This case report describes dermatologic similarities among family members with the familial medullary thyroid carcinoma variant of multiple endocrine neoplasia type 2A syndrome and cutaneous manifestations of multiple endocrine neoplasia type 2B and PTEN hamartoma-tumor syndromes.
Key Points
Question
Is there a phenotypic association between the cutaneous manifestations of multiple endocrine neoplasia type 2A and PTEN (phosphatase and tensin homologue) hamartoma-tumor syndromes?
Findings
A kindred of 11 individuals with familial medullary thyroid carcinoma variant of multiple endocrine neoplasia type 2A syndrome showed a moderate risk RET p.Val804Met (protein valine at residue 804 replaced by methionine) genetic mutation. Two family members had dermal hyperneury lesions characteristically seen in multiple endocrine neoplasia type 2B syndrome; 1 family member also showed multiple sclerotic fibromas, a cutaneous manifestation previously described in PTEN hamartoma-tumor syndrome, but not in multiple endocrine neoplasia type 2A syndrome.
Meaning
PTEN hamartoma-tumor and multiple endocrine neoplasia type 2 syndromes show considerable overlap in the signaling pathways regulated by PTEN and RET genes, respectively, which may explain the clinical overlap between the 2 syndromes.
Abstract
Importance
Multiple endocrine neoplasia type 2 (MEN 2) syndrome is an autosomal dominant, hereditary cancer disorder caused by germline mutations in the RET (formerly MEN2A, MEN2B) proto-oncogene located on chromosomal band 10q11.21. Two distinct clinical forms have been described as the following phenotypes: multiple endocrine neoplasia type 2A (MEN 2A) and multiple endocrine neoplasia type 2B (MEN 2B) syndromes. The common and necessary nexus that defines these 2 phenotypes is the presence of medullary thyroid carcinoma (MTC). The familial MTC type of MEN 2 syndrome was included within the spectrum of MEN 2A syndrome. Cutaneous manifestations of MEN 2A syndrome include macular amyloidosis, whereas MEN 2B syndrome is traditionally linked to multiple mucosal neuromas.
Objectives
To describe a family with cutaneous manifestations not previously described in patients with MEN 2A syndrome and to discuss the association of this disorder with Cowden syndrome.
Design, Setting, and Participants
Clinicopathologic correlation of cutaneous lesions and genetic studies in 11 members of a family with familial MTC.
Interventions
Cutaneous lesions were histopathologically and immunohistochemically studied. Genetic screening for a germline mutation at the RET gene was performed in 11 family members.
Main Outcomes and Measures
Identification of cutaneous lesions not previously described in patients with MEN 2A syndrome.
Results
This family of 11 individuals with familial MTC type of MEN 2A syndrome demonstrated the moderate risk RET p.Val804Met (protein valine at residue 804 replaced by methionine) genetic mutation, with 2 of the relatives presenting with dermal hyperneury, cutaneous lesions classically described in MEN 2B syndrome, and 1 relative also showing multiple sclerotic fibromas, a cutaneous manifestation of PTEN (phosphatase and tensin homologue) hamartoma-tumor syndrome.
Conclusions and Relevance
Dermal hyperneury and multiple sclerotic fibromas should be added to the list of cutaneous manifestations of patients with the familial MTC type of MEN 2A syndrome.
Introduction
Multiple endocrine neoplasia type 2 (MEN2) (OMIM 171400) syndrome is an autosomal dominant, hereditary cancer disorder resulting from germline mutations in the RET (formerly MEN2A, MEN2B) proto-oncogene located on chromosomal band 10q11.21. Two variants of MEN 2 syndrome, MEN 2A syndrome (OMIM 171400) and MEN 2B syndrome (OMIM 162300), have been described and result from different RET gene mutations with good genotype-phenotype correlation. Both variants are characterized by the triad: medullary thyroid carcinoma (MTC), pheochromocytoma, and hyperparathyroidism. Hyperparathyroidism is much less common in MEN 2B syndrome than in MEN 2A syndrome. The common nexus of these 2 variants is MTC, a rare calcitonin-secreting tumor of the parafollicular cells (C-cells) of the thyroid, which presents earlier and more aggressively in MEN 2B syndrome than in MEN 2A syndrome. Familial MTC was included within the spectrum of MEN 2A syndrome because of the nearly exact concordance of the involved mutations with variable allelic penetrance. According to the American Thyroid Association, the risk of RET gene mutations for developing aggressive MTC may be classified into the following categories: highest risk for RET codon M918T mutation; high risk for RET codon C634 and A883F mutations; and moderate risk for RET codon V804M and E768D mutations and excluding M918T, C634, and A883F mutations.
