Key Points
Question
Does a prophylactic levosimendan infusion reduce the incidence of postoperative low cardiac output syndrome in patients with impaired left ventricular function who are undergoing isolated or combined coronary artery bypass grafting surgery under cardiopulmonary bypass?
Findings
In this randomized clinical trial involving 335 patients, levosimendan compared with placebo did not result in a significant difference in the composite end point of prolonged catecholamine infusion, use of left ventricular mechanical assist device, or renal replacement therapy (52% in the levosimendan group vs 61% in the placebo group).
Meaning
Levosimendan was not effective in reducing the incidence of postoperative low cardiac output syndrome in patients such as these.
Abstract
Importance
Low cardiac output syndrome after cardiac surgery is associated with high morbidity and mortality in patients with impaired left ventricular function.
Objective
To assess the ability of preoperative levosimendan to prevent postoperative low cardiac output syndrome.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled trial conducted in 13 French cardiac surgical centers. Patients with a left ventricular ejection fraction less than or equal to 40% and scheduled for isolated or combined coronary artery bypass grafting with cardiopulmonary bypass were enrolled from June 2013 until May 2015 and followed during 6 months (last follow-up, November 30, 2015).
Interventions
Patients were assigned to a 24-hour infusion of levosimendan 0.1 µg/kg/min (n = 167) or placebo (n = 168) initiated after anesthetic induction.
Main Outcomes and Measures
Composite end point reflecting low cardiac output syndrome with need for a catecholamine infusion 48 hours after study drug initiation, need for a left ventricular mechanical assist device or failure to wean from it at 96 hours after study drug initiation when the device was inserted preoperatively, or need for renal replacement therapy at any time postoperatively. It was hypothesized that levosimendan would reduce the incidence of this composite end point by 15% in comparison with placebo.
Results
Among 336 randomized patients (mean age, 68 years; 16% women), 333 completed the trial. The primary end point occurred in 87 patients (52%) in the levosimendan group and 101 patients (61%) in the placebo group (absolute risk difference taking into account center effect, −7% [95% CI, −17% to 3%]; P = .15). Predefined subgroup analyses found no interaction with ejection fraction less than 30%, type of surgery, and preoperative use of β-blockers, intra-aortic balloon pump, or catecholamines. The prevalence of hypotension (57% vs 48%), atrial fibrillation (50% vs 40%), and other adverse events did not significantly differ between levosimendan and placebo.
Conclusions and Relevance
Among patients with low ejection fraction who were undergoing coronary artery bypass grafting with cardiopulmonary bypass, levosimendan compared with placebo did not result in a significant difference in the composite end point of prolonged catecholamine infusion, use of left ventricular mechanical assist device, or renal replacement therapy. These findings do not support the use of levosimendan for this indication.
Trial Registration
EudraCT Number: 2012-000232-25; clinicaltrials.gov Identifier: NCT02184819
This randomized clinical trial compares the effect of preoperative levosimendan vs placebo on the incidence of postoperative low cardiac output syndrome in patients with low ventricular ejection fraction who were undergoing coronary artery bypass grafting with cardiopulmonary bypass.
Introduction
Mortality after coronary artery bypass grafting (CABG) surgery declined from 2.4% to less than 1.5% between 1994 and 2009, but remained much higher (17% to 24%) in the subgroup of patients who developed a postoperative low cardiac output syndrome. The prevalence of low cardiac output syndrome after CABG surgery varies from 3% to 14%, but the presence of a preoperative left ventricular dysfunction, ie, a left ventricular ejection fraction less than or equal to 40%, is associated with an odds ratio of 2.0 (95% CI 1.7-2.4). The low cardiac output syndrome is also associated with more frequent complications, including pulmonary impairment, myocardial infarction, stroke, and renal failure.
The therapeutic management of low cardiac output syndrome involves inotropic agents as first-line treatment and mechanical assist devices when pharmacologic support fails to restore adequate perfusion. However, inotropic agents have been associated with an increase in morbidity and mortality after cardiac surgery. Therefore, new drugs with less deleterious adverse effects could be useful. Several studies and meta-analyses have suggested that levosimendan (Orion Pharma) could help prevent low cardiac output syndrome and reduce morbidity and even mortality after cardiac surgery. However, 2 recent, large, randomized, clinical trials did not confirm these findings when levosimendan was used prophylactically, ie, before cardiac surgery, or implemented in the presence of an already manifest postoperative low cardiac output syndrome. The Levosimendan in Coronary Artery Revascularization trial was conducted during the same period as these 2 studies.
The aim of this trial was to assess whether a 24-hour levosimendan infusion started at anesthetic induction in patients with impaired left ventricular function (left ventricular ejection fraction ≤40%) who were undergoing CABG (alone or combined) with the use of cardiopulmonary bypass could reduce the prevalence and severity of postoperative low cardiac output syndrome.
Methods
The trial was approved by an independent National Research Ethics Committee (CPP Ile de France VI). The study protocol was published previously and is available in Supplement 1. All patients provided written informed consent to participate.
