Key Points
Question
Does azithromycin given at the time of the conditioning regimen improve airflow decline–free survival after an allogeneic hematopoietic stem cell transplant?
Findings
In this randomized clinical trial that included 465 patients, 2-year airflow decline–free survival was significantly worse for the azithromycin group than for the placebo group (hazard ratio, 1.3). The trial was terminated early for a significant increased risk in the azithromycin group of hematological relapses, which was a nonprespecified adverse event.
Meaning
Azithromycin resulted in worse airflow decline–free survival, but findings are limited by early termination, and the potential for harm requires further evaluation.
Abstract
Importance
Bronchiolitis obliterans syndrome has been associated with increased morbidity and mortality after allogeneic hematopoietic stem cell transplant (HSCT). Previous studies have suggested that azithromycin may reduce the incidence of post–lung transplant bronchiolitis obliterans syndrome.
Objective
To evaluate if the early administration of azithromycin can improve airflow decline–free survival after allogeneic HSCT.
Design, Setting, and Participants
The ALLOZITHRO parallel-group trial conducted in 19 French academic transplant centers and involving participants who were at least 16 years old, had undergone allogeneic HSCT for a hematological malignancy, and had available pretransplant pulmonary function test results. Enrollment was from February 2014 to August 2015 with follow-up through April 26, 2017.
Interventions
Patients were randomly assigned to receive 3 times a week either 250 mg of azithromycin (n = 243) or placebo (n = 237) for 2 years, starting at the time of the conditioning regimen.
Main Outcomes and Measures
The primary efficacy end point was airflow decline–free survival at 2 years after randomization. Main secondary end points were overall survival and bronchiolitis obliterans syndrome at 2 years.
Results
Thirteen months after enrollment, the independent data and safety monitoring board detected an unanticipated imbalance across blinded groups in the number of hematological relapses, and the treatment was stopped December 26, 2016. Among 480 randomized participants, 465 (97%) were included in the modified intention-to-treat analysis (mean age, 52 [SD, 14] years; 75 women [35%]). At the time of data cutoff, 104 patients (22%; 54 azithromycin vs 50 placebo) had experienced an airflow decline; 138 patients (30%) died (78 azithromycin vs 60 placebo). Two-year airflow decline–free survival was 32.8% (95% CI, 25.9%-41.7%) with azithromycin and 41.3% (95% CI, 34.1%-50.1%) with placebo (unadjusted hazard ratio [HR], 1.3; 95% CI, 1.02-1.70; P = .03). Of the 22 patients (5%) who experienced bronchiolitis obliterans syndrome, 15 (6%) were in the azithromycin group and 7 (3%) in the placebo group (P = .08). The azithromycin group had increased mortality, with a 2-year survival of 56.6% (95% CI, 50.2%-63.7%) vs 70.1% (95% CI, 64.2%-76.5%) in the placebo group (unadjusted HR, 1.5; 95% CI, 1.1-2.0; P = .02). In a post hoc analysis, the 2-year cumulative incidence of hematological relapse was 33.5% (95% CI, 27.3%-39.7%) with azithromycin vs 22.3% (95% CI, 16.4%-28.2%) with placebo (unadjusted cause-specific HR, 1.7; 95% CI, 1.2-2.4; P = .002).
Conclusions and Relevance
Among patients undergoing allogeneic HSCT for hematological malignancy, early administration of azithromycin resulted in worse airflow decline–free survival than did placebo; these findings are limited by early trial termination. The potential for harm related to relapse requires further investigation.
Trial Registration
clinicaltrials.gov Identifier: NCT01959100
This clinical trial, which was stopped early, seeks to evaluate whether the early administration of azithromycin would improve airflow decline–free survival among patients who had undergone an allogeneic hematopoietic stem cell transplant.
Introduction
Bronchiolitis obliterans syndrome after transplants, characterized by a new-onset airflow obstruction after either lung transplant or allogeneic hematopoietic stem cell transplant (HSCT), is a serious complication associated with increased morbidity and mortality. The pathophysiology of bronchiolitis obliterans syndrome occurring after either lung transplant or HSCT is likely similar. After HSCT, bronchiolitis obliterans syndrome is closely linked to chronic graft-vs-host disease (GVHD) and typically develops within 2 years of the procedure; bronchiolitis obliterans syndrome has been reported to occur in 4% to 6% of allogeneic HSCT recipients and in up to 14% of patients with chronic GVHD. The diagnosis of bronchiolitis obliterans syndrome is based on the presence of obstructive lung disease demonstrated by pulmonary function testing. Although early diagnosis is challenging, a growing body of evidence suggests that a decrease in the forced expiratory volume in the first second of expiration (FEV1) may occur before the onset of obstructive lung disease. Bronchiolitis obliterans syndrome, once diagnosed, is usually irreversible despite immunosuppressive treatment. To avoid the adverse effects of steroids, new strategies targeting the airways are increasingly being investigated. Given the disease severity and lack of reliable risk factors, prophylactic protocols are needed.
