Key Points
Question
What is known about cutaneous adverse events associated with anti–programmed cell death 1 (anti–PD-1) and anti–programmed cell death ligand 1 (anti–PD-L1 ) therapies for patients with lung cancer?
Findings
In this case series we report 14 patients with hair repigmentation during their anti–PD-1/anti–PD-L1 treatment for lung cancer. Thirteen of the 14 patients remained in treatment with stable disease or partial response, and only 1 had to discontinue therapy owing to disease progression after 4 cycles of treatment.
Meaning
Hair repigmentation may be a good response marker in patients receiving anti–PD-1/anti–PD-L1 therapy for lung cancer.
This case series study examines hair repigmentation in patients receiving anti–programmed cell death 1 and anti–programmed cell death ligand 1 therapy for treatment of lung cancer.
Abstract
Importance
New targeted therapies for cancer have been released in recent years, opening new horizons in the treatment of patients with cancer. However, their related adverse events (AE) are not fully characterized. Hair repigmentation (HR) is a nondescribed effect secondary to anti–programmed cell death 1 (anti–PD-1) and anti–programmed cell death ligand 1 (anti–PD-L1 ) therapy for treatment of lung cancer (LC), in opposition to the vitiligo reactions that develop during melanoma treatment.
Objective
To describe a new adverse event occurring during anti–PD-1/anti–PD-L1 therapy for LC.
Design, Setting, and Participants
A case series from a descriptive observation of 14 patients with HR after anti–PD-1/anti–PD-L1 treatment, recruited between September and December, 2016, who were followed up to detect whether they developed cutaneous AE at the time HR was detected. The patients had all been treated in the dermatology department at Hospital Universitari Germans Trias i Pujol, Badalona, Spain.
Main Outcomes and Measures
Clinical observation of HR during anti–PD-1/anti–PD-L1 therapy for LC, proved by comparing old pictures provided by the patients and recent pictures taken during the follow-up.
Results
Fourteen patients (13 men and 1 woman; mean age, 64.9 years) receiving anti–PD-1 or anti–PD-L1 therapy for non–small-cell lung cancer (NSCLC) presented hair repigmentation during follow-up. This hair repigmentation consisted in a diffuse darkening of the hair in 13 of 14 patients, or in black patches between white hairs in 1. Thirteen of 14 patients presented a good clinical response to the treatment, with at least stable disease, and only 1 had to stop the therapy after only 4 cycles of treatment owing to a life-threatening progression of the disease.
Conclusions and Relevance
We present to our knowledge the first report of hair repigmentation owing to anti–PD-1/anti–PD-L1 therapy for lung cancer in a series of 14 patients. Hair repigmentation may be a good response marker in patients receiving anti-PD1/anti–PD-L1 therapy for LC.
Introduction
In the past few years some new targeted therapies have been developed. The programmed death receptor-1 (PD-1) and PD ligand-1 (PD-L1) are immune checkpoints that prevent the immune system from acting against the patient’s own tissues, thus by blocking these mediators it is possible to prevent tumors from escaping the immune system response. Now 2 anti–PD-1 (nivolumab and pembrolizumab) and 1 anti–PD-L1 (atezolizumab) drug is approved for metastatic non–small-cell lung cancer (NSCLC).
Owing to the blocking of an immune checkpoint that prevents autoimmunity, several immune-related adverse events have been described. The skin is the most frequently affected organ. Although only 5% of patients develop severe reactions, about half will develop mild to moderate cutaneous adverse events. These include cutaneous eruption, vitiligo, and pruritus, but no cases of hair repigmentation (HR) have been reported so far.
Report of Cases
In our dermatology department there are 52 patients with lung cancer (LC) in an ongoing prospective study to see whether they develop cutaneous toxic effects while undergoing anti-PD1/anti–PD-L1 therapy. The Hospital Universitari Germans Trias i Pujol institutional review board approved the study and patients gave written informed consent. They were not compensated for participating. We present 14 cases (13 men and 1 woman; mean age, 64.9 years) of HR during anti–PD-1/anti–PD-L1 treatment for LC. Four patients were affected by squamous cell lung cancer and 10 by lung adenocarcinoma (28.6% and 71.4% respectively). Twelve patients were undergoing treatment with an anti–PD-1 therapy (11 nivolumab, 1 pembrolizumab). Two of them were receiving atezolizumab.
All the patients that presented HR remain in treatment with a partial response or stable disease (SD) except 1, who suspended the treatment after 4 cycles owing to progression of the disease (PD) and finally died. Clinical details are summarized in the Table.
