Key Points
Question
Is the rate of hypoglycemia noninferior or lower with insulin degludec vs insulin glargine U100 in insulin-treated patients with type 1 diabetes?
Findings
In this randomized crossover trial of 501 patients, insulin degludec compared with insulin glargine U100 resulted in a significantly lower rate of overall symptomatic hypoglycemic episodes over a 16-week maintenance period (2201 vs 2463 episodes per 100 patient-years of exposure).
Meaning
Patients with type 1 diabetes treated with insulin degludec, compared with insulin glargine U100, had a reduced risk of overall symptomatic hypoglycemia.
Abstract
Importance
Hypoglycemia, common in patients with type 1 diabetes, is a major barrier to achieving good glycemic control. Severe hypoglycemia can lead to coma or death.
Objective
To determine whether insulin degludec is noninferior or superior to insulin glargine U100 in reducing the rate of symptomatic hypoglycemic episodes.
Design, Setting, and Participants
Double-blind, randomized, crossover noninferiority trial involving 501 adults with at least 1 hypoglycemia risk factor treated at 84 US and 6 Polish centers (January 2014-January 12, 2016) for two 32-week treatment periods, each with a 16-week titration and a 16-week maintenance period.
Interventions
Patients were randomized 1:1 to receive once-daily insulin degludec followed by insulin glargine U100 (n = 249) or to receive insulin glargine U100 followed by insulin degludec (n = 252) and randomized 1:1 to morning or evening dosing within each treatment sequence.
Main Outcomes and Measures
The primary end point was the rate of overall severe or blood glucose-confirmed (<56 mg/dL) symptomatic hypoglycemic episodes during the maintenance period. Secondary end points included the rate of nocturnal symptomatic hypoglycemic episodes and proportion of patients with severe hypoglycemia during the maintenance period. The noninferiority criterion for the primary end point and for the secondary end point of nocturnal hypoglycemia was defined as an upper limit of the 2-sided 95% CI for a rate ratio of 1.10 or lower; if noninferiority was established, 2-sided statistical testing for superiority was conducted.
Results
Of the 501 patients randomized (mean age, 45.9 years; 53.7% men), 395 (78.8%) completed the trial. During the maintenance period, the rates of overall symptomatic hypoglycemia were 2200.9 episodes per 100 person-years’ exposure (PYE) in the insulin degludec group vs 2462.7 episodes per 100 PYE in the insulin glargine U100 group for a rate ratio (RR) of 0.89 (95% CI, 0.85-0.94; P < .001 for noninferiority; P < .001 for superiority; rate difference, −130.31 episodes per 100 PYE; 95% CI, −193.5 to −67.16). The rates of nocturnal symptomatic hypoglycemia were 277.1 per 100 PYE in the insulin degludec group vs 428.6 episodes per 100 PYE in the insulin glargine U100 group, for an RR of 0.64 (95% CI, 0.56-0.73; P < .001 for noninferiority; P < .001 for superiority; rate difference, −61.94 episodes per 100 PYE; 95% CI, −83.85 to −40.03). A lower proportion of patients in the insulin degludec than in the insulin glargine U100 group experienced severe hypoglycemia during the maintenance period (10.3%, 95% CI, 7.3%-13.3% vs 17.1%, 95% CI, 13.4%-20.8%, respectively; McNemar P = .002; risk difference, −6.8%; 95% CI, −10.8% to −2.7%).
Conclusions and Relevance
Among patients with type 1 diabetes and at least 1 risk factor for hypoglycemia, 32 weeks’ treatment with insulin degludec vs insulin glargine U100 resulted in a reduced rate of overall symptomatic hypoglycemic episodes.
Trial Registration
clinicaltrials.gov Identifier: NCT02034513
This crossover nonineriority trial compared the effects of insulin degludec with insulin glargine U100 in reducing on rates of symptomatic hypoglycemic episodes among adult patients with type 1 diabetes.
Introduction
Hypoglycemic episodes in type 1 diabetes are frequent, occurring both during the day and at night, and can result in significant adverse events including death. Concern about hypoglycemia is a well-recognized barrier to achieving good glycemic control, which reduces the risk of long-term complications.
First-generation basal insulin analogues have longer half-lives and reduced glycemic variability than intermediate-acting insulins. These differences translate into a clinical benefit in reducing hypoglycemia in people with type 1 diabetes. Insulin degludec is an ultralong–acting basal insulin with a half-life of more than 24 hours and a lower day-to-day variability than insulin glargine U100 and U300. Two phase 3a open-label trials and a prespecified meta-analysis involving patients with type 1 diabetes demonstrated lower rates of confirmed nocturnal hypoglycemia and no difference in overall hypoglycemia with insulin degludec vs insulin glargine U100. The SWITCH 1 trial tested whether treatment with insulin degludec was noninferior to insulin glargine U100 with respect to rate of overall symptomatic hypoglycemic episodes in patients with type 1 diabetes.
Methods
Trial Design and Participants
The SWITCH 1 trial was conducted in accordance with the Declaration of Helsinki and International Conference of Harmonisation Good Clinical Practice. Prior to trial initiation, the study design, protocol, consent form, and patient information sheet were reviewed and approved by appropriate health authorities, and an independent ethics committee and institutional review board at each site (trial protocol in Supplement 1). The review panel, which operated independently from the investigators and study sponsor, was responsible for ensuring the protection of the rights, safety, and well-being of trial participants. All protocol amendments were reviewed and approved as required according to local regulations, prior to implementation. Informed written consent was obtained from all participating patients before they entered the trial. This randomized, double-blind, 2-period crossover, multicenter, treat-to-target clinical trial involved patients with type 1 diabetes and who had at least 1 hypoglycemia risk factor (eFigure 1 in Supplement 2), across 84 sites in the United States and 6 sites in Poland between January 2014 and January 12, 2016. The trial spanned 65 weeks, consisting of treatment with once-daily insulin degludec or insulin glargine U100, both with insulin aspart 2- to 4-times daily for 2 consecutive 32-week periods and 1 week of follow-up (eFigure 1 in Supplement 2). Each 32-week treatment period consisted of a 16-week titration period (to reduce potential carry-over effects and to obtain stable glycemic control) and a 16-week maintenance period (to compare the difference in hypoglycemia when glycemic control and dose are stable).
