This systematic review and meta-analysis evaluates the treatment responses of vitiligo to phototherapy based on all relevant prospective studies.
Key Points
Question
How much response to phototherapy might patients with vitiligo expect?
Findings
In this meta-analysis of 35 studies comprising 1428 patients, an at least mild response to narrowband UV-B phototherapy occurred in 74% at 6 months and 75% at 12 months, and a marked response was achieved in 19% at 6 months and 36% at 12 months. Marked responses were achieved in 44% on the face and neck, 26% on the trunk, 17% on the extremities, and none on the hands and feet after at least 6 months of narrowband UV-B phototherapy.
Meaning
Long-duration phototherapy should be encouraged to enhance the treatment response, with the greatest response anticipated on the face and neck.
Abstract
Importance
References to the expected treatment response to phototherapy would be helpful in the management of vitiligo because phototherapy requires long treatment durations over several months.
Objective
To estimate the treatment response of vitiligo to phototherapy.
Data Sources
A comprehensive database search of MEDLINE, EMBASE, and the Cochrane library from inception to January 26, 2016, was performed for all prospective studies. The main keywords used were vitiligo, phototherapy, psoralen, PUVA, ultraviolet, NBUVB, and narrowband.
Study Selection
All prospective studies reporting phototherapy outcome for at least 10 participants with generalized vitiligo were included. Of 319 studies initially identified, the full texts of 141 studies were assessed for eligibility, and 35 were finally included in the analysis. Of these, 29 studies included 1201 patients undergoing narrowband UV-B (NBUVB) phototherapy, and 9 included 227 patients undergoing psoralen–UV-A (PUVA) phototherapy.
Data Extraction and Synthesis
Two reviewers independently extracted the following data: study design, number and characteristics of the participants, phototherapy protocol, and rate of repigmentation based on the quartile scale. Single-arm meta-analyses were performed for the NBUVB and PUVA groups. Sample size–weighted means were calculated using a random-effects model for the repigmentation rates of the included studies.
Main Outcomes and Measures
The primary outcomes were at least mild (≥25%), at least moderate (≥50%), and marked (≥75%) responses on a quartile scale. Response rates were calculated as the number of participants who showed the corresponding repigmentation divided by the number of all participants enrolled in the individual studies.
Results
The meta-analysis included 35 unique studies (1428 unique patients). For NBUVB phototherapy, an at least mild response occurred in 62.1% (95% CI, 46.9%-77.3%) of 130 patients in 3 studies at 3 months, 74.2% (95% CI, 68.5%-79.8%) of 232 patients in 11 studies at 6 months, and 75.0% (95% CI, 60.9%-89.2%) of 512 patients in 8 studies at 12 months. A marked response was achieved in 13.0% (95% CI, 2.1%-23.9%) of 106 patients in 2 studies at 3 months, 19.2% (95% CI, 11.4%-27.0%) of 266 patients in 13 studies at 6 months, and 35.7% (95% CI, 21.5%-49.9%) of 540 patients in 9 studies at 12 months. For PUVA phototherapy, an at least mild response occurred in 51.4% (95% CI, 28.1%-74.7%) of 103 patients in 4 studies at 6 months and 61.6% (95% CI, 20.2%-100%) of 72 patients in 3 studies at 12 months. In the subgroup analyses, marked responses were achieved on the face and neck in 44.2% (95% CI, 24.2%-64.2%), on the trunk in 26.1% (95% CI, 8.7%-43.5%), on the extremities in 17.3% (95% CI, 8.2%-26.5%), and on the hands and feet in none after at least 6 months of NBUVB phototherapy.
Conclusions and Relevance
Long-duration phototherapy should be encouraged to enhance the treatment response in vitiligo. The greatest response is anticipated on the face and neck.
Introduction
Vitiligo is a common, chronic, acquired cutaneous depigmentation disorder causing loss of melanocytes in the skin and mucosa. The reported prevalence rate is 1% to 2% of the population for both sexes and all races. Vitiligo is one of the best-known autoimmune diseases, and depigmentation can evolve throughout life in affected persons, especially in the case of generalized vitiligo. Vitiligo has major effects on self-esteem and social life, and quality of life is highly impaired in patients with this disease.
Although several interventions are available to treat patients with vitiligo, no definite cure has yet been developed. Phototherapy, including psoralen–UV-A (PUVA) and narrowband UV-B (NBUVB) therapy, constitutes the principal treatment modality for generalized vitiligo, whereas excimer laser therapy and various topical agents are used to treat localized disease. However, phototherapy demands frequent clinic visits and requires long treatment durations for several months to years, sometimes resulting in disappointing outcomes. Thus, management of vitiligo is quite challenging, and patient adherence and clinician confidence are crucial for successful phototherapy treatment. References to expected treatment responses of vitiligo to phototherapy would be helpful in the management of this disease.
Since Njoo et al first reviewed the effectiveness of nonsurgical therapeutic methods for vitiligo in 1998, to our knowledge, no comprehensive systematic reviews have been performed to estimate treatment responses to phototherapy for vitiligo. In the present study, we update the results of the previous study with subsequent accumulated experiences. We performed a systematic review and meta-analysis of all relevant prospective studies to determine the repigmentation rates of NBUVB and PUVA phototherapy across different treatment durations. Additional meta-analyses were performed to delineate the treatment responses to NBUVB phototherapy by body site.
Methods
We performed a systematic review and meta-analysis to estimate the treatment response of vitiligo to phototherapy. The study was conducted according to the PRISMA guidelines and was registered with PROSPERO, an international prospective register of systematic reviews (https://www.crd.york.ac.uk/PROSPERO/).
Search Strategy
A comprehensive database search using predefined search terms (eTable 1 in the Supplement) was performed in MEDLINE, EMBASE, and the Cochrane library from inception to January 26, 2016. The main keywords used were vitiligo, phototherapy, psoralen, PUVA, ultraviolet, NBUVB, and narrowband. All prospective studies were included with no language restriction, and the reference lists in relevant review articles were scanned manually. All identified articles were screened independently by 2 reviewers (J.M.B. and H.M.J.).