Participants
Thirteen cardiac surgical centers participated in this study. Patients scheduled for CABG surgery with cardiopulmonary bypass alone or combined with valve surgery procedure were eligible if they had a left ventricular ejection fraction of 40% or lower. Patients undergoing isolated valve surgery were not eligible. Exclusion criteria were younger than 18 years, pregnancy, renal failure (creatinine clearance <30 mL/min), liver failure (prothrombin ratio <50%), emergency surgery, hypotension (mean arterial pressure <60 mm Hg), or tachycardia (heart rate >120/min). Patients could be enrolled if they were receiving preoperative inotropes or if the surgeon decided to insert an intra-aortic balloon pump as a prophylactic measure before cardiopulmonary bypass.
Trial Design and Randomization
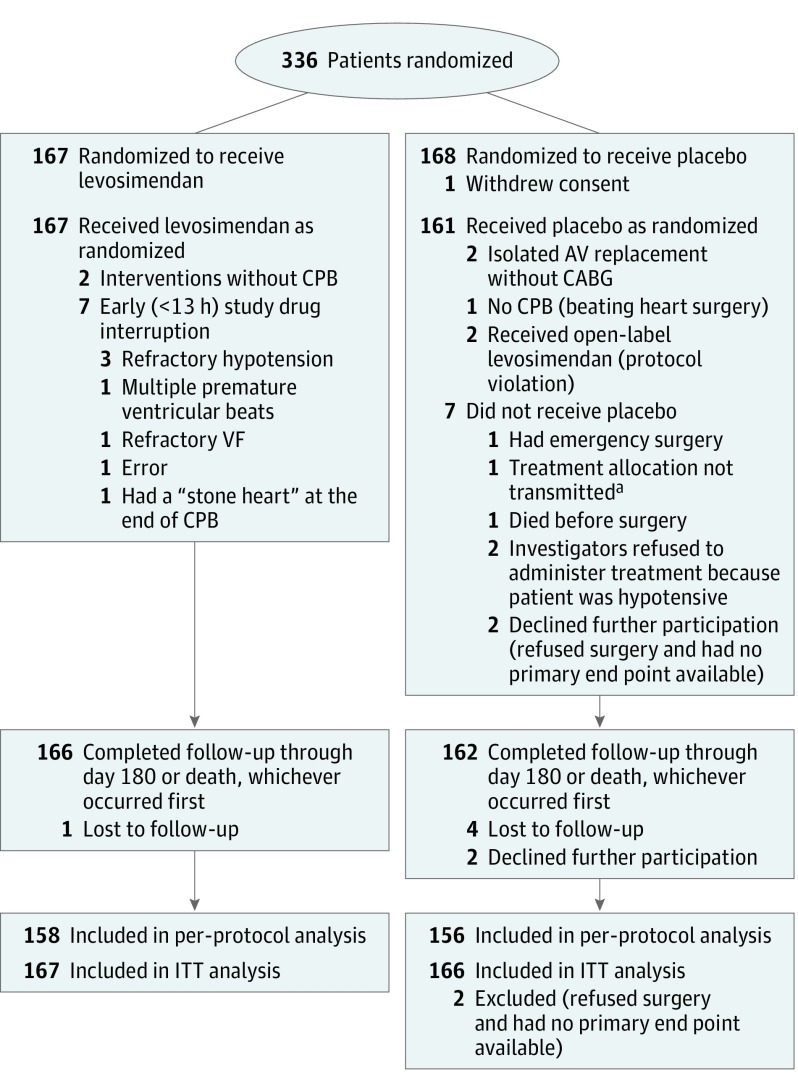
This trial was a randomized, double-blind, 2-arm, parallel-group, placebo-controlled study (Figure 1). Patients were assigned to receive prophylactic levosimendan or placebo in a 1:1 ratio with a deterministic dynamic allocation. The procedure took into account the center, the surgery performed (CABG alone or combined surgery), left ventricular ejection fraction (<30%, or between 30% and 40%), preoperative treatment by inotropes or intra-aortic balloon pump, and preoperative β-blocker therapy. This minimization procedure was expected to balance the distribution of prognostic factors between the 2 groups and within each center. A random component was introduced to reduce the predictability of allocation. An independent statistician established the randomization algorithm, which was installed on a website with secure access (as described in the protocol (Supplement 1).
Figure 1. Flow of Participants Through the Study.
CABG indicates coronary artery bypass graft; CPB, cardiopulmonary bypass; ITT, intention to treat; and VF, ventricular fibrillation. The number of patients screened for eligibility was not available. “Stone heart” is a lethal complication that corresponds to postbypass ischemically-induced myocardial contracture.
aTreatment allocation was not transmitted as a result of a communication failure with the website.
Intervention
We stored the study drugs at 5°C±3°C until reconstitution with 500 mL of 5% dextrose solution immediately before administration to the patient. The placebo was made of riboflavin to reproduce the yellow color of levosimendan and was indistinguishable from the intervention drug. No loading dose was administered. A continuous prophylactic infusion of 0.1 µg/kg/min was started just after anesthetic induction and was maintained over 24 hours in the absence of serious adverse reactions. Study drug administration was discontinued in the event of an anaphylactic reaction, refractory hypotension (defined as a mean arterial pressure <60 mm Hg despite optimal therapeutic management at the discretion of the anesthesiologist), or intractable arrhythmias.