Beyond their antibiotic effects, macrolides have immunomodulatory and anti-inflammatory properties for which they are broadly used in the management of several chronic respiratory diseases. A randomized clinical trial showed that azithromycin prophylaxis improved bronchiolitis obliterans syndrome–free survival among patients who had undergone a lung transplant with a significant reduction in bronchiolitis obliterans syndrome prevalence after 2 years. The ALLOZITHRO study investigated whether prophylactic azithromycin would improve airflow decline–free survival 2 years after HSCT.
Methods
Study Design and Oversight
The study was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki. The Institutional Ethics Committee Ile-de-France study IV approved the research protocol (Supplement 1). Written informed consent was obtained from all participants before randomization. The ALLOZITHRO trial was a multicenter, randomized, double-blind, placebo-controlled phase 3 superiority trial. Participants were recruited from 19 French bone marrow transplant sites from February 2014 to August 2015. An independent data and safety monitoring board periodically reviewed blinded data to assess safety. A steering committee blindly reviewed diagnoses of noninfectious lung complications.
In September 2016, 13 months after completing recruitment, the independent data and safety monitoring board detected an unexpected imbalance across blinded groups in the number of hematological relapses reported as severe adverse events and alerted the trial steering committee and the study sponsor. Analysis of these data led to early termination of the trial in December 2016 for safety concerns. Then, as requested by national authorities, the protocol was amended to include follow-up assessments for up to 5 years after randomization to evaluate the occurrence of late-onset hematological relapses. We present herein the results as of April 26, 2017, at which time 91 patients included after April 2015 had not had the opportunity to complete 2 years of treatment (48 in the azithromycin group; 43 in the placebo group), including 24 who had already experienced airflow decline (15 in the azithromycin group; 9 in the placebo group).
Participants
Eligible participants were at least 16 years old, had undergone an allogeneic HSCT for a hematological malignancy, and had available pretransplant pulmonary function test results according to standard practice. Exclusion criteria were an allergy or intolerance to azithromycin, macrolides, ketolides, or the excipient used; an indication for the use of macrolides as a prophylaxis to prevent infection, according to a 2014 protocol amendment (Supplement 1); a prolonged corrected QT (QTc) interval (>450 milliseconds); the use of medications prolonging the QTc interval; a family history of a prolonged QTc interval; the use of ergotamine or dihydroergotamine; the use of colchicine; a history of congestive heart failure; severe liver insufficiency; and history of nontuberculous mycobacterial infection.
Randomization and Intervention
Participants scheduled to undergo an allogeneic HSCT were randomly assigned (the day the pretransplant conditioning regimen was started), in a 1:1 allocation ratio, to receive azithromycin (a generic drug) at a dose of 250 mg orally or an identical-appearing placebo 3 times a week for 2 years. An interactive web-response system was used to randomize patients. The randomization was stratified by center on potential confounders—FEV1/forced vital capacity (FVC) ratio (≤0.85 or >0.85) and age (≤50 or >50 years)—with prespecified lists based on permutation blocks of fixed size that remained blinded to investigators.
Outcomes
The primary composite end point of the study was initially airflow obstruction–free survival at 2 years after randomization, that is, time to airflow obstruction or to death from any cause. Airflow obstruction was defined as a rate of a predicted FEV1 decline of more than 5% per year (airflow decline) and an FEV1/FVC ratio of less than 0.8, which were associated with both significant overall and attributable mortality. In 2015, the protocol was amended due to new data deemphasizing the importance of the FEV1/FVC ratio in these patients, and only airflow decline and death were retained as events in the primary composite end point. Secondary end points were overall survival, bronchiolitis obliterans syndrome and other late-onset pulmonary noninfectious complications, change in pulmonary function parameters (FEV1, FVC, total lung capacity, residual volume, and forced midexpiratory flow of 25%-75%), acute and extrathoracic chronic GVHD, quality of life, and tolerance of azithromycin, all assessed at 2 years. Late-onset pulmonary noninfectious complications, including bronchiolitis obliterans syndrome, were determined according to the National Institutes of Health (NIH) criteria, and interstitial lung disease criteria were determined as previously defined. Diagnostic criteria and severity of acute and extrathoracic chronic GVHD were assessed according to the NIH criteria, with a grading scale from 1 to 4 for acute GVHD and a limited to extensive grading scale for chronic GVHD. The St George’s Respiratory Questionnaire was used to evaluate quality of life. According to the recommendation of the data and safety monitoring board and the sponsor, in October 2016, the protocol was amended to include hematological relapse up to 2 years after randomization as a post hoc end point.