Study Selection
Selection was performed based on the following inclusion criteria: (1) prospective study, including randomized and nonrandomized clinical trials and open trials; (2) participants of all age groups with a diagnosis of generalized or symmetrical vitiligo; (3) at least 1 phototherapy group, including NBUVB or PUVA; (4) at least 10 participants in each treatment arm, regardless of the dropout rate; (5) treatment duration of at least 12 weeks or at least 24 treatment sessions; (6) outcomes measured based on all vitiligo lesions on the participant’s whole body or at least half of the body; and (7) outcomes measured according to the degree of repigmentation based on the quartile scale (≥25%, ≥50%, and ≥75%). Exclusion criteria consisted of (1) duplicate publication; (2) retrospective or observational study; (3) segmental or focal vitiligo; (4) vitiligo refractory to previous conventional treatment; (5) phototherapy other than NBUVB and PUVA; (6) receiving therapies in addition to phototherapy; and (7) outcomes based on separate patches. The types of phototherapy evaluated in this review were restricted to NBUVB and PUVA because other phototherapies have not been widely used for treatment of vitiligo. We also excluded targeted phototherapy, such as excimer laser and light, which are usually used to treat localized vitiligo. Combination therapies with any other intervention, such as topical agents, systemic corticosteroids, and antioxidants, were also not included.
Two reviewers (J.M.B. and H.M.J.) independently identified relevant articles by searching the titles and abstracts. If the abstract did not provide enough information to include or exclude the study, full-text evaluation was performed to determine eligibility. The reviewers compared the results, and discrepancies were resolved through discussion or, if necessary, by arbitration by a third reviewer (B.Y.H.). All included studies were evaluated with levels of evidence as suggested by Shekelle et al.
Outcomes of Interest
The outcome of interest was the repigmentation rate. Repigmentation was graded based on a quartile scale with at least mild (≥25% repigmentation), at least moderate (≥50% repigmentation), and marked (≥75% repigmentation) responses. The rates (percentages) were calculated as the number of participants who achieved the corresponding degree of repigmentation divided by the total number of enrolled participants in each study. The degree of repigmentation was evaluated based on all lesions in each participant or in at least half of the participant’s body. We excluded outcomes based on individual patches and other measurements. The primary authors of included studies were contacted for further information when necessary.
Data Extraction
The following information was extracted independently by 2 reviewers (J.M.B. and H.M.J.) from the eligible reports meeting the inclusion criteria: study design, number and characteristics of the participants, subtype and duration of vitiligo, type of phototherapy, initial dose, treatment frequency and duration, and numbers of participants with repigmentation based on the quartile scale. An intention-to-treat analysis was planned, and dropouts were included in the analysis, if possible. Otherwise, we included patients who were described in the final assessment.
Data Synthesis
Meta-analyses were performed separately according to the type of phototherapy (NBUVB and PUVA) and duration of treatment (≤3, ≤6, and ≤12 months). We included oral and topical PUVA in the PUVA group in this review.
Subgroup Analyses
We performed subgroup analyses to investigate the treatment response to NBUVB phototherapy by body site, categorized as (1) face and neck, (2) trunk, (3) extremities, and (4) hands and feet. The outcomes by body site for subgroups containing at least 10 participants, were included, and we excluded data pertaining to other body parts. Only the treatment responses to NBUVB were evaluated owing to the rarity of reports on PUVA phototherapy. We restricted treatment duration to at least 6 months because a period of less than 6 months was not sufficient to evaluate treatment response.
Statistical Analyses
The statistical methods followed the procedure used by Njoo et al, which was adapted from the method of Einarson, in which data across studies were combined to produce a point estimate and a 95% CI. Sample size–weighted means were calculated using a random-effects model for each phototherapy type by dividing the total numbers of participants who achieved the corresponding repigmentation by the total number of participants in the included studies. The means and 95% CIs were calculated using Microsoft Excel 2010 (version 14.0; Microsoft Corp) and R (version 3.3.1; R Foundation for Statistical Computing).
