comparison with Ca(OH)2 in suspension form and DFDBA groups (Figure 4).
Figure4.

Osteogenic differentiation assay. Alizarin red staining results qualitatively showed osteogenic differentiation in marrow-derived mesenchymal stem cells in four designed groups. a: The red mineralized nodule formation were observed in osteogenic media containing DFDBA, b: Ca(OH)2 solution + DFDBA, c: Ca (OH)2 suspension + DFDBA. d: The MSCs in osteogenic media was cultured as positive control.
Discussion
Ca(OH)2 suspension in 1.0 mg/ml dose was cytotoxic to the cells and significantly decreased the cell proliferation compared with the baseline. Combination of 1 mg/ml Ca(OH)2 suspension with DFDBA increased the viability of cell proliferation. This was probably due to the buffering ability of DFDBA through a reduction in Ca(OH)2 pH.
Narita et al.[19] demonstrated that dentine powder and hydroxyapatite (both comprised of mineralized compounds similar to DFDBA) had buffering effect on Ca(OH)2; this is beneficial when using a mixture of DFDBA and Ca(OH)2.
The results of the present study were consistent with some previous studies. Narita et al.[19] used different doses of Ca(OH)2 at different pH levels. They concluded that high dose of Ca(OH)2 (2.5 mg/ml) was cytotoxic to the primary osteoblast cells (in both pH-adjusted and non-pH-adjusted groups); whereas, in lower doses (0.25 and 0.025mg/ml), proliferation was comparable to the control group. In addition, 0.25mg/ml Ca(OH)2 had better mineralization potency and this was significantly higher for 7.4 pH in comparison to 8.5 pH.[19] Paula-Silva et al.[15] evaluated the effect of different concentrations of Ca(OH)2 on cementoblastic differentiation of PDL mesenchymal cells, observed that Ca(OH)2 with the concentration below 15mM (1.1mg/ ml) led to cell viability of more than 90%. A 10-mM (0.74mg/ml) suspension of Ca(OH)2 decreased the cell proliferation at post-culture 48-72 hours; meanwhile, it led to significant cementoblastic differentiation of PDL cells, increasing mineralization. In their study, the pH was adjusted to 7.4 by the buffering agents of culture environment and 5% CO2 incubation.[15]
Ji et al.[20] evaluated the effect of low doses of Ca(OH)2 (1-10 µg/ml) on cell proliferation of periodontal stem cells. They concluded that low doses of Ca(OH)2 positively affected the proliferation of pulp and PDL-derived stem cells. They also found that the combination of 10µg/ml Ca(OH)2 and osteogenic media increased the mineralization in vitro in comparison with each of them per se.[20] This was not consistent with the study conducted by Narita et al.[19] who found that low dose of Ca(OH)2 (0.025 mg/ml) did not influence the mineralization. This could be due to applying Ca(OH)2 with osteogenic media, as well as different in vitro conditions in different studies.
The present study, in line with Narita et al.[19] and Paula-Silva et al.,[15] showed that the pH-adjusted solution of Ca(OH)2 yielded better outcomes compared with the suspension form. They found that the Ca(OH)2- induced mineralization was strictly dose-dependent and responsible for osteoblast differentiation through providing extracellular and intracellular Ca2+ ions which led to the activation of calcium/ calmodulin pathway.[15,19]
However, previously the induction of hard tissue formation by Ca(OH)2 was mostly attributed to its alkaline pH (hydroxyl ions).
The elevated pH by Ca(OH)2 may not be a problem in pulp-capping or apexification, so that applying Ca(OH)2 adjacent to the pulpal structure leads to the necrosis (2mm), followed by an inflammation zone and eventually hard-tissue formation. Yet, the elevated pH should be considered in periodontal regeneration in order not to disturb the cell viability, migration, and differentiation.[13] The main and interesting result of the study was that combination of 0.5 mg/ml pH-adjusted solution of Ca(OH)2 and DFDBA significantly increased the proliferation and mineralization compared with the DFDBA per se. Further studies are suggested to test it in vivo.
Moghadam et al.[21] evaluated the effect of adding 0.15% by weight Ca(OH)2 to demineralized bone material (DBM) gel on bone defects in rabbit calvaria. They found no beneficial effect in adding Ca(OH)2 to DBM gel.[21] This could be because of the improper dose or form of Ca(OH)2, which is highly dose-dependent regarding both cell proliferation and induction of hard tissue formation.
In the present study, the results of cell proliferation in DFDBA group revealed that 10mg/ml concentration of DFDBA caused lower cell proliferation than the control group, and did not induce mineralization. This was in agreement with Carnes et al.’s study[22] on 2T9 cells (preosteoblastic cell line derived from transgenic mice calvariae). They reported that the exposure of these cells to DFDBA did not result in mineralization. However, exposure of these cells to soluble BMP-2 led to increased mineralization and alkaline phosphatase activity. Accordingly, they concluded that DFDBA was not likely to liberate soluble BMPs towards osteoinduction, or in vivo studies might be more appropriate to determine the osteoinductive properties.[22]
Vaziri et al.[23] in a study on the evaluation of osteoinductive property in three different commercial brands of DFDBA (one of them Cenobone- the same brand as the present study) reported that 8 and 16 mg/ml DFDBA decreased the proliferation of osteoblast-like cells. However, in contrast to the present study, they observed increased mineralization in 16mg/ml DFDBA.[23] These differences could be due to different cell lines and different doses administered.
In order to adopt the appropriate dose of DFDBA in in vitro assessment, the present study followed Vaziri et al.[23] and adopted 16mg/ml at the beginning. However, this dose resulted in very low cell viability and proliferation in hBM-MSCs. Hence, 10mg/ml was chosen according Dereka et al.’s study.[24] Other factors likely to be involved in the variability of osteoinductive property of DFDBA were the donor’s age, improper processing of DFDBA, and not exposing adequate amount of osteoinductive portions such as BMPs or inadequate Ca2+ ions remained in its structure.[9,25]
This study observed that the low solubility of Ca(OH)2 led to the formation of a suspension when mixing Ca(OH)2 with culture medium. Therefore, Ca(OH)2 was used in two forms of suspension and solution. The amount of ionic dissociation in the suspension form was different from the solution form. The ions in the suspension form were released into the environment over time in a non-linear manner; while, in the solution form, Ca(OH)2 was solved by applying acid, yielding a consistent solution with a higher level of Ca2+ ions available.
The present study showed that combination of Ca(OH)2 solution and DFDBA enhanced the osteoinductive activity. However, regarding its use in clinical conditions, especially in regeneration of osseous defects, one should consider the following issues. First, an appropriate combination is required to provide adequate Ca2+ ions during the active phase of osseous regeneration in vivo in the presence of blood and inflammatory exudate. Second, proper pH for regeneration is necessary as alterations in pH may impair the regeneration process.2 There is a specific kind of oil-based Ca(OH)2 called Osteora (previously Osteoinductal), which is introduced for periodontal purposes considered to have a milder pH increment. There are controversies about the impact of this product on improving the periodontal regeneration and whether it could release the appropriate content of Ca2+ ions in favor of increasing mineralization or not.[14,26] Therefore, the impact of adding this product to DFDBA on osteoinduction could be assessed in future studies.
Despite the promising findings of this study, it still has some limitations including the undetermined content of Ca2+ and OH- ions in different time spans in Ca(OH)2 suspension groups as well as the Ca2+ content in DFDBA group to verify the hypothesis of the study.
Despite the previously conducted studies on the properties of Ca(OH)2 in cell proliferation and osteoblast differentiation, the strength of the present study lied in evaluation of the most effective dosages of Ca(OH)2 with respect to the previous studies. Furthermore, the current study evaluated both solution and suspension forms in order to investigate different aspects of this issue and to examine the previously proposed hypotheses regarding the mechanism of mineralization induction by Ca(OH)2. Besides, the recruited cell line was hBM-MSCs, which are one of the most important cells involved in the process of bone regeneration.
Conclusion
Within the limitations of this study, it seems that the addition of 0.5mg/ml Ca(OH)2 solution to DFDBA is beneficial for improving cell proliferation as well as osteoblast differentiation compared with DFDBA per se. Owing to the availability and low price of Ca(OH)2, it is recommended that the manufacturers consider Ca2+ content of DFDBA and apply DFDBA in intraosseous defects or bone reconstruction surgeries in the combination form to provide appropriate content of Ca2+ ions.
Acknowledgement
The authors thank the Vice-Chancellery of Shiraz University of Medical Science for supporting this research (grant#93-01-03-8172). This article was based on the thesis by Dr. Nazila Lashkarizadeh (thesis# 1734). The authors would also like to thank Dr. Ehya Amalsaleh for improving English in the manuscript and Dr. Vossough from the Dental Research Development Center of the School of Dentistry for the statistical analysis.
Conflict of Interest:The authors of this manuscript certify that they have no conflict of interest.
References
- 1.Araújo AC, Gusmão ES, Batista JE, Cimões R. Impact of periodontal disease on quality of life. Quintessence Int. 2010; 41: e111–e118. [PubMed] [Google Scholar]
- 2.Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontalintrabony and furcation defects. A systematic review. Ann Periodontol. 2003; 8: 266–302. doi: 10.1902/annals.2003.8.1.266. [DOI] [PubMed] [Google Scholar]
- 3.Darby I, Chen ST, Buser D. Ridge preservation techniques for implant therapy. Int J Oral Maxillofac Implants. 2009; 24 Suppl: 260–271. [PubMed] [Google Scholar]
- 4.Yamada Y, Ueda M, Naiki T, Takahashi M, Hata KI, Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: Tissue-engineered bone regeneration. Tissue Engin. 2004; 10: 955–964. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Washio K, Iwata T, Okano T, Ishikawa I. Current status and future development of cell transplantation therapy for periodontal tissue regeneration. Int J Dent. 2012; 2012: 307024. doi: 10.1155/2012/307024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao ST, Scott DD. A review of bone substitutes. Oral Maxillofac Surg Clin North Am. 2007; 19: 513–521. doi: 10.1016/j.coms.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Miron RJ, Zhang YF. Osteoinduction: a review of old concepts with new standards. J Dent Res. 2012; 91: 736–744. doi: 10.1177/0022034511435260. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Pujic Z, Xiao Y, Bartold PM. Identification of bone morphogenetic proteins 2 and 4 in commercial demineralized freeze-dried bone allograft preparations: pilot study. Clin Implant Dent Relat Res. 2000; 2: 110–117. doi: 10.1111/j.1708-8208.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Powers RM Jr, Wolfinbarger L Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J Periodontol. 1997; 68: 1085–1092. doi: 10.1902/jop.1997.68.11.1085. [DOI] [PubMed] [Google Scholar]
- 10.Wei L, Miron RJ, Shi B, Zhang Y. Osteoinductive and Osteopromotive Variability among Different Demineralized Bone Allografts. Clin Implant Dent Relat Res. 2015; 17: 533–542. doi: 10.1111/cid.12118. [DOI] [PubMed] [Google Scholar]
- 11.Fujioka-Kobayashi M, Schaller B, Zhang Y, Kandalam U, Hernandez M, Miron RJ. Recombinant human bone morphogenetic protein (rhBMP)9induces osteoblast differentiation when combined with demineralized freeze-dried bone allografts (DFDBAs) or biphasic calcium phosphate (BCP) Clin Oral Investig. 2017; 21: 1883–1893. doi: 10.1007/s00784-016-1983-0. [DOI] [PubMed] [Google Scholar]
- 12.Miron RJ, Bosshardt DD, Laugisch O, Dard M, Gemperli AC, Buser D, et al. In vitro evaluation of demineralized freeze-dried bone allograft in combination with enamel matrix derivative. J Periodontol. 2013; 84: 1646–1654. doi: 10.1902/jop.2013.120574. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodonticsand dental traumatology. Int Endod J. 2011; 44: 697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 14.Eick S, Strugar T, Miron RJ, Sculean A. In vitro-activity of oily calcium hydroxide suspension on microorganisms as well as on human alveolar osteoblasts and periodontal ligament fibroblasts. BMC Oral Health. 2014; 14: 9. doi: 10.1186/1472-6831-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paula-Silva FW, Ghosh A, Arzate H, Kapila S, da Silva LA, Kapila YL. Calcium hydroxide promotes cementogenesis and inducescementoblastic differentiation of mesenchymal periodontalligament cells in a CEMP1- and ERK-dependent manner. Calcif Tissue Int. 2010; 87: 144–157. doi: 10.1007/s00223-010-9368-x. [DOI] [PubMed] [Google Scholar]
- 16.Stockmann P, Park J, von Wilmowsky C, Nkenke E, Felszeghy E, Dehner JF, et al. Guided bone regeneration in pig calvarial bone defects using autologous mesenchymal stem/progenitor cells- a comparison of different tissue sources. J Craniomaxillofac Surg. 2012; 40: 310–320. doi: 10.1016/j.jcms.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Ayatollahi M, Geramizadeh B, Zakerinia M, Ramzi M, Yaghobi R, Hadadi P, et al. Human Bone Marrow-derived Mesenchymal Stem Cell: A Source for Cell-Based Therapy. Int J Organ Transplant Med. 2012; 3: 32–41. [PMC free article] [PubMed] [Google Scholar]
- 18.Ayatollahi M, Soleimani M, Geramizadeh B, Imanieh MH. Insulin-like growth factor 1 (IGF-I) improves hepaticdifferentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011; 35: 1169–1676. doi: 10.1042/CBI20110016. [DOI] [PubMed] [Google Scholar]
- 19.Narita H, Itoh S, Imazato S, Yoshitake F, Ebisu S. An explanation of the mineralization mechanism in osteoblastsinduced by calcium hydroxide. Acta Biomater. 2010; 6: 586–590. doi: 10.1016/j.actbio.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Ji YM, Jeon SH, Park JY, Chung JH, Choung YH, Choung PH. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng Part A. 2010; 16: 1823–1833. doi: 10.1089/ten.TEA.2009.0054. [DOI] [PubMed] [Google Scholar]
- 21.Moghadam HG, Sándor GK, Holmes HH, Clokie CM. Histomorphometric evaluation of bone regeneration using allogeneic and alloplastic bone substitutes. J Oral Maxillofac Surg. 2004; 62: 202–213. doi: 10.1016/j.joms.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Carnes DL J, Fontaine JDL, Cochran D, Mellonig J, Keogh B, Harris S. Evaluation of 2 novel approaches for assessing the ability of demineralized freeze-dried bone allograft to induce new bone formation. J Periodontolo. 1999; 70: 353–363. doi: 10.1902/jop.1999.70.4.353. [DOI] [PubMed] [Google Scholar]
- 23.Vaziri S, Vahabi S, Torshabi M, Hematzadeh S. In vitro assay for osteoinductive activity of differentdemineralized freeze-dried bone allograft. J Periodontal Implant Sci. 2012; 42: 224–230. doi: 10.5051/jpis.2012.42.6.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dereka XE, Markopoulou CE, Mamalis A, Vrotsos IA. Effect of rhBMP-7 combined with two bone grafts on humanperiodontal ligament cell differentiation. Growth Factors. 2009; 27: 274–279. doi: 10.1080/08977190903112721. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz Z, Mellonig J, Carnes D, Fontaine JDL, Cochran DL, Dean DD. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation. J Periodontol. 1996; 67:918–926. doi: 10.1902/jop.1996.67.9.918. [DOI] [PubMed] [Google Scholar]
- 26.Stratul SI, Schwarz F, Becker J, Willershausen B, Sculean A. Healing of intrabony defects following treatment with an oilycalcium hydroxide suspension (Osteoinductal) A controlled clinical study. Clin Oral Investig 2006; 10: 55–60. doi: 10.1007/s00784-005-0024-1. [DOI] [PubMed] [Google Scholar]