Patients were included if they were at least 18 years or older, diagnosed with type 1 diabetes for 52 weeks or more, treated with either a basal-bolus regimen or continuous subcutaneous insulin infusion for 26 weeks or more; had hemoglobin A1c (HbA1c) levels of 10% or less and a body mass index of 45 or less (calculated as weight in kilograms divided by height in meters squared); fulfilled at least 1 of the following pretrial risk criteria for developing hypoglycemia: (1) experienced 1 or more severe hypoglycemic episodes within the last year (based on American Diabetes Association [ADA] definition); (2) had moderate chronic renal failure (estimated glomerular filtration rate 30-59 mL/min/1.73 m2); (3) were unaware of their hypoglycemic symptoms; (4) had diabetes for more than 15 years; or (5) had an episode of hypoglycemia (symptoms, blood glucose level of ≤70 mg/dL [to convert glucose from mg/dL to mmol/L, multiply by 0.0555], or both) within the last 12 weeks. The determination of whether a patient had hypoglycemia unawareness was based on a patient’s history of impaired autonomic responses (tremulousness, sweating, palpitations, and hunger) during hypoglycemia. Patients were excluded if they had received insulin degludec or insulin glargine U100 within the last 26 weeks before screening. Self-reported race/ethnicity was based on fixed categories. Noninferiority of the primary end point was assessed initially because the overall number of hypoglycemic episodes could be influenced by the concomitant use of bolus insulin.
Interventions
Patients were randomized 1:1 with a block size of 8 using a trial-specific central interactive voice or web-response system that used a simple sequential allocation randomization schedule without stratifying factors, which could be accessed at any time by authorized persons. Patients were randomized 1:1 to one of the treatment sequences (insulin degludec followed by insulin glargine U100 or insulin glargine U100 followed by insulin degludec) in a blinded manner. There was a regulatory concern that the difference in the pharmacokinetic and pharmacodynamic profiles of the insulins could affect the relative hypoglycemia; therefore, to eliminate confounding, within each treatment sequence patients were randomized 1:1 to administer basal insulin in either the morning (from waking up to breakfast) or the evening (from main evening meal to bedtime, Figure 1). Assigned administration timing was maintained throughout the trial. The trial was double-blinded; as such, all involved parties were blinded to insulin treatment allocation throughout the trial. To maintain blinding, insulin degludec 100 U/mL (Novo Nordisk) and insulin glargine 100 U/mL (Sanofi) were both administered subcutaneously from identical vials via syringes. Insulin aspart 100 U/mL was administered using a prefilled pen (FlexPen; Novo Nordisk). The starting dose of basal insulin and total bolus insulin (algorithm users only) was reduced by 20% at randomization and at crossover (ie, after 32 weeks).
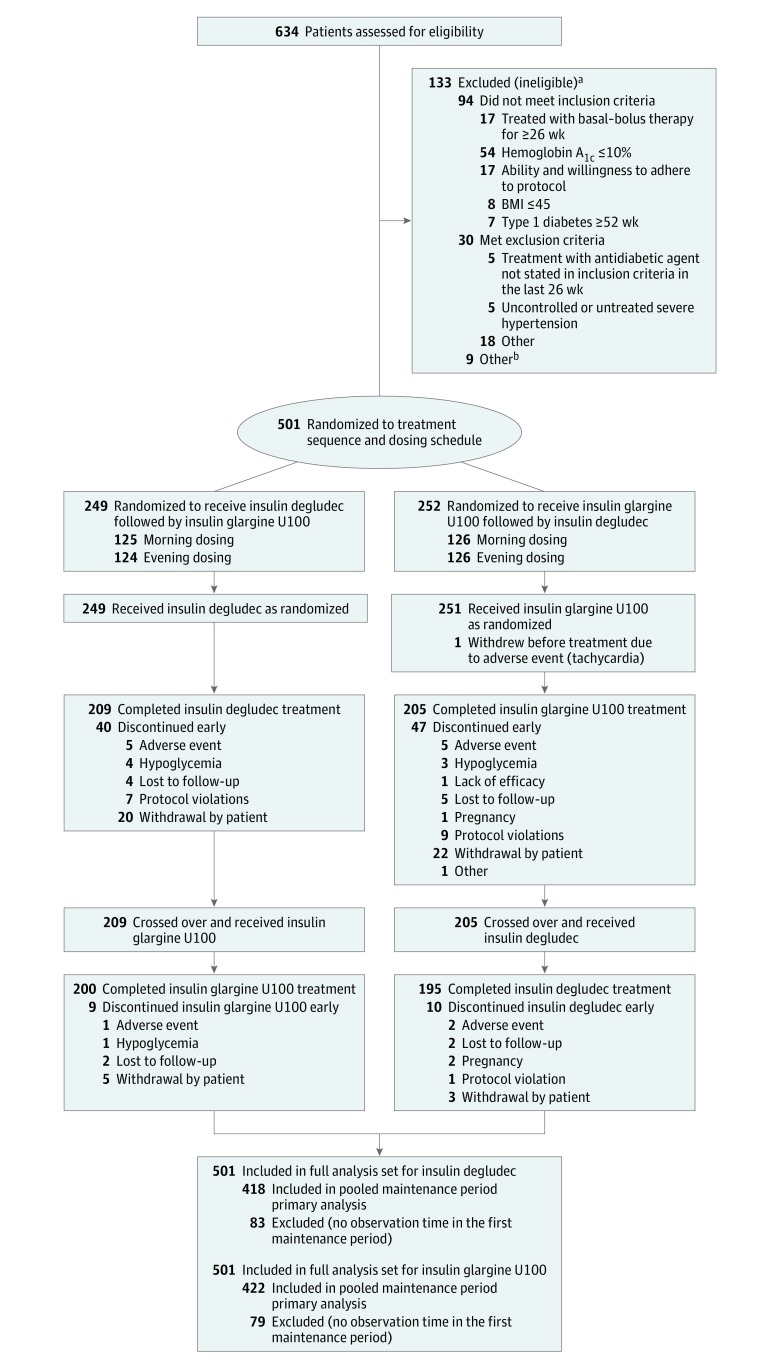
Figure 1. Patient Flow Through the SWITCH 1 Randomized Clinical Trial.
aSome patients fulfilled more than one inclusion or exclusion criterion.
bWithdrawal at the request of the patient or investigator or patient was unavailable at randomization visit following screening.
BMI, indicates body mass index, calculated as weight in kilograms divided by height in meters squared.