Results
Search Results
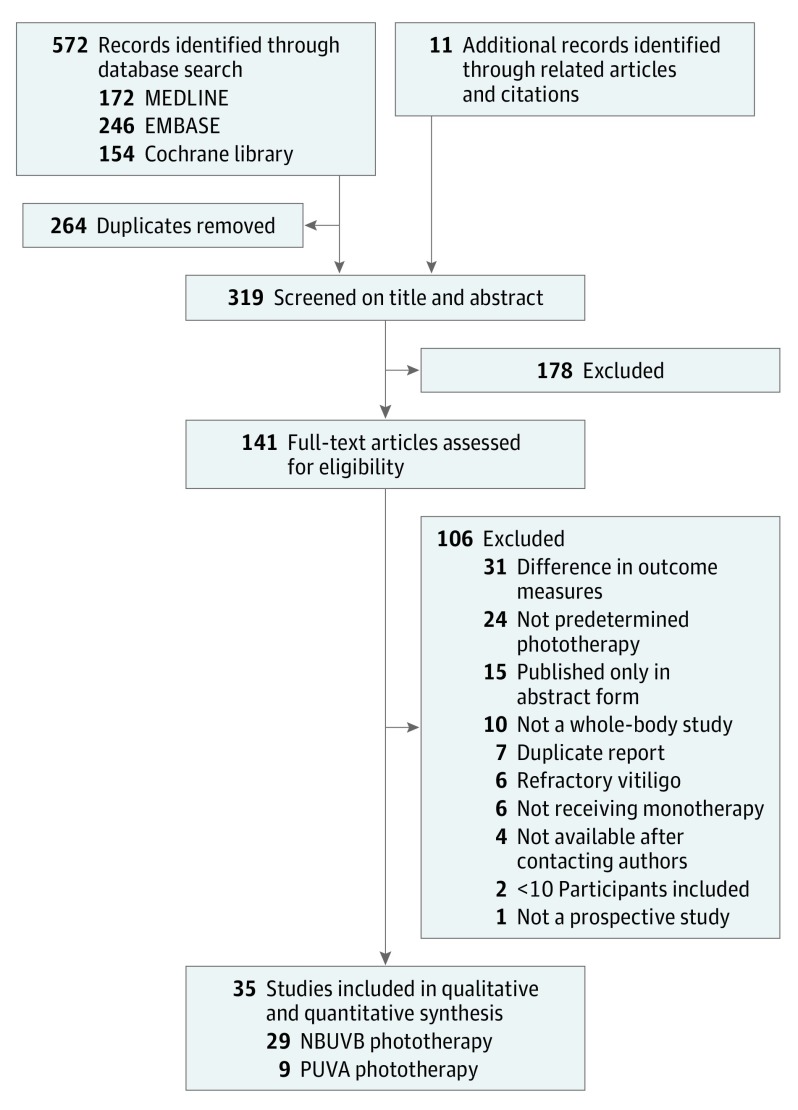
A total of 572 records were identified through computerized database searches, and 141 articles remained after the independent reviewers screened the titles and abstracts (Figure 1). A total of 141 full-text articles were assessed for eligibility, 106 of which were excluded for the following reasons: (1) duplicate report (n = 7); (2) published only in abstract form (n = 15); (3) not a prospective study (n = 1); (4) not predetermined phototherapy, such as UV-A phototherapy without psoralen, broadband UV-B phototherapy, targeted phototherapy, and home-based phototherapy (n = 24); (5) participants did not receive monotherapy (n = 6); (6) difference in outcome measures (n = 31); (7) not a whole-body study (n = 10); (8) fewer than 10 participants included (n = 2); (9) refractory vitiligo (n = 6); or (10) not available after contacting authors (n = 4). Finally, 35 unique studies involving 1428 unique patients fulfilled the inclusion criteria for this review. Of these, 29 studies with 1201 patients were included in the NBUVB group and 9 studies with 227 patients were included in the PUVA group (eTable 2 in the Supplement).
Figure 1. Flow Diagram for Identification of the Eligible Studies.
NBUVB indicates narrowband UV-B; PUVA, psoralen–UV-A.
Description of the Included Studies
All included studies were prospective studies in which patients with generalized vitiligo were treated using NBUVB or PUVA (Table 1). Of the 35 articles, 11 were single-arm studies, 9 were within-patient trials, and 15 were parallel trials. Five studies compared the efficacy of NBUVB and PUVA phototherapy, and 12 compared the efficacy of phototherapy and combination therapy with topical agents. or systemic antioxidants. Three studies targeted children, 8 targeted adults, and the remaining 24 targeted participants of all ages.
Table 1. Characteristics of the Included Studies.
| Source | Country | Study Design | Level of Evidencea | Subtype, Body Surface Area | Treatment Duration | Fitzpatrick Skin Type | Intervention | No. of Patients | Age, Mean (Range), y |
|---|---|---|---|---|---|---|---|---|---|
| al-Aboosi and Ajam, 1995 | Iraq | Nonblind single-arm | IV | Generalized vitiligo, ND | 6-18 mo | III, IV | PUVA | 29 | 23.0 (14-32) |
| Westerhof and Nieuweboer-Krobotova, 1997 | The Netherlands | Nonblind parallel trial | IIA | Active, extensive, and generalized vitiligo, ND | 3-12 mo | II, III, IV, V | 1: NBUVB 2: Topical PUVA |
1: 51 2: ND |
1: 36.0 (7-70) 2: 36.7 (8-63) |
| Njoo et al, 2000 | The Netherlands | Single-arm open trial | IV | Generalized vitiligo, ≥5% | 12 mo | II, III, IV, V | NBUVB | 51 | 9.9 (4-16) |
| Cestari et al, 2001 | Brazil | Double-blind parallel RCT | IB | Vitiligo, <2% | 3 mo | II, III, IV, V | 1: Topical PUVA (4-dimethoxyamoidina, 2%) 2: Topical PUVA (8-MOP) |
1: 14 2: 13 |
1: 23.9 (ND) 2: 15.2 (ND) |
| Ermis et al, 2001 | Turkey | Double-blind within-patient RCT | IB | Generalized vitiligo, ≥5% | 3 mo | II, III, IV | 1: PUVA 2: PUVA + topical calcipotriol, 0.005% |
1: 35 2: 35 |
29.8 (16-64) |
| Al Rubaie, 2002 | United Arab Emirates | Nonblind parallel RCT | IB | Generalized vitiligo, ND | 6-12 mo | IV, V | 1: NBUVB 2: PUVA 3: PUVA + topical calcipotriol |
1: 13 2: 9 3: 11 |
28.6 (9-65) |
| Cherif et al, 2003 | Tunisia | Nonblind within-patient trial | III | Bilateral and symmetrical NSV, ND | 15 wk | IV, V | 1: PUVA 2: PUVA + topical calcipotriol, 0.005% |
1: 23 2: 23 |
36 (19-73) |
| Park et al, 2003 | Korea | Nonblind single-arm | IV | Vitiligo, ND | >6 mo | III, IV, V | NBUVB | 13 | 36.6 (11-66) |
| Hamzavi et al, 2004 | Canada | Nonblind within-patient RCT | IB | Vitiligo on the trunk and extremities, >5% | 6 mo | II, III, IV, V | 1: NBUVB 2: No treatment |
22 | 47 (23-77) |
| Valkova et al, 2004 | Bulgaria | Nonblind within-patient trial | III | Various vitiligo, ND | 4 mo | II, III, IV | 1: PUVA 2: Topical khellin + UV-A |
17 | 26.1 (12-59) |
| Brazzelli et al, 2005 | Italy | Nonblind single-arm open trial | IV | Vitiligo in children, ND | 6 mo | II, III, IV | NBUVB | 10 | 9.7 (6-14) |