Table. Characteristics of 14 Patients With Hair Repigmentation.
| Patient No./Sex/Age | Neoplasm | Drug | Clinical Response | Sessions, No. | Previous RT | Previous Chemotherapy | Hair Loss During Chemotherapy/Radiotherapy |
|---|---|---|---|---|---|---|---|
| 1/M/60s | Lung squamous cell carcinoma | Nivolumab | Partial response | 28 | No | Cisplatin + vinorelbine | No |
| 2/M/60s | Lung squamous cell carcinoma | Nivolumab | Disease progression | 4 | No | Cisplatin + gemcitabine | Partial loss |
| 3/M/60s | Lung squamous cell carcinoma | Pembrolizumab | Stable disease | 35 | Lung | Carboplatin + vinorelbine | No |
| 4/M/70s | Lung squamous cell carcinoma | Nivolumab | Partial response | 27 | No | First-line therapy: carboplatin + paclitaxel; second-line therapy: carboplatin + gemcitabine | No |
| 5/M/50s | Lung adenocarcinoma | Nivolumab | Partial response | 17 | Lung, holocraneal | Cisplatin + vinorelbine | Yes (RT) |
| 6/M/70s | Lung adenocarcinoma | Atezolizumab | Partial response | 38 | No | Carboplatin + pemetrexed | No |
| 7/F/60s | Lung adenocarcinoma | Atezolizumab | Stable disease | 35 | No | First-line therapy: cisplatin + vinorelbine; second-line therapy: cisplatin + pemetrexed | Partial loss |
| 8/M/50s | Lung adenocarcinoma | Nivolumab | Stable disease | 15 | Lung | First-line therapy: cisplatin + docetaxel; change to: cisplatin + pemetrexed | Partial loss |
| 9/M/60s | Lung adenocarcinoma | Nivolumab | Partial response | 9 | Iliac crest (pain treatment) | Cisplatin + gemcitabine | No |
| 10/M/70s | Lung adenocarcinoma | Nivolumab | Partial response | 12 | No | Carboplatin + paclitaxel | No |
| 11/M/60s | Lung adenocarcinoma | Nivolumab | Stable disease | 12 | No | First-line therapy: cisplatin + vinorelbine; second-line therapy: carboplatin + pemetrexed + demcizumab | No |
| 12/M/70s | Lung adenocarcinoma | Nivolumab | Stable disease | 7 | No | No | No |
| 13/M/50s | Lung adenocarcinoma | Nivolumab | Partial response | 13 | Lung | Cisplatin + vinorelbine | No |
| 14/M/60s | Lung adenocarcinoma | Nivolumab | Partial response | 19 | No | Carboplatin + gemcitabine | No |
Abbreviation: RT, radiotherapy.
Most patients who did not present HR had no whitish hair before the treatment, and some of them did not experience HR during therapy.
The HR usually occurred starting at the occipital and temporal regions and progressing to the frontal and parietal areas, or in previously absent patches of black hair appearing between the remaining white hairs (Figure 1 and Figure 2). In all cases the patients recovered their previous hair color.
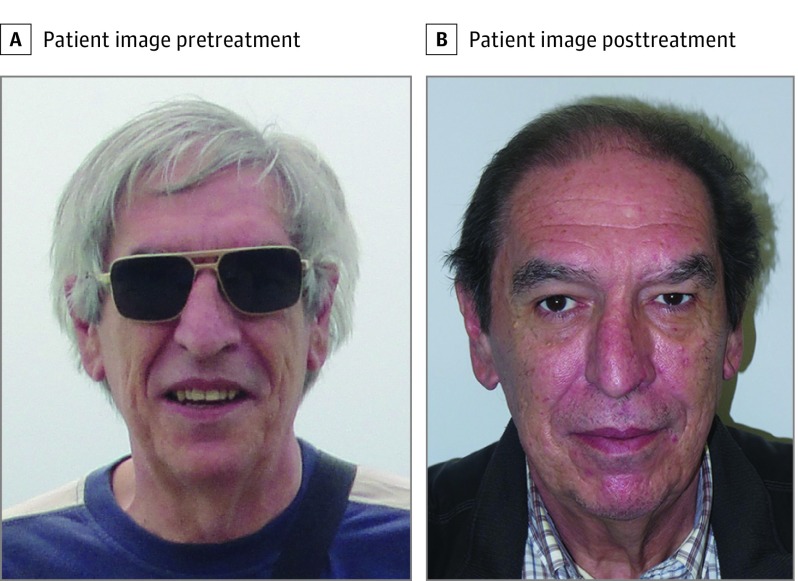
Figure 1. Hair Repigmentation in Patient Number 5.
A, Picture provided by the patient from before starting therapy. B, Picture taken during the follow up with a clear repigmentation.
