Key Points
Question
Does high-dose vitamin D supplementation (2000 IU/d) help to prevent wintertime viral upper respiratory tract infections compared with standard-dose vitamin D supplementation (400 IU/d) among preschool children?
Findings
In this multisite randomized clinical trial that included 703 children, the number of wintertime laboratory-confirmed viral upper respiratory tract infections was higher in the high-dose group than the standard-dose group, not a statistically significant difference.
Meaning
Vitamin D dosing higher than 400 IU/d may not be indicated for preventing wintertime viral upper respiratory tract infections in children.
Abstract
Importance
Epidemiological studies support a link between low 25-hydroxyvitamin D levels and a higher risk of viral upper respiratory tract infections. However, whether winter supplementation of vitamin D reduces the risk among children is unknown.
Objective
To determine whether high-dose vs standard-dose vitamin D supplementation reduces the incidence of wintertime upper respiratory tract infections in young children.
Design, Setting, and Participants
A randomized clinical trial was conducted during the winter months between September 13, 2011, and June 30, 2015, among children aged 1 through 5 years enrolled in TARGet Kids!, a multisite primary care practice–based research network in Toronto, Ontario, Canada.
Interventions
Three hundred forty-nine participants were randomized to receive 2000 IU/d of vitamin D oral supplementation (high-dose group) vs 354 participants who were randomized to receive 400 IU/d (standard-dose group) for a minimum of 4 months between September and May.
Main Outcome Measures
The primary outcome was the number of laboratory-confirmed viral upper respiratory tract infections based on parent-collected nasal swabs over the winter months. Secondary outcomes included the number of influenza infections, noninfluenza infections, parent-reported upper respiratory tract illnesses, time to first upper respiratory tract infection, and serum 25-hydroxyvitamin D levels at study termination.
Results
Among 703 participants who were randomized (mean age, 2.7 years, 57.7% boys), 699 (99.4%) completed the trial. The mean number of laboratory-confirmed upper respiratory tract infections per child was 1.05 (95% CI, 0.91-1.19) for the high-dose group and 1.03 (95% CI, 0.90-1.16) for the standard-dose group, for a between-group difference of 0.02 (95% CI, −0.17 to 0.21) per child. There was no statistically significant difference in number of laboratory-confirmed infections between groups (incidence rate ratio [RR], 0.97; 95% CI, 0.80-1.16). There was also no significant difference in the median time to the first laboratory-confirmed infection: 3.95 months (95% CI, 3.02-5.95 months) for the high-dose group vs 3.29 months (95% CI, 2.66-4.14 months) for the standard-dose group, or number of parent-reported upper respiratory tract illnesses between groups (625 for high-dose vs 600 for standard-dose groups, incidence RR, 1.01; 95% CI, 0.88-1.16). At study termination, serum 25-hydroxyvitamin D levels were 48.7 ng/mL (95% CI, 46.9-50.5 ng/mL) in the high-dose group and 36.8 ng/mL (95% CI, 35.4-38.2 ng/mL) in the standard-dose group.
Conclusions and Relevance
Among healthy children aged 1 to 5 years, daily administration of 2000 IU compared with 400 IU of vitamin D supplementation did not reduce overall wintertime upper respiratory tract infections. These findings do not support the routine use of high-dose vitamin D supplementation in children for the prevention of viral upper respiratory tract infections.
Trial Registration
clinicaltrials.gov Identifier: NCT01419262
This randomized clinical trial compares the effects of 2000 IU vs 400 IU of vitamin D supplementation on the number of upper respiratory tract infections among preschool children during winter months in Canada.
Introduction
Viral upper respiratory tract infections are the most common infectious illnesses of childhood. From the mid-1980s and onward, vitamin D has received attention for its potential to prevent disease through its role in both innate and adaptive immune responses. Both observational and clinical trial data have suggested a link between low levels of serum 25-hydroxyvitamin D and increased rates of respiratory tract infections. In addition to the morbidity they cause, upper respiratory tract infections place a considerable economic burden on health care systems and societies.
Vitamin D increases the synthesis of the antimicrobial peptide cathelicidin in respiratory epithelium, which has been shown to reduce disease severity and replication of the influenza virus in vitro. Although a number of clinical trials have attempted to assess the effect of vitamin D supplementation on the prevention of respiratory tract infections among adults and children, conclusions have been hampered by small sample sizes, short trial duration, and lack of laboratory-confirmed outcomes. Likewise, a meta-analysis of 7 clinical trials found no association between vitamin D and childhood acute respiratory infections, but limitations included a shortage of studies and heterogeneity in terms of populations and end points. To our knowledge, only 1 trial has assessed the effect of vitamin D on laboratory-confirmed upper respiratory tract infections in children specifically. It included 334 children in Japan and found a significant reduction in influenza A, but not influenza B, among children receiving 1200 IU/d of vitamin D supplementation. It remains unclear if vitamin D supplementation can prevent all-cause upper respiratory tract infections among children.
The vitamin D Outcomes and Interventions in Toddlers (DO IT) trial was conducted to examine the effect of high-dose oral vitamin D supplementation (2000 IU/d) vs the currently recommended supplemental dose for children (400 IU/d) over 4 to 8 winter months on all-cause laboratory-confirmed viral upper respiratory tract infection rates among children aged 1 to 5 years.