| Kanwar and Dogra, 2005 | India | Uncontrolled single-arm open trial | IV | Generalized vitiligo, ≥5% | ≤12 mo | IV, V | NBUVB | 26 | 10.6 (5-14) |
| Kanwar et al, 2005 | India | Nonblind single-arm trial | IV | Vitiligo vulgaris, ≥5% | 12 mo | IV, V | NBUVB | 15 | ND (12-56) |
| Anbar et al, 2006 | The Netherlands | Uncontrolled single-arm open trial | IV | NSV, ND | >6 mo | II, III, IV | NBUVB | 135 | 24.5 (4-65) |
| Arca et el, 2006 | Turkey | Nonblind parallel RCT | IB | Stable NSV, ≥10% | 10 wk | ND | 1: NBUVB 2: NBUVB + topical calcipotriol, 0.05% |
1: 24 2: 13 |
1: 22.0 (ND) 2: 21.5 (ND) |
| Goktas et al, 2006 | Turkey | Nonblind within-patient trial | IIA | Generalized symmetrical NSV, ≥20% | 6 mo | II, III | 1: NBUVB 2: NBUVB + topical calcipotriol, 0.005% |
1: 28 2: 28 |
34.2 (16-53) |
| Bhatnagar et al, 2007 | India | Single-blind parallel RCT | IB | NSV, ≥5% | 12 mo | IV, V | 1: NBUVB 2: PUVA |
1: 25 2: 25 |
1: 29.0 (ND) 2: 26.6 (ND) |
| Dell’Anna et al, 2007 | The Netherlands | Double-blind parallel RCT | IB | Generalized vitiligo, ≥15% | 6 mo | II, III, IV, V | 1: NBUVB 2: NBUVB + systemic antioxidant |
1: 14 2: 21 |
39.9 (24-61) |
| Nicolaidou et al, 2007 | Greece | Single-arm open trial | IV | NSV, ≥5% | 12 mo | I, II, III, IV, V | NBUVB | 84 | 39.5 (8-68) |
| Sitek et al, 2007 | Norway | Single-arm open trial | IV | Generalized vitiligo, ND | ≤12 mo | II, III, IV, V | NBUVB | 34 | ND (ND) |
| Yones et al, 2007 | England | Double-blind parallel RCT | IB | NSV, ≥20% | I, II, III, IV, V, VI | 1: NBUVB 2: PUVA |
1: 25 2: 25 |
1: 38 (18-64) 2: 36 (18-70) |
|
| Percivalle et el, 2008 | Italy | Single-arm open trial | IV | Localized or generalized vitiligo, ND | ≤12 mo | II, III, IV, V, VI | NBUVB | 53 | 36.5 (3-74) |
| Elgoweini and Nour El Din, 2009 | Egypt | Nonblind parallel RCT | IB | Stable vitiligo, ≥20% | 6 mo | II, III, IV | 1: NBUVB 2: NBUVB + oral antioxidant |
1: 12 2: 12 |
1: ND (19-48) 2: ND (20-50) |
| Esfandiarpour et al, 2009 | Iran | Double-blind parallel RCT | IB | NSV, ND | 3 mo | ND | 1: NBUVB 2: NBUVB + topical pimecrolimus, 1% |
1: 25 2: 25 |
1: 34.6 (15-72) 2: 25.9 (16-56) |
| Kishan Kumar et al, 2009 | India | Single-arm open trial | IV | Localized and generalized vitiligo, ND | ≤12 mo | IV, V | NBUVB | 150 | ND (3-70) |
| Stinco et al, 2009 | Italy | Nonblind parallel RCT | IB | Stable vitiligo, ND | 6 mo | II, III, IV | 1: NBUVB 2: Topical pimecrolimus, 1% 3: Topical tacrolimus, 0.1% |
1: 13 2: 15 3: 16 |
1: 48.8 (27-72) 2: 42.9 (27-56) 3: 43.2 (30-61) |
| Yuksel et al, 2009 | Turkey | Nonblind parallel trial | IIA | Generalized NSV, ≥20% | 6 mo | ND | 1: NBUVB 2: NBUVB + topical antioxidant |
1: 15 2: 15 |
1: 28 (18-67) 2: 33 (20-54) |
| Nordal et al, 2011 | Norway | Double-blind within-patient RCT | IB | Stable NSV, ND | 3 mo | II, III, IV, V, VI | 1: NBUVB 2: NBUVB + topical tacrolimus, 0.1% |
46 | 44.8 (23-69) |
| Sapam et al, 2012 | Nepal | Single-blind parallel RCT | IB | Stable NSV, >5% | 6 mo | IV, V III, IV, V |
1: NBUVB 2: PUVA |
1: 28 2: 28 |
1: 31.3 (ND) 2: 29.2 (ND) |
| Bansal et al, 2013 | India | Nonblind parallel RCT | IB | NSV, ≥5% | 5 mo | ND | 1: NBUVB 2: Psoralen-NBUVB |
1: 20 2: 20 |
29.9 (ND) |
| El-Mofty et al, 2013 | Egypt | Single-blind parallel RCT | IB | Bilateral and symmetrical NSV, >30% | 4 mo | III, IV | 1: NBUVB 2: Broadband UV-B |
1: 20 2: 20 |
1: 26.9 (ND) 2: 33.3 (ND) |
| Satyanarayan et al, 2013 | India | Nonblind within-patient RCT | IB | Generalized NSV, 5%-50% | 36 wk | III, IV | 1: NBUVB 2: NBUVB + topical tacrolimus, 0.1% |
25 | ND (14-36) |
| Singh et al, 2013 | India | Nonblind parallel RCT | IB | NSV, ≥2% | 36 wk | III, IV, V | 1: PUVA (8-MOP) 2: PUVA sol |
1: 18 2: 17 |
1: 27.3 (16-41) 2: 31.8 (12-49) |
| Baldo et al, 2014 | Italy | Nonblind within-patient RCT | IB | Stable vitiligo, ND | 36 wk | ND | 1: NBUVB 2: Topical tacrolimus, 0.1% |
48 | 27.0 (6-67) |
| Khullar et al, 2014 | India | Nonblind within-patient RCT | IB | Slowly progressive NSV, 5%-50% | 24 wk | III, IV, V | 1: NBUVB 2: NBUVB + topical calcipotriol, 0.005% |
27 | 24.4 (12-37) |
Abbreviations: MOP, methoxypsoralen; NBUVB, narrowband UV-B; ND, not determined; NSV, nonsegmental vitiligo; PUVA, psoralen–UV-A; RCT, randomized clinical trial.