Regarding cutaneous manifestations, MEN 2A syndrome is associated with macular amyloidosis, whereas MEN 2B syndrome patients have multiple mucosal neuromas. This report describes a family with MEN 2A syndrome consisting of 2 members with dermal hyperneury, a cutaneous manifestation previously described in people with MEN 2B syndrome, and 1 member with multiple sclerotic fibromas, a cutaneous manifestation of PTEN (phosphatase and tensin homologue) hamartoma-tumor syndrome (PHTS). These findings support phenotypic overlap between MEN 2A syndrome and PHTS.
Report of Cases
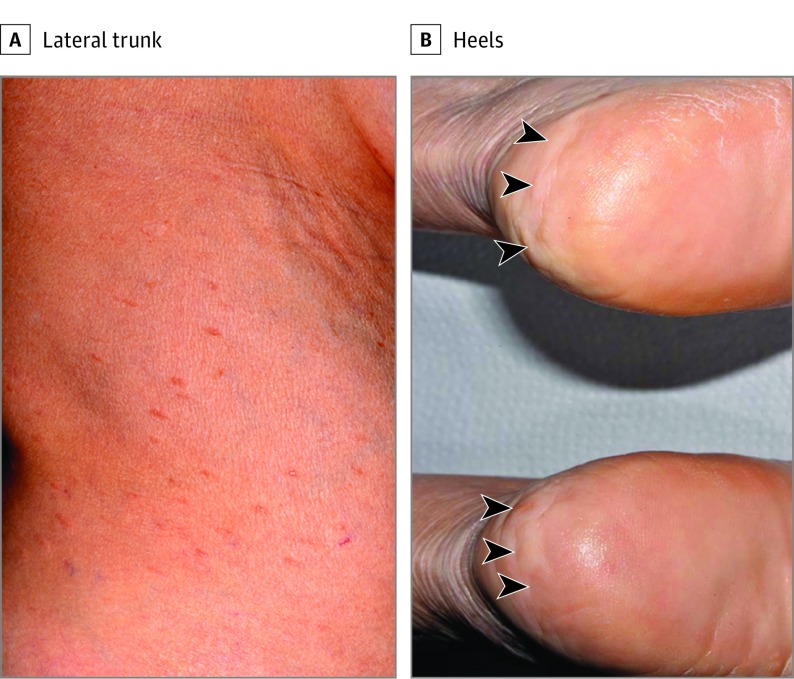
A woman in her 60s presented with approximately 50 slightly tender, hyperpigmented, 3- to 5-mm-diameter papules on the trunk and forearms (Figure 1A). The lesions showed a linear configuration along the lines of skin tension. The lesions steadily increased in number by 2 to 3 lesions per year. The patient also had asymptomatic, firm, flesh-colored, 5- to 10-mm-diameter nodular lesions on the heels (Figure 1B). No mucosal lesions or ophthalmologic anomalies were found.
Figure 1. Clinical Images of the Lesions.
A, Elongated papules located on the lateral aspect of the trunk. B, Papular and nodular lesions located on the bilateral heels (arrowheads).