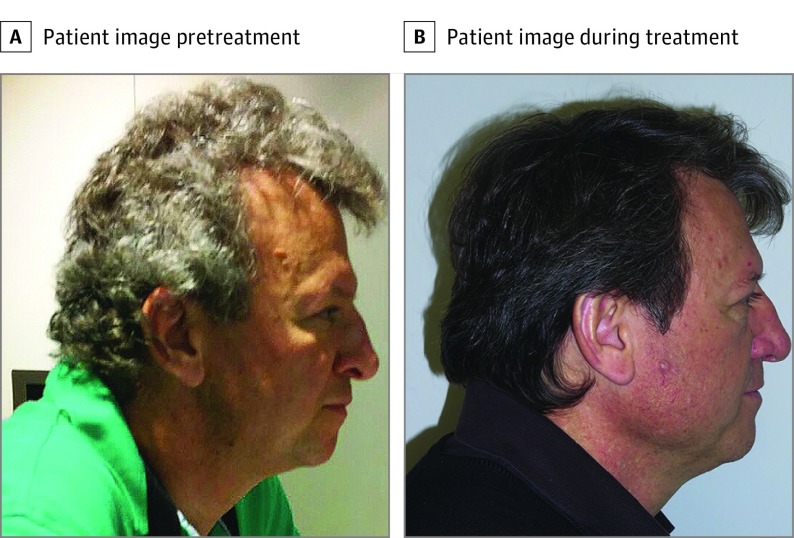
Figure 2. Hair Repigmentation in Patient Number 8.
A, Picture provided by the patient from before the therapy. B, Picture taken a few months after the treatment had begun.
Discussion
Hair graying is a physiological process associated with aging, with some differences between ethnicities. Hair repigmentation is rare, and it has been reported only in a few publications related to multiple drugs, such as thalidomide, lenalidomide, erlotinib, adalimumab, or etretinate. The mechanisms are not clear, although some hypotheses have been proposed. Some authors propose that different drugs can inhibit proinflamatory cytokines (tumor necrosis factor-α, tumor necrosis factor-β, interleukin 1 and interleukin 6) that act as negative regulators of melanogenesis, explaining this repigmentation. Other authors propose an almost opposed hypothesis: melanocytes in hair follicles might be activated through inflammatory mediators, such as cytokines and reactive oxygen species, explaining the localized HR after an induced folliculitis.
Patients with melanoma receiving anti–PD-1 therapies may develop vitiligo involving their hair. Nevertheless, HR after anti–PD-1/anti–PD-L1 has not been described. It is worth remembering that all of the patients with HR were receiving treatment for LC.
The mechanisms of melanocyte aging are not yet fully understood. With age, melanocytes are progressively lost from the skin and hair, nevi, and the retinal pigment epithelium of the eye. A recent study reported that the follicular units of gray hair present an increased melanocyte death by apoptosis and oxidative stress compared with matched normally pigmented hair follicles. Unlike “senile white” follicles, gray hair follicles still preserve a reduced number of differentiated and functioning melanocytes located in the hair bulb. This reduced number of melanocytes may explain the possibility of HR under appropriate conditions.
Of note, all patients with HR but 1 maintained the treatment with at least SD. The patient who had PD, had only received 4 doses. However, clinical trials consider the mean time until clinical response 2.1 months (1.2-7.6 months), thus we cannot consider this case as a therapy failure.
Conclusions
We report 14 cases of HR in patients with LC undergoing anti–PD-1/anti–PD-L1 treatment. We propose that HR might be a marker of clinical response, although further studies with longer series of patients are required to gain insights into the physiopathology of the follicle aging and mechanisms of HR.
References
- 1.Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer CJ. Emerging immunotherapies in the treatment of non-small cell lung cancer (NSCLC): the role of immune checkpoint inhibitors. Am J Clin Oncol. 2015;38(4):422-430. [DOI] [PubMed] [Google Scholar]
- 3.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270-1271. [DOI] [PubMed] [Google Scholar]
- 5.Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74(3):455-61.e1. [DOI] [PubMed] [Google Scholar]
- 6.Tobin DJ. Aging of the hair follicle pigmentation system. Int J Trichology. 2009;1(2):83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovering S, Miao W, Bailie T, Amato D. Hair repigmentation associated with thalidomide use for the treatment of multiple myeloma. BMJ Case Rep. 2016;2016:bcr2016215521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasanu CA, Mitsis D, Alexandrescu DT. Hair repigmentation associated with the use of lenalidomide: graying may not be an irreversible process! J Oncol Pharm Pract. 2013;19(2):165-169. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YP, Chen HJ, Chiu HC. Erlotinib-induced hair repigmentation. Int J Dermatol. 2014;53(1):e55-e57. [DOI] [PubMed] [Google Scholar]
- 10.Tintle SJ, Dabade TS, Kalish RA, Rosmarin DM. Repigmentation of hair following adalimumab therapy. Dermatol Online J. 2015;21(6):13030/qt6fn0t1xz. [PubMed] [Google Scholar]
- 11.Nagase K, Inoue T, Narisawa Y. Manifest hair repigmentation associated with etretinate therapy. J Dermatol. 2017;44(3):e34-e35. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann L, Forschner A, Loquai C, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti–PD-1 therapy. Eur J Cancer. 2016;60:190-209. [DOI] [PubMed] [Google Scholar]
- 13.Arck PC, Overall R, Spatz K, et al. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006;20(9):1567-1569. [DOI] [PubMed] [Google Scholar]
- 14.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330. [DOI] [PubMed] [Google Scholar]