Methods
Study Design and Participants
This study was a multisite pragmatic randomized clinical superiority trial conducted in Toronto, Ontario, Canada (latitude 43° north). The study was designed as a parallel group trial with a 1:1 allocation over the 2011-2015 winter seasons. No changes to methods were made after trial commencement. Ethical approval was granted by St Michael’s Hospital and Hospital for Sick Children Research Ethics Boards. Written, informed consent was obtained from parents willing to participate and provide baseline measures. Trial procedures have been described in detail elsewhere (see Supplement 1).
Eligible participants were healthy children aged 1 to 5 years. Exclusion criteria were gestational age younger than 32 weeks and chronic illness (other than asthma). The study took place at 8 pediatric or family medicine group practices participating in TARGet Kids!, a primary care research network. Each site has between 3 and 10 practicing physicians. Parents of children scheduled for a well-child visit prior to the viral season (September through November) each year between 2011 and 2015 were approached to participate in the study.
Randomization and Blinding
Children were randomized to receive 1 of 2 formulations: the standard dose, 400 IU/d of vitamin D, or the high dose, 2000 IU/d. The standard dose was chosen to be consistent with vitamin D guidelines from the American Academy of Pediatrics (AAP) and high dose was chosen to be within the tolerable upper vitamin D intake specified by the Institute of Medicine. Children could only be randomized into the trial during 1 winter season. Parents of participants received a drop-based formulation in order to ease administration of the study drug (Kids Ddrops containing Vitamin D3). From the time of enrollment (September-November) until follow-up (April-May), parents were instructed to administer 1 drop of the provided solution to their child by mouth once each day. Drops were identical in taste, volume, and color between the intervention groups; they differed only in concentration of vitamin D. Drops could be given at any time during the day. The recruitment time window was chosen with the knowledge that the circulation of respiratory viruses peaks during the winter months and serum 25-hydroxyvitamin D levels are known to stabilize within 8 weeks. The duration of the dosing regimen (4-8 months) was selected to follow the routine practice of supplementing preschoolers with vitamin D over the winter months.
The randomization sequence was generated using a computer-based random-number generator by the SickKids research pharmacy. The study biostatistician (K.T.) was unaware of the randomization sequence. Randomization was stratified by practice site with blocks of size 4. The research pharmacy prepared the vitamin D formulations in sealed, serially numbered bottles identical in appearance and weight to maintain allocation concealment. Study personnel, parents, attending physicians, laboratory personnel, investigators, and data analysts were all blinded to group allocation throughout the study period. Research assistants at each site approached participants for entry into the study.
Procedures
After providing informed consent, parents completed a standardized data collection form with questions adapted from the Canadian Community Health Survey. These included age; sex; birth weight; enrollment date; skin pigmentation; parents’ race/ethnicity; maternal age, educational level, and health status; duration of breastfeeding; bottle use; current and past vitamin D supplementation; influenza immunization status; physical activity; outdoor time; and sun exposure. Skin pigmentation and ethnicity were captured in this study since previous work has suggested that both factors are related to the cutaneous production of vitamin D. Ethnicity was reported by the parent using fixed categories and skin pigmentation was assessed by trained research assistants through fixed categories using the Fitzpatrick scale. Research assistants also recorded child height and weight using standardized techniques described elsewhere. Baseline serum 25-hydroxyvitamin D levels were measured using the Roche ELECSYS Vitamin D total assay (Roche Diagnostics Ltd) with a functional sensitivity of 1.60 ng/mL (to convert from ng/mL to nmol/L, multiply by 2.496) (coefficient of variance, 18.5%) and an analytical specificity of 100% for 25-hydroxyvitamin D3.
After receiving bottles containing the assigned formulation, parents were informed that concomitant interventions such as over-the-counter multivitamins that contain vitamin D, over-the-counter vitamin D preparations, and prescription vitamin D were prohibited. Throughout the winter months, parents were asked to complete a symptom checklist and collect viral nasal swabs for every upper respiratory tract infection and were reminded through monthly telephone calls. Parents were instructed on the proper technique for obtaining nasal swabs, including storing swabs in the provided transport media in the refrigerator until couriered within 24 hours to the study laboratory. Nasal swabs collected by parents have been shown to be as effective as those obtained by health professionals in detecting respiratory viruses. Parents were not required to visit their child’s physician to report an upper respiratory tract infection episode.
In the months of April and May the following year (4-8 months after randomization), participants and their parents returned to the physician’s office, at which point a follow-up data collection form was completed and follow-up 25-hydroxyvitamin D levels were measured from venous blood samples. Parents were asked to return the bottles, and the amount of vitamin D administered was calculated by measuring the amount of remaining formulation.
The primary outcome was the number of all-cause laboratory-confirmed viral upper respiratory tract infections per child. This was ascertained by detecting the presence of respiratory viruses in parent-collected nasal swabs using the Luminex xMAP ID-Tag RVP assay system (Luminex Corp), which tested for 18 common viruses including influenza A and B, adenoviruses, respiratory syncytial virus, picornaviruses, coronavirus, human metapneumovirus, and parainfluenza viruses with an overall clinical sensitivity that exceeds 92% for all targets and an overall specificity that exceeds 97% for all targets. Defining respiratory infection using laboratory confirmation avoids the uncertainty associated with symptoms and illness presentations through parent reported measures.
Secondary outcomes included time to first laboratory-confirmed, total parent-reported, laboratory-confirmed influenza, and noninfluenza upper respiratory tract infections and serum 25-hydroxyvitamin D levels. Other secondary outcomes not presented in this article included asthma exacerbations among children with asthma, physician-diagnosed otitis media and pneumonia, emergency department visits, and hospitalizations.