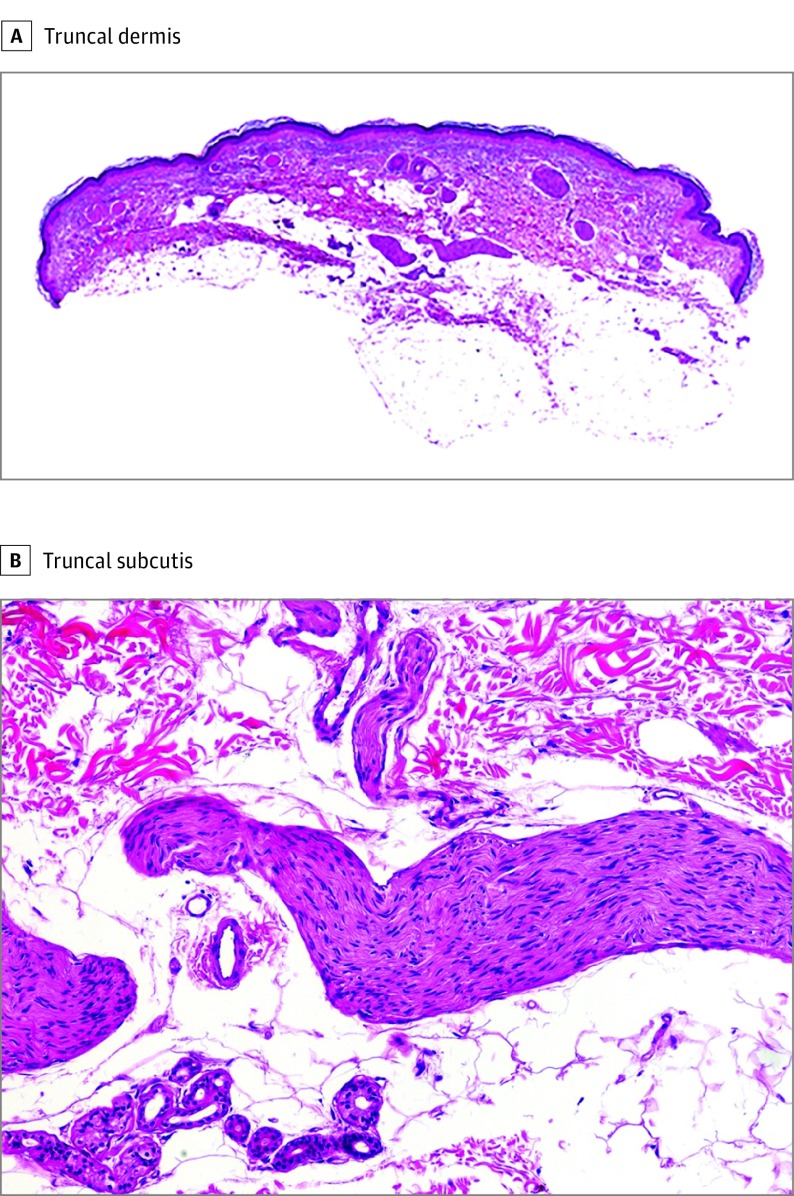
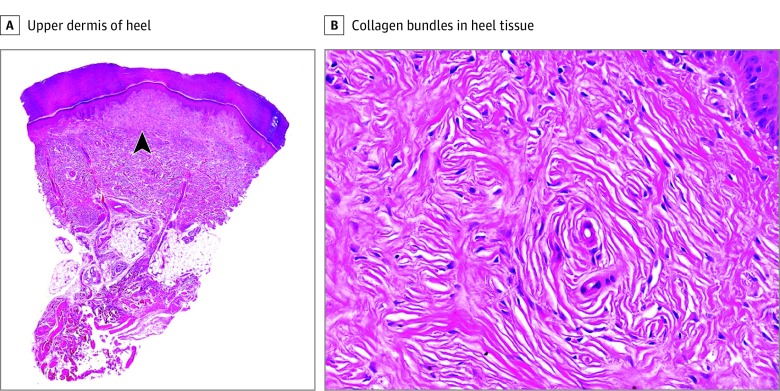
Immunohistochemical studies of the cutaneous lesions included CD34, S-100 protein, neurofilaments, and epithelial membrane antigen. Screening of the RET proto-oncogene was performed by Sanger sequencing of exons 8, 10, 11, and 13 to 16 in DNA extracted from peripheral blood samples. By histopathologic description, a papule on the forearm showed tortuous nerve bundles that were increased in size and number, scattered throughout the entire thickness of the dermis, and extended to the superficial subcutis (Figure 2). Each bundle was composed of broad fascicles of monomorphous spindle cells with wavy nuclei and was surrounded by a capsule of perineurium. The spindle cells showed S-100 protein positivity, and the stain for neurofilaments revealed abundant axonal content. The cells of the capsule surrounding each nerve bundle expressed epithelial membrane antigen. A nodule from each heel showed identical findings consisting of a well-demarcated, nonencapsulated, hypocellular dermal nodule composed of thick, eosinophilic sclerotic collagen bundles separated by clefts and arranged in storiform pattern with sparse fibroblasts (Figure 3). Immunohistochemical study for CD34, CD99, S-100 protein, neurofilaments, and epithelial membrane antigen demonstrated a few scattered CD34-positive cells and negative results for the remaining markers.
Figure 2. Histopathologic Features of the Truncal Lesions.
A, Multiple large-diameter nerve fibers in the dermis (hematoxylin-eosin, original magnification ×10). B, Large-diameter nerve fibers in the subcutis (hematoxylin-eosin, original magnification ×200).
Figure 3. Histopathologic Features of the Heel Lesions.
A, Well-circumscribed lesion (arrowhead) involving the upper dermis (hematoxylin-eosin, original magnification ×10). B, Characteristic onion-like configuration of the collagen bundles (hematoxylin-eosin, original magnification ×200).