IB indicates randomized controlled studies; IIA, nonrandomized controlled studies; III, comparative studies, correlation studies, and case-control studies; and IV, expert committee reports or opinions and case reports.
Treatment Response to NBUVB Phototherapy
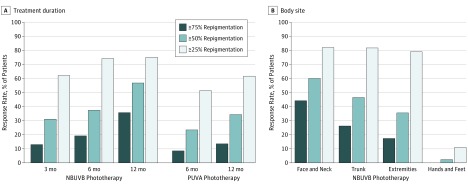
An at least mild response (≥25% repigmentation) to NBUVB phototherapy occurred in 62.1% (95% CI 46.9%-77.3%) of 130 patients in 3 studies at 3 months, in 74.2% (95% CI, 68.5%-79.8%) of 232 patients in 11 studies at 6 months, and 75.0% (95% CI, 60.9%-89.2%) of 512 patients in 8 studies at 12 months (Figure 2A and Table 2). A marked response (≥75% repigmentation) to NBUVB phototherapy was achieved in 13.0%; (95% CI, 2.1%-23.9%) of 106 patients in 2 studies at 3 months, 19.2% (95% CI, 11.4%-27.0%) of 266 patients in 13 studies at 6 months, and 35.7% (95% CI, 21.5%-49.9%) of 540 patients in 9 studies at 12 months.
Figure 2. Treatment Response of Phototherapy for Vitiligo.
A, Treatment response to narrowband UV-B (NBUVB) and psoralen–UV-A (PUVA) phototherapy according to treatment duration. B, Treatment response to NBUVB phototherapy depending on body site.
Table 2. Summary of Findings for Phototherapy for Vitiligo.
| Condition | Treatment Response Rate, % (95% CI) | Quality of Evidencea | Grade of Recommendationb | ||
|---|---|---|---|---|---|
| ≥75% Repigmentation | ≥50% Repigmentation | ≥25% Repigmentation | |||
| NBUVB phototherapy, duration | |||||
| 3 mo | 13.0 (2.1-23.9) | 31.1 (14.0-48.1) | 62.1 (46.9-77.3) | A | 1 |
| 6 mo | 19.2 (11.4-27.0) | 37.4 (27.1-47.8) | 74.2 (68.5-79.8) | A | 1 |
| 12 mo | 35.7 (21.5-49.9) | 56.8 (40.9-72.6) | 75.0 (60.9-89.2) | A | 1 |
| PUVA phototherapy, duration | |||||
| 6 mo | 8.5 (0-18.3) | 23.5 (9.5-37.4) | 51.4 (28.1-74.7) | A | 1 |
| 12 mo | 13.6 (4.2-22.9) | 34.3 (23.4-45.2) | 61.6 (20.2-100) | A | 1 |
| NBUVB phototherapy, 6-12 mo | |||||
| Face and neck | 44.2 (24.2-64.2) | 60.0 (43.9-76.1) | 82.0 (68.2-95.8) | A | 1 |
| Trunk | 26.1 (8.7-43.5) | 46.5 (18.6-74.4) | 81.7 (70.8-92.6) | A | 1 |
| Extremities | 17.3 (8.2-26.5) | 35.5 (14.6-56.5) | 79.0 (65.9-92.2) | A | 1 |
| Hands and feet | 0 (NA) | 2.30 (NA) | 11.0 (5.1-16.9) | A | 1 |
Abbreviations: NA, not applicable; NBUVB, narrowband UV-B; PUVA, psoralen–UV-A.
Indicates quality of evidence as described by Robinson et al, where A indicates systematic review and meta-analysis, randomized clinical trial with consistent findings, or all-or-none observational study.
Indicates grade of recommendation as described by Robinson et al, where 1 indicates strong recommendation with high-quality, patient-oriented evidence.
Treatment Response to PUVA Phototherapy
An at least mild response to PUVA phototherapy was achieved in 51.4% (95% CI, 28.1%-74.7%) of 103 patients in 4 studies at 6 months and 61.6% (95% CI, 20.2%-100%) of 72 patients in 3 studies at 12 months. A marked response to PUVA phototherapy was achieved in 8.5% (95% CI, 0%-18.3%) of 88 patients in 3 studies at 6 months and 13.6% (95% CI, 4.2%-22.9%) of 72 patients in 3 studies at 12 months (Figure 2A and Table 2).
Treatment Response to NBUVB Phototherapy Depending on Body Site
After at least 6 months of NBUVB phototherapy, an at least mild response occurred on the face and neck in 82.0% (95% CI, 68.2%-95.8%) of 153 patients in 5 studies, on the trunk in 81.7% (95% CI, 70.8%-92.6%) of 134 patients in 5 studies, on the extremities (excluding hands and feet) in 79.0% (95% CI, 65.9%-92.2%) of 162 patients in 5 studies, and on the hands and feet in 11.0% (95% CI, 5.1%-16.9%) of 172 patients in 6 studies. Marked responses were achieved on the face and neck in 44.2% (95% CI, 24.2%-64.2%) of 153 patients in 5 studies, on the trunk in 26.1% (95% CI, 8.7%-43.5%) of 134 patients in 5 studies, on the extremities in 17.3% (95% CI, 8.2%-26.5%) of 162 patients in 5 studies, and on the hands and feet in none of 172 patients in 6 studies (Figure 2B and Table 2).