Sample Size and Statistical Analysis
It was assumed that participants would have an average of 1 laboratory-confirmed upper respiratory tract infection per month during the minimum 4-month winter period. Considering that the infections in children can be stressful and disruptive for families and that studies suggest that a reduction of infectious burden between 25% and 30% is relevant to patients, we assumed a reduction of 1 per winter would have clinical importance. Under these assumptions, it was estimated that a sample of 300 participants per group would be required to detect this effect with 90% power at the .05 level of significance. This number was increased to 375 children per group to compensate for loss to follow-up. The sample size assumed a Poisson distributed outcome and was based on asymptotic methods for a likelihood ratio test.
The number of upper respiratory tract infection events per child was summed and the mean infection rates (per child) per group were calculated. For the primary analysis comparing the number of laboratory-confirmed infections per group, a negative-binomial model using the variable length of follow-up as an offset was computed. An offset variable is the length of time a participant was observed. The offset was used to adjust for varying lengths of participant follow-up. The negative binomial model produces an incidence rate ratio (RR), which provides a relative measure of the effect of high-dose vs standard-dose vitamin D supplementation. It is derived as the number of incident infections per season for the 2000-IU/d group divided by the number of incident infections per season for the 400-IU/d group. Parental-reported infections were compared using a similar method. Time to first infection was examined using Kaplan-Meier curves and the log-rank test. Prespecified analyses of influenza and noninfluenza incidence were conducted using Poisson and negative-binomial models respectively using length of follow-up as an offset. Complete case analysis was performed for all primary and secondary outcomes in which only cases with available data were analyzed. Adverse events were monitored by educating collaborating community physicians about the signs and symptoms of vitamin D toxicity and asking them to report any adverse events to the study. In addition, serum calcium, alkaline phosphatase, and parathyroid hormone were measured at a follow-up study visit.
All analyses were conducted using the intention-to-treat principle. The α level was 2-sided with statistical significance set at P = .05. All analyses were conducted using SAS version 9.2 (SAS Institute Inc) and R v 3.2.5.
Results
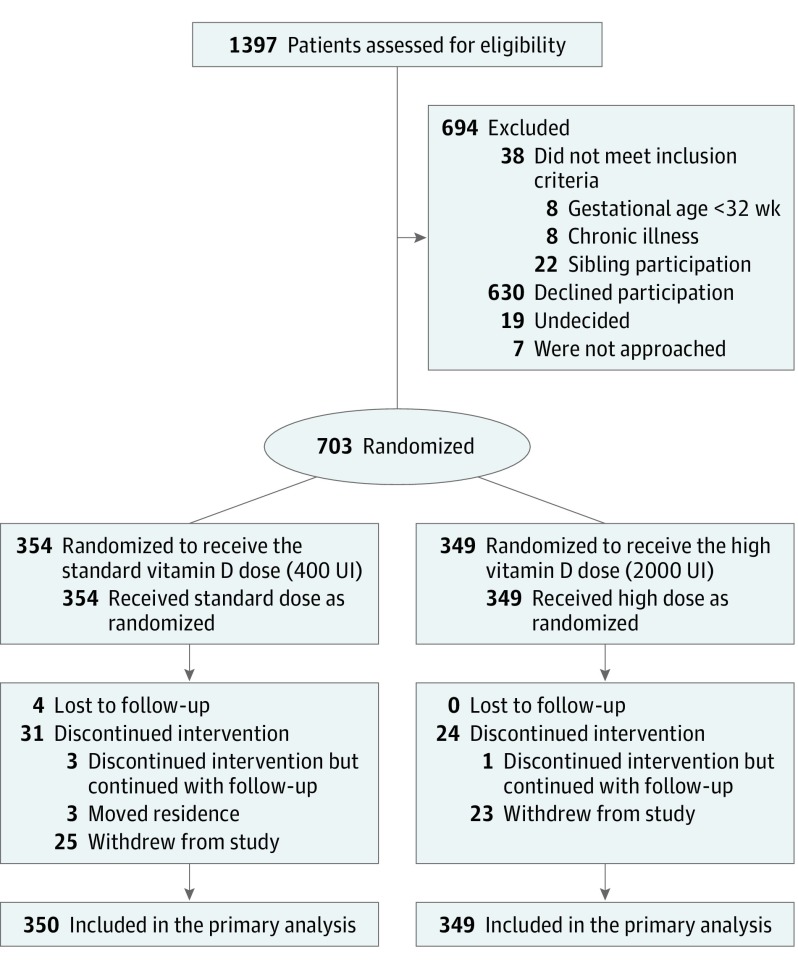
Of 1397 participants screened, 703 eligible participants consented to participate and were randomly assigned to receive 1 of the 2 vitamin D formulations (Figure 1). There were no clinically important differences between the enrolled and nonenrolled participants (eTable in Supplement 2). Three hundred fifty-four participants (50.4%) were allocated to receive the standard dose, and 349 participants (49.6%) were allocated to receive the high dose. Six hundred ninety-nine (99.4%) participants had data available for the primary analysis; 4 (0.6%) were lost to follow-up. Fifty-five (7.9%) discontinued treatment but completed follow-up. The mean follow-up length and exposure duration was 6.2 months (95% CI, 5.1-7.2 months) for the standard-dose group and 6.3 months (95% CI, 5.8-7.4 months) for the high-dose group. All participants with primary outcome data were included in the intention-to-treat analysis (n = 699). Four hundred sixty-five participants (66.1%) returned bottles at the end of the study. Among bottles returned, quantities of administered formula were comparable between groups: the high-dose mean formulation administered was 5.39 g (95% CI, 5.11-5.66 g); the standard dose mean was 5.01 g (95% CI, 4.72-5.29 g). The average proportion of the intended dose received was also comparable between groups: the high dose was 98% (95% CI, 93%-102%); the standard dose, 100% (95% CI, 92%-105%).