On examination, the patient had a goiter with thyroid indices within the normal range. Routine laboratory results, chest radiographic findings, and serum levels of calcium, phosphate, and vanillylmandelic acid were within normal limits. The serum calcitonin level was 258 pg/mL (normal range, 0-30 pg/mL [to convert to picomoles per liter, multiply by 0.292]) after pentagastrin stimulation. Total thyroidectomy was performed. Histopathologic study of the left lobe of the thyroid demonstrated diffuse nodular hyperplasia with foci of lymphocytic thyroiditis, 2 small microadenomatous foci, C-cell hyperplasia, and 4-mm-diameter micro-MTC. No involved lymph nodes were found, and results from computed tomography failed to demonstrate metastases. Genetic screening results revealed a germline mutation at RET exon 14, p.Val804Met (protein valine at residue 804 replaced by methionine); no other germline mutations were found. Germline PTEN (cytogenic location: 10q23.3) inactivating mutations were also absent.
The patient’s daughter, who was in her 40s, was a carrier of the same mutation and presented with similar linear lesions on the trunk. Histopathologic features of dermal hyperneury were noted with no other clinical manifestation. Ophthalmologic examination showed no anomalies. She underwent a total thyroidectomy and a micro-MTC was found. In an extended family screening, 16 first- and second-degree relatives were genetically tested. Eleven relatives were also carriers of the germline mutation at RET exon 14, p.Val804Met. Of these 11 carriers, 6 relatives underwent thyroidectomy procedures and 5 of these 6 relatives were diagnosed with MTC. The 5 remaining relatives designated as carriers, who did not undergo a thyroidectomy, had results within the normal range for periodic calcitonin levels after pentagastrin stimulation. There were no mucocutaneous lesions, Marfanoid phenotype, solid organ hamartomas, or other endocrinopathies diagnosed in the remaining 9 relatives studied. After more than 10 years of follow-up, there was no clinical, laboratory, or imaging evidence of pheochromocytoma or hyperparathyroidism in the kindred; recently, a granddaughter of the index patient, who was also a p.Val804Met carrier, was diagnosed with a thyroid nodule. This patient is awaiting further study results.
Discussion
In 1982, Winkelmann and Carney described dermal hyperneury in clinically normal skin of patients with MEN 2B syndrome and recognized these neurocutaneous lesions as multiple mucosal neuromas. Histopathologic differences between neuromas and dermal hyperneury consist of the nodular and well-circumscribed configuration of neuromas and the poor delimitation of dermal hyperneury, which results from hyperplastic proliferation and an increased number of preexisting cutaneous nerve fibers. In 1992, Requena et al remarked on the possibility of Cowden syndrome in patients with multiple sclerotic fibromas. These cutaneous lesions were linked to their respective genetic syndrome: neurocutaneous lesions with MEN 2B syndrome and multiple sclerotic fibromas with Cowden syndrome, as well as with other PHTSs, namely, Bannayan–Ruvalcaba–Riley syndrome (BRRS). Dermal hyperneury, located in cutaneous lesions and normal skin, and mucosal or cutaneous multiple neuromas were described in a single case of the MTC type of MEN 2A syndrome, in patients with MEN 2B syndrome, in patients with pure mucosal neuroma syndrome, in individuals without any other abnormality, and in the context of PHTS. Patients with pure mucosal neuroma syndrome possess features of MEN 2B syndrome, such as mucosal neuromas, thickened corneal nerves, or Marfanoid phenotype without associated endocrinopathy (MTC or pheochromocytoma) and no RET gene mutation, although a heterozygous SOS1 gene chromosome band 2p22.1 frameshift mutation has recently been described. Some authors considered predominantly acral neuromas as an underrecognized manifestation of PHTS.
Multiple sclerotic fibromas are considered markers of PHTS, an autosomal dominant spectrum of disorders that includes Cowden syndrome, BRRS, Lhermitte-Duclos syndrome, and autism spectrum disorders associated with macrocephaly. They are caused by germline inactivating mutations in the PTEN tumor suppressor gene located on chromosomal band 10q23.3. Multiple sclerotic fibromas may appear in any cutaneous area. Patients with PHTS have increased risk for breast, thyroid, endometrium, colorectal, and kidney carcinomas; melanoma; multiple colonic polyposis; and colonic ganglioneuromatosis, with the latter also described in the context of MEN 2B syndrome. In these patients, the main histopathologic subtype of thyroid carcinoma is papillary carcinoma (60%-80%); C-cell hyperplasia was also reported, but MTC has not yet been described, to our knowledge.