Discussion
Phototherapy has been the mainstay of treatment for vitiligo for decades. Since PUVA phototherapy was first introduced for the treatment of vitiligo in 1948, it has been widely adopted as a promising therapeutic modality. Although PUVA phototherapy is effective, it has several limitations, including phototoxic effects, nausea, and the potential risk for skin cancer. Moreover, PUVA phototherapy cannot be applied to children or pregnant women because of the systemic use of psoralen. Since NBUVB phototherapy was first reported to be effective for treatment of vitiligo in 1997, it has gradually taken the place of PUVA phototherapy. The lack of a photosensitizer, the lower cumulative dose, and fewer adverse effects are considered to be major advantages of NBUVB over PUVA, and NBUVB even showed superior efficacy over PUVA. Narrowband UV-B phototherapy is also associated with adverse events such as erythema, itching, and mild burning or pain, which are well tolerated and spontaneously disappear a few hours after treatment in most cases. Therefore, NBUVB phototherapy is now considered to be the criterion standard therapy for generalized vitiligo, whereas PUVA phototherapy is still considered under special conditions, such as cases of spreading vitiligo with deeper penetration of UV-A.
In the present study, we reveal the treatment response of vitiligo to phototherapy by treatment duration. We verify that phototherapy requires at least 1 year to achieve a maximal treatment response, although we could not determine the appropriate treatment duration based on our results. For example, 56.8% achieved an at least moderate response (≥50% repigmentation) to 12 months of NBUVB phototherapy, although 62.1% of patients achieved an at least mild response (≥25% repigmentation) within 3 months. Furthermore, 37.4% of patients achieved an at least moderate response (≥50% repigmentation) within 6 months of NBUVB phototherapy, with 35.7% achieving a marked response (≥75% repigmentation) within 12 months. A longer treatment duration was assumed to enhance the treatment response. In a disappointing finding, 25.8% and 25.0% of patients did not achieve a mild response (≥25% repigmentation) within 6 or 12 months of NBUVB phototherapy, respectively. We postulated that some patients would not respond to NBUVB phototherapy despite 12 months of treatment. However, 3 months is not sufficient to discriminate nonresponders from late responders because 37.9% of patients did not achieve a mild response within 3 months. Our results suggest that at least 6 months of treatment is required to determine the responsiveness to NBUVB phototherapy.
With PUVA phototherapy, we also showed that the treatment response after 12 months of treatment was better than that after 6 months. However, the overall treatment response to PUVA phototherapy was inferior to that to NBUVB, although statistical comparisons were not conducted in our study. Five studies compared the efficacy of NBUVB with that of PUVA phototherapy. Westerhof and Nieuweboer-Krobotova first reported that NBUVB phototherapy was more effective than topical PUVA but without statistical significance. Yones et al demonstrated the superiority of NBUVB phototherapy to oral PUVA therapy in their randomized clinical trial. In their study, the rate of more than 50% repigmentation was significantly higher in the NBUVB group (64%) than in the PUVA group (36%) after 6 months of treatment. Moreover, the repigmented skin showed excellent color match in all patients in the NBUVB group but only 44% of those in the PUVA group.
We also examined the treatment response to NBUVB phototherapy by different body sites in all relevant studies that presented the outcomes of more than 10 patients per body site treated for at least 6 months. The most responsive body site was the face and neck, for which the marked repigmentation rate was 44.2%, followed by the trunk (26.1%), extremities (17.3%), and hands and feet (0%). The treatment responses on hands and feet were extremely low, and a mild response was observed in only 11.0% of patients. Meanwhile, the rates of an at least mild response were 82.0% on the face and neck, 81.7% on the trunk, and 79.0% on the extremities, and the proportion of enrolled patients who failed to show a response was similar (approximately 20%), regardless of body site, except for the hands and feet. Certain shared host factors might hinder repigmentation, such as disease activity, autoimmune state, large involved body surface area, and presence of poliosis.
The present study demonstrated the treatment response of vitiligo to phototherapy according to phototherapy type, treatment duration, and body site. In the clinical setting, treatment outcome might be better than our results because this review exclusively included studies of phototherapy alone. Various adjuvant treatments, including topical calcipotriol, topical calcineurin inhibitors, and systemic antioxidants, could be used in addition to phototherapy to enhance the treatment response in practice. Nevertheless, our findings would be a useful guide for clinicians and patients for establishment of the treatment strategy. Because phototherapy usually requires a long duration, reassuring and encouraging patients to achieve the maximal treatment response are critical.
Limitations
Our systematic review had some limitations. First, the study design, characteristics of the enrolled patients, and phototherapy protocol had considerable heterogeneity. The included studies may have been conducted with different objectives and different comparisons, even within a single arm. Therefore, we excluded retrospective studies to minimize unidentified biases and assumed that the degree of repigmentation after a given protocol would represent the efficacy of phototherapy in prospective studies. Second, the quantitative quartile scale may be somewhat arbitrary. Moreover, the degree of repigmentation itself cannot indicate treatment success in vitiligo management. However, the quartile scale is the most commonly used measure to date, and the overall treatment response should be estimated as objectively as possible based on the degree of repigmentation. Finally, a meta-analysis of a single arm could have methodologic weaknesses. However, we attempted to integrate the outcomes of all relevant prospective studies and used the statistical methods validated in the previous studies. Furthermore, our results were supported by the high quality of evidence and strong grade of recommendation (Table 2).