Outcomes
The primary end point was a composite of 3 elements reflecting low cardiac output syndrome: catecholamine (ie, dobutamine, epinephrine, norepinephrine, or milrinone) infusion persisting beyond 48 hours after the initiation of the study drug, the need for circulatory mechanical assist devices in the postoperative period (for patients who had an intra-aortic balloon pump inserted preventively, it was considered that they met the outcome measure if they were not weaned from the balloon within 96 hours after study treatment initiation), or the need for renal replacement therapy at any time during intensive care unit stay. If a patient had at least 1 of these criteria, he or she was considered as meeting the primary end point.
The secondary end points were in-hospital mortality, mortality at days 28 and 180, each component of the primary end point, number of days with circulatory mechanical assist device, number of days with catecholamine infusion, number of days with renal replacement therapy and number of renal replacement therapy kits (ie, disposable circuits and filters) that were used for each patient, and number of ventilator-free days, out-of-intensive care unit and out-of-hospital days at day 28, and total hospital and intensive care unit lengths of stay. The study drug safety was assessed by recording of the incidence of hypotension, defined as mean arterial pressure less than 60 mm Hg occurring after study drug initiation. Data on fluids (volume and type of fluid) or vasopressors used to reverse hypotension, as well as arrhythmias, conduction disturbances, and the treatments they required, were also recorded. Refractory hypotension was defined as a failure to increase mean arterial pressure above 60 mm Hg despite optimal management. The myocardial damage was reflected by the highest troponin value measured between hours 24 and 48.
All adverse events and serious adverse events were prospectively recorded and reported to an independent data and safety monitoring board, which had full access to unblinded data and periodically reviewed the safety results.
Sample Size
The number of patients required was estimated to be 340 (170 per group) according to Nquery Advisor version 4.0 statistical software, with the following assumptions: (1) prevalence of the primary end point in the control group: 65% (proportion observed in the same population between 2002 and 2004 at center 1; ); (2) prevalence of the primary end point in the levosimendan group: 50% (ie, 15% absolute risk difference, more conservative than that observed by Levin et al but attesting for a clinical benefit); and α level of 5% and power of 80%.
Statistical Analysis
The primary analysis was performed on an intention-to-treat basis for all patients included in the trial except 3 (1 withdrew consent and 2 refused surgery, declined further participation, and had no criteria of judgment available). We analyzed the composite primary end point and its components according to the following strategy: first, absolute risk differences were estimated by fitting a binomial regression model using the identity link function, including a random effect to account for center effect. In case of nonconvergence of the model, a binomial regression model with a robust variance was used. Second, relative risks were estimated by fitting a log-binomial regression model. In case of nonconvergence of the model, a log-binomial regression model with a robust variance was used. Mortality data were analyzed with the same methods used for the primary end point. The other secondary end points were analyzed as count data with a Poisson model including a random effect to take into account center effect. In case of overdispersion, a negative binomial distribution was used. The log-rank test was used to compare survival curves. Cumulative incidences were compared with the Gray test.
Planned subgroup analyses assessed whether the effect of levosimendan varied according to type of surgical procedure performed (CABG alone or combined surgery); left ventricular ejection fraction less than 30%, or 30% to 40%; preoperative catecholamine or mechanical assist device; and preoperative β-blockers. The heterogeneity of the treatment effect was evaluated by an interaction test and is presented with a forest plot. The P value for interaction was obtained from the log-binomial model.
For all hypotheses testing, 2-sided tests were used and P < .05 was considered to indicate statistical significance. No adjustment was made for multiple comparisons; therefore, all secondary outcomes should be considered exploratory. Because of the small amount of missing data, multiple imputation was not performed. All calculations were performed with SAS version 11.3.
Results
Patient Population
A total of 336 patients were eligible and randomized from June 2013 until May 2015 and followed up during 6 months (last follow-up November 30, 2015). A total of 333 patients completed the trial, as detailed in Figure 1. Vital status was missing for 6 patients. Levosimendan and placebo groups were well balanced: mean (SD) age (69 [10] vs 67 [10] years), male sex (83% vs 85%), mean (SD) ejection fraction (33% [6%] vs 33% [6%]), and Euroscore II (3.1% vs 3.4%), respectively. The baseline characteristics are presented in Table 1 and the surgical procedures are described in Table 2. In both groups, 74% of patients had isolated CABG and 26% had combined surgery. The proportion of patients with left ventricular ejection fraction of 30% or lower was 20% in the levosimendan group and 23% in the placebo group (P = .44). The complete details of the Euroscore II are presented in eTable 1 in Supplement 2). Ninety-one percent of the participants received an infusion of study drug during 24 hours (SD, 3 hours).