Figure 1. Flow Diagram for Treatment Effect of High-Dose vs Standard-Dose Vitamin D Oral Supplementation on Laboratory-Confirmed Upper Respiratory Tract Infection.
Balance was achieved on all measured baseline characteristics. Two hundred ninety-six participants (42.3%) were girls. The mean age at recruitment was 2.70 years (95% CI, 2.58-2.81 years). The mean baseline serum 25-hydroxyvitamin D level for the high-dose group was 35.9 ng/mL (SD, 12.3 ng/mL) and was 36.9 ng/mL (SD, 11.8 ng/mL) for the standard-dose group (Table 1).
Table 1. Sample Baseline Characteristics.
| Vitamin D Dose | ||
|---|---|---|
| 400 IU/d (n = 354) |
2000 IU/d (n = 349) |
|
| Sex, No (%) | ||
| Girl | 139 (39.4) | 157 (45.2) |
| Boy | 214 (60.6) | 190 (54.8) |
| Age, mean (SD), y | 2.76 (1.54) | 2.70 (1.52) |
| Serum 25-hydroxyvitamin D, ng/mL | ||
| Mean (SD) | 36.9 (11.7) | 35.9 (12.3) |
| <30, No. (%) | 108 (32.66) | 114 (30.50) |
| Regular vitamin D supplementation, No. (%) | ||
| No | 131 (40.6) | 125 (39.3) |
| Yes | 192 (59.4) | 193 (60.7) |
| IU per day, median (IQR) | 0 (0-400) | 0 (0-400) |
| Average minutes in unstructured free play outdoors per week, median (IQR) | 60 (30-73) | 35 (20-60) |
| Influenza vaccination, No. (%) | ||
| No | 264 (77.6) | 256 (78.3) |
| Yes | 76 (22.4) | 71 (21.7) |
| Fitzpatrick skin type, No. (%) | ||
| I, Pale white | 39 (11.2) | 50 (14.9) |
| II, White | 116 (33.4) | 105 (31.3) |
| III, Light brown | 115 (33.1) | 114 (34.0) |
| IV, Moderate brown | 41 (11.8) | 35 (10.4) |
| V, Dark brown | 22 (6.3) | 18 (5.4) |
| VI, Deeply pigmented dark brown-black | 14 (4.0) | 13 (3.9) |
| Birth weight, mean (SD), kg | 3.41 (0.64) | 3.96 (11.50) |
| Currently using bottle, No. (%) | ||
| No | 204 (71.8) | 198 (72.3) |
| Yes | 80 (28.2) | 76 (27.7) |
| Currently breastfed, No. (%) | ||
| No | 196 (78.7) | 200 (82.0) |
| Yes | 53 (21.3) | 44 (18.0) |
| Weight, mean (SD), kg | 14.71 (3.98) | 13.94 (4.46) |
| Height, mean (SD), cm | 94.74 (12.39) | 93.26 (14.71) |
| Body mass index, z score, mean (SD) | 0.08 (1.25) | 0.18 (1.11) |
| Mother’s education level, No. (%) | ||
| College or university | 307 (89.8) | 302 (91.2) |
| High school | 32 (9.4) | 24 (7.3) |
| Public school | 3 (0.9) | 5 (1.5) |
| Mother’s ethnicity, No. (%) | ||
| European | 206 (66.9) | 196 (67.1) |
| East Asian | 15 (4.9) | 21 (7.2) |
| South Asian | 13 (4.2) | 12 (4.1) |
| Southeast Asian | 15 (4.9) | 14 (4.8) |
| Arab | 4 (1.3) | 7 (2.4) |
| African | 22 (7.1) | 14 (4.8) |
| Latin American | 9 (2.9) | 10 (3.4) |
| Mixed ethnicity | 21 (6.8) | 18 (6.2) |
| Other | 3 (1.0) | 0 (0.0) |
| Father’s ethnicity, No. (%) | ||
| European | 193 (66.3) | 207 (68.8) |
| East Asian | 13 (4.5) | 8 (2.7) |
| South Asian | 19 (6.5) | 14 (4.6) |
| Southeast Asian | 7 (2.4) | 12 (4.0) |
| Arab | 9 (3.1) | 5 (1.7) |
| African | 20 (6.9) | 29 (9.6) |
| Latin American | 11 (3.8) | 13 (4.3) |
| Mixed ethnicity | 16 (5.5) | 12 (4.0) |
| Other | 3 (1.0) | 1 (0.3) |
Abbreviation: IQR, interquartile range.
SI conversion factor: to convert serum 25-hydroxyvitamin D from ng/mL to nmol/L, multiply by 2.496.