Sample Size and Statistical Analysis
The trial was designed as a superiority trial. The sample size computation was initially based on airflow obstruction–free survival, which required 460 patients (see the trial protocol in Supplement 1). After amending the protocol in 2015, the primary outcome was changed to airflow decline–free survival. Overall, 218 events and 460 patients (230 in each group) were needed to demonstrate a clinically relevant 15% improvement in the primary outcome at 2 years for patients treated with azithromycin compared with an expected rate of 45% for patients receiving placebo based on previous data on airflow decline and mortality data from the French Biomedicine Agency (https://www.agence-biomedecine.fr), with a type I error of .05 and a power of 0.9 based on a 2-sided log-rank test. To account for an estimated 5% dropout rate due to consent withdrawals, 480 patients had to be recruited.
Efficacy analysis of data at 2 years was based on a modified intention-to-treat analysis, excluding only patients who withdrew their consent according to French regulation. All analyses used April 26, 2017, as the time of data cutoff, and patients with less than 2 years of follow-up were administratively censored at this time. Airflow decline–free survival and overall survival after randomization were displayed using Kaplan-Meier plots, compared using log-rank tests. Cumulative incidences of each event were described separately. The Cox proportional-hazards model was used to compute hazard ratios (HRs) as a measure of effect size, with associated 2-sided 95% CIs. Adjusted HRs were secondarily computed, including stratification variables as the variables known a priori to be associated with the primary outcome, and variables selected as associated with the outcome based on univariable prognostic analyses. Center effect was tested using a frailty term. Cumulative incidences of bronchiolitis obliterans syndrome as well as acute and chronic GVHD were estimated in a competing-risk setting, for which death free of the event of interest was a competing event, and were compared across randomized groups with the Gray test. To summarize pulmonary function data, a k-means approach specifically designed to analyze longitudinal data was used (eAppendix in Supplement 2).
In a post hoc analysis, the cumulative incidence of hematological relapse was estimated as described for GVHD. A cause-specific Cox proportional-hazards model with adjustment for imbalanced prognostic factors was secondarily used; those variables were selected as prognostic based on previous available data. Post hoc exploratory treatment-by-covariate interaction tests based on Gail and Simon statistics were performed in relation to well-known prognostic covariates to better understand the effects of treatment on the cause-specific hazards of hematological relapse, and chronic GVHD.
Because the trial was stopped early, a tipping-point analysis was also performed to assess the likelihood that the primary outcome could have become null or better had the study been completed (eAppendix in Supplement 2).
Safety data, including serious adverse events, were summarized based on randomized groups.
In the regression models, missing values of the disease risk index were imputed using the modal class. A 2-sided P value of <.05 was considered statistically significant. For the secondary end point analyses, there was no adjustment for multiple comparisons. Accordingly, these findings should be considered exploratory. The statistical analysis was performed using R version 3.3.2 (https://www.R-project.org/).
Results
In September 2016, the independent data and safety monitoring board detected an unexpected imbalance across blinded groups in the number of hematological relapses reported as severe adverse events. The data and safety monitoring board requested that the sponsor globally unblind the study and requested additional data. An intensive monitoring of all relapse data was performed, and other imbalanced risk factors for hematological relapses were investigated. Based on these analyses, on December 26, 2016, both the data and safety monitoring board and the sponsor decided to terminate the trial early.