Patients were supplied with a blood glucose meter and instructed to measure their blood glucose before breakfast, lunch, main evening meal, and bedtime on all days throughout the trial. Their blood glucose levels were also measured whenever a hypoglycemic episode was suspected. Titration of basal insulin was performed once weekly according to the trial algorithm, based on the lowest of 3 previous prebreakfast self-measured blood glucose measurements, aiming for a fasting target of between 71 and 90 mg/dL (eTable 1 in Supplement 2). Titration of bolus insulin was either performed twice weekly based on the previous 3 or 4 days’ readings according to the provided algorithm (eTable 2 in Supplement 2), or several times daily based on the insulin:carbohydrate ratio and insulin sensitivity factor, to achieve a preprandial blood glucose target of between 71 and 108 mg/dL. Only those patients experienced in carbohydrate counting could use the latter approach. During the initial 8 weeks of the first treatment period, patients could change from carbohydrate counting to use of the bolus algorithm, but not vice versa.
End Points
The primary end point was the rate of overall severe or blood glucose–confirmed (<56 mg/dL) symptomatic hypoglycemic episodes during the maintenance period (weeks 16-32 and 48-64). Severe hypoglycemia was defined according to the ADA definition, an episode requiring assistance of another person to actively administer carbohydrate, glucagon, or take other corrective actions, neurological recovery following the return of plasma glucose to normal, or both. The hypoglycemia definition is illustrated in eFigure 2 in Supplement 2.
The secondary end points were the rate of nocturnal (severe or blood glucose–confirmed episodes between 12:01 am and 5:59 am, both inclusive) symptomatic hypoglycemic episodes, and the proportion of patients experiencing severe hypoglycemia, both occurring during the maintenance period. Other hypoglycemic end points included rates of severe hypoglycemia; overall symptomatic and nocturnal symptomatic hypoglycemia in the full treatment period; rate of severe hypoglycemia in the maintenance period; and proportion of patients with severe hypoglycemia, overall symptomatic, and nocturnal symptomatic hypoglycemia during the maintenance period and the full treatment period. All severe hypoglycemic episodes reported by investigators or identified by a predefined Medical Dictionary for Regulatory Activities version 18.1 (MedDRA) search of safety data were adjudicated prospectively by an external committee; only confirmed episodes were analyzed (eTable 3 in Supplement 2).
The efficacy end points measured were change in HbA1c, fasting plasma glucose, and prebreakfast self-measured blood glucose levels after 32 weeks of treatment. Safety end points included daily basal, bolus, and total insulin doses; change from baseline in body weight; incidence of adverse events; vital signs (including blood pressure and pulse); funduscopy and electrocardiogram results; and standard biochemical parameters.
Statistical Analysis
Analyses of all end points were based on the full analysis set (all randomized patients) following the intention-to-treat principle using SAS statistical software version 9.4 (SAS Institute Inc). Efficacy end points were summarized based on the full analysis set. Safety end points were summarized based on the safety analysis set (patients exposed to at least 1 dose of investigational product or comparator).
Missing data were explored to ascertain whether the patients who dropped out before the first maintenance period differed from those exposed during the first maintenance period because information from these patients was not included in the primary analysis. Missing data were also investigated to identify any differences in dropouts between the 2 treatments. The effects of missing data on the primary analysis were investigated with a post hoc tipping-point analysis. Missing data were imputed assuming that the rate of hypoglycemia for patients who had not completed the trial was similar to that of patients who completed the same treatment period and who had a similar number of episodes prior to withdrawal. The imputed number of episodes for patients withdrawing from insulin degludec was gradually increased until the treatment contrast between the 2 insulins was no longer significant.
A hierarchical testing procedure was specified to adjust for multiplicity and control the type I error in the strong sense for the primary and secondary end points. Noninferiority of reduction in HbA1c with a noninferiority margin of 0.4% in both treatment periods was a prerequisite to initiation of the test hierarchy (eFigure 3 in Supplement 2).
The test hierarchy specified that following the noninferiority criterion for HbA1c reduction, the primary end point was tested for noninferiority. If this criterion was achieved, then the primary end point was subsequently tested for superiority. This was also the case for the first secondary end point. Noninferiority was defined as the upper limit of the 95% CI for the estimated rate ratio of 1.10 or less. Superiority was achieved if the upper limit of the 95% CI for the rate ratio was less than 1.0. The last secondary end point, proportion with severe hypoglycemia, was directly tested for superiority, which was confirmed if the McNemar test was significant on a 5% significance level. This margin was selected based on ADA guidance defining a 10% to 20% reduction in hypoglycemia as clinically relevant. The primary and first secondary multiplicity-adjusted analyses were prespecified to be tested with 1-sided tests on a 2.5% level. Other analyses were tested with 2-sided tests on a 5% level.
The trial was powered to show noninferiority of the primary end point. Based on the assumption that up to 10% of the randomized patients may not contribute to the analysis, 400 patients needed to contribute to the analysis if 446 patients were randomized to ensure a power of 94%, to demonstrate noninferiority with an expected rate of overall symptomatic hypoglycemia of 5.0 episodes per patient-years’ exposure (PYE).
A Poisson model with patients as a random effect; treatment, period, sequence, and dosing time as fixed effects; and logarithm of the observation time (100 years) as offset was prespecified as the primary analysis to estimate the rate ratio of overall symptomatic hypoglycemia during the maintenance period. Only patients with positive observation time during the maintenance contributed to the estimated rate ratio.
Sensitivity analyses were performed to test the robustness of the results, using patients exposed in both maintenance periods only, completers only, and using a negative binomial model; further details are available in the statistical analysis plan provided as part of Supplement 1.
A post hoc analysis of the absolute difference in hypoglycemia rate was conducted using a nonlinear Poisson model with a specified mean parameter, measuring the difference between average nonexisting patients taking insulin degludec followed by insulin glargine U100, respectively (50% treatment period 1, 50% evening dose, 50% treatment sequence insulin degludec followed by insulin glargine U100).
The McNemar nonparametric test was prespecified to compare the 2 treatments with respect to the secondary outcome of the proportion of patients experiencing severe hypoglycemia, using 2-sided testing and a 5% significance level. In order to quantify the differences in proportions with 95% CIs post hoc, a binomial distribution with correlated measurements was assumed.