Conclusions
The present systematic review and meta-analysis revealed the treatment response to phototherapy for vitiligo based on all relevant prospective studies in the literature. A longer treatment duration should be encouraged to enhance the treatment response, and a period of at least 6 months is required to assess the responsiveness to phototherapy. The overall treatment response to NBUVB phototherapy was better than that to PUVA therapy. The most effective response is anticipated on the face and neck, whereas the hands and feet show minimal response.
eTable 1. Predetermined Search Terms in Each Database
eTable 2. Interventions and Clinical Outcomes of Phototherapy for Vitiligo
References
- 1.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview, part I: introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473-491. [DOI] [PubMed] [Google Scholar]
- 2.Ongenae K, Beelaert L, van Geel N, Naeyaert JM. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol. 2006;20(1):1-8. [DOI] [PubMed] [Google Scholar]
- 3.Felsten LM, Alikhan A, Petronic-Rosic V. Vitiligo: a comprehensive overview, part II: treatment options and approach to treatment. J Am Acad Dermatol. 2011;65(3):493-514. [DOI] [PubMed] [Google Scholar]
- 4.Njoo MD, Spuls PI, Bos JD, Westerhof W, Bossuyt PM. Nonsurgical repigmentation therapies in vitiligo: meta-analysis of the literature. Arch Dermatol. 1998;134(12):1532-1540. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. [DOI] [PubMed] [Google Scholar]
- 6.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318(7183):593-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Einarson TR. Pharmacoeconomic applications of meta-analysis for single groups using antifungal onychomycosis lacquers as an example. Clin Ther. 1997;19(3):559-569. [DOI] [PubMed] [Google Scholar]
- 8.Westerhof W, Nieuweboer-Krobotova L. Treatment of vitiligo with UV-B radiation vs topical psoralen plus UV-A. Arch Dermatol. 1997;133(12):1525-1528. [PubMed] [Google Scholar]
- 9.Njoo MD, Bos JD, Westerhof W. Treatment of generalized vitiligo in children with narrow-band (TL-01) UVB radiation therapy. J Am Acad Dermatol. 2000;42(2, pt 1):245-253. [DOI] [PubMed] [Google Scholar]
- 10.Al Rubaie S. An open randomized study of treatment of 39 patients of generalized vitiligo with narrow-band UVB vs topical calcipotriol + PUVA vs PUVA therapy for 6-12 months. J Eur Acad Dermatol Venereol. 2002;16(suppl S1):270. [Google Scholar]
- 11.Park JH, Kim HJ, Lee MH. Efficacy of narrow-band UVB phototherapy in vitiligo patients. Korean J Dermatol. 2003;41(8):1022-1027. [Google Scholar]
- 12.Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: the Vitiligo Area Scoring Index. Arch Dermatol. 2004;140(6):677-683. [DOI] [PubMed] [Google Scholar]
- 13.Brazzelli V, Prestinari F, Castello M, et al. . Useful treatment of vitiligo in 10 children with UV-B narrowband (311 nm). Pediatr Dermatol. 2005;22(3):257-261. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar AJ, Dogra S. Narrow-band UVB for the treatment of generalized vitiligo in children. Clin Exp Dermatol. 2005;30(4):332-336. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar AJ, Dogra S, Parsad D, Kumar B. Narrow-band UVB for the treatment of vitiligo: an emerging effective and well-tolerated therapy. Int J Dermatol. 2005;44(1):57-60. [DOI] [PubMed] [Google Scholar]
- 16.Anbar TS, Westerhof W, Abdel-Rahman AT, El-Khayyat MA. Evaluation of the effects of NB-UVB in both segmental and non-segmental vitiligo affecting different body sites. Photodermatol Photoimmunol Photomed. 2006;22(3):157-163. [DOI] [PubMed] [Google Scholar]
- 17.Arca E, Taştan HB, Erbil AH, Sezer E, Koç E, Kurumlu Z. Narrow-band ultraviolet B as monotherapy and in combination with topical calcipotriol in the treatment of vitiligo. J Dermatol. 2006;33(5):338-343. [DOI] [PubMed] [Google Scholar]
- 18.Goktas EO, Aydin F, Senturk N, Canturk MT, Turanli AY. Combination of narrow band UVB and topical calcipotriol for the treatment of vitiligo. J Eur Acad Dermatol Venereol. 2006;20(5):553-557. [DOI] [PubMed] [Google Scholar]
- 19.Bhatnagar A, Kanwar AJ, Parsad D, De D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: an open prospective study. J Eur Acad Dermatol Venereol. 2007;21(5):638-642. [DOI] [PubMed] [Google Scholar]
- 20.Dell’Anna ML, Mastrofrancesco A, Sala R, et al. . Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32(6):631-636. [DOI] [PubMed] [Google Scholar]
- 21.Nicolaidou E, Antoniou C, Stratigos AJ, Stefanaki C, Katsambas AD. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. J Am Acad Dermatol. 2007;56(2):274-278. [DOI] [PubMed] [Google Scholar]
- 22.Sitek JC, Loeb M, Ronnevig JR. Narrowband UVB therapy for vitiligo: does the repigmentation last? J Eur Acad Dermatol Venereol. 2007;21(7):891-896. [DOI] [PubMed] [Google Scholar]
- 23.Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen–UV-A therapy vs narrowband–UV-B therapy. Arch Dermatol. 2007;143(5):578-584. [DOI] [PubMed] [Google Scholar]
- 24.Percivalle S, Piccino R, Caccialanza M, Forti S. Narrowband UVB phototherapy in vitiligo: evaluation of results in 53 patients. G Ital Dermatol Venereol. 2008;143(1):9-14. [PubMed] [Google Scholar]
- 25.Elgoweini M, Nour El Din N. Response of vitiligo to narrowband ultraviolet B and oral antioxidants. J Clin Pharmacol. 2009;49(7):852-855. [DOI] [PubMed] [Google Scholar]
- 26.Esfandiarpour I, Ekhlasi A, Farajzadeh S, Shamsadini S. The efficacy of pimecrolimus 1% cream plus narrow-band ultraviolet B in the treatment of vitiligo: a double-blind, placebo-controlled clinical trial. J Dermatolog Treat. 2009;20(1):14-18. [DOI] [PubMed] [Google Scholar]
- 27.Kishan Kumar YH, Rao GR, Gopal KV, Shanti G, Rao KV. Evaluation of narrow-band UVB phototherapy in 150 patients with vitiligo. Indian J Dermatol Venereol Leprol. 2009;75(2):162-166. [DOI] [PubMed] [Google Scholar]
- 28.Stinco G, Piccirillo F, Forcione M, Valent F, Patrone P. An open randomized study to compare narrow band UVB, topical pimecrolimus and topical tacrolimus in the treatment of vitiligo. Eur J Dermatol. 2009;19(6):588-593. [DOI] [PubMed] [Google Scholar]
- 29.Yuksel EP, Aydin F, Senturk N, Canturk T, Turanli AY. Comparison of the efficacy of narrow band ultraviolet B and narrow band ultraviolet B plus topical catalase-superoxide dismutase treatment in vitiligo patients. Eur J Dermatol. 2009;19(4):341-344. [DOI] [PubMed] [Google Scholar]
- 30.Nordal EJ, Guleng GE, Rönnevig JR. Treatment of vitiligo with narrowband-UVB (TL01) combined with tacrolimus ointment (0.1%) vs placebo ointment, a randomized right/left double-blind comparative study. J Eur Acad Dermatol Venereol. 2011;25(12):1440-1443. [DOI] [PubMed] [Google Scholar]
- 31.Sapam R, Agrawal S, Dhali TK. Systemic PUVA vs narrowband UVB in the treatment of vitiligo: a randomized controlled study. Int J Dermatol. 2012;51(9):1107-1115. [DOI] [PubMed] [Google Scholar]
- 32.Bansal S, Sahoo B, Garg V. Psoralen-narrowband UVB phototherapy for the treatment of vitiligo in comparison to narrowband UVB alone. Photodermatol Photoimmunol Photomed. 2013;29(6):311-317. [DOI] [PubMed] [Google Scholar]
- 33.El-Mofty M, Mostafa W, Youssef R, et al. . BB-UVA vs NB-UVB in the treatment of vitiligo: a randomized controlled clinical study (single blinded). Photodermatol Photoimmunol Photomed. 2013;29(5):239-246. [DOI] [PubMed] [Google Scholar]
- 34.Satyanarayan HS, Kanwar AJ, Parsad D, Vinay K. Efficacy and tolerability of combined treatment with NB-UVB and topical tacrolimus versus NB-UVB alone in patients with vitiligo vulgaris: a randomized intra-individual open comparative trial. Indian J Dermatol Venereol Leprol. 2013;79(4):525-527. [DOI] [PubMed] [Google Scholar]
- 35.Baldo A, Lodi G, Di Caterino P, Monfrecola G. Vitiligo, NB-UVB and tacrolimus: our experience in Naples. G Ital Dermatol Venereol. 2014;149(1):123-130. [PubMed] [Google Scholar]
- 36.Khullar G, Kanwar AJ, Singh S, Parsad D. Comparison of efficacy and safety profile of topical calcipotriol ointment in combination with NB-UVB vs NB-UVB alone in the treatment of vitiligo: a 24-week prospective right-left comparative clinical trial. J Eur Acad Dermatol Venereol. 2015;29(5):925-932. [DOI] [PubMed] [Google Scholar]
- 37.al-Aboosi MM, Ajam ZA. Oral photochemotherapy in vitiligo: follow-up, patient compliance. Int J Dermatol. 1995;34(3):206-208. [DOI] [PubMed] [Google Scholar]
- 38.Cestari TF, Dias M, Fernandes EI, Albaneze R. Comparative study of two psoralens in topical phototherapy for vitiligo. An Bras Dermatol. 2001;76(5):683-692. [Google Scholar]
- 39.Ermis O, Alpsoy E, Cetin L, Yilmaz E. Is the efficacy of psoralen plus ultraviolet A therapy for vitiligo enhanced by concurrent topical calcipotriol? a placebo-controlled double-blind study. Br J Dermatol. 2001;145(3):472-475. [DOI] [PubMed] [Google Scholar]
- 40.Cherif F, Azaiz MI, Ben Hamida A, Ben O, Dhari A. Calcipotriol and PUVA as treatment for vitiligo. Dermatol Online J. 2003;9(5):4. [PubMed] [Google Scholar]
- 41.Valkova S, Trashlieva M, Christova P. Treatment of vitiligo with local khellin and UVA: comparison with systemic PUVA. Clin Exp Dermatol. 2004;29(2):180-184. [DOI] [PubMed] [Google Scholar]
- 42.Singh S, Khandpur S, Sharma VK, Ramam M. Comparison of efficacy and side-effect profile of oral PUVA vs oral PUVA sol in the treatment of vitiligo: a 36-week prospective study. J Eur Acad Dermatol Venereol. 2013;27(11):1344-1351. [DOI] [PubMed] [Google Scholar]
- 43.Monem El Mofty A. A preliminary clinical report on the treatment of leucodermia with Ammi majus Linn. J Egypt Med Assoc. 1948;31(8):651-665. [PubMed] [Google Scholar]
- 44.Pacifico A, Leone G. Photo(chemo)therapy for vitiligo. Photodermatol Photoimmunol Photomed. 2011;27(5):261-277. [DOI] [PubMed] [Google Scholar]
- 45.Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32(2):66-80. [DOI] [PubMed] [Google Scholar]
- 46.Robinson JK, Dellavalle RP, Bigby M, Callen JP. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol. 2008;144(1):97-99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Predetermined Search Terms in Each Database
eTable 2. Interventions and Clinical Outcomes of Phototherapy for Vitiligo