Study Participants
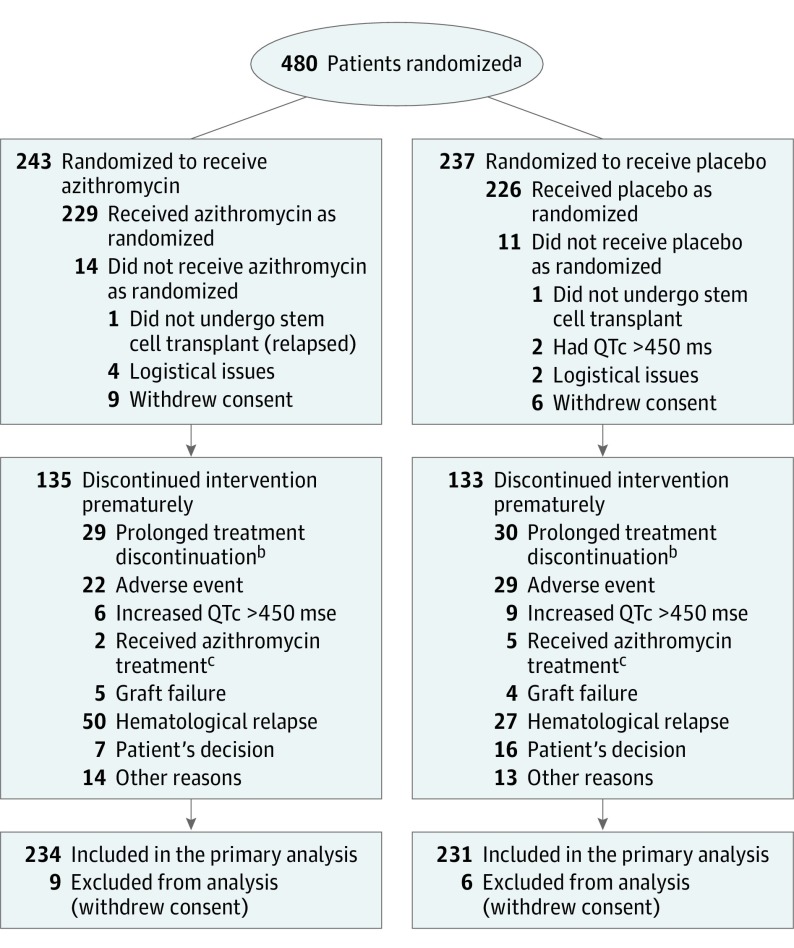
From February 2014 through August 2015, 480 patients were randomized in 19 centers; 15 patients withdrew their consent and were subsequently excluded from the analysis (Figure 1). The baseline characteristics of the remaining 465 patients are shown in Table 1. More high-risk patients, more patients with acute lymphoblastic leukemia, and more patients older than 50 years with an FEV1/FVC ratio greater than 0.85 were randomized into the azithromycin group. Treatment of the last randomized patient was initially expected to be completed in August 2017; treatment was actually stopped for all patients in December 2016.
Figure 1. Flow of Patients Through the ALLOZITHRO Trial.
QTc indicates corrected QT interval.
aThe number of patients screened for eligibility was not available.
bThe protocol required that the intervention treatment not be discontinued for more than 4 weeks.
cSome patients received azithromycin as a curative treatment.
Table 1. Baseline Characteristics of the Study Participants.
| Characteristics | No. (%) of Participants | |
|---|---|---|
| Azithromycin (n = 234) |
Placebo (n = 231) |
|
| Women | 85 (36.3) | 77 (33.3) |
| Age, median (IQR), y | 57.5 (45.0-63.6) | 55.6 (40.3-63.2) |
| Randomization strata | ||
| FEV1/FVC ≤0.85 | ||
| ≤50 y | 47 (20.1) | 57 (24.7) |
| >50 y | 122 (52.1) | 126 (54.5) |
| FEV1/FVC >0.85 | ||
| ≤50 y | 37 (15.8) | 31 (13.4) |
| >50 y | 28 (12.0) | 17 (7.4) |
| FEV1/FVC, median (IQR) | 0.77 (0.72-0.82) | 0.77 (0.72-0.82) |
| Diagnosis | ||
| Acute leukemiaa | 121 (51.7) | 116 (50.2) |
| Lymphoid malignanciesb | 51 (21.8) | 48 (20.8) |
| Myelodysplastic/myeloproliferative disordersc | 39 (16.7) | 43 (18.6) |
| Other malignancies | 23 (9.8) | 24 (10.4) |
| Disease risk indexd | ||
| Low | 34 (14.6) | 34 (15.0) |
| Intermediate | 157 (67.4) | 168 (74.3) |
| High | 42 (18.0) | 24 (10.6) |
| Disease status at transplant | ||
| First complete remission | 109 (46.6) | 95 (41.1) |
| Other complete remission | 49 (20.9) | 49 (21.2) |
| Othere | 76 (32.5) | 87 (37.7) |
| Prior autologous stem cell transplant | 38 (16.2) | 31 (13.4) |
| Donor typef | ||
| Related | 58 (24.9) | 67 (29.1) |
| Haploidentical | 38 (16.3) | 33 (14.3) |
| Unrelatedg | 137 (58.8) | 130 (56.6) |
| Donor/recipient sexf,h | ||
| Male/male | 108 (46.6) | 97 (42.5) |
| Male/female | 48 (20.7) | 47 (20.6) |
| Female/male | 40 (17.2) | 56 (24.6) |
| Female/female | 36 (15.5) | 28 (12.3) |
| Donor/recipient cytomegalovirus serologyf,i | ||
| −/− | 92 (40.0) | 90 (40.2) |
| −/+ | 55 (23.9) | 37 (16.5) |
| +/+ | 32 (13.9) | 28 (12.5) |
| +/− | 51 (22.2) | 69 (30.8) |
| Source of stem cellsf | ||
| Peripheral blood | 186 (79.8) | 184 (80.0) |
| Bone marrow | 35 (15.0) | 33 (14.3) |
| Cord blood | 12 (5.2) | 13 (5.7) |
| Conditioning regimenf | ||
| Myeloablative | 70 (30.0) | 62 (27.0) |
| Nonmyeloablative | 163 (70.0) | 168 (73.0) |
| Details of conditioningf | ||
| Cyclophosphamide-total body irradiation | 17 (7.3) | 17 (7.4) |
| Busulfan-cyclophosphamide | 18 (7.7) | 16 (7.0) |
| Busulfan-fludarabine | 142 (60.9) | 135 (58.7) |
| Busulfan-total body irradiation | 37 (15.9) | 36 (15.6) |
| Other | 19 (8.2) | 26 (11.3) |
| Antithymocyte globulin | 148 (63.5) | 146 (63.5) |
| Graft-vs-host disease prophylaxisf | ||
| Cyclosporine-methotrexate | 67 (28.8) | 62 (27.0) |
| Cyclosporine-mycophenolate mofetil | 95 (40.8) | 86 (37.4) |