Change from baseline in HbA1c after 32 weeks of treatment was analyzed separately for each treatment period, with a mixed model for repeated measurements including treatment, visit, sex, region, pretrial insulin regimen, and dosing time as fixed effects, and age and baseline HbA1c as covariates. All fixed factors and covariates are nested within visit. Dosing time was a factor with 2 levels: morning and evening; region was also a factor with 2 levels: Poland and the United States. Pretrial insulin regimen was a factor with 3 levels: continuous subcutaneous insulin infusion, once-daily basal insulin injections, or twice-daily basal insulin injections.
Post hoc statistical analysis of the estimated treatment difference for the difference in absolute fasting plasma glucose values was performed using an analysis of covariance model with treatment, period, sex, region, pretrial insulin treatment, and dosing time as fixed effects, patient as random effect, and age and fasting plasma glucose at randomization as covariates.
The post hoc analysis of insulin dose was conducted on patients with observation time in the first maintenance period, with a mixed model for repeated measurements with treatment, period, dosing time, and visit as fixed effects; patient as random effect; and the log-transformed baseline dose as covariate. All fixed effects and the covariate were nested within visit.
Results
Of 634 patients screened, 501 were randomized. Two hundred forty-nine patients were randomized to receive insulin degludec followed by insulin glargine U100, and 252 patients were randomized to receive insulin glargine U100 followed by insulin degludec, with 50.1% randomized to the morning and 49.9% to the evening dosing schedule, all of whom were included in the full analysis set. One patient withdrew before treatment exposure. Overall, 395 (78.8%) patients completed the trial (Figure 1). The proportion of patients and the reasons for withdrawing from the trial were similar between treatments (insulin degludec, 11.0%; insulin glargine U100, 12.2%). The most common reasons for withdrawal in both treatment groups were withdrawal by patient and adverse events (Figure 1). Patients discontinuing before the first maintenance period were similar to those with observation time during the first maintenance period.
Baseline characteristics and insulin treatment at screening are summarized in Table 1. Patients were a mean age of 45.9 years (SD, 14.2) and had a mean duration of diabetes of 23.4 years (SD, 13.4). At screening, 19.4% were using continuous subcutaneous insulin infusion, 44.7% were using once-daily basal insulin, and 35.7% were using twice-daily basal insulin (both combined with 2-4 bolus insulin injections).
Table 1. Baseline Characteristics.
| Characteristic | No. (%) of Patients | |||
|---|---|---|---|---|
| Insulin Degludec Followed by Insulin Glargine U100 | Insulin Glargine U100 Followed by Insulin Degludec | All Patients | Completers | |
| Full analysis set | 249 | 252 | 501 | 395 |
| Men | 126 (50.6) | 143 (56.7) | 269 (53.7) | 221 (55.9) |
| Race | ||||
| White | 233 (93.6) | 229 (90.9) | 462 (92.2) | 365 (92.4) |
| Black | 13 (5.2) | 19 (7.5) | 32 (6.4) | 25 (6.3) |
| Asian | 1 (0.4) | 1 (0.4) | 2 (0.4) | 2 (0.5) |
| Other | 2 (0.8) | 3 (1.2) | 5 (1.0) | 3 (0.9) |
| Ethnicity | ||||
| Hispanic or Latino | 23 (9.2) | 28 (11.1) | 51 (10.2) | 39 (9.9) |
| Age, mean (SD), y | 45.4 (13.7) | 46.4 (14.6) | 45.9 (14.2) | 46.2 (14.0) |
| Physical characteristics, mean (SD) | ||||
| Body weight | ||||
| Kilograms | 82.1 (18.4) | 78.9 (16.2) | 80.5 (17.4) | 80.6 (17.4) |
| Pounds | 181.0 (40.5) | 174.0 (35.7) | 177.5 (38.3) | 177.6 (38.3) |
| BMI | 27.9 (5.1) | 27.0 (4.5) | 27.5 (4.8) | 27.4 (4.8) |
| Duration of diabetes, y | 23.2 (13.5) | 23.6 (13.4) | 23.4 (13.4) | 22.9 (13.3) |
| Laboratory values, mean (SD) | ||||
| Hemoglobin A1c, % | 7.7 (1.0) | 7.5 (1.0) | 7.6 (1.0) | 7.5 (1.0) |
| Fasting plasma glucose | ||||
| Milligrams per deciliter | 165.1 (77.3) | 174.4 (81.7) | 169.8 (79.6) | 168.6 (77.8) |
| Millimoles per liter | 9.2 (4.3) | 9.7 (4.5) | 9.4 (4.4) | 9.4 (4.3) |
| eGFR, mL/min/1.73 m2 | 89.9 (21.2) | 90.0 (20.9) | 90.0 (21.1) | 89.7 (20.9) |
| Smoking status | ||||
| Never | 152 (61.0) | 159 (63.1) | 311 (62.1) | 250 (63.3) |
| Previous | 65 (26.1) | 55 (21.8) | 120 (24.0) | 95 (24.1) |
| Current | 32 (12.9) | 38 (15.1) | 70 (14.0) | 50 (12.7) |
| Pretrial treatment regimen | ||||
| CSII | 43 (17.3) | 54 (21.4) | 97 (19.4) | 73 (18.5) |
| Basal + 2-4 bolus injections | ||||
| Once daily | 106 (42.6) | 118 (46.8) | 224 (44.7) | 184 (46.6) |
| Twice daily | 99 (39.8) | 80 (31.7) | 179 (35.7) | 138 (34.9) |
| Pretrial insulin | ||||
| Rapid-acting insulin (CSII) | 43 (17.3) | 54 (21.4) | 97 (19.4) | 73 (18.5) |
| Insulin detemir | ||||
| Once daily | 89 (35.7) | 101 (40.1) | 190 (37.9) | 157 (39.7) |
| Twice daily | 69 (27.7) | 46 (18.3) | 115 (23.0) | 89 (22.5) |
| Neutral protamine Hagedorn | ||||
| Once daily | 17 (6.8) | 16 (6.3) | 33 (6.6) | 26 (6.6) |
| Twice daily | 30 (12.0) | 34 (13.5) | 64 (12.8) | 49 (12.4) |
| Insulin glargine U100a | 0 | 1 (0.4) | 1 (0.2) | 1 (0.3) |
| Inclusion criterion | ||||
| Fulfilling ≥1 of the following 4 criteria | 187 (75.1) | 201 (79.8) | 388 (77.4) | 302 (76.5) |
| ≥1 Severe hypoglycemic episode in the last y | 62 (24.9) | 63 (25.0) | 125 (25.0) | 94 (23.8) |
| Moderate chronic renal failure | 25 (10.0) | 17 (6.7) | 42 (8.4) | 33 (8.4) |
| Hypoglycemia unawareness | 53 (21.3) | 51 (20.2) | 104 (20.8) | 78 (19.7) |
| Diabetes for ≥15 y | 156 (62.7) | 176 (69.8) | 332 (66.3) | 259 (65.6) |
| Hypoglycemic episode within the last 12 wk | 237 (95.2) | 222 (88.1) | 459 (91.6) | 367 (92.9) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CSII, continuous subcutaneous insulin infusion; eGFR, estimated glomerular filtration rate.