PTEN hamartoma-tumor and MEN 2 syndromes share several clinical features, including neuromas, dermal hyperneury, prominent corneal nerves, gastrointestinal tract ganglioneuromatosis, and thyroid C-cell hyperplasia. There is also considerable overlap in the signaling pathways regulated by PTEN and RET, mostly those associated with neural and neural crest–derived tissue homeostasis. These overlaps in signaling pathways may explain the clinical overlap in some of the phenotypic features between the 2 syndromes.
Conclusions
This family with the familial MTC type of MEN 2A syndrome, carried a moderate-risk RET p.Val804Met mutation. Two relatives presented with dermal hyperneury, a cutaneous manifestation classically described in MEN 2B syndrome, and 1 relative also possessed multiple sclerotic fibromas. To our knowledge, multiple sclerotic fibromas not associated with PHTS have not been previously described. Although this association may be casual, this description of these lesions in a patient with the familial MTC type of MEN 2A syndrome supported a phenotypic overlap between these 2 syndromes.
References
- 1.de Groot JW, Links TP, Plukker JT, Lips CJ, Hofstra RM. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev. 2006;27(5):535-560. [DOI] [PubMed] [Google Scholar]
- 2.Raue F, Frank-Raue K. Genotype-phenotype correlation in multiple endocrine neoplasia type 2. Clinics (Sao Paulo). 2012;67(suppl 1):69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu YH, Lloyd RV. Medullary thyroid carcinoma: recent advances including microRNA expression. Endocr Pathol. 2016;27(4):312-324. [DOI] [PubMed] [Google Scholar]
- 4.Wells SA Jr, Asa SL, Dralle H, et al. ; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma . Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkelmann RK, Carney JA. Cutaneous neuropathology in multiple endocrine neoplasia, type 2b. J Invest Dermatol. 1982;79(5):307-312. [DOI] [PubMed] [Google Scholar]
- 6.Requena L, Gutiérrez J, Sánchez Yus E. Multiple sclerotic fibromas of the skin: a cutaneous marker of Cowden’s disease. J Cutan Pathol. 1992;19(4):346-351. [DOI] [PubMed] [Google Scholar]
- 7.Al-Daraji WI, Ramsay HM, Ali RB. Storiform collagenoma as a clue for Cowden disease or PTEN hamartoma tumour syndrome. J Clin Pathol. 2007;60(7):840-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baykal C, Buyukbabani N, Boztepe H, Barahmani N, Yazganoglu KD. Multiple cutaneous neuromas and macular amyloidosis associated with medullary thyroid carcinoma. J Am Acad Dermatol. 2007;56(2)(suppl):S33-S37. [DOI] [PubMed] [Google Scholar]
- 9.Owens M, Kivuva E, Quinn A, et al. SOS1 frameshift mutations cause pure mucosal neuroma syndrome, a clinical phenotype distinct from multiple endocrine neoplasia type 2B. Clin Endocrinol (Oxf). 2016;84(5):715-719. [DOI] [PubMed] [Google Scholar]
- 10.Truchot F, Grézard P, Wolf F, Balme B, Perrot H. Multiple idiopathic mucocutaneous neuromas: a new entity? Br J Dermatol. 2001;145(5):826-829. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer JV, Kamino H, Witkiewicz A, McNiff JM, Orlow SJ. Mucocutaneous neuromas: an underrecognized manifestation of PTEN hamartoma-tumor syndrome. Arch Dermatol. 2006;142(5):625-632. [DOI] [PubMed] [Google Scholar]
- 12.Ferran M, Bussaglia E, Lázaro C, Matias-Guiu X, Pujol RM. Acral papular neuromatosis: an early manifestation of Cowden syndrome. Br J Dermatol. 2008;158(1):174-176. [DOI] [PubMed] [Google Scholar]
- 13.Cameselle-Teijeiro J, Fachal C, Cabezas-Agrícola JM, et al. Thyroid pathology findings in Cowden syndrome: a clue for the diagnosis of the PTEN hamartoma tumor syndrome. Am J Clin Pathol. 2015;144(2):322-328. [DOI] [PubMed] [Google Scholar]
- 14.Wagner SM, Zhu S, Nicolescu AC, Mulligan LM. Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2. Clinics (Sao Paulo). 2012;67(suppl 1):77-84. [DOI] [PMC free article] [PubMed] [Google Scholar]