| Posttransplant cyclophosphamide | 33 (14.2) | 29 (12.6) |
| Other | 38 (16.3) | 53 (23.0) |
Abbreviations: FEV1/FVC, forced expiratory volume in the first second/forced vital capacity ratio; IQR, interquartile range.
Includes 55 patients with acute lymphoblastic leukemia (32 vs 23) and 182 (89 vs 93) with acute myeloid leukemia; 6 patients with acute leukemia had a low disease index (2 vs 4), 185 had an intermediate disease risk index (90 vs 95), 44 had a high disease risk index (28 vs 16), and 2 were unknown (1 in each).
Includes 43 patients with non-Hodgkin lymphomas (23 vs 20), 25 with Hodgkin lymphoma (11 vs 14), 20 with multiple myeloma (11 vs 9), and 11 with chronic lymphocytic leukemia (6 vs 5); 54 patients with lymphoid malignancies had a low disease risk index (29 vs 25), and 45 had an intermediate disease risk index (22 vs 23).
Includes 8 patients with chronic myeloid leukemia (5 vs 3) and 74 with myelodysplastic syndrome (38 vs 36); 8 patients with myelodysplastic/myeloproliferative disorders had a low disease risk index (3 vs 5), 55 had an intermediate disease risk index (25 vs 30), and 19 had a high disease risk index (11 vs 8).
The disease risk index for patients undergoing allogeneic stem cell transplant was defined according to hematological disease and disease status at the time of transplant. Six had missing data (1 in the azithromycin group and 5 in the placebo group).
Any situation other than complete remission.
Two patients did not actually undergo allogeneic hematopoietic stem cell transplant (1 in each randomization group).
Nine/10 human leukocyte antigen (HLA)–match in 50 unrelated donors (31 vs 19), 10/10 HLA-match in 189 unrelated donors (93 vs 96), and missing data in 28 unrelated donors (13 vs 15).
Three patients who underwent allograft (1 vs 2) had missing data for donor sex.
Nine patients who underwent allograft (3 vs 6) had missing data for cytomegalovirus donor serology.
Efficacy Analysis
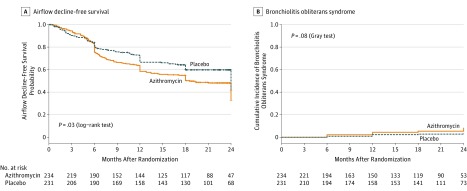
At the time of data cutoff for analysis (April 26, 2017) in the modified intention-to-treat population, 242 patients (52%) had experienced a primary event: 104 patients (22%) had experienced an airflow decline (54 in the azithromycin group and 50 in the placebo group), and 138 patients (30%) died (78 in the azithromycin group and 60 in the placebo group) (eFigure 1 in Supplement 2). The airflow decline–free survival was significantly lower in the azithromycin group than in the placebo group, with a 2-year estimate of 32.8% (95% CI, 25.9%-41.7%) in the azithromycin group vs 41.3% (95% CI, 34.1%-50.1%) in the placebo group (P = .03 by the log-rank test) (Figure 2A). The unadjusted HR for airflow decline–free survival was 1.3 (95% CI, 1.02-1.70). In a secondary multivariable analysis adjusted by randomization strata, acute lymphoblastic leukemia, a high disease risk index, and a random effect of center (though not significant), the HR for airflow decline–free survival was 1.2 (95% CI, 0.9-1.6) (Table 2). A total of 22 patients (5%) experienced bronchiolitis obliterans syndrome, 15 (6.4%) in the azithromycin group and 7 (3.0%) in the placebo group (P = .08) (Figure 2B). At year 2, no significant differences in variation of FEV1 (eFigure 2 and eFigure 3 in Supplement 2) nor in the change in the other pulmonary function parameters were noted between groups (eFigure 3 in Supplement 2).