One patient was randomized in error.
Primary End Point
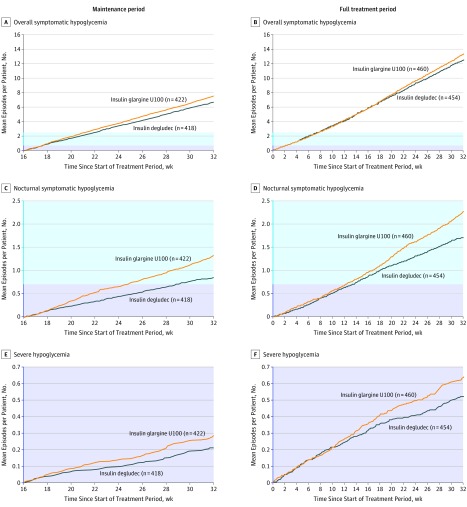
The rates of overall symptomatic hypoglycemia during the maintenance period were significantly lower with insulin degludec (2200.9 episodes per 100 PYE) than with insulin glargine U100 (2462.7 episodes per 100 PYE), for a rate ratio of 0.89 (95% CI, 0.85-0.94; P < .001). Because the upper bound of the 95% CI was lower than 1.00, noninferiority was confirmed (P < .001) and superiority was demonstrated (P < .001), meeting the primary objective (Figure 2A, Table 2). An analysis of the rate difference was also significant (−130.31 episodes per 100 PYE; 95% CI, −193.5 to −67.16), with a similar proportion of patients experiencing episodes (77.3% vs 79.9%; risk difference, –2.6%; 95% CI, –6.9% to 1.7%). Sensitivity analyses supported the findings of the primary analysis of the primary end point (eFigure 4 in Supplement 2). The post hoc tipping-point analysis showed that the statistically significant difference between the 2 treatments remained until each noncompleter taking insulin degludec was assumed to have experienced an additional 12 episodes compared with 0 additional episodes for noncompleters taking insulin glargine. The additional 12 events for noncompleters taking insulin degludec corresponded to a rate of 5316 episodes per 100 PYE compared with the observed rate of 2212 episodes per 100 PYE for insulin degludec completers (mean number of events, 17.1 vs 6.8; eTable 4 in Supplement 2).
Figure 2. Cumulative Rates of Hypoglycemia per Patient.
Data are based on safety analysis set. The tinted region in blue indicates the range from y = 0.7 to 2.5, the mean cumulative number of episodes per person; the tinted region in purple, y = 0 to 0.7, the mean cumulative number of episodes per person.
Table 2. Analysis of Hypoglycemia in the Maintenance and Full Treatment Periods.
| Definition | Insulin Degludec | Insulin Glargine U100 | Insulin Degludec vs Insulin Glargine U100 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence, No. (%) | No. of Events | Rate/100 PYE | Incidence, No. (%) | No. of Events | Rate/100 PYE | ERR (95% CI) | P Value | Absolute Rate Differences/100 PYE (95% CI) | Difference in % PYE (95% CI) | ||||||
| Maintenance Period | |||||||||||||||
| Included in the analysisa | |||||||||||||||
| No. | 418 | 422 | |||||||||||||
| PYE | 126.0 | 126.9 | |||||||||||||
| Overall symptomatic hypoglycemiab | 323 (77.3) | 2772 | 2200.9 | 337 (79.9) | 3126 | 2462.7 | 0.89 (0.85 to 0.94) |
<.001c | −130.31 (−193.5 to −67.16) |
−2.6 (−6.9 to 1.7) |
|||||
| Nocturnal symptomatic hypoglycemia | 137 (32.8) | 349 | 277.1 | 182 (43.1) | 544 | 428.6 | 0.64 (0.56 to 0.73) |
<.001c | −61.94 (−83.85 to −40.03) |
−10.4 (−15.8 to −4.9) |
|||||
| Severe hypoglycemia | 43 (10.3) | 87 | 69.1 | 72 (17.1) | 117 | 92.2 | 0.65 (0.48 to 0.89) |
.007 | −13.65 (−23.66 to −3.65) |
−6.8 (−10.8 to −2.7) |
|||||
| Full Treatment Period | |||||||||||||||
| Included in the analysisd | |||||||||||||||
| No. | 454 | 460 | |||||||||||||
| PYE | 259.2 | 261.4 | |||||||||||||
| Overall symptomatic hypoglycemia | 377 (83.0) | 5300 | 2044.6 | 398 (86.5) | 5668 | 2168.4 | 0.94 (0.91 to 0.98) |
.002 | −66.17 (−108.8 to −23.55) |
−3.5 (−7.4 to 0.4) |
|||||
| Nocturnal symptomatic hypoglycemia | 210 (46.3) | 729 | 281.2 | 248 (53.9) | 972 | 371.9 | 0.75 (0.68 to 0.83) |
<.001 | −39.35 (−54.09 to −24.61) |
−7.7 (−12.9 to −2.4) |
|||||
| Severe hypoglycemia | 90 (19.8) | 225 | 86.8 | 119 (25.9) | 275 | 105.2 | 0.74 (0.61 to 0.90) |
.003 | −6.84 (−11.73 to −1.96) |
−6.0 (−10.8 to −1.3) |
|||||
Abbreviations: ERR, estimated rate ratio; PYE, patient-years of exposure.
Only patients who were exposed in the first maintenance period contributed to the maintenance period analysis (for insulin degludec 83 patients and for insulin glargine 79 patients did not contribute to the analysis because there was no observation time in the maintenance period).
Primary end point. No. (%), No. of events and rate data are for the safety analysis set; RR was analyzed for the full analysis set (all patients randomized).
For superiority.