Table 1. Baseline Patient Characteristics.
| No. (%) | ||
|---|---|---|
| Levosimendan (n = 167) |
Placebo (n = 168) |
|
| Age, mean (SD), y | 69 (10) | 67 (10) |
| Male sex | 139 (83) | 143 (85) |
| BMI, mean (SD) | 27 (5) | 27 (5) |
| ASA classa | ||
| 1-2 | 7 (4) | 4 (2) |
| 3 | 126 (75) | 120 (71) |
| 4 | 34 (20) | 44 (26) |
| Euroscore II,b median (IQR) [range], % | 3.1 (2-5.9) [0.7-31.1] |
3.4 (2-6.4) [0.9-29.5] |
| LVEF | ||
| 30%-40% | 134 (80) | 129 (77) |
| <30% | 33 (20) | 39 (23) |
| Creatinine clearance, mL/min | ||
| <50 | 36 (22) | 31 (18) |
| >85 | 60 (36) | 58 (35) |
| ≥50 and ≤85 | 71 (42) | 79 (47) |
| NYHA class | ||
| I | 18 (11) | 19 (11) |
| II | 87 (52) | 59 (35) |
| III | 57 (34) | 84 (50) |
| IV | 5 (3) | 6 (4) |
| Cancer | 6 (4) | 13 (8) |
| Cirrhosis | 0 | 4 (2) |
| Preoperative medication | ||
| IABP | 8 (5) | 4 (2) |
| Catecholamine infusion | 2 (1) | 4 (2) |
| β-Blockers | 134 (80) | 136 (81) |
| Statin | 150 (90) | 142 (85) |
| Anticoagulant therapy | 62 (37) | 65 (39) |
| Antiplatelet therapy | 154 (92) | 160 (95) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
ASA class 1, normal healthy patient; class 2, patient with mild systemic disease; class 3, patient with severe systemic disease; class 4, patient with severe systemic disease that is a constant threat to life; and class 5, moribund patient who is not expected to survive without the operation.
Euroscore II is a risk model that provides the risk of death after cardiac surgery (range, 0.5% to 99.7%).
Table 2. Surgical Procedures.
| Levosimendan (n = 167) |
Placebo (n = 168)a |
|
|---|---|---|
| CABG only, No. (%) | 123 (74) | 123 (74) |
| Grafts among CABG only, No. (%) | ||
| 1 | 6 (5) | 3 (2) |
| 2 | 48 (39) | 37 (30) |
| ≥3 | 69 (56) | 83 (68) |
| All-arterial revascularization | 51 (41) | 56 (46) |
| Left anterior descending artery involved | 116 (94) | 119 (97) |
| Off-pump surgery | 2 | 1 |
| CABG combined with other cardiac repair, No. (%) | 44 (26) | 43 (26) |
| CABG not performed, No. (%) | 0 | 3 (7) |
| CABG grafts performed, No. (%) | ||
| 1 | 13 (30) | 9 (21) |
| 2 | 19 (43) | 19 (44) |
| ≥3 | 12 (27) | 12 (28) |
| Mitral valve repair | 8 (18) | 12 (28) |
| Biological mitral replacement | 4 (9) | 3 (7) |
| Tricuspid valve repair | 0 | 3 (7) |
| Aortic valve repair | 0 | 3 (7) |
| Biological aortic valve replacement | 24 (55) | 19 (44) |
| Mechanical aortic valve replacement | 4 (9) | 0 |
| Mitral and tricuspid valve repair | 0 | 1 (2) |
| Other associated proceduresb | 5 (11) | 7 (16) |
| CPB time, median (IQR) [range], min | 89 (68-112) [27-251] |
92 (72-119) [19-226] |
| Aortic cross-clamp time, median (IQR) [range], min | 61 (44-81) [0-196] |
62 (48-81) [0-168] |
| Post-CPB catecholamine infusion, No. (%) | 150 (90) | 140 (83) |
Abbreviations: CABG, coronary artery bypass graft; CPB cardiopulmonary bypass.
Two patients in the placebo group refused participation after randomization.
Surgical treatment of arrhythmia, carotid endarterectomy, pacemaker, or internal defibrillator implantation.
Primary and Secondary End Points
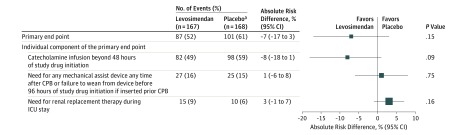
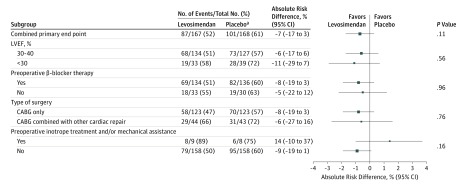
In the intention-to-treat population, the primary end point occurred in 87 levosimendan patients (52%) and 101 placebo patients (61%) (absolute risk difference, −7%; 95% CI, −17% to 3%; P = .15) (Figure 2). Among the 3 components of the primary end point, the need for catecholamine infusion beyond 48 hours of study drug initiation was the most frequent (n = 180; 54%) but was not significantly affected by levosimendan administration, with an absolute risk difference of −8% (95% CI, −18% to 1%; P = .09). The need for mechanical assist device (or failure to wean from intra-aortic balloon pump at hour 96 of study drug initiation, when the balloon was inserted preoperatively) and the need for renal replacement therapy were also not affected by levosimendan (Figure 2). In the per-protocol population, the primary end point occurred in 81 levosimendan patients (51%) and 95 placebo patients (61%) (absolute risk difference, −8%; 95% CI, −18% to 3%; P = .14). Predefined subgroup analysis found no interaction with left ventricular ejection fraction lower than 30%, preoperative treatment with β-blockers, type of surgery, or preoperative intra-aortic balloon pump or preoperative catecholamine infusion (Figure 3). Relative risks in regard to the primary end point and its components, as well as for the predefined subgroups, are presented in the supplement (eFigures 1 and 2 in Supplement 2, respectively).