Figure 2. Airflow Decline–Free Survival and Bronchiolitis Obliterans Syndrome.
In this modified intention-to-treat analysis set, data cutoff was April 26, 2017.
A, Crosses on the curves indicate censored observations. The median (interquartile range [IQR]) follow-up for the occurrence of airflow decline–free survival among those in the azithromycin group was 15.5 months (IQR, 4.4-23.1) and was 18.8 months (IQR, 6.0-23.8) for those in the placebo group.
B, The median (IQR) follow-up for occurrence of bronchiolitis obliterans syndrome among those in the azithromycin group was 10.0 (IQR, 7.0-23.0) months and 19.0 (IQR, 9.0-24.0) for those in the placebo group.
Table 2. Follow-up Data With Primary and Secondary Outcomes.
| No. (%) of Participants | P Value for Unadjusted Dataa | Adjusted HR (95% CI)b | ||
|---|---|---|---|---|
| Azithromycin | Placebo | |||
| All participants, No. | 234 | 231 | ||
| Actual duration, median (IQR), mo | ||||
| Follow-up | 18.6 (7.2-23.5) | 20.6 (9.0-24.0) | ||
| Treatment | 9.4 (3.6-20.9) | 10.3 (3.7-21.3) | ||
| Primary Outcome | ||||
| Airflow decline–free survival, No. (%) with eventc | 132 (56.4) | 110 (47.6) | .03d | 1.2 (0.9-1.6) |
| Secondary Outcomes | ||||
| Bronchiolitis obliterans syndrome | 15 (6.4) | 7 (3.0) | .08e | 2.7 (1.1-6.8) |
| Acute GVHDf | 97 (41.4) | 94 (40.7) | .98e | 1.0 (0.7-1.3) |
| Chronic GVHDg | 66 (28.2) | 75 (32.5) | .30e | 0.9 (0.6-1.2) |
| Death | 95 (40.6) | 66 (28.6) | .02d | 1.3 (1.0-1.8) |
| Post Hoc Outcomes | ||||
| Acute GVHD grade ≥2f | 64 (27.4) | 58 (25.1) | .65e | 1.1 (0.7-1.5) |
| Chronic, moderate, or severe GVHDg | 36 (15.4) | 45 (19.5) | .26e | 0.8 (0.5-1.3) |
| Relapse | 77 (32.9) | 48 (20.8) | .002e | 1.6 (1.1-2.3) |
Abbreviations: GVHD, graft-vs-host disease; IQR, interquartile range.
Empty cells for P values indicate absence of any statistical tests.
For each outcome, hazard ratios (HRs) are based on multivariable cause-specific Cox models including randomization strata, acute lymphoblastic leukemia, high disease risk index, and center effect as frailty; missing data on disease risk index were imputed as not high. Empty cells indicate that there was no regression modeling.
Events accounting for airflow decline–free survival were 104 airflow declines (54 vs 50), and 138 deaths before any decline (78 vs 60).
Log-rank test.
Gray test.
Acute GVHD includes erythema, maculopapular rash, nausea, vomiting, anorexia, profuse diarrhea, ileus, or cholestatic liver disease. Acute GVHD was graded from 1 to 4, according to the severity, using the National Institutes of Health consensus criteria.
Chronic GVHD is a syndrome of variable clinical features resembling autoimmune and other immunologic disorders such as scleroderma, Sjögren syndrome, primary biliary cirrhosis, wasting syndrome, bronchiolitis obliterans, immune cytopenias, and chronic immunodeficiency. Manifestations of chronic GVHD may be restricted to a single organ or site or may be widespread. The global score of mild, moderate, and severe reflects the degree of organ impact and functional impairment due to chronic GVHD. It has been established according National Institutes of Health consensus criteria.
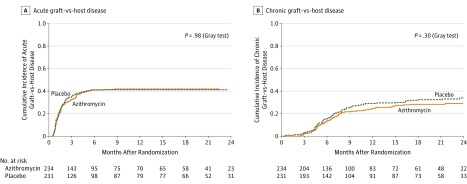
The cumulative incidence of acute GVHD did not significantly differ across randomized groups, with a 2-year estimate of 41.9% (95% CI, 35.7%-48.1%) in the azithromycin group vs 41.2% (95% CI, 35.0%-47.4%) in the placebo group (P = .98) (Table 2, Figure 3A). The cumulative incidence of chronic GVHD was not significantly different between the randomized groups with a 2-year estimate of 29.0% (95% CI, 23.1%-34.9%) in the azithromycin group vs 34.0% (95% CI, 27.8%-40.2%) in the placebo group (P = .30) (Table 2, Figure 3B).