Only patients exposed during the full treatment period contributed to the analysis (for insulin degludec 47 patients and for insulin glargine 41 patients did not contribute to the analysis because there was no observation time in the full treatment period). All episodes of severe hypoglycemia were confirmed by the external adjudication committee. Incidence defined as the number (No.) and proportion (%) of patients experiencing a treatment-emergent hypoglycemic episode that met the classification criteria. The prespecified analysis of hypoglycemia was conducted using a Poisson model with patient as a random effect; treatment, period, sequence and dosing time as fixed effects; and logarithm of the exposure time (100 y) as offset. A post hoc analysis of the rate difference was conducted using a nonlinear Poisson model and a post hoc analysis comparing the proportion of patients with events was conducted using a regression analysis which assumed binomial distribution.
Secondary End Points
The rate of nocturnal symptomatic hypoglycemia during the maintenance period was 277.1 episodes per 100 PYE for insulin degludec vs 428.6 episodes per 100 PYE for insulin glargine U100, for a rate ratio of 0.64 (95% CI, 0.56-0.73; P < .001 for noninferiority), meeting criteria for noninferiority and also demonstrating a significant difference (P < .001) for superiority, with a rate difference of −61.94 episodes per 100 PYE (95% CI, −83.85 to −40.03), and similarly a significantly lower proportion of patients with episodes with insulin degludec than with insulin glargine U100 (32.8% vs 43.1%; risk difference, –10.4%; 95% CI, –15.8% to –4.9%; Figure 2B, Table 2). Sensitivity analyses supported the findings of the primary analysis of this secondary end point (eFigure 4 in Supplement 2).
The proportion of patients experiencing a severe hypoglycemic episode during the maintenance period was significantly lower in the insulin degludec group than in the insulin glargine U100 group (10.3%; 95% CI, 7.3%-13.3% vs 17.1%; 95% CI, 13.4%-20.8%, respectively; P = .002). An analysis of the difference in proportion was also statistically significant (−6.8%; 95% CI, −10.8% to −2.7%).
Other End Points
Hypoglycemia
During the full 32-week treatment period, use of insulin degludec had fewer overall symptomatic hypoglycemic episodes than insulin glargine (2044.6 vs 2168.4 episodes per 100 PYE) with a rate ratio of 0.94 (95% CI, 0.91-0.98; P = .002) and a rate difference of −66.17 (95% CI, −108.8 to −23.55) and had fewer nocturnal symptomatic hypoglycemic episodes than insulin glargine (281.2 vs 371.9 episodes per 100 PYE) for a rate ratio of 0.75 (95% CI, 0.68-0.83: P < .001) and a rate difference of −39.35 (95% CI, −54.09 to −24.61; Figure 2B and D, Table 2).
The rate for episodes of severe hypoglycemia was significantly lower during the maintenance period among those treated with insulin degludec than those treated with insulin glargine U100 (69.1 vs 92.2 episodes per 100 PYE) for a rate ratio of 0.65 (95% CI, 0.48-0.89, P = .007) and a rate difference of −13.65 (95% CI, −23.66 to −3.65). This trend continued during the full treatment period (86.8 vs 105.2 episodes per 100 PYE) for a rate ratio of 0.74 (95% CI, 0.61-0.90; P = .003) and a rate difference of −6.84 (95% CI, −11.73 to −1.96; Figure 2E and F, Table 2). The difference in proportions of patients experiencing 1 or more episodes during the maintenance period was not significantly different overall but was significantly different for nocturnal symptomatic hypoglycemia. The results were consistent for the full treatment period (Table 2).
Glycemic Control
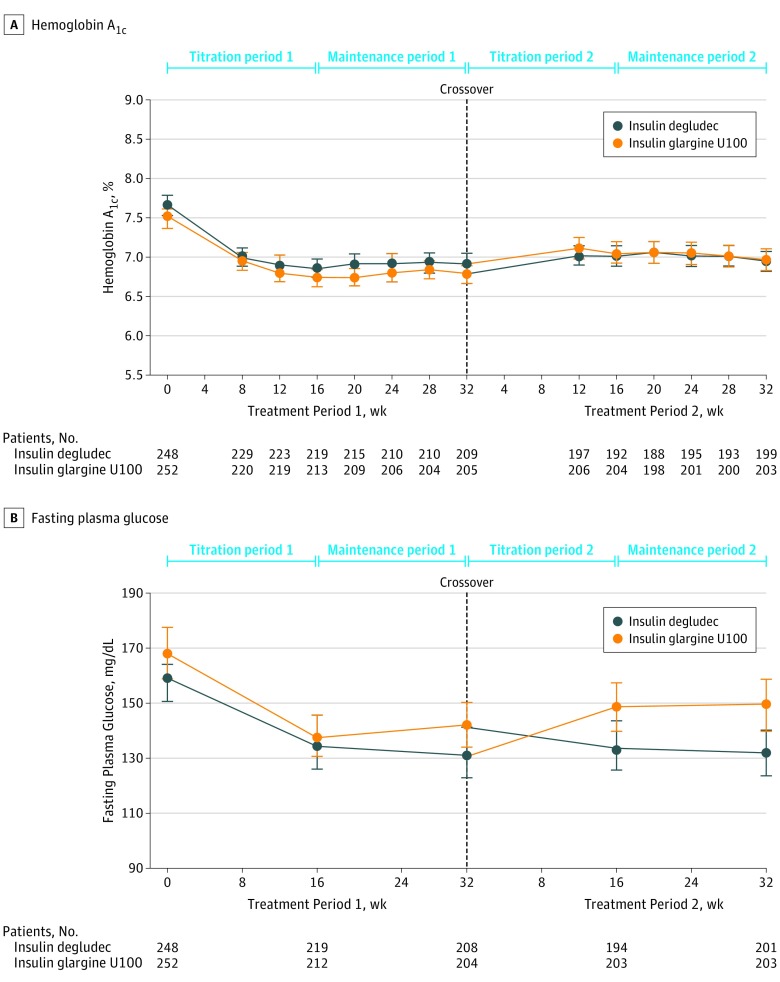
Observed mean HbA1c levels at the end of the first treatment period were 6.92% for insulin degludec vs 6.78% for insulin glargine U100 (estimated treatment difference, 0.03 percentage-points; 95% CI, –0.10 to 0.15). At the end of the second treatment period, the mean HbA1c levels were 6.95% for insulin degludec vs 6.97% for insulin glargine U100 (estimated treatment difference, 0.11 percentage-points; 95% CI, –0.00 to 0.23; Figure 3A). Noninferiority of insulin degludec to insulin glargine U100 with respect to change in HbA1c values from baseline was confirmed for both treatment periods.