A total of 728 laboratory-confirmed upper respiratory tract infections occurred throughout the study period. Eight hundred eighty-three swabs were submitted by 415 participants; 155 of these swabs from 130 participants tested negative for any infection (Table 2). In total, 322 participants (46.0%) had no laboratory-confirmed infections. The mean number of infections per child was 1.03 (95% CI, 0.90-1.16; the median, 1; interquartile range [IQR], 0-2) for the standard-dose group and was 1.05 (95% CI, 0.91-1.19; median, 1; IQR, 0-2) for the high-dose group, for a between-group difference of 0.02 (95% CI, −0.17 to 0.21). In the negative binomial model, there was no statistically significant difference in the rate of all-cause upper respiratory tract infections between the groups (incidence RR, 0.97; 95% CI, 0.80-1.16; Table 2). Site-specific incidence RRs were symmetrically distributed around the overall incidence RR (eFigure in Supplement 2).
Table 2. Laboratory-Confirmed Upper Respiratory Tract Infection by Study Group.
| Vitamin D Dose | Incidence Rate Ratio (95% CI) | P Value | ||
|---|---|---|---|---|
| 400 IU/d (n = 354) |
2000 IU/d (n = 349) |
|||
| Primary Outcome Measure | ||||
| Laboratory-confirmed upper respiratory tract infections | ||||
| Parent-report per child, No. | ||||
| 0 | 157 | 165 | ||
| 1 | 94 | 87 | ||
| 2 | 56 | 41 | ||
| ≥3 | 43 | 56 | ||
| Mean (95% CI) | 1.03 (0.90 to 1.16) |
1.05 (0.91 to 1.19) |
||
| Median (IQR) | 1 (0 to 2) |
1 (0 to 2) |
||
| No. of person-years | 177.3 | 184.6 | ||
| Incidence per person-year (95% CI) | 2.04 (1.83 to 2.26) |
1.99 (1.79 to 2.20) |
0.97 (0.80 to 1.16)a |
.71 |
| −0.05 (−0.34 to 0.24)b |
.74 | |||
| Other Measures | ||||
| Children, No. with laboratory-confirmed upper respiratory tract infections | 193 | 184 | ||
| Swabs submitted for testing, No. | 430 | 453 | ||
| Result, No. (%) | ||||
| Positive for upper respiratory tract infections | 361 (84.0) | 367 (81.0) | ||
| Negative for upper respiratory tract infections | 69 (16.0) | 86 (19.0) | ||
| Swabs positive for respiratory virus, No. (%) | ||||
| Adenovirus | 15 (4.2) | 11 (3.0) | ||
| Coronavirus | 26 (7.2) | 34 (9.3) | ||
| Enterovirus or rhinovirus | 216 (59.8) | 232 (63.2) | ||
| Metapneumovirus | 18 (5.0) | 14 (3.8) | ||
| Parainfluenza | 27 (7.5) | 24 (6.5) | ||
| Respiratory syncytial virus | 28 (7.8) | 36 (9.8) | ||
| Any noninfluenza virus | 330 (91.4) | 351 (95.6) | 1.01 (0.83 to 1.23)a |
.91 |
| Influenza A | 20 (5.5) | 7 (1.9) | ||
| Influenza B | 11 (3.0) | 9 (2.5) | ||
| Any influenza virus | 31 (8.5) | 16 (4.4) | 0.50 (0.28 to 0.89)c |
.02 |
Abbreviation: IQR, interquartile range.
Incidence rate ratio from negative binomial model.
Incidence rate difference.
Incidence rate ratio from Poisson model.
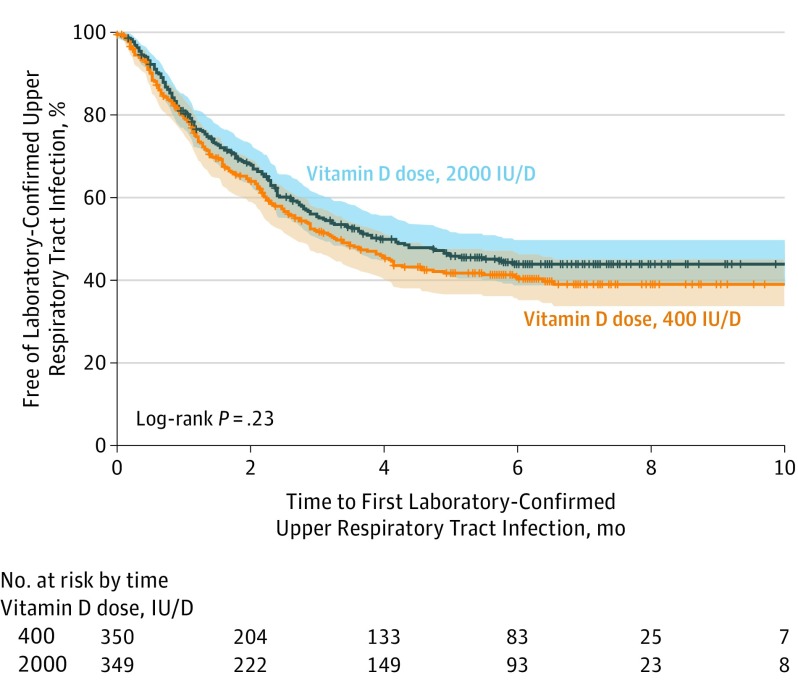
The median time to the first laboratory-confirmed upper respiratory tract infection was 3.29 months (95% CI, 2.66-4.14 months) for the standard-dose group and 3.95 months (95% CI, 3.02-5.95 months) for the high-dose group. There was no statistically significant difference in the groups with respect to time to first laboratory-confirmed infection (Figure 2).