Figure 2. Forest Plot of the Absolute Risk Difference in the Primary End Point and Its Individual Components.
CPB indicates cardiopulmonary bypass; ICU, intensive care unit. The size of the data markers reflects the precision of the estimation. The error bars indicate 95% CIs. Mechanical assist devices consisted of 22 intra-aortic balloon pumps in each group and 8 venoarterial extracorporeal life supports (5 in the levosimendan group and 3 in the placebo group). The decision for renal replacement therapy was at the discretion of the physicians in charge.
aTwo patients in the placebo group who refused participation after randomization could not be assessed for the primary end point and were excluded from the intention-to-treat analysis.
Figure 3. Forest Plot of the Absolute Risk Difference in the Primary End Point According to Predefined Subgroups.
CABG indicates coronary artery bypass graft; LVEF, left ventricular ejection fraction. The size of the data markers reflects the precision of the estimation. The error bars indicate 95% CIs. P values for subgroup comparisons correspond to test for interaction.
aTwo patients in the placebo group who refused participation after randomization could not be assessed for the primary end point and were excluded from the intention-to-treat analysis.
No significant difference was found in any secondary end point between the 2 groups, including mortality at days 28 and 180 (Table 3). Absolute risk difference for mortality at day 180 was −1% (95% CI, −6% to 3%; P = .59). Relative risks for mortality and the actuarial survival curve are presented in eTable 2 and eFigure 3 in Supplement 2, respectively. Cumulative incidence curves representing probability of intensive care unit discharge at day 28 are shown in eFigure 4 in Supplement 2 and weaning from mechanical ventilation at day 28 in eFigure 5 in Supplement 2.
Table 3. Secondary End Points.
| Levosimendan (n = 167) |
Placebo (n = 168)a |
P Value | |
|---|---|---|---|
| Mortality, No. (%) | |||
| In-hospital death | 10 (6) | 12 (7) | .59 |
| Mortality at day 28 | 12 (7) | 9 (5) | .39 |
| Mortality at day 180 | 14 (8) | 16 (10) | .59 |
| Survival, length of stay, and treatment outcomes, median (IQR) [range], d | |||
| Days alive out-of-hospital at day 28 | 16 (9-20) [0-24] | 16 (11-19) [0-23] | .31 |
| Days alive out of ICU at day 28 | 24 (21-26) [0-28] | 24 (22-25) [0-27] | .33 |
| Length of ICU stay | 4 (2-7) [0-61] | 4 (3-6) [1-42] | .41 |
| Length of hospital stay | 7 (5-10) [1-134] | 7 (5-10) [2-86] | .73 |
| Days without ventilator at day 28 | 11 (8-16) [3-138] | 10 (8-14) [2-87] | .46 |
| Duration of catecholamine treatment | 2 (1-4) [0-23] | 3 (2-5) [0-32] | .18 |
| Duration of renal replacement therapy | 4 (3-5) [1-9] | 4 (3-6) [1-30] | .43 |
| No. of days with circulatory mechanical assist device | 4 (2-5) [1-17] | 4 (2-5) [0-32] | .18 |
| No. of renal replacement therapy kits used, mean (SD) [range]b | 1.7 (1.0) [1-4] | 1.8 (1.0) [1-4] | .84 |
Abbreviation: ICU, intensive care unit.
Two patients in the placebo group refused participation after randomization.
A renal replacement kit corresponds to disposable circuit and filter used for dialysis.
Safety Outcomes
There was no significant difference in the incidence of adverse events or serious adverse events between the 2 groups (Table 4). Severe hypotension occurred in 57% of patients receiving levosimendan and 48% of those receiving placebo (P = .11). The management of hypotension was comparable between the 2 groups, except for the use of epinephrine. Refractory hypotension requiring study drug interruption occurred in 5 patients from the levosimendan group vs 1 from the placebo group (P = .12). There was no significant between-group difference in the incidence of postoperative atrial fibrillation (50% vs 40%, levosimendan vs placebo, respectively; P = .09), or third-degree atrioventricular blocks (4% vs 9%, respectively; P = .08). During the hospital stay, the use of antiarrhythmic agents was comparable between the 2 groups, but more levosimendan patients received amiodarone in the operating room (19 [11%] vs 6 [4%] placebo patients; P = .007). The peak troponin value measured between hours 24 and 48 was not significantly different between the 2 groups (Table 4).