Figure 3. Mean Hemoglobin A1c and Fasting Plasma Glucose Levels Over Time.
Data are observed means. Error bars indicate 95% CIs for the full analysis set. Statistical analyses were performed using a mixed-model repeated measures with treatment, sex, region, dosing time, pretrial insulin treatment, and visit as factors and with baseline hemoglobin A1c (HbA1c) and age as covariates. All fixed factors and covariates are nested within visit. Analysis of treatment period 1 only included patients having observation time in maintenance period 1; for treatment period 2, all patients having any HbA1c measurements after crossover contributed to the analysis. Severe hypoglycemia was defined according to the ADA definition (see the Methods section). The numbers of patients represent those contributing to the data at that time point.
At the end of the first treatment period, the observed mean (SD) fasting plasma glucose levels decreased in the group receiving insulin degludec followed by insulin glargine U100 from 165.1 mg/dL (77.3) at baseline to 134.3 mg/dL (64.4), with an increase when switched to insulin glargine U100 in the second treatment period to 155.3 mg/dL (76.4). A decrease in fasting plasma glucose levels was also observed in the first treatment period for the group treated with insulin glargine U100 followed by insulin degludec, from 174.4 mg/dL (81.7) at baseline to 146.3 mg/dL (64.1), which was further decreased when switched to insulin degludec in the second treatment period to 135.9 mg/dL (66.3; Figure 3B).
Post hoc analysis showed a significant reduction in fasting plasma glucose with insulin degludec compared with insulin glargine U100 after 32 weeks of treatment for an estimated treatment difference of –17.0 mg/dL (95% CI, –25.5 to –8.41 mg/dL; P < .001). The mean prebreakfast self-measured blood glucose level (used for basal dose adjustment) increased for both groups during the first week after randomization, reflecting the recommended 20% dose reduction, and decreased throughout titration period 1 before stabilizing. Insulin degludec decreased self-measured blood glucose levels more rapidly than insulin glargine U100 (eFigure 5 in Supplement 2). In the second treatment period, mean self-measured blood glucose levels for those switching from insulin degludec to insulin glargine U100 increased during the first 3 weeks (again corresponding to the recommended 20% dose reduction) and declined thereafter, before stabilizing; in contrast, mean self-measured blood glucose values for those switching from insulin glargine U100 to insulin degludec remained at a similar level throughout (eFigure 5 in Supplement 2).
Insulin Dose and Body Weight
Mean basal, bolus, and total insulin doses are summarized in eTable 5 in Supplement 2. At the end of the first treatment period, the observed mean dose increased in the group receiving insulin degludec followed by insulin glargine U100 from 29 U at baseline to 39 U. After switching to insulin glargine U100, the dose at the end of the second treatment period was 41 U. An increase in dose was also observed in the first treatment period for the group receiving insulin glargine U100 followed by insulin degludec, from 24 U at baseline to 36 U. After switching to insulin degludec, the dose at the end of the second treatment period was 37 U (eTable 5 in Supplement 2). The bolus insulin dose was stable throughout the trial in both treatment groups. Consequently, the total insulin dose followed the same pattern as the basal insulin dose. Post hoc analysis showed a 3% lower basal insulin dose and a 3% lower total insulin dose with insulin degludec than with insulin glargine U100 after 32 weeks of treatment; both results were significant, with an estimated treatment ratio of 0.97 (95% CI, 0.95-0.99; P = .02) and 0.97 (95% CI, 0.95-0.99; P = .01), respectively.
Weight changes were not significantly different between groups during the first treatment period (2.6 kg vs 2.7 kg; difference, –0.25 kg; 95% CI, –0.99 kg to 0.49 kg; P = .51) and the second treatment period (0.7 kg vs 0.0 kg; difference, 0.75 kg; 95% CI, –0.04 kg to 1.55 kg; P = .06).
Adverse Events
Rates of adverse events were 356.8 and 358.5 events per 100 PYE with insulin degludec and insulin glargine U100, and rates of serious adverse events were 39.0 and 45.1 events per 100 PYE, respectively (eTable 6 in Supplement 2). The most commonly reported adverse events experienced by 5% or more patients in the insulin degludec and insulin glargine U100 treatment groups were nasopharyngitis (15.0% and 13.3%), upper respiratory tract infection (6.4% and 8.5%), and hypoglycemia (3.7% and 7.2%), respectively.
In total, 4 patients died during the trial. One patient taking insulin degludec died as a result of smoke inhalation. Three deaths among patients taking insulin glargine U100 were reported: 1 occurred during treatment, resulting from acute coronary syndrome; the other 2 (1 from pneumonia, 1 cardiac death) occurred during follow-up. There were 2 major adverse cardiovascular events confirmed by adjudication for insulin degludec (1 nonfatal myocardial infarction, 1 nonfatal stroke). For insulin glargine U100, 2 nonfatal myocardial infarctions were confirmed.
There were no clinically relevant differences in physical examinations, blood pressure, pulse, electrocardiograms, funduscopy, or biochemical parameters between treatments.
Discussion
In this double-blind, treat-to-target, crossover trial, insulin degludec compared with insulin glargine U100 resulted in lower rates of overall symptomatic hypoglycemic episodes and nocturnal symptomatic hypoglycemia in the 16-week maintenance period and a lower proportion of patients with severe episodes in the 16-week maintenance period. These findings were consistent when analyzed over the full 32-week treatment period. The reduction of hypoglycemia in this trial, reflected in both the rates and the proportions of severe hypoglycemia, were similar in size to those observed in a meta-analysis of patients with type 1 diabetes comparing long-acting analogs (insulin glargine and detemir) with neutral protamine Hagedorn (severe hypoglycemia odds ratio, 0.73; 95% CI, 0.60-0.89) and in a recently conducted randomized trial (severe hypoglycemia odds ratio, 0.51; 95% CI, 0.19-0.84).