Figure 2. Kaplan-Meier Curve for Time to First Laboratory-Confirmed Upper Respiratory Tract Infection.
Ticks on the curves indicate participants who have been right-censored due to end of follow-up. Tinted regions indicate 95% confidence intervals around the proportion of participants free of laboratory-confirmed upper respiratory tract infection. The median follow-up length of time for the standard-dose group was 6.2 months (interquartile range [IQR], 5.1-7.2 months) and 6.3 months (IQR, 5.8-7.4 months) for the high-dose group.
Parents reported a total of 1225 respiratory tract illness episodes, with an average of 1.91 illnesses (95% CI, 1.72-2.09) per child (median, 2; IQR, 1-3) for the standard-dose group and 1.97 illnesses (95% CI, 1.78-2.16) per child (median, 2; IQR, 1-3) for the high-dose group, for a between-group difference of 0.06 (95% CI, −0.22 to 0.33) illnesses per child. There was no statistically significant difference in the incidence of parent-reported upper respiratory tract illnesses between groups (incidence RR, 1.01; 95% CI, 0.88-1.16; Table 3).
Table 3. Secondary Outcome Measures by Study Group.
| Vitamin D Dose | Incidence Rate Ratio or Coefficient (95% CI) | P Value | ||
|---|---|---|---|---|
| 400 IU/d (n = 354) |
2000 IU/d (n = 349) |
|||
| Time to first laboratory-confirmed upper respiratory tract infections, median (95% CI), mo | 3.29 (2.66 to 4.14) |
3.95 (3.02 to 5.95) |
.23 | |
| Parent-report per child, No. | ||||
| 0 | 76 | 73 | ||
| 1 | 69 | 76 | ||
| 2 | 75 | 71 | ||
| 3 | 39 | 47 | ||
| ≥4 | 58 | 47 | ||
| Mean per child (95% CI) | 1.91 (1.72 to 2.09) |
1.97 (1.78 to 2.16) |
||
| Median per child (IQR) | 2 (1 to 3) | 2 (1 to 3) | ||
| No. of person-years | 177.7 | 184.6 | ||
| Incidence rate for parent-reported respiratory tract illnesses (95% CI) per person-year | 3.39 (3.12 to 3.67) |
3.39 (3.12 to 3.66) |
1.01 (0.88 to 1.16)a |
.89 |
| 0.00 (−0.38 to 0.38)b |
.99 | |||
| Children with any parent-reported episodes, No. | 241 | 241 | ||
| Overall No. of parent-reported episodes | 600 | 625 | ||
| Serum 25-hydroxyvitamin D level, ng/mL | ||||
| At study termination, mean (95% CI) | 36.8 (35.4 to 38.2) |
48.7 (46.9 to 50.5) |
12.3 (10.3 to 14.3)c |
<.001 |
| Children with serum 25-hydroxyvitamin D level <30 ng/mL, No. (%) | 78 (22.2) | 20 (5.7) | ||
SI conversion factor: to convert serum 25-hydroxyvitamin D from ng/mL to nmol/L, multiply by 2.496.
Incidence rate ratio from negative binomial model.
Incidence rate difference.
B, coefficient from linear regression.
There was a statistically significant difference in serum 25-hydroxyvitamin D levels between the groups (P < .001) at study termination. Mean 25-hydroxyvitamin D levels were 48.7 ng/mL (95% CI, 46.9-50.5 ng/mL) in the high-dose group and 36.8 ng/mL (95% CI, 35.4-38.2 ng/mL) in the standard-dose group. After adjusting for baseline 25-hydroxyvitamin D serum levels, the high-dose group demonstrated a 12.3 ng/mL (95% CI, 10.3-14.3 ng/mL) increase in serum 25-hydroxyvitamin D levels compared with the standard-dose group at study termination (Table 3). No adverse events were reported.
The prespecified comparison of the incidence of influenza (influenza A and influenza B combined) revealed that the incidence of influenza infections in the high-dose group was reduced by 50% (incidence RR, 0.50; 95% CI, 0.28-0.89; Table 2). However, relative to other infection types there were few influenza infections that occurred in each group: the number of influenza infections in the high-dose group was 16 (4.4% of all infections) vs 31 (8.5% of all infections) in the standard-dose group (mean, 0.05; 95% CI, 0.03-0.07 in the high-dose group vs 0.09; 95% CI, 0.06-0.12 in the standard-dose group), for a between-group difference of 0.04 (95% CI, 0.01-0.08). This reduction was smaller than the minimal clinically important difference. No difference between groups was detected for incidence of noninfluenza viruses (all viruses except influenza, combined) (incidence RR, 1.01; 95% CI, 0.83-1.23). Enterovirus or rhinovirus was the most prevalent respiratory virus (n = 448 out of 728 swabs that tested positive for a virus). There were no clinically important differences between the groups for the number of upper respiratory tract infection episodes for adenovirus, coronavirus, human metapneumovirus, parainfluenza, and respiratory syncytial virus.
Discussion
In this study involving 703 healthy children aged 1 to 5 years, supplementation with high-dose vitamin D compared with standard dose vitamin D did not reduce all-cause upper respiratory tract infections during the winter season despite a 12.3 ng/mL increase in serum 25-hydroxyvitamin D concentration between groups. This conclusion remained whether the outcome was defined as number of laboratory-confirmed upper respiratory tract infections, time to first upper respiratory tract infection or parent-reported upper respiratory tract illness.