Figure 3. Graft-vs-Host Disease .
In this modified intention-to-treat analysis set, data cutoff was April 26, 2017.
A, The median (interquartile range [IQR]) follow-up for the occurrence of acute graft-vs-host disease in the azithromycin group was 8.5 months (IQR, 1.4-17.6) and 3.7 months (IQR, 1.4- 18.9) in the placebo group. Acute graft-vs-host disease includes symptoms of erythema, maculopapular rash, nausea, vomiting, anorexia, profuse diarrhea, ileus, or cholestatic liver disease.
B, The median (IQR) follow-up for the occurrence of chronic graft-vs-host disease, 7.1 months (IQR, 4.2-18.6) for the azithromycin group and was 7.9 months (IQR, 4.5-21.0) for the placebo group. Chronic graft-vs-host disease is a syndrome of variable clinical features resembling autoimmune and other immunologic disorders such as scleroderma, Sjögren syndrome, primary biliary cirrhosis, wasting syndrome, bronchiolitis obliterans, immune cytopenias, and chronic immunodeficiency; manifestations of chronic graft-vs-host disease may be restricted to a single organ or site or may be widespread.
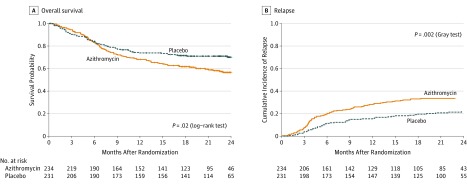
A total of 161 patients (35%) died (95 in the azithromycin group vs 66 in the placebo group), with a 2-year survival rate of 56.6% (95% CI, 50.2%-63.7%) in the azithromycin group vs 70.1% (95% CI, 64.2%-76.5%) in the placebo group (unadjusted HR, 1.5, 95% CI, 1.1-2.0, P = .02) (Figure 4A), including 75 deaths after hematological relapse (52 in the azithromycin group vs 23 in the placebo group) (eFigure 4 in Supplement 2). Adjusting for factors associated with death (eTable 1 in Supplement 2) did not significantly modify the results, with an adjusted HR for death in the azithromycin group of 1.3 (95% CI, 1.0-1.8) (Table 2).
Figure 4. Overall Survival and Hematological Relapse.
In this modified intention-to-treat analysis set, data cutoff was April 26, 2017. Crosses on the curves indicate censored observations.
A, The median (interquartile range [IQR]) follow-up for overall survival in the azithromycin group was 18.6 months (IQR, 7.2-23.5) and was 20.6 months (IQR, 9.0-24.1) for placebo group.
B, The median (IQR) follow-up for hematologic relapse was 15.5 months (IQR, 4.4-23.1) for the azithromycin group and was 18.8 months (IQR, 6.0-23.8) for the placebo group.
Serious Adverse Events
Serious adverse events that occurred during the study period are summarized in the eTable 2 in Supplement 2. Cardiovascular events, 5.6% in the azithromycin group vs 3.0% in the placebo group, were not significantly different (P = .25).
Post Hoc Analyses
In a post hoc analysis, relapse of the underlying hematological disease occurred in 77 patients (32.9%) in the azithromycin group and 48 patients (20.8%) in the placebo group (Table 2), with a 2-year cumulative incidence of 33.5% (95% CI, 27.3%-39.7%) in the azithromycin group and 22.3% (95% CI, 16.4%-28.2%) in the placebo group (cause-specific HR, 1.7; 95% CI, 1.2-2.4; P = .002) (Figure 4B). Adjustment for factors associated with relapse (eTable 3 in Supplement 2) did not significantly modify the estimated HR at 1.6 (95% CI, 1.1-2.3).
Additional post hoc analyses of the incidence of acute GVHD grade 2 or higher and of moderate or severe chronic GVHD, as well as post hoc subgroup analyses of GVHD and of hematological relapse are presented in Table 2, the eAppendix, eTable 4 and eFigures 5 through 8 in Supplement 2.
A tipping-point analysis suggested that the effect of azithromycin on the primary end point could not be reversed to demonstrate benefit of azithromycin (eTable 5 in Supplement 2).