Severe hypoglycemia has been associated with an increased risk of subsequent mortality, morbidity, and cardiovascular events and, for patients with diabetes, is the most serious adverse effect of insulin therapy, and can result in costly hospitalization. Therefore, reducing the risk of severe hypoglycemia could represent a clinically important improvement. Less hypoglycemia was observed in the context of achieving an HbA1c level lower than 7% during treatment with both insulin degludec and with insulin glargine U100, a target recommended by the ADA. In addition, several mechanisms were established to confirm the validity of reported hypoglycemic episodes. The trial was designed as a double-blinded, crossover, treat-to-target design that supported the objective of capturing all episodes, and all episodes of severe hypoglycemia were evaluated by an external blinded adjudication committee.
This trial has several limitations. First, intensive patient monitoring occurred in the trial setting and may have affected the frequency with which hypoglycemia was collected and reported compared with the actual clinical setting. However, this type of intensive monitoring may have provided a more accurate representation of hypoglycemia rates in a population including patients with recurrent hypoglycemia than would be derived from observational studies or randomized clinical trials from which such patients are typically excluded. Second, the crossover design has an inherent potential for carryover; however, specifying the primary and secondary end points during the maintenance period aimed to eliminate the carryover effect following a 16-week wash-out and titration period. Third, the higher-than-expected withdrawal rate may have been a result of the demanding nature of the trial, including its 64-week duration, 2 different treatments, and the use of vial and syringe.
Conclusions
Among patients with type 1 diabetes and at least 1 risk factor for hypoglycemia, treatment for 32 weeks with insulin degludec compared with insulin glargine U100 resulted in a reduced rate of overall symptomatic hypoglycemic episodes.
Protocol and Statistical Plan
eFigure 1. Trial design
eFigure 2. Hypoglycemia definition used in this trial
eFigure 3. Hierarchical analysis model
eFigure 4. Sensitivity analyses for the number of overall symptomatic and nocturnal symptomatic hypoglycemic episodes in the maintenance periods
eFigure 5. Self-measured blood glucose (SMBG) over time
eTable 1. Basal insulin titration algorithm
eTable 2. Bolus insulin titration algorithm
eTable 3. Medical Dictionary for Regulatory Activities (MedDRA) predefined search of safety data
eTable 4. Multiple imputation tipping analysis for primary endpoint
eTable 5. Summary of mean basal, bolus, and total insulin doses
eTable 6. Summary of adverse events occurring during treatment periods 1 and 2
References
- 1.Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711-722. [DOI] [PubMed] [Google Scholar]
- 2.Leiter LA, Yale J-F, Chiasson J-L, Harris S, Kleinstiver P, Sauriol L. Assessment of the impact of fear of hypoglycemic episodes on glycemic and hypoglycemia management. Can J Diabetes. 2005;29(3):186-192. [Google Scholar]
- 3.Nathan DM, Genuth S, Lachin J, et al. ; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM; DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heise T, Nosek L, Rønn BB, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53(6):1614-1620. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen-Bjergaard U, Kristensen PL, Beck-Nielsen H, et al. Effect of insulin analogues on risk of severe hypoglycaemia in patients with type 1 diabetes prone to recurrent severe hypoglycaemia (HypoAna trial): a prospective, randomised, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol. 2014;2(7):553-561. [DOI] [PubMed] [Google Scholar]
- 7.Agesen RM, Kristensen PL, Beck-Nielsen H, et al. Effect of insulin analogues on frequency of non-severe hypoglycaemia in patients with type 1 diabetes prone to severe hypoglycaemia: The HypoAna trial. Diabetes Metab. 2016;42(4):249-255. [DOI] [PubMed] [Google Scholar]
- 8.Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29(8):2104-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14(9):859-864. [DOI] [PubMed] [Google Scholar]
- 10.Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14(10):944-950. [DOI] [PubMed] [Google Scholar]
- 11.Novo Nordisk Co Announcement Tresiba demonstrated lower day-to-day and within-day variability in glucose-lowering effect compared with insulin glargine U300. http://www.novonordisk.com/media/news-details.2056385.html. Posted November 16, 2016. Accessed January 6, 2017.
- 12.Heller S, Buse J, Fisher M, et al. ; BEGIN Basal-Bolus Type 1 Trial Investigators . Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 1 diabetes (BEGIN Basal-Bolus Type 1): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1489-1497. [DOI] [PubMed] [Google Scholar]
- 13.Bode BW, Buse JB, Fisher M, et al. ; BEGIN Basal-Bolus Type 1 trial investigators . Insulin degludec improves glycaemic control with lower nocturnal hypoglycaemia risk than insulin glargine in basal-bolus treatment with mealtime insulin aspart in Type 1 diabetes (BEGIN Basal-Bolus Type 1): 2-year results of a randomized clinical trial. Diabet Med. 2013;30(11):1293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15(2):175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 16.International Conference on Harmonisation. ICH Harmonised Tripartite Guideline. Good Clinical Practice 01 May 1996. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed January 6, 2017.
- 17.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245-1249. [DOI] [PubMed] [Google Scholar]
- 19.Khunti K, Alsifri S, Aronson R, et al. ; HAT Investigator Group . Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs NPH human insulin in type 1 diabetes: a meta-analysis. Diabetes Obes Metab. 2009;11(4):372-378. [DOI] [PubMed] [Google Scholar]
- 21.Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes. 2014;63(5):1738-1747. [DOI] [PubMed] [Google Scholar]
- 22.Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. [DOI] [PubMed] [Google Scholar]
- 23.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35(9):1897-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoungas S, Patel A, Chalmers J, et al. ; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363(15):1410-1418. [DOI] [PubMed] [Google Scholar]
- 25.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012). J Med Econ. 2014;17(3):176-183. [DOI] [PubMed] [Google Scholar]
- 27.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28(5):1245-1249. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association Standards of Medical Care in Diabetes–2017. Diabetes Care. 2017;40(suppl 1):S1-S133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol and Statistical Plan
eFigure 1. Trial design
eFigure 2. Hypoglycemia definition used in this trial
eFigure 3. Hierarchical analysis model
eFigure 4. Sensitivity analyses for the number of overall symptomatic and nocturnal symptomatic hypoglycemic episodes in the maintenance periods
eFigure 5. Self-measured blood glucose (SMBG) over time
eTable 1. Basal insulin titration algorithm
eTable 2. Bolus insulin titration algorithm
eTable 3. Medical Dictionary for Regulatory Activities (MedDRA) predefined search of safety data
eTable 4. Multiple imputation tipping analysis for primary endpoint
eTable 5. Summary of mean basal, bolus, and total insulin doses
eTable 6. Summary of adverse events occurring during treatment periods 1 and 2