Table 4. Safety Outcomes.
| No. (%) | P Value | ||
|---|---|---|---|
| Levosimendan (n = 167) |
Placebo (n = 168)a |
||
| Patients with any adverse event | 160 (96) | 155 (92) | .17 |
| Patients with any serious adverse eventb | 149 (89) | 145 (86) | .40 |
| Hypotension after treatment initiation before CPB | 95 (57) | 80 (48) | .11 |
| Patients receiving fluids to correct hypotension | 49 (50) | 39 (47) | .69 |
| Colloid | 9 (18) | 5 (13) | .31 |
| Crystalloid | 35 (71) | 33 (85) | |
| Crystalloid and colloid | 5 (10) | 1 (3) | |
| Total fluid volume administered, median (IQR) [range], mL | 500 (325-538) [250-2500] | 500 (500-750) [250-2500] | .13 |
| Patients receiving vasoconstrictors to correct hypotension | 81 (49) | 66 (39) | .11 |
| Ephedrine | 53 (32) | 48 (29) | .58 |
| Phenylephrine | 34 (20) | 24 (14) | .16 |
| Norepinephrine | 34 (20) | 25 (15) | .21 |
| Epinephrine | 8 (5) | 1 (1) | .04 |
| Highest troponin value between hours 24 and 48 after surgery, median (IQR) [range], µg/Lc | 2.9 (0.7-6.5) [0.1-657] |
3.0 (0.8-9.3) [0.1-399] |
.40 |
| Arrhythmia or conduction disturbance during hospital stay | 104 (62) | 94 (56) | .24 |
| Postoperative atrial fibrillation | 83 (50) | 68 (40) | .09 |
| Ventricular tachycardia | 20 (12) | 18 (11) | .72 |
| Ventricular fibrillation | 24 (14) | 27 (16) | .67 |
| Third-degree atrioventricular block | 7 (4) | 15 (9) | .08 |
| No. of times patients required electrical cardioversion in the OR | |||
| 1 | 18 (56) | 21 (64) | .20 |
| 2 | 6 (19) | 9 (27) | |
| ≥3 | 8 (25) | 3 (9) | |
| Reasons for study drug interruption before end of treatment | 14 (8) | 5 (3) | .03 |
| Refractory hypotension | 5 (3) | 1 (1) | .12 |
| Death during study drug infusion | 2 (1) | 0 | .25 |
Abbreviations: CPB, cardiopulmonary bypass; OR, operating room.
Two patients in the placebo group refused participation after randomization.
The complete list of serious adverse events is provided as a supplement (eTable 3 in Supplement 2).
Troponin value measured between hours 24 and 48 after the end of the surgery.
Discussion
In this randomized trial of patients with ejection fraction of 40% or lower who were undergoing CABG with cardiopulmonary bypass, levosimendan compared with placebo did not result in a significant difference in the composite end point of prolonged catecholamine infusion, use of left ventricular mechanical assist device, or renal replacement therapy.
The low cardiac output syndrome is a postoperative complication defined as the need for catecholamine infusion or mechanical assist device to maintain adequate arterial blood pressure and cardiac index. Patients with low cardiac output syndrome also have a high rate of renal failure (≈ 20%) as a consequence of inadequate tissue perfusion. Therefore, if a patient does not require prolonged postoperative catecholamine infusion, mechanical assist device, or renal replacement therapy, it is unlikely that he or she has experienced low cardiac output syndrome. This is why the combination of these 3 clinical markers was chosen as a simple and robust primary end point to evaluate the efficacy of levosimendan.
Administering inotropic support to patients with impaired left ventricular ejection fraction at cardiopulmonary bypass weaning is common even if there is no measurement attesting for a low cardiac output. However, these infusions are usually tapered and stopped quickly in the intensive care unit when there is no persistent cardiac dysfunction, whereas their maintenance beyond 48 hours postoperatively reflects a true impairment in myocardial contractility. Inotropic support at hour 48 was required in 49% of levosimendan patients as opposed to 59% of those who received placebo (P = .09).
These findings are consistent with those of Mehta et al (the LEVO-CTS study [Levosimendan in Patients with Left Ventricular Systolic Dysfunction Undergoing Cardiac Surgery Requiring Cardiopulmonary Bypass]), who recently reported, in a similar cohort of patients in whom levosimendan infusion was started at anesthetic induction, a lack of significant reduction in their primary end point, composed of mortality at day 30; the need for mechanical assist device at day 5; the need for renal replacement therapy through day 30; and myocardial infarction through day 5. Likewise, in another recent randomized trial (the CHEETAH study [Levosimendan to Reduce Mortality in High Risk Cardiac Surgery Patients: A Multicenter Randomized Controlled Trial]), Landoni et al did not find a significant difference in mortality at day 30 (primary end point) in their population, in which levosimendan was administered only after the onset of severe cardiac dysfunction after cardiopulmonary bypass.
None of the predefined factors (prophylactic intra-aortic balloon pump or catecholamine support, preoperative β-blockers, severity of left ventricular ejection fraction alteration, or type of surgery) was found to influence the effect of levosimendan. The lack of significant difference according to preoperative β-blockade does not corroborate previous observations. To explain this discrepancy, it is likely that β-blockers were not reintroduced in the early postoperative period in patients with hemodynamic compromise who were treated with the β-agonist dobutamine.
No significant difference in mortality (up to day 180) was found between the levosimendan and placebo-treated groups, a result in sharp contradiction with the conclusions of several meta-analyses but confirming the findings of the 2 abovementioned recent large randomized clinical trials. Similarly, the need for renal replacement therapy was not reduced by the use of levosimendan in this study, as well as in the LEVO-CTS and CHEETAH trials, a finding that contradicts results of previous studies supporting the improvement in renal function with levosimendan after cardiac surgery.