Conclusions of systematic reviews and meta-analyses of clinical trials that have assessed the association of vitamin D in children with respiratory infections have varied and were limited by the variability of dosing regimens (bolus vs daily supplementation), age ranges (preschoolers vs adults), populations (both healthy and unhealthy participants), types of respiratory tract infections (aggregating upper and lower, bacterial and viral respiratory tract infections), outcome measurement (severity, duration, number) and laboratory confirmation of respiratory tract infection. Meta-analyses of trials have demonstrated both positive and null associations of vitamin D with respiratory infections. However, all systematic reviews included very few studies (<5) involving children, and only one of these studies examined viral upper respiratory tract infections in children specifically.
Although the present study did not reveal a protective effect of high-dose vitamin D supplementation over a standard dose on overall upper respiratory tract infection frequency, it is possible that high-dose supplementation is effective in preventing upper respiratory tract infections in certain subpopulations. Bergman et al found that symptoms were significantly reduced among participants with antibody deficiency receiving daily 4000 IU vitamin D3 vs placebo. Several groups have suggested a link between vitamin D and asthma exacerbations among children. Asthma exacerbations are known to be associated with upper respiratory tract infections suggesting a possibly protective effect of vitamin D among children with asthma.
The secondary finding that vitamin D may be associated with a lower risk of influenza is limited in its interpretation given the small number of influenza infections and should be more formally examined in future studies. Furthermore, the reduction in influenza infections was less than the minimal clinically important difference of 1 infection per winter season. However, this finding is consistent with the only other randomized clinical trial that investigated vitamin D supplementation on laboratory-confirmed upper respiratory tract infection in children. Urashima et al investigated the effect of 1200 IU/d of vitamin D supplementation vs placebo on the incidence of seasonal influenza A and B among children aged 6 to 15 years. They found that vitamin D supplementation reduced the incidence of influenza A but not the incidence of influenza B. In the present study, although the incidence was statistically reduced in the high-dose group, the difference in the mean number of influenza infections per child between groups was minimal (0.05 infections per season in the high-dose group vs 0.09 infections per season in the standard-dose group). Investigation of vitamin D supplementation for prevention of influenza infection in populations or seasons during which influenza is epidemic would be necessary to evaluate these findings.
The study has a number of strengths including testing the currently recommended American Academy of Pediatrics standard dose of vitamin D against the safe upper limit as recommended by the Institute of Medicine. Also, it was a trial designed to mimic the routine practice of supplementing children with daily vitamin D in the wintertime as recommended by many practitioners and performed in households across North America. Also, the use of laboratory-confirmed upper respiratory tract infection as the primary outcome is well-defined and unbiased. Furthermore, the trial was conducted across multiple sites including both family physician and primary care pediatric physicians suggesting generalizability across primary care settings, and it was conducted over 5 winter seasons, capturing yearly variation in upper respiratory tract infection frequency.
There are several limitations to this study. First, although parents are as capable as clinicians in administering nasal swabs, children may have had upper respiratory tract infections without swabs being submitted. Because daily monitoring of infections was not feasible, monthly contact with each family revealed a similar null effect of vitamin D on parent-reported upper respiratory tract illnesses. Second, this study was also not able to make comparisons to a placebo group; this was prohibited by the research ethics board due to the American Academy of Pediatrics suggested dose of 400 IU/d. It is possible that 400 IU/daily may have been sufficient for a protective effect against infections. Third, like other national studies of children, baseline 25-hydroxyvitamin D serum levels at the end of the summer were relatively high and this could have contributed to the lack of effect of high-dose vitamin D supplementation. Vitamin D supplementation may provide greater benefit among children with lower baseline serum 25-hydroxyvitamin D levels. Previous work in healthy adults has suggested that a 25-hydroxyvitamin D concentration of 30 ng/mL is sufficient to reduce the incidence of upper respiratory tract infections. Fourth, the sample size calculation was powered to detect a reduction of 1 infection per season assuming 1 infection per month, but the data demonstrated that children experienced on average 1 laboratory-confirmed upper respiratory tract infection per season. Preliminary calculations using a Poisson distribution showed that fewer infections per season required even smaller sample sizes; thus, the study was powered to detect differences in the primary outcome.
Conclusions
Among healthy children aged 1 to 5 years, daily administration of 2000 IU compared with 400 IU of vitamin D supplementation did not reduce overall wintertime upper respiratory tract infections. These findings do not support the routine use of high-dose vitamin D supplementation in children for the prevention of viral upper respiratory tract infections.
Trial Protocol
eTable. Nonenrolled vs enrolled baseline characteristics results of quality assessment per study
eFigure. Forest plot of site-specific treatment effect of high dose vitamin D (2000 IU/day) vs standard dose vitamin D (400 IU/ Day) on URTI
References
- 1.Taylor S, Lopez P, Weckx L, et al. Respiratory viruses and influenza-like illness: epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J Infect. 2017;74(1):29-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65(5 pt 2):106R-113R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman P, Lindh AU, Björkhem-Bergman L, Lindh JD. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8(6):e65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charan J, Goyal JP, Saxena D, Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J Pharmacol Pharmacother. 2012;3(4):300-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487-494. [DOI] [PubMed] [Google Scholar]
- 6.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909-2912. [DOI] [PubMed] [Google Scholar]
- 7.Barlow PG, Svoboda P, Mackellar A, et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6(10):e25333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camargo CA Jr, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012;130(3):e561-e567. [DOI] [PubMed] [Google Scholar]
- 9.Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2(6):e001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li-Ng M, Aloia JF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect. 2009;137(10):1396-1404. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308(13):1333-1339. [DOI] [PubMed] [Google Scholar]
- 12.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91(5):1255-1260. [DOI] [PubMed] [Google Scholar]
- 13.Grant CC, Kaur S, Waymouth E, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial. Acta Paediatr. 2015;104(4):396-404. [DOI] [PubMed] [Google Scholar]
- 14.Xiao L, Xing C, Yang Z, et al. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. Br J Nutr. 2015;114(7):1026-1034. [DOI] [PubMed] [Google Scholar]
- 15.Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition . Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142-1152. [DOI] [PubMed] [Google Scholar]
- 16.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes for Calcium And Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 17.Kids Ddrops. Ddrops website. http://ca.vitaminddrops.com/products/kids/. Published 2016. Accessed August 8, 2016.
- 18.Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222-230. [DOI] [PubMed] [Google Scholar]
- 19.Aloia JF, Talwar SA, Pollack S, Feuerman M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr. 2006;84(3):602-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Nooijer J, Jansen R, van Assema P. The use of implementation intentions to promote vitamin D supplementation in young children. Nutrients. 2012;4(10):1454-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor JA, Geyer LJ, Feldman KW. Use of supplemental vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125(1):105-111. [DOI] [PubMed] [Google Scholar]
- 22.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M; Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society . Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122(2):398-417. [DOI] [PubMed] [Google Scholar]
- 23.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76. [DOI] [PubMed] [Google Scholar]
- 24.Hintzpeter B, Scheidt-Nave C, Müller MJ, Schenk L, Mensink GB. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutr. 2008;138(8):1482-1490. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869-871. [DOI] [PubMed] [Google Scholar]
- 26.Onis M; WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450(S450):76-85. [DOI] [PubMed] [Google Scholar]
- 27.Elecsys vitamin D total: product characteristics. Cobas website. http://www.cobas.com/home/product/clinical-and-immunochemistry-testing/elecsys-vitamin-d-total-assay.html. Published August 2012. Accessed September 1, 2016.
- 28.Jacobs B, Young NL, Dick PT, et al. Canadian Acute Respiratory Illness and Flu Scale (CARIFS): development of a valid measure for childhood respiratory infections. J Clin Epidemiol. 2000;53(8):793-799. [DOI] [PubMed] [Google Scholar]
- 29.Esposito S, Molteni CG, Daleno C, et al. Collection by trained pediatricians or parents of mid-turbinate nasal flocked swabs for the detection of influenza viruses in childhood. Virol J. 2010;7(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.xTAG respiratory viral panel (RVP) clinical brochure. Luminex website. https://www.luminexcorp.com/clinical/infectious-disease/respiratory-viral-panel/resources/. Published 2016. Accessed August 14, 2016.
- 31.Lambert SB, Allen KM, Druce JD, et al. Community epidemiology of human metapneumovirus, human coronavirus NL63, and other respiratory viruses in healthy preschool-aged children using parent-collected specimens. Pediatrics. 2007;120(4):e929-e937. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz B, Giebink GS, Henderson FW, Reichler MR, Jereb J, Collet JP. Respiratory infections in day care. Pediatrics. 1994;94(6 pt 2):1018-1020. [PubMed] [Google Scholar]
- 33.Heikkinen T, Järvinen A. The common cold. Lancet. 2003;361(9351):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett B, Harahan B, Brown D, Zhang Z, Brown R. Sufficiently important difference for common cold: severity reduction. Ann Fam Med. 2007;5(3):216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Signorini DF. Sample size for Poisson regression. Biometrika. 1991;78(2):446-450. [Google Scholar]
- 36.Vuichard Gysin D, Dao D, Gysin CM, Lytvyn L, Loeb M. Effect of Vitamin D3 supplementation on respiratory tract infections in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2016;11(9):e0162996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao S, Huang S. Vitamin D supplementation and risk of respiratory tract infections: a meta-analysis of randomized controlled trials. Scand J Infect Dis. 2013;45(9):696-702. [DOI] [PubMed] [Google Scholar]
- 38.Litonjua AA, Carey VJ, Laranjo N, et al. Effect of prenatal supplementation with vitamin D on asthma or recurrent wheezing in offspring by age 3 years: the VDAART randomized clinical trial. JAMA. 2016;315(4):362-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chawes BL, Bønnelykke K, Stokholm J, et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353-361. [DOI] [PubMed] [Google Scholar]
- 40.Rawlinson WD, Waliuzzaman Z, Carter IW, Belessis YC, Gilbert KM, Morton JR. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J Infect Dis. 2003;187(8):1314-1318. [DOI] [PubMed] [Google Scholar]
- 41.Mansbach JM, Ginde AA, Camargo CA Jr. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics. 2009;124(5):1404-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langlois K, Green-Finestone L, Little J, Hidiroglou N, Whiting S Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2010:21(1)47-55. http://publications.gc.ca/collections/collection_2010/statcan/82-003-X/82-003-x2010001-eng.pdf. Published March 2010. Accessed August 3, 2016. [PubMed]
- 43.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5(6):e11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Belle G. Statistical Rules of Thumb. 2nd ed Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Nonenrolled vs enrolled baseline characteristics results of quality assessment per study
eFigure. Forest plot of site-specific treatment effect of high dose vitamin D (2000 IU/day) vs standard dose vitamin D (400 IU/ Day) on URTI