Discussion
In this randomized clinical trial involving patients undergoing allogeneic HSCT for hematological malignancy, early administration of azithromycin compared with placebo resulted in worse airflow decline–free survival, although these findings were limited by the early trial termination. Azithromycin administration also was associated with decreased survival at 2 years and in post hoc analysis was associated with hematological relapse at 2 years. These adverse outcomes require further investigation.
With the increasing number of transplants, chronic GVHD has emerged as a significant complication of allogeneic HSCT. Among the target organs of chronic GVHD, lung involvement with bronchiolitis obliterans syndrome has been associated with increased morbidity and mortality. Thus, prophylactic and therapeutic interventions aimed at decreasing the incidence and severity of bronchiolitis obliterans syndrome are clearly needed.
Azithromycin, a macrolide derivative, has been widely used for years both for its antibacterial and immunomodulatory properties. Azithromycin has been associated with improved pulmonary function results following lung transplant in randomized, placebo-controlled trials. Following allogeneic HSCT, azithromycin has also been shown to decrease GVHD and mortality and to prevent noninfectious pneumonia in murine models of allogeneic HSCT, and the association of fluticasone, azithromycin, and montelukast has been generally used in the United States to treat bronchiolitis obliterans syndrome in humans following results from a phase 2 open-label trial.
These factors were considered in designing this randomized, placebo-controlled trial which demonstrated that airflow decline–free survival was significantly decreased in the azithromycin group compared with the placebo group. Adjusting for imbalanced prognostic factors yielded a nonsignificant estimated adjusted HR of 1.2 (95% CI, 0.9-1.6) for the primary outcome. The number of patients with bronchiolitis obliterans syndrome in the azithromycin group was not significantly different from that of the placebo group. No significant differences in the trajectories of FEV1 and the other pulmonary function tests were found between the 2 groups. Overall, the results of this study suggest it is unlikely that azithromycin could reduce the risk of bronchiolitis obliterans syndrome after allogeneic HSCT. Moreover, survival was decreased in the azithromycin group.
This trial had to be stopped prematurely due to an unanticipated increase in the hematological relapse rate in the azithromycin group. Although other factors may have contributed to hematological relapse, adjusting for factors associated with relapse in univariable analysis did not significantly modify the effect on hematological relapse observed in the azithromycin group.
The mechanism for increased hematological relapse in the azithromycin group is unclear. The graft-vs-leukemia effect after allogeneic HSCT is a complex phenomenon that is intrinsically associated with GVHD. A link between the increased relapse risk in the azithromycin group and differences in GVHD incidence was considered, but no differences in GVHD incidence (either acute or chronic) between the 2 study groups was noted. Azithromycin has pleiotropic but poorly characterized effects on the immune system that might partly explain the increased hematological relapse rate observed in this trial, particularly in the early posttransplant period. It is also possible that azithromycin interfered with the conditioning regimen, although the current understanding of the antileukemic power of allogeneic HSCT is thought to be mediated more by the immune-mediated graft-vs-leukemia effect than by the cytotoxic role of the pretransplant conditioning regimen. Another potential explanation may be that azithromycin, as a macrolide derivative, might disturb the microbiome and influence the risk of subsequent hematological relapse since commensal bacterial species have recently been associated with both GVHD and graft-vs-leukemia in the transplant setting or in response to checkpoint inhibitors.
Based on the hypothesis that the first event leading to bronchiolitis obliterans syndrome would be the epithelial aggression induced by conditioning, azithromycin was started at the time of conditioning in this study. However, the effect of azithromycin may be different if administered after engraftment. Although less than other macrolides, azithromycin may interact with the many other drugs that are administered in allogeneic HSCT recipients, including chemotherapy, and thus, the effective concentration of azithromycin may have varied during the study.
Limitations
This study has several limitations. First, the trial was stopped prematurely, which may have affected the primary outcome findings because it resulted in a decreased statistical power. Nevertheless, the observed number of events was higher than expected, likely due to the increased death rate in the azithromycin group. Tipping-point analysis conducted to assess the potential effect of premature stopping of the study suggested that the primary end point findings could not be reversed to demonstrate a beneficial effect of azithromycin. Moreover, the administrative censoring of patients with less than 2 years of follow-up argue against any bias in the analyses of the primary end point.
Second, this trial used airflow-decline criteria as a component of the primary end point. Although well recognized in predicting the occurrence of bronchiolitis obliterans syndrome, airflow decline has not been validated for use previously. Nevertheless, no evidence of any effect on pulmonary function tests was observed.
Third, the use of a composite end point, including death as another primary event, assumed that azithromycin could act on mortality from other causes. This relied on the reported effect of FEV1 decline on mortality as well as on the reported benefit in animal models of azithromycin prophylaxis on mortality.
Fourth, we had to exclude patients who withdrew their consent, and this may have introduced some selection bias, although this only concerