Abstract
Statement of the Problem:
Regional lymph nodes are the nodes draining lymph from region around the tumor and presence of metastatic tumor in regional lymph nodes is the most important prognostic factor for malignant tumors of epithelial origin. Lymphangiogenesis is associated with an increased incidence of regional lymph nodes metastasis and is possibly an essential step for metastasis. Tumor cells secrete lymphangiogenic cytokines, which results in formation of lymphatic vessels within and around the tumor and act as portals for tumor spread.
Purpose:
The present study aims to investigate and quantify lymphatic vessel density (LVD) in oral squamous cell carcinoma (OSCC) and determine whether LVD can predict the risk of sentinel lymph node metastasis.
Material and Method:
50 specimens of OSCC, without and with lymph node metastasis (25 each) along with 25 specimens of normal oral mucosa were assessed and LVD was quantified by D2-40 immunostaining. Discrete hotspots of intratumoral lymphatics were identified in superficial and deep areas in all carcinomas to calculate LVD.
Results:
When compared to normal mucosa, LVD decreased in the superficial intratumoral areas and decreased further in deep areas. In addition, LVD in the superficial and deep areas of OSCC without nodal metastasis was significantly higher than that in OSCC with nodal metastasis.
Conclusion:
Our study provides evidence that lymphangiogenesis varies within the tumor. Lymphatic vessels are either compressed, collapsed, destroyed or absent in deep intratumoral region. Decrease in LVD predicts cervical lymph node metastasis both in superficial and deep areas. Lymphatics in superficial areas are probably major conduit for nodal metastasis in OSCCs.
Keywords: Mouth neoplasms , Lymphatic vessels , Lymph nodes , Lymphangiogenesis , Immunohistochemistry
Introduction
The role of lymphatic vascular system in promoting cancer metastasis has received intense interest, research effort and clinical attention in the past 15 years. In clinical oncology, sentinel lymph nodes play a vital role in diagnosis, staging and management of disease and is considered a marker for dissemination, increased stage and worse prognosis.[1] For oral squamous cell carcinoma (OSCC), lymphatic spread is more important than other routes because malignant cells preferentially metastasize to roughly 400 lymph nodes in the cervical region.[2] Sentinel lymph nodes are the first nodes to receive drainage from primary tumor and hence first sites to develop metastasis. This pattern of metastasis is central to the usefulness of sentinel lymphadenectomy as a surgical technique.[3]
Although not common in adults, lymphangiogenesis can be induced in pathological processes such as inflammation, wound healing, and cancer.[4] Angiogenesis is well known to be essential for tumor growth and hematogenous metastasis and has been extensively documented. Due to lack of efficient methods and selective markers, research on lymphatic vessels lags far behind that of blood vessels. With the advent of selective and specific lymphatics markers, differentiation of lymphatics from blood vessels has become possible in tissue sections; thus, providing new areas of research in cancer metastasis. Since then, immunohistochemical studies demonstrating tumor lymphangiogenesis have been performed. One of the problems with these studies is that lymphatic vessel density (LVD) has been measured in intratumoral region, disregarding intratumoral variations.[3,5]
In the present study, we investigated lymphatic vessel density of the tumor dividing it in topographic areas (superficial and deep areas), using D2-40 and its relationship with clinicopathological parameters. We also explored the association of LVD in superficial and deep areas of tumor with lymph node metastasis. D2-40 is lymphatic endothelial cell-specific antibody and is a highly selective marker of lymphatic endothelium in sections of both frozen and formalin-fixed paraffin-embedded normal and neoplastic tissues.[5]
Materials and Method
Clinical data
The study population consisted of 50 OSCC patients who underwent tumour resection with either modified radical or suprahyoid neck dissection at Rashtrasant Tukadoji Regional Cancer Hospital, Nagpur from 2012 to 2014. This retrospective study conformed to the institutional Ethics Committee guidelines conducted in Government Dental College and Hospital, Nagpur as per the guidelines of Maharahtra University of Health Sciences. Patient’s charts were reviewed to obtain information such as age, gender, tumour location, treatment modalities, and lymph node status. Patients who had previously received any treatment in the form of radiotherapy or chemotherapy and recurrences were excluded from the study. Lymph nodes were examined histopathologically for reactive inflammatory response or neoplastic infiltration. Accordingly, 25 cases of OSCC without cervical lymph node metastasis and 25 cases of OSCC with cervical lymph node metastasis were selected and study groups were formed. Twenty-five specimens of normal oral mucosa were obtained from the Department of Oral Pathology, Government Dental College and Hospital, Nagpur.
Immunohistochemistry
Sections of 4-µm thickness were cut and mounted on silane-coated slides. The sections were deparaffinized in xylene and rehydrated by immersion in graded series of ethanol dilutions. A universal Super Sensitive Polymer-HRP IHC Detection System (BioGenex) was used for immunohistochemical staining. Heat induced antigen retrieval was performed using microwave at 97°C for 20 min in 0.01 M sodium citrate buffer (pH 6.0). The immersed slides were then allowed to cool at room temperature for 20 min. After antigen retrieval procedure, sections were incubated in hydrogen peroxide for 20 minutes to block endogenous peroxidase activity. Serum-blocking solutions were applied for 10 minutes to block antibody-binding sites. The sections were then incubated with D2-40 antibody (FLEX Monoclonal Mouse Antihuman D2-40, Ready-to-use, DAKO AS/AS+, USA) and incubated in a humidifying chamber for 60 minutes according to manufacturer’s instructions. After adequate binding of the primary antibodies, biotinylated secondary antibody was applied to the tissue sections for 30 minutes. Tissue sections were washed with phosphate buffer to remove any unbound secondary antibodies and then treated with horseradish peroxidase-conjugated streptavidin complex solution for 30 minutes. 0.01% Diaminobenzidine solution was applied for five minutes for colorization, after which the sections were counter-stained with Harris’s hematoxylin. Phosphate buffer solution was applied to wash and preserve the tissue sections in between steps. Adjacent normal appearing lymphatic vessels within the section served as positive internal control. Internal negative control specimens were obtained by omitting primary antibodies.
Lymphatic Vessel Density
Each slide was evaluated for LVD using a modification of Weidner’s method.[6] For measuring LVD of OSCC, the intratumoral tissue area was divided into superficial and deep areas, resulting in two portions. Intratumoral area was defined as the stromal tissue with two or more neoplastic aggregates. In brief, the area with the highest vascular density (hot spot) was selected at a low magnification 40X and the number of positively stained vessels was counted in each of three microscopic fields using 400X magnification lenses. In the absence of apparent hot spots, three or more randomly selected areas were counted. The examination of each microscopic field at 400X magnification corresponds to a number of vessels confined to an area of 0.17mm2. The mean number of vessels from the three fields was converted to the number of vessels per mm2 area and was defined as LVD. D2-40 immunohistochemical positive reactions were independently counted in lymphatic vessels from superficial and deep portions of intratumoral areas. Any single, brown stained cell or cluster of endothelial cells that clearly was separated from the adjacent blood vessels, tumor cells, and other connective tissue elements was considered positive for the antibodies that were used. Distorted and overlapped lymphatics are common in cancer and assumed as one lymphatic unit. All hematoxylin and eosin (H&E) and immunohistochemical stained sections were evaluated by two observers to eliminate inter-observer bias, and any disagreements were resolved with a pentahead microscope.
Statistical analysis
All the analyses were performed in SPSS version 18.0 and the statistical significance was tested at 5% level. The comparison of mean LVD between groups was performed using t-test for independent samples. Further, the comparison of mean LVD as per tumor sizes and site for each area was carried out using t-test for independent samples. Any differences resulting in a P-value of less than 0.05 were considered statistically significant.
Results
The mean age of 35 male and 15 female OSCC patients at the time of diagnosis was 45.7 years, ranging from 26 to 70 years.
D2-40 as a specific lymphatic marker
It was observed that lymphatics are positively stained with D2-40, thin-walled and irregularly shaped vessels, clearly distinguished from adjacent blood vessels, which are negatively stained, regular in shape, surrounded by smooth muscle and containing erythrocytes (Figure 1A and B).
Figure1.
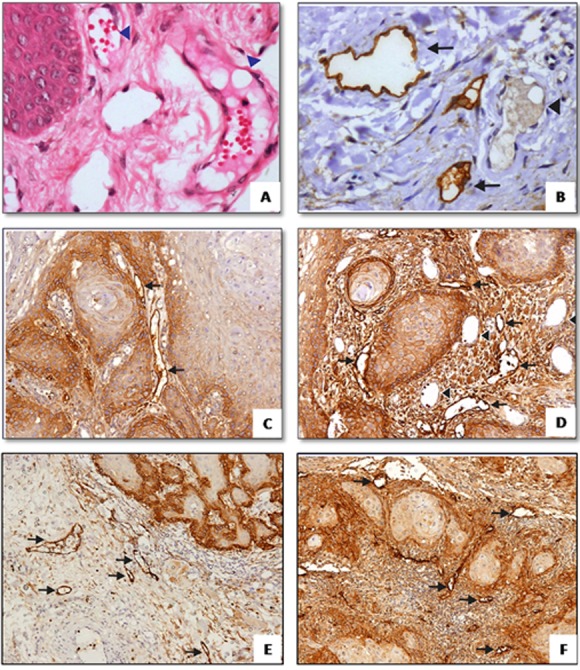
a: H&E staining of normal mucosa showing blood vessels (arrowhead) (400X). b: D2-40 immunostaining of normal mucosa, lymphatics are positively stained (arrow) and distinguished from blood vessels which are negatively stained (400X). D2-40 immunostaining of intratumoral lymphatics in superficial c and d: and deep, e and f: region (100X)
Expression of podoplanin in tumor cells
Podoplanin is a transmembrane mucoprotein recognized by D2-40 monoclonal antibody.[5] Incidentally, we observed podoplanin expression in basal cells of histologically normal oral squamous epithelium. In some of the hyperplastic and dysplastic epithelial areas adjacent to the tumors, D2-40 was highly expressed in the basal and suprabasal cell layers. In OSCC tumors, D2-40 expression was generally heterogeneous and displayed two patterns: diffuse expression in most tumor cells and focal expression at the proliferating periphery of the tumor cell nests with no/weak expression in the central areas. In the latter cases, the central areas often contained more differentiated cells, mimicking the pattern seen in the hyperplastic and dysplastic epithelium. Some of the tumors exhibited weak podoplanin expression.
LVD between tumor groups based on size for superficial and deep areas
For statistical analysis, T1, T2 size tumors were grouped together and T3, T4 size tumors were grouped together. Intratumoral LVD in deep areas of tumor decreased in T3 and T4 group when compared with T1 and T2 group and was statistically significant (p= 0.021; Figure 2).
Figure2.
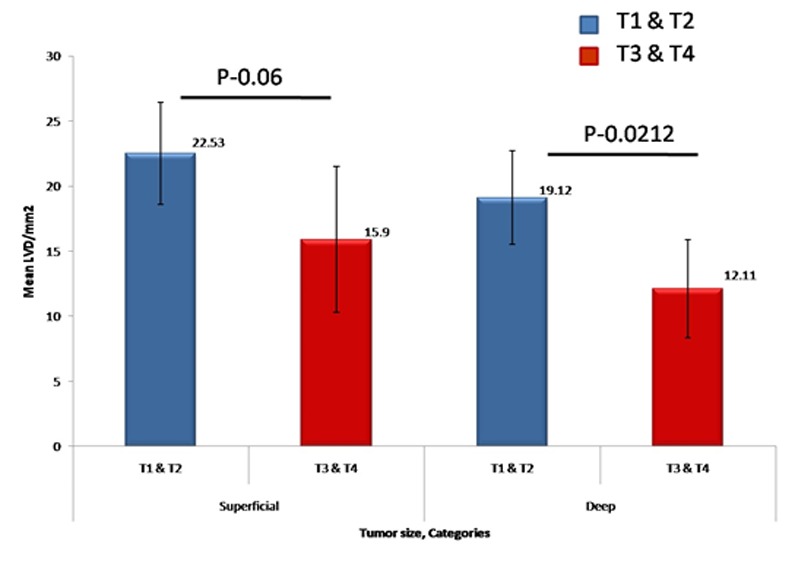
A bar chart representation of mean LVD between tumor groups for superficial and deep areas based on tumor size
LVD between normal oral mucosa group and OSCC without metastasis group and OSCC with metastasis group for superficial and deep areas
Normal oral mucosa contains dilated lymphatic vessels, most abundantly in the subepithelial layer. On comparing LVD of normal mucosa with OSCC without lymph node metastasis group, we found intratumoral LVD decreased significantly in both superficial (p= 0.03) and deep areas (p= 0.0008). On comparing LVD of normal mucosa and OSCC with lymph node metastasis group, we found intratumoral LVD decreased significantly in both superficial (p< 0.0001) and deep areas (p< 0.0001; Figure 1 and 3).
Figure3.
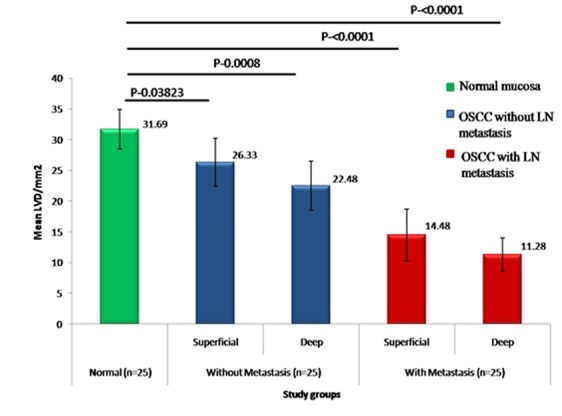
A bar chart representation of mean LVD between normal group and OSCC without / with metastasis groups, for superficial and deep areas
LVD between OSCC without lymph node metastasis and OSCC with lymph node metastasis for superficial and deep areas
We found that intratumoral LVD in the lymph node metastasis group was significantly lower in both superficial area (p= 0.0001) and deep area (p= 0.00003) when compared with that in the group without lymph node metastasis (Figure 4).
Figure4.
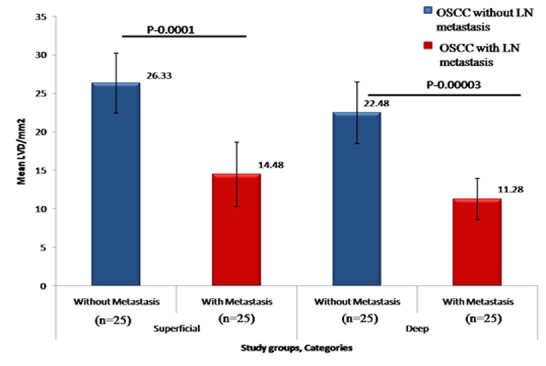
A bar chart representation of the mean LVD for OSCC without and with metastasis groups for superficial and deep areas
Characteristics of lymphatic vessels
In our study material, we observed that intratumoral lymphatics in the superficial areas often had a distinctive architecture, and were dilated with wide-open lumina (Figure 1D). Interestingly, lymphatics in the deeper regions of the tumor were usually small, flattened, compressed or irregular with tiny or ill-defined lumen and were almost lacking in a few cases (Figure 1F). Importantly, characteristics of peritumoral lymphatics were similar to those of superficial intratumoral lymphatics, i.e., they were dilated with large open lumina (Figure 5).
Figure5.
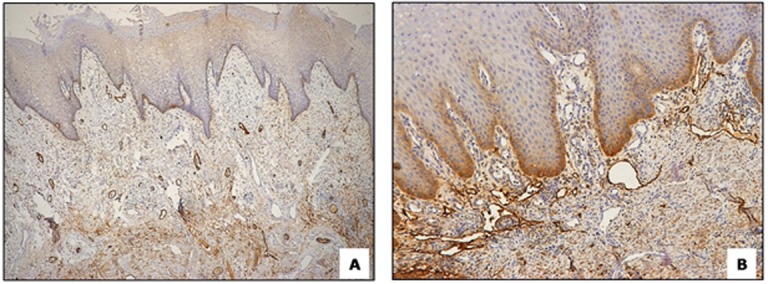
a:Peritumoral region showing numerous lymphatics with dilated,b: open lumen (100X)
Interestingly, in few slides, lymphovascular invasion events were clearly seen in D2-40 stained lymphatics in superficial areas of intratumoral tissue. Proliferating tumor cell nests seem to contact lymphatic vessels in clumps directly and break through the thin walled lymphatic vessel to enter the lumen (Figure 6a and b).
Figure6.
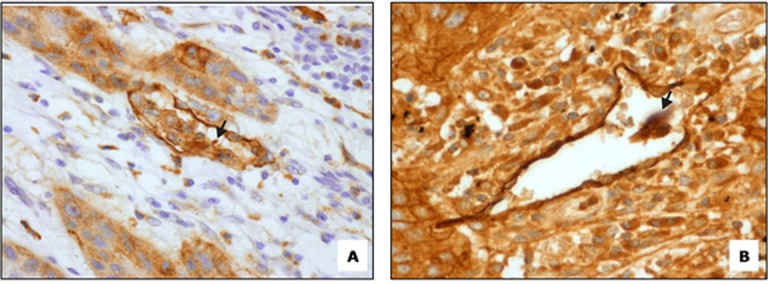
a:Tumor nest invasion into D2-40 stained lymph vessel (400X). b: Initiation of lymphovascular invasion (400X)
Discussion
Cancer treatment aims at providing the most effective treatment to the right people, i.e., to tailor cancer treatment for individual patients as closely as possible.
Identification of characteristics of primary tumor and spread patterns of tumor cells would help in treatment planning and prognosis. Many studies have documented conflicting relationship between lymphangiogenesis and nodal metastasis, prognostic factors and patient survival in different tumor types.[7-9] In our study, we observed local variation of LVD within the tumor tissue and low LVD in deep areas of tumor than in superficial areas. In addition, low intratumoral LVD was associated with cervical lymph node metastasis. Most of the studies reported have measured lymphatic density using the hot spot method in which areas of highest lymphatic density are evaluated irrespective of its location within the tumor.[5-6] However, considering the varied tumor microenvironment and locoregional variations, this poses the risk of bias or oversimplification. In present study, we divided the intratumoral tissue into superficial and deep areas to study LVD in detail.
We found that large tumor size correlated with low lymphatic density in deep areas of tumors. Very few studies have published results similar to ours in HNSCCs.[8] Brundler et al.[9] proposed that low LVD in the center of tumors could be an underestimation if lymphatic vessels are collapsed given the increased interstitial pressure within tumors or it could be a real absence of lymphatics. It is suggested that brisk growth of tumor causes tissue edema, which results in mechanical forces causing compression of lymphatics. Additionally, tumor cells might destroy the lymphatic structure, and the newly formed valves of intratumoral lymphatic are then incomplete and nonfunctional.[2]
In the present study, LVD was higher in the normal mucosa than OSCC. Matsumoto et al.[10] had similar observations of decreased LVD in tumor-associated stroma when compared with normal areas. Probably, lymphangiogenesis did not occur immediately in tumor-associated stroma. These findings may suggest that tumor invaded into normal submucosa and subsequently destroyed the lymphatic vessels. Ohno et al.[11] reported significant increase in LVD in superficial area of intratumoral tissue when compared to normal mucosa and significant decrease in LVD in deep area, which may be attributed to the fact that lymphangiogenesis is impaired or inhibited rather than induced. This can be confirmed by the finding of decreased Vascular endothelial growth factor C (VEGF-C) expression, a potent mitogen for lymphangiogenesis, in deep intratumoral tissue when compared with normal mucosa.[7,11]
The primary objective of our study was to assess LVD and correlate it with sentinel lymph node metastasis. LVD in the superficial and deep areas of OSCC with lymph node metastasis was significantly lower than that in the OSCC without lymph node metastasis. Fewer studies have published results similar to ours.[7,10-12]
However, Beasley et al.[13] reported significant correlations between high intratumoral LVD and lymph node metastasis in oropharyngeal carcinoma but not in OSCC or laryngeal carcinomas. In contrast to our findings, Franchi et al.[14] observed that HNSCCs presenting with lymph node metastasis have a significantly higher LVD, both within the tumor mass and in the peritumoral areas. High peritumoral LVD correlated with a significantly higher risk of lymph node metastasis.
Destruction of the lymphatic vessels due to proteolytic enzymes like matrix metalloproteinase (MMP-2 and MMP-9) and Cathepsin D probably secreted by the OSCC might be more or less responsible for the lowered LVD in deeper regions of the tumor.[15-16] High levels of Cathepsin D expression were observed in OSCCs with regional lymph nodes metastasis compared with node-negative tumors.[17] Leu AJ et al.[18] proposed that destruction of lymphatics in the tumor is due to collapse under the pressure (mechanical stress) of growing cancer cells. Presence of an antilymphangiogenic molecule(s) in tumors cannot be excluded. These factors may be partly responsible for lowered LVD in tumors with lymph node metastasis in our study.
Lymph node invasion of tumor cells may occur through destruction and penetration into preexisting or newly generated lymphatic vessels. Another hypothesis is that cancer cells secrete factors leading to activation of endothelial cells and thus facilitating their entry into the lymphatics and lymph node invasion.[10,19]
There is still unsettled argument whether the major contributors of metastasis are intratumoral or peritumoral lymphatic vessels. Franchi et al.[14] found that peritumoral lymphatics are more dilated with open lumina, had a larger average size, and occupied a greater relative area compared with intratumoral lymphatics. When we observed the characteristics of intratumoral lymphatics in our samples, small compressed lymphatics were observed only in the deeper areas of tumor. However, the characteristics of superficial intratumoral lymphatics were similar to those of peritumoral lymphatics as described by Franchi et al.,[14] i.e., they were dilated with large open lumina.
Padera et al.[20] reported that since lymphatics in the center of tumor are nonfunctional, peritumoral lymphatics and functional lymphatics at the tumor margins play an important role in tumor metastasis. Our observations are in agreement with those of Padera et al. as such nonfunctional lymphatics were observed in the deep areas of tumor whereas lymphatics in the superficial intratumoral areas were functional. In addition, disorganized intratumoral lymphatics would act as poor portal for metastasis, limiting its role in tumor metastasis.[21] In our series, we found such compressed and disorganized lymphatics only in the intratumoral deep areas as opposed to functional lymphatics in the intratumoral superficial areas. Ohno et al.[11] had somewhat similar observations, that tumor cells utilize pre-existing dilated lymphatic vessels in the superficial zone of the intratumoral and peritumoral tissue to enter into lymphatic circulation rather than those in the deep area.
We therefore speculate that the spread of OSCC tumor cells to lymph node may involve invasion of superficial intratumoral vessels, along with peritumoral vessels as reported in the literature. In any event, the link between lymphangiogenesis / lymphoproliferation and dissemination to lymph nodes is likely to be complex. Various tumors may vary in their capacity to invade lymphatics. This could be attributable to differential production of factors such as MMP-2, MMP-9 that help in invasion of tumor cells or growth factors, such as VEGF, which promotes angiogenesis and lymph vessel permeability. If a positive correlation between decreased lymphangiogenesis and cervical lymph node involvement is confirmed in further studies, this parameter could be useful for selecting OSCC patients who are more susceptible to metastatic spread via lymphatic pathways to undergo elective cervical lymph node dissection. Recent data shows enhanced lymphangiogenesis in sentinel lymph nodes even before cancer metastasis.[22] Inhibition of tumor lymphangiogenesis and arresting lymphatic invasion can be an effective option in treatment of cancer.[23] One possibility would be to use inhibitors of lymphangiogenic factors and destroy lymphatic vessels, which could inhibit lymphatic metastasis.
Conclusion
In conclusion, we provide evidence that intratumoral lymphangiogenesis varies depending on the area, and is probably not induced in the deep intratumoral tissue in OSCC. Based on our findings, we propose that decrease in LVD, in both superficial and deep areas of intratumoral stroma, predict cervical lymph node metastasis. We also suggest that the poor lymphatics in the deeper areas of tumor do not play a role in nodal metastasis. Lymphatic channels in the superficial intratumoral areas are most likely involved in lymphatic dissemination.
Acknowledgement
The authors are thankful to Mr. Maroti Chopade for his technical support and guidance through immunohistochemical procedures.
Conflict of Interest:The authors of this manuscript certify that they have no conflict of interest.
References
- 1.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003; 98: 413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelial cells and lymphatic metastasis in head and neck cancer--a review of mechanisms. Int J Oral Sci. 2010; 2: 5–14. doi: 10.4248/IJOS10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002; 16: 922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 4.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31:4499–4508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 5.Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006; 95: 1611–125. doi: 10.1038/sj.bjc.6603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991; 324: 1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 7.Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007; 25: 4298–4307. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- 8.Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003; 63: 1920–1926. [PubMed] [Google Scholar]
- 9.Brundler MA, Harrison JA, de Saussure B, de Perrot M, Pepper MS. Lymphatic vessel density in the neoplastic progression of Barrett's oesophagus to adenocarcinoma. J Clin Pathol. 2006; 59: 191–195. doi: 10.1136/jcp.2005.028118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto N, Mukae S, Tsuda H, Sawada A, Okazaki Y, Nagai K, et al. Prognostic value of LYVE-1-positive lymphatic vessel in tongue squamous cell carcinomas. Anticancer Res. 2010; 30: 1897–1903. [PubMed] [Google Scholar]
- 11.Ohno F, Nakanishi H, Abe A, Seki Y, Kinoshita A, Hasegawa Y, et al. Regional difference in intratumoral lymphangiogenesis of oral squamous cell carcinomas evaluated by immunohistochemistry using D2-40 and podoplanin antibody: an analysis in comparison with angiogenesis. J Oral Pathol Med. 2007; 36: 281–289. doi: 10.1111/j.1600-0714.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe S, Kato M, Kotani I, Ryoke K, Hayashi K. Lymphatic Vessel Density and Vascular Endothelial Growth Factor Expression in Squamous Cell Carcinomas of Lip and Oral Cavity: A Clinicopathological Analysis with Immunohistochemistry Using Antibodies to D2-40, VEGF-C and VEGF-D. Yonago Acta Med. 2013; 56: 29–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002; 62: 1315–1320. [PubMed] [Google Scholar]
- 14.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometric study with clinical correlations. Cancer. 2004; 101: 973–978. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 15.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013; 4: 66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortesina G, Martone T. Molecular metastases markers in head and neck squamous cell carcinoma: review of the literature. Acta Otorhinolaryngol Ital. 2006; 26: 317–325. [PMC free article] [PubMed] [Google Scholar]
- 17.Vigneswaran N, Zhao W, Dassanayake A, Muller S, Miller DM, Zacharias W. Variable expression of cathepsin B and D correlates with highly invasive and metastatic phenotype of oral cancer. Hum Pathol. 2000; 31: 931–937. doi: 10.1053/hupa.2000.9035. [DOI] [PubMed] [Google Scholar]
- 18.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000; 60: 4324–4327. [PubMed] [Google Scholar]
- 19.Shayan R, Achen MG, Stacker SA. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis. 2006; 27: 1729–1738. doi: 10.1093/carcin/bgl031. [DOI] [PubMed] [Google Scholar]
- 20.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002; 296: 1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 21.Kyzas PA, Geleff S, Batistatou A, Agnantis NJ, Stefanou D. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J Pathol. 2005; 206: 170–177. doi: 10.1002/path.1776. [DOI] [PubMed] [Google Scholar]
- 22.Wakisaka N, Hasegawa Y, Yoshimoto S, Miura K, Shiotani A, Yokoyama J, et al. Primary Tumor-Secreted Lymphangiogenic Factors Induce Pre-Metastatic Lymphvascular Niche Formation at Sentinel Lymph Nodes in Oral Squamous Cell Carcinoma. PLoS One. 2015; 10: 0144056. doi: 10.1371/journal.pone.0144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato Y, Kaneko MK, Kuno A, Uchiyama N, Amano K, Chiba Y, et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem Biophys Res Commun. 2006; 349: 1301–1307. doi: 10.1016/j.bbrc.2006.08.171. [DOI] [PubMed] [Google Scholar]