The total numbers of adverse events and serious adverse events were not significantly different between the 2 groups. Refractory hypotension leading to study drug interruption was observed in 5 patients in the levosimendan group and 1 in the placebo group (P = .12). Nonsignificant excess in hypotension was also observed in the LEVO-CTS trial, in which patients received the same regimen of levosimendan as that used in this study, but not in the CHEETAH trial, in which the average dose was 0.07 µg/kg/min. The prevalence of postoperative atrial fibrillation was not significantly higher in the levosimendan group (50% vs 40%; P = .09), but an excess of postoperative atrial fibrillation has been observed in medical patients with advanced left ventricular heart failure, as well as in surgical patients after cardiac procedures. The role of levosimendan in protecting the myocardium was not specifically addressed in this study, but no difference was observed in peak troponin values, unlike what had been reported previously. This lack of clinically relevant anti-ischemic effect was also suggested by the 2 other randomized clinical trials that found no difference in postoperative myocardial infarction.
Limitations
This study has several limitations. First, the use of a small dose of levosimendan without an initial bolus was deliberately chosen to avoid an excess of vasodilatation in this fragile population, as currently recommended. This might explain, in part, the minimal effect of levosimendan observed in this study. Second, the timing of administration was also very close to the “myocardial injury” resulting from cardiopulmonary bypass and cardioplegic arrest, thus limiting the potential protective effect of the drug related to the opening of cardiac mitochondrial adenosine triphosphate–dependent potassium channels. Starting levosimendan several hours before surgery would have required an environment that was not available routinely at most institutions to monitor the effect of the infusion. Third, the end point of the study involved decisions that may vary according to the center and to caregivers, which is why we performed statistical comparisons with models taking into account the variability related to the center. Fourth, this study failed to enroll the anticipated number of participants by 5 (335 instead of 340) because recruitment had to be stopped when study drugs reached their expiration date. However, this small gap is unlikely to have severely reduced the anticipated power of the study, especially because only 2 patients had no data on the primary end point and had to be removed from the intention-to-treat analysis. The number of patients to include in this trial was estimated according to an expected prevalence of the composite primary end point of 65% in the target population. The observed prevalence was 61% in the placebo group, attesting that the initial assumption was realistic. We hypothesized that levosimendan would reduce this figure to 50%, but the observed prevalence was 52% in the intention-to-treat population (51% in the per-protocol population). Because the study was powered according to a 15% absolute risk reduction and the point estimate favored levosimendan (by 7%) but included a reduction of 17%, it is possible that the study was underpowered to definitely rule out a clinically important benefit for the drug for the primary composite outcome.
Conclusions
Among patients with low ejection fraction who were undergoing CABG with cardiopulmonary bypass, levosimendan compared with placebo did not result in a significant difference in the composite end point of prolonged catecholamine infusion, use of left ventricular mechanical assist device, or renal replacement therapy. These findings do not support the use of levosimendan for this indication.
Protocol for LICORN.
eFigure 1: Forest plot of relative risk differences for the primary end point and its components.
eFigure 2: Forest plot of the absolute risk difference in the primary end point according to predefined subgroups.
eFigure 3: Kaplan-Meier estimates of the probability of survival at Day 180.
eFigure 4: Cumulative incidence of patients “out of ICU and alive” at Day 28.
eFigure 5: Cumulative incidence of patients “off ventilator” at Day 28.
eTable 1 Euroscore II.
eTable 2: Relative Risks for in-hospital mortality, mortality at Day 28 and Day 180.
eTable 3: Complete list of serious adverse events in the levosimendan and placebo groups.
References
- 1.Algarni KD, Maganti M, Yau TM. Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 2011;92(5):1678-1684. [DOI] [PubMed] [Google Scholar]
- 2.Landoni G, Bove T, Crivellari M, et al. Acute renal failure after isolated CABG surgery: six years of experience. Minerva Anestesiol. 2007;73(11):559-565. [PubMed] [Google Scholar]
- 3.Fellahi JL, Parienti JJ, Hanouz JL, Plaud B, Riou B, Ouattara A. Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity-adjusted analyses. Anesthesiology. 2008;108(6):979-987. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen DV, Hansen MK, Johnsen SP, Hansen M, Hindsholm K, Jakobsen CJ. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120(5):1098-1108. [DOI] [PubMed] [Google Scholar]
- 5.De Hert SG, Lorsomradee S, Cromheecke S, Van der Linden PJ. The effects of levosimendan in cardiac surgery patients with poor left ventricular function [erratum in Anesth Analg.2007;104(6):1544]. Anesth Analg. 2007;104(4):766-773. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson HI, Jalonen JR, Heikkinen LO, et al. Levosimendan facilitates weaning from cardiopulmonary bypass in patients undergoing coronary artery bypass grafting with impaired left ventricular function. Ann Thorac Surg. 2009;87(2):448-454. [DOI] [PubMed] [Google Scholar]
- 7.Tritapepe L, De Santis V, Vitale D, et al. Levosimendan pre-treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth. 2009;102(2):198-204. [DOI] [PubMed] [Google Scholar]
- 8.Erb J, Beutlhauser T, Feldheiser A, et al. Influence of levosimendan on organ dysfunction in patients with severely reduced left ventricular function undergoing cardiac surgery. J Int Med Res. 2014;42(3):750-764. [DOI] [PubMed] [Google Scholar]
- 9.Levin R, Degrange M, Del Mazo C, Tanus E, Porcile R. Preoperative levosimendan decreases mortality and the development of low cardiac output in high-risk patients with severe left ventricular dysfunction undergoing coronary artery bypass grafting with cardiopulmonary bypass. Exp Clin Cardiol. 2012;17(3):125-130. [PMC free article] [PubMed] [Google Scholar]
- 10.Baysal A, Yanartas M, Dogukan M, Gundogus N, Kocak T, Koksal C. Levosimendan improves renal outcome in cardiac surgery: a randomized trial. J Cardiothorac Vasc Anesth. 2014;28(3):586-594. [DOI] [PubMed] [Google Scholar]
- 11.Landoni G, Biondi-Zoccai G, Greco M, et al. Effects of levosimendan on mortality and hospitalization: a meta-analysis of randomized controlled studies. Crit Care Med. 2012;40(2):634-646. [DOI] [PubMed] [Google Scholar]
- 12.Harrison RW, Hasselblad V, Mehta RH, Levin R, Harrington RA, Alexander JH. Effect of levosimendan on survival and adverse events after cardiac surgery: a meta-analysis. J Cardiothorac Vasc Anesth. 2013;27(6):1224-1232. [DOI] [PubMed] [Google Scholar]
- 13.Lim JY, Deo SV, Rababa’h A, et al. Levosimendan reduces mortality in adults with left ventricular dysfunction undergoing cardiac surgery: a systematic review and meta-analysis. J Card Surg. 2015;30(7):547-554. [DOI] [PubMed] [Google Scholar]
- 14.Mehta RH, Leimberger JD, van Diepen S, et al. ; LEVO-CTS Investigators . Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med. 2017;376(21):2032-2042. [DOI] [PubMed] [Google Scholar]
- 15.Landoni G, Lomivorotov VV, Alvaro G, et al. ; CHEETAH Study Group . Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med. 2017;376(21):2021-2031. [DOI] [PubMed] [Google Scholar]
- 16.Caruba T, Hourton D, Sabatier B, et al. Rationale and design of the multicenter randomized trial investigating the effects of levosimendan pretreatment in patients with low ejection fraction (≤40 %) undergoing CABG with cardiopulmonary bypass (LICORN study). J Cardiothorac Surg. 2016;11(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330(7495):843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin RL, Degrange MA, Porcile R, et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome [in Spanish]. Rev Esp Cardiol. 2008;61(5):471-479. [PubMed] [Google Scholar]
- 19.Cuzick J. Forest plots and the interpretation of subgroups. Lancet. 2005;365(9467):1308. [DOI] [PubMed] [Google Scholar]
- 20.Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112(1):38-51. [DOI] [PubMed] [Google Scholar]
- 21.Maganti M, Badiwala M, Sheikh A, et al. Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg. 2010;140(4):790-796. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, Teng J, Xu J, et al. Dynamic predictive scores for cardiac surgery–associated acute kidney injury. J Am Heart Assoc. 2016;5(8):e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Follath F, Cleland JG, Just H, et al. ; Steering Committee and Investigators of the Levosimendan Infusion Versus Dobutamine (LIDO) Study . Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360(9328):196-202. [DOI] [PubMed] [Google Scholar]
- 24.Bragadottir G, Redfors B, Ricksten SE. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: a randomized placebo-controlled study. Crit Care Med. 2013;41(10):2328-2335. [DOI] [PubMed] [Google Scholar]
- 25.Zhou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2016;67(3):408-416. [DOI] [PubMed] [Google Scholar]
- 26.Mebazaa A, Nieminen MS, Packer M, et al. ; SURVIVE Investigators . Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA. 2007;297(17):1883-1891. [DOI] [PubMed] [Google Scholar]
- 27.Grieshaber P, Lipp S, Arnold A, et al. Impact of prophylactic administration of levosimendan on short-term and long-term outcome in high-risk patients with severely reduced left-ventricular ejection fraction undergoing cardiac surgery—a retrospective analysis. J Cardiothorac Surg. 2016;11(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomivorotov VV, Boboshko VA, Efremov SM, et al. Levosimendan versus an intra-aortic balloon pump in high-risk cardiac patients. J Cardiothorac Vasc Anesth. 2012;26(4):596-603. [DOI] [PubMed] [Google Scholar]
- 29.Toller W, Heringlake M, Guarracino F, et al. Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int J Cardiol. 2015;184:323-336. [DOI] [PubMed] [Google Scholar]
- 30.Papp Z, Édes I, Fruhwald S, et al. Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 2012;159(2):82-87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol for LICORN.
eFigure 1: Forest plot of relative risk differences for the primary end point and its components.
eFigure 2: Forest plot of the absolute risk difference in the primary end point according to predefined subgroups.
eFigure 3: Kaplan-Meier estimates of the probability of survival at Day 180.
eFigure 4: Cumulative incidence of patients “out of ICU and alive” at Day 28.
eFigure 5: Cumulative incidence of patients “off ventilator” at Day 28.
eTable 1 Euroscore II.
eTable 2: Relative Risks for in-hospital mortality, mortality at Day 28 and Day 180.
eTable 3: Complete list of serious adverse events in the levosimendan and placebo groups.