Abstract
Sinusoidal obstruction syndrome (SOS) is a severe complication of hematopoietic stem cell transplantation (HSCT) that can be fatal, often attributed to the conditioning regimen prior to HSCT. We evaluated the association of SOS risk with gene variants in cystathionase (CTH), an enzyme involved in glutathione synthesis, in 76 children receiving intravenous busulfan (Bu) before HSCT. Our results indicated an association with CTHc.1364 G>T (ORTT=10.6, 95% confidence interval (CI)=2.16, 51.54) and SOS risk, which was sex dependent (female patients, ORTT=21.82, 95% CI=3.590–132.649). The interaction between CTHc.1364 G>T and another risk variant (GSTA1*B) was explored. A recessive model with the use of GSTA1*B*B and CTH c.1364 TT genotypes proved to be useful at predicting SOS occurrence, indicating the possibility of using these gene variants as markers of SOS occurrence and to further individualize preemptive treatment aimed at reducing SOS incidence.
Introduction
Sinusoidal obstruction syndrome (SOS) is characterized by the clinical features of rapid weight gain, ascites, painful hepatomegaly and jaundice.1 The incidence of SOS in the pediatrics transplant population ranges between 7% and 27% and is higher than in adults.2, 3 The use of a busulfan (Bu) and cyclophosphamide (Cy) based myeloablative conditioning regimen is associated with SOS.4 High Bu and high Cy metabolites concentrations often lead to increased risk of SOS.5 Furthermore, large interindividual and intraindividual variability of Bu and Cy plasma concentrations have been observed after the same first dose administration, thought to be in part owing to metabolizing enzymes.6
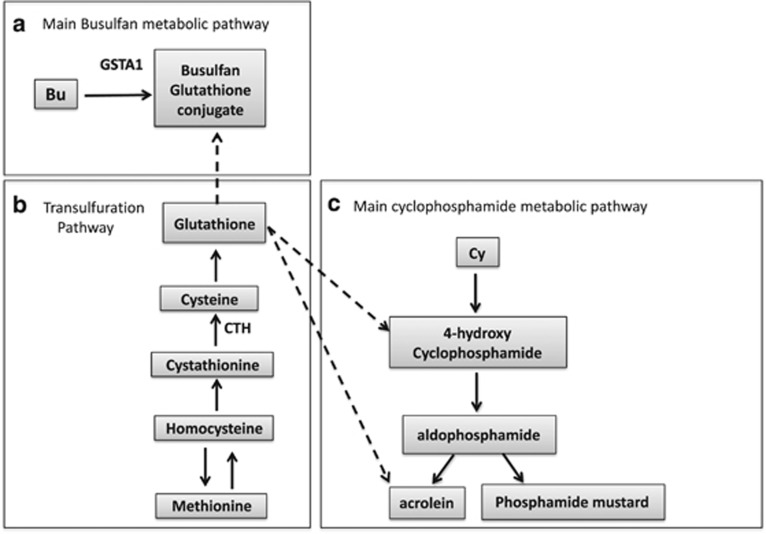
Glutathione S-transferase alpha 1 (GSTA1) is the main metabolic enzyme involved in Bu’s clearance (see Figure 1a). We have previously identified GSTA1*B (variant with reduced enzymatic activity) as a potential predictor of SOS, suggesting its potential for individualizing Bu treatment.7 High Bu doses alone does not explain the occurrence of SOS8 and GSTA1*B’s contribution is fairly small. As one of the main hypothesis of SOS occurrence involves the interaction between Bu and Cy causing depletion of glutathione (GSH),9, 10, 11 we here investigated the variation in cystathionine γ-lyase (CTH) gene, which encodes an enzyme involved in the synthesis of GSH (Figure 1b). More specifically, this gene encodes an enzyme in the trans-sulfuration pathway that converts cystathionine derived from methionine into cysteine, which is one of the three amino acids that make up GSH (see Figure 1b). GSH is an important antioxidant that prevents damage to cellular components caused by oxidative stress.12 If an association with CTH is found, the aim is to also investigate whether it can add predictive value to GSTA1 by performing gene–gene interaction models.
Figure 1.
A simple diagram to describe the depletion of glutathione (GSH) when busulfan (Bu) is administered first before cyclophosphamide (Cy) and the role of glutathione S-transferase alpha 1 (GSTA1) and cystathionine gamma-lyase (CTH) within this hypothesis of sinosoidal obstruction syndrome (SOS) risk. (a) Shows the main Bu metabolic pathway, where Bu conjugates with GSH and where GSTA1 is the main catalytic enzyme. (b) Shows the transulfuration pathway where methionine is converted to cysteine, the main rate-limiting step for the formation of GSH. CTH is needed for the conversion of cystathionine into cysteine, which is one of the components of GSH. (c) Shows the main Cy metabolic pathway and its metabolites acrolein and 4-hydroxycyclophosphamide, two compounds known to be toxic to liver cells.
Materials and Methods
Patients
The study included 76 pediatric patients who underwent myeloablative allogeneic hematopoietic stem cell transplantation (HSCT) with intravenous Bu- and Cy-based myeloablative conditioning regimen at CHU Sainte-Justine between May 2000 and August 2012. The Institutional Review Board approved the study and all patients/parents provided an informed consent form (IRB number: 2450). This study was fully registered in a public trial registry as part of an ongoing prospective European Blood and Marrow Transplantation multicenter study (registered at Clinical Trials.gov, NTC01257854). The demographic characteristics of the patients, details of disease and conditioning regimen are described in Table 1.
Table 1. Demographics of the study subjects (N=76).
| Characteristics |
Patients |
|
|---|---|---|
| N | % | |
| Gender | ||
| Male | 35 | 46.0 |
| Female | 41 | 54.0 |
| Ethnicity | ||
| Caucasian | 60 | 79 |
| African | 11 | 14.5 |
| Asian | 3 | 3.9 |
| American Indian | 2 | 2.6 |
| Diagnosis | ||
| AML | 24 | 31.6 |
| ALL | 6 | 7.9 |
| MDS | 17 | 22.3 |
| Non-malignanta | 29 | 38.2 |
| HLA compatibility | ||
| MRD | 28 | 36.8 |
| MUD | 13 | 17.1 |
| MMRD | 3 | 4.0 |
| MMUD | 32 | 42.1 |
| Stem cell source | ||
| Bone marrow | 31 | 40.8 |
| Peripheral blood | 1 | 1.3 |
| Cord blood | 44 | 57.9 |
| Conditioning | ||
| BuCy | 68 | 89.5 |
| BuCyVP16 | 7 | 9.2 |
| BuCyMel | 1 | 1.3 |
| Serotherapy | ||
| No | 19 | 25 |
| ATG | 57 | 75 |
| SOS prophylaxis | ||
| Urso | 70 | 92.1 |
| Defibrotide | 1 | 1.3 |
| None | 5 | 6.6 |
| Median | Range | |
|---|---|---|
| CD34+ cells (× 108 kg−1) | 0.007 | (0.00019–1.3) |
| Age (years) | 6.35 | (0.1–19.9) |
| Weight (kg) | 23.8 | (4–95.6) |
| Height (cm) | 122.5 | (52.5–183) |
| BMI (kg m−2) | 0.88 | (0.54–1.45) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, antithymocyte globulin; BMI, body mass index; HLA, human leukocyte antigen; MDS, myelodysplastic syndrome; MMD, HLA-mismatched donor; MMRD, mismatched related donor; MMUD, mismatched unrelated donor; MRD, matched related donor; SOS, sinosoidal obstruction syndrome.
Non-maligant diseases included: myeloproliferative syndrome (N=1), hemoglobinopathies (N=10), immunodeficiency (N=9), metabolic disease (N=5), and hemophagocytic syndrome (N=4).
Treatment regimen
Intravenous Bu (Busulfex, Otsuka Pharmaceuticals, Tokyo, Japan) was administered as a 2 h infusion every 6 h from day −9 to −6. Bu first dose was based on the age of the patient and pharmacokinetic-guided dose adjustment was performed from fifth dose onwards with a target Css of 600–900 ng ml−1. Details of pharmacokinetic analysis and validation of technique is described elsewhere.13 Acid ursodeoxycholic (Urso, Axcan Pharma, Quebec, Canada) was given for prophylaxis against SOS to 75 patients who received from 7.5 mg kg−1 per dose, max 500 mg (PO, BID). Urso was given the first day of conditioning till day +28 after HSCT. One patient had a second transplant with abnormal liver enzymes and received Defibrotide (Defitelio, Jazz Pharmaceuticals, Palo Alto, CA, USA) 25 mg kg−1 day−1 intravenously given in four divided doses each infused over 2 h from the first day of conditioning till day +30 after HSCT as a SOS prophylaxis. During the period of HSCT, Defibrotide was not approved in the United States and Canada but was available as an investigational drug candidate in the United States.
Definition of clinical outcome
SOS was diagnosed according to the Seattle criteria, as proposed by McDonald et al.14 in 1984 with the day of onset extended to 50 days. The clinical course or severity of SOS was evaluated according to the description provided by McDonald et al.15 in 1985.
Genotyping
DNA was extracted from blood collected before conditioning. CTH1364G>T rs1021737 and rs648743 single-nucleotide polymorphisms (SNPs) were chosen owing to their high minor allele frequencies (0.21 and 0.47, respectively) and potential functionality. CTH1364G>T is predicted as damaging (score=0.04) by the SIFT protein analysis software (http://sift.jcvi.org),16 whereas rs648743 is a promoter SNP (−1320 C>T) that could potentially abolish a glucocorticoid receptor-alpha-binding site. Furthermore, these SNPs have prior associations in pharmacogenetic studies related to homocysteine levels and stroke.17, 18 Genotyping was performed using TaqMan-based assays (C_8369524_10 and C_998383_10, respectively) on a StepOne Plus real-time PCR system under standard life technology SNP genotyping Taqman assay conditions (https://www.lifetechnologies.com).
Statistical analysis
Non-parametric (for continuous variables) and chi-square test (for categorical variables) were used to explore correlations between patient characteristics (that is, age, gender, weight, SOS prophylaxis, conditioning regimen, Cy dose and Bu pharmacokinetic parameters) with SOS risk. Cumulative incidence of SOS in relation to the genotypes was estimated using a 1−Kaplan–Meier curves and compared using log-rank test, in a univariate analysis. The interaction between the two gene variants was also explored, as well as the sensitivity and specificity when combined.19 The power of the sample was calculated using G power version 3.1 (http://www.ats.ucla.edu/stat/sas/notes2) using a Goodness-of-fit test. With an expected effect size of 0.6, an alpha error probability of 0.05 and power (1−β error probability) of 95%, this study required a total sample size of 55 patients.
Protein sequence analysis
In order to understand the effect of the mutation on CTH at the sequential level, the physiochemical properties were investigated and protein sequence analysis was performed for both wild-type and mutant form using ExPASy Proteomics Tools (www.expasy.org/tools).
Molecular docking simulation
To further understand the functionality of the CTH1364G>T variant (rs1021737 or Ser403Ile), molecular docking simulation was performed. The full-length structure of CTH is not available in the Protein Data Bank (PDB) (http://www.rcsb.org) (Available residues: 10–400, PDB ID: 3COG), thus hybrid homology modeling and ab initio approaches were used to predict the full-length model of CTH using Robetta web server (http://robetta.bakerlab.org/). Then the in silico mutant form of CTH (Ser403Ile) was generated using the COOT program.20 Once the models were obtained, they were energy minimized by modrefiner21 and validated by Rampage programs.22 The energy minimized models were then subsequently subjected into protein preparation steps that included (i) addition of polar hydrogens and (ii) assignment of kollman charges and record file into the Protein Data Bank+charge+atom (PDBQT) format. The three-dimensional structure of cystathionine (CID 439258) was retrieved from PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and PDB format of the substrate was obtained by the Open Babel program23 followed by the addition of gasteiger charges, merging non-polar hydrogens, set up rotatable bonds and finally recorded into PDBQT format. The above-mentioned protein and ligand preparation steps were performed by Auto Dock Tools.24 Auto Dock Vina was used to perform the docking calculation of substrate into both native and mutant (Ser403Ile) form of CTH.25 For the docking calculation, the grid box size was set at 22 × 28 × 20 Å and centered on the coordinates x=0.322, y=0.565 and z=0.219. Once the docking analysis was completed, the best protein–substrate complexes were selected on the basis of binding affinity and exported into the PDB format. The LigPlus26 and PyMOL (https://www.pymol.org) programs were used for analyzing and to compare the docking results of both the native and mutant forms of CTH.
Results
The overall incidence of SOS was 11.5%. Genotype frequencies for CTH c.1364G>T within our sample were GG (61.8%), GT (34.2%) and TT (3.9%), while for rs648743 they were TT (28.4%), CT (49.3%) and CC (22.4%). Both SNPs were in Hardy–Weinberg equilibrium and the minor allele frequency resembled Hapmap populations. No significant differences in Bu clearance and Css were observed between the CTH c.1364G>T or rs648743 genotype groups. There were no significant correlations between SOS risk and other features (SOS prophylaxis, conditioning regimen, weight, age, Cy dose, sex, Bu, Css).
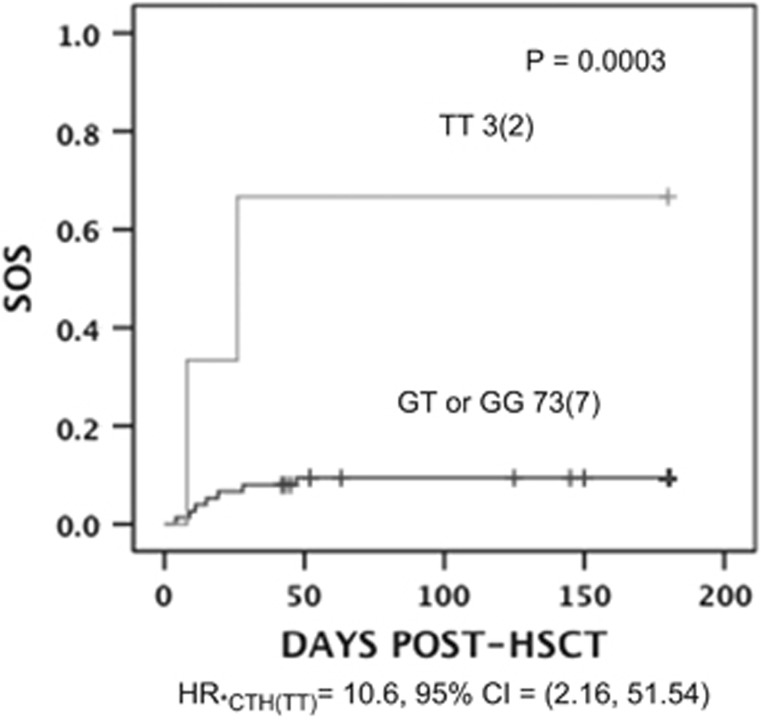
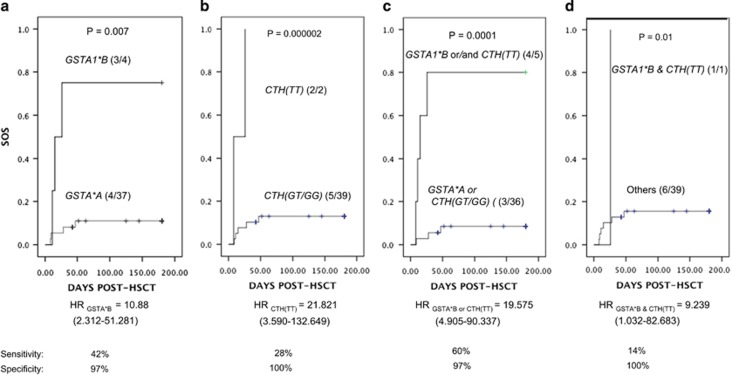
A significant association was found between CTH c.1364 TT genotype and SOS, (odds ratio of 10.6 (95% confidence interval (CI); 1.5–51.54) P=0.003; Figure 2). As in our previous study the association between GSTA1 and SOS incidence was mainly in female patients, we also performed the analysis following stratification by sex. In girls only, there was a relationship between SOS risk and CTH c.1364 TT (odds ratio of 22.40, 95% CI=3.69, 136.17), P=0.000001). In fact in this sample, the association seen with CTH c.1364 TT is exclusively in girls. No significant association was found between CTH rs648743 and SOS risk. We extended this analysis by performing a gene–gene analysis using a recessive model for both CTH c.1364G>T and GSTA1*B in female patients only. The analysis demonstrated that these two variants combined (Figures 3c and d) provided good predictability of SOS risk that was slightly better than the individual models (see Figures 3a and b) in terms of sensitivity and accuracy although they appear to be independent risk factors.
Figure 2.
Association of sinosoidal obstruction syndrome (SOS) risk with CTH c.1364G>T. The plot shows the number of patients in each curve with and without CTH c.1364 TT and the number of individuals with SOS given in parenthesis. The P-value is shown above the curves, estimated by log-rank test for the cumulative SOS incidence between the genotype groups. Risk of SOS associated with CTH TT carriers in expressed as hazard ratio (HR) with 95% confidence interval (CI) indicated below the plot. HSCT, hematopoietic stem cell transplantation.
Figure 3.
Association of sinosoidal obstruction syndrome (SOS) risk with different combinations of GSTA1*B and CTH c.1364G>T or alone. The plots show the number of patients in each curve with GSTA1*B (a), CTH c.1364 TT (b), GSTA1*B and or CTH c.1324 TT (c), GSTA1*B and CTH c.1324 TT (d) and the number of individuals with SOS is given in the parenthesis. The P-value is shown above the curves, estimated by log-rank test for cumulative SOS incidence between the genotype groups. Risk of SOS associated is expressed as hazard ratio (HR) with 95% confidenc interval (CI) indicated below the plot for each.
For protein sequence analysis, the main differences are observed in two posttranslational modification sites, namely, O-GalNAc (mucin type) glycosylation and serine-dependent phosphorylation. Further exploration of these two posttranslational modifications using GlycoEP27 and IsoGlyp28 revealed no evidence for differences in the glycocylation sites. On the other hand, for phosphorylation using DisPhos29 it is suggested that Ser403 can be phosphorylated and this is lost with Ile403.
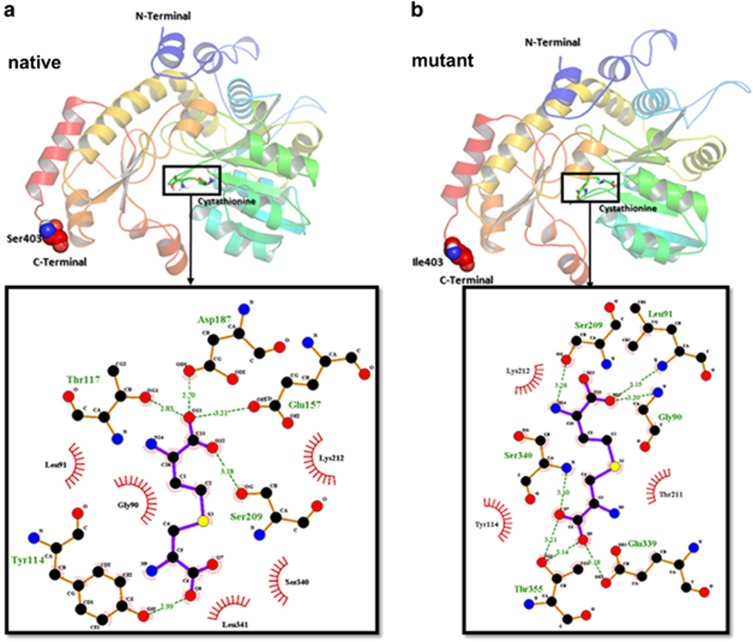
Docking calculation suggested that the native form (−5.0 kcal mol−1, see Figure 4a) of CTH showed higher binding affinity toward the substrate in comparison to the mutant form (−4.7 kcal mol−1, see Figure 4b).
Figure 4.
Binding orientation of cystathionine with cystathionine gamma-lyase (CTH). Two-dimensional representation of CTH (native (a) and mutant (b)) with substrate. The three-dimensional representation of protein–ligand complex was prepared by PyMOL. The LigPlot shows the amino-acid residues of target enzyme around the ligand molecule with hydrogen bond and hydrophobic contacts.
Discussion
The proposed mechanism by which Bu may increase the risk of SOS is GSH depletion at high doses.30 Mice experiments suggested that activation of GSH synthesis through the increase of liver cysteine content is essential for clearance and detoxification of Bu.11 Our previous analysis showed a tendency of higher SOS incidence with a first Bu dose Css >600 ng ml−1.7 Furthermore, we reported the association between GSTA1*B and SOS, where GSTA1*B carriers had a greater risk of SOS. In accordance with the functional effect of this variant and its impact on Bu clearance, the association is likely to be due to the reduced rate of Bu metabolism.7 Other proposed risk factors include the depletion of GSH caused by Bu conjugation influencing elimination of Cy and its toxic metabolites (4-hydroxycyclophosphamide and acrolein, see Figure 1c).31, 32 Cy’s toxic metabolites if not cleared tend to accumulate in area 3 of the hepatic acinus as this area is rich in P450 but poor in GSH, consequently causing damage to hepatocyte and sinusoidal endothelium.33 CTH is vital for the production of cysteine, which is one of the components of GSH and also the rate-limiting amino acid in GSH biosynthesis.34 Interestingly, an increase in plasma levels of homocysteine and cysteine are strongly correlated with severity of liver damage in children, whereas total GSH levels were significantly lower in these subjects.35 The CTH c.1364G>T variant is located in exon 12 and causes a serine to isoleucine change but the functional role of this change is unknown. However, there are several studies that suggest a functionality of this variant. Two studies in adults found a significant increase of homocysteine in TT carriers compared with non-TT carriers.17, 36 Elevated homocysteine concentrations are often associated with elevated cystathionine concentrations, suggesting an increase in production or decrease in degradation possibly linking this variant to GSH depletion. Given a conserved residue of this polymorphism, its functional effect is suggested and patients with T allele may be at a greater risk of SOS owing to lower synthesis of GSH. Exploration of posttranslational modifications with S403I points to a possible loss of two phosphorylation sites when Ile403 is present. Furthermore, the docking simulation suggests a functional change for TT genotype. Essentially, the wild type of CTH has a higher affinity (−5.0 kcal mol−1) toward cystathionine compared with the mutant form (−4.7 kcal mol−1). However, because the Ser403 or Ile403 C-terminus is not directly involved in the active site interaction, it can be hypothesized that it is the stability of the substrate that is decreased in the case of the mutant allele. Further experimental studies are necessary to validate these theoretical calculations.
Our study also demonstrated that the SOS risk linked to the CTH variant was limited to girls. An explanation for this association is unclear but may have something to do with the general sex differences in amino-acid levels produced by the tran-sulfuration pathway (that is, methionine, homocysteine, cystathionine, cysteine). For example, in a study comparing serum total homocysteine levels, boys were seen to have significantly higher homocysteine levels compared with girls starting as early as 10 years, suggesting higher amino-acid turnover for boys.37 The significance of this is unclear but it does demonstrate sex difference within the pediatric population of amino-acid concentrations within this pathway, which may explain why girls might respond differently to drug toxicities.
As the clearance of Bu is mainly determined by hepatic GSTA1 activity and conjugation with GSH, which also contributes a great deal to Cy detoxification, it seems likely that cellular depletion of GSH occurs if Bu is administered first. Thus, as all but one of the patients who had occurrences of SOS in our study were given 200 mg kg−1 of Cy after Bu, the SOS seen in our patients could be related to Cy toxicity potentiated by Bu depletion of glutathione. This may be further exacerbated in patients who possessed either CTH c. 1364 TT or GSTA1*B variants.
Furthermore, the gene–gene interaction analysis revealed that the presence of either risk factor or in combination might predispose individuals to developing SOS after receiving Bu followed by Cy. Thus these results show that either of the models shown in Figures 3a–d could be used to predict the occurrence of SOS, some with more sensitivity than others. The importance of genotyping both variants may be linked to the severity (see Table 2). The main limitation of this study is the small sample size as well as it being a non-homogenous disease cohort and thus the inability to perform more complex multivariate analysis. A larger pediatric study is on going in order to confirm these results.
Table 2. SOS severity vs CTH c.1364 G>T and GSTA1*B −69 C>T variants in females only.
| SOS severity | CTH c.1364 G>T and GSTA1*B C>T |
Total | |
|---|---|---|---|
| No risk genotype | At least one risk genotype | ||
| Mild | 2 | 0 | 2 |
| Moderate | 0 | 2 | 2 |
| Severe | 1 | 2 | 3 |
| Total | 3 | 4 | 7 |
Abbreviation: SOS, sinosoidal obstruction syndrome.
In the early 1990s, it was shown that adding a third alkylating agent to the conditioning regimen increased the incidence of SOS significantly.9 In the present population, 10% of the children were given three alkylating agents, either BU/CY/VP16 (N=7) or BU/CY/Mel (N=1). The incidence of SOS was 1 out of 8 (12.5%). This patient had neither CTH nor GST risk genotype, which might suggest that three alkylating agents increases the risk of SOS; however, it also suggests that three alkylating agents were not a confounding factor to the genetic effects.
Conclusion
We hypothesize that the association with SOS in this cohort involves the following combinations: (1) high first Bu dose, (2) the slow metabolism of Bu by the GSTA1*B variant, (3) the interaction between Bu and Cy, (4) the slow synthesis of GSH in CTH c.1364 TT carriers, and (5) the lower baseline GSH levels in girls. This study suggests that GSTA1 and CTH gene variants may identify female patients at risk of SOS who might need a better SOS prophylaxis, for example, by administering defibrotide (Defitelio, Jazz Pharmaceuticals).2 The administration of Cy before Bu or its replacement by Fludarabine could be also considered to avoid or reduce toxicity.
Acknowledgments
We thank all patients and their parents for consenting to participate in this study. This investigation was supported by a grant provided by CANSEARCH and Dr Dubois-Ferrière Dinu Lipatti Foundations as well as the Geneva Cancer League and the Swiss National Funds (320030_153389/1). We thank the Swiss Oncology Group as our sponsors and the European Blood and Marrow Transplantation Paediatric (EBMT) working disease group for their support and for labeling this study as an EBMT trial (ClinicalTrials.gov Identifier, NCT01257854, EudraCT number: 2009-018105-41). We also thank Catherine Deseau, Marie France Vachon and Mary Boudal Khoshbeen for their help with this study. JM thanks UCIBIO (UID/Multi/04378/2013), Fundação para a Ciência e a Tecnologia (FCT) (PTDC/QUI-BIQ/117799/2010), (SFRH/BPD/97719/2013) Portugal for providing support to carry out in silico part of the research work.
Footnotes
The authors declare no conflict of interest.
References
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005–3020. [PubMed] [Google Scholar]
- Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet 2012; 379: 1301–1309. [DOI] [PubMed] [Google Scholar]
- Cesaro S, Pillon M, Talenti E, Toffolutti T, Calore E, Tridello G et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Haematologica 2005; 90: 1396–1404. [PubMed] [Google Scholar]
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267. [DOI] [PubMed] [Google Scholar]
- Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M, Andersson BS. Role of pharmacogenetics in busulfan/cyclophosphamide conditioning therapy prior to hematopoietic stem cell transplantation. Pharmacogenomics 2013; 14: 75–87. [DOI] [PubMed] [Google Scholar]
- Ansari M, Rezgui MA, Théoret Y, Uppugunduri CR, Mezziani S, Vachon MF et al. Glutatione S-Transferase gene variations influence BU pharmacokinetics and outcome of hematopoietic SCT in pediatric patients. Bone Marrow Transplant 2013; 48: 939–946. [DOI] [PubMed] [Google Scholar]
- Peters WP, Henner WD, Grochow LB, Olsen G, Edwards S, Stanbuck H et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res 1987; 47: 6402–6406. [PubMed] [Google Scholar]
- Meresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135–141. [PubMed] [Google Scholar]
- DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology 1996; 23: 589–599. [DOI] [PubMed] [Google Scholar]
- Bouligand J, Deroussent A, Simonnard N, Opolon P, Morizet J, Connault E et al. Induction of glutathione synthesis explains pharmacodynamics of high-dose busulfan in mice and highlights putative mechanisms of drug interaction. Drug Metab Dispos 2007; 35: 306–314. [DOI] [PubMed] [Google Scholar]
- DeLeve LD, Wang X. Role of oxidative stress and glutathione in busulfan toxicity in cultured murine hepatocytes. Pharmacology 2000; 60: 143–154. [DOI] [PubMed] [Google Scholar]
- Ansari M, Lauzon-Joset JF, Vachon MF, Duval M, Theoret Y, Champagne MA et al. Influence of GST gene polymorphisms on busulfan pharmacokinetics in children. Bone Marrow Transplant 2010; 45: 261–267. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122. [DOI] [PubMed] [Google Scholar]
- McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. The clinical course of 53 patients with venocclusive disease of the liver after marrow transplantation. Transplantation 1985; 39: 603–608. [DOI] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 2009; 4: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Wang J, Huff AM, Spence JD, Hegele RA. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin Genet 2004; 65: 483–486. [DOI] [PubMed] [Google Scholar]
- Tzeng JY, Lu W, Hsu FC. Gene-level pharmacogenetic analysis on survival outcomes using gene-trait similarity regression. Ann Appl Stat 2014; 8: 1232–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn LW, Ritchie MD, Moore JH. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics 2003; 19: 376–382. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of COOT. Acta Crystallogr D Biol Crystallogr 2010; 66 (Pt 4): 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhang Y. Improving the physical realism and structural accuracy of protein models by a two-step atomic-level energy minimization. Biophys J 2011; 101: 2525–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell SC, Davis IW, Arendall WB3rd, de Bakker PI, Word JM, Prisant MG et al. Structure validation by Calpha geometry: phi,psi and Cbeta deviation. Proteins 2003; 50: 437–450. [DOI] [PubMed] [Google Scholar]
- O'Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. J Cheminform 2011; 3: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GM GD, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem 1999; 19: 1639–1662. [Google Scholar]
- Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010; 31: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 2011; 51: 2778–2786. [DOI] [PubMed] [Google Scholar]
- Chauhan JS, Rao A, Raghava GP. In silico platform for prediction of N-, O- and C-glycosites in eukaryotic protein sequences. PLoS One 2013; 8: e67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken TA, Raman J, Fritz TA, Jamison O. Identification of common and unique peptide substrate preferences for the UDP-GalNAc:polypeptide alpha-N-acetylgalactosaminyltransferases T1 and T2 derived from oriented random peptide substrates. J Biol Chem 2006; 281: 32403–32416. [DOI] [PubMed] [Google Scholar]
- Diella F, Cameron S, Gemund C, Linding R, Via A, Kuster B et al. Phospho.ELM: a database of experimentally verified phosphorylation sites in eukaryotic proteins. BMC Bioinformatics 2004; 5: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61. [DOI] [PubMed] [Google Scholar]
- Hassan M, Ljungman P, Ringden O, Hassan Z, Oberg G, Nilsson C et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000; 25: 915–924. [DOI] [PubMed] [Google Scholar]
- Busulfex. [prescribing information]. Accessed 17 Februay 2012 (cited; available from: http://www.ema.europa.eu.
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22: 27–42. [DOI] [PubMed] [Google Scholar]
- Meister A. New aspects of glutathione biochemistry and transport—selective alteration of glutathione metabolism. Nutr Rev 1984; 42: 397–410. [DOI] [PubMed] [Google Scholar]
- Pastore A, Alisi A, di Giovamberardino G, Crudele A, Ceccarelli S, Panera N et al. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int J Mol Sci 2014; 15: 21202–21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JP, Hasek J, Kozich V, Collard R, Venezia S, Janosikova B et al. Cystathionine gamma-lyase: clinical, metabolic, genetic, and structural studies. Mol Genet Metab 2009; 97: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Jacques PF, Rogers G, Rosenberg IH, Selhub J. Serum total homocysteine concentrations in children and adolescents: results from the third National Health and Nutrition Examination Survey (NHANES III). J Nutr 2003; 133: 2643–2649. [DOI] [PubMed] [Google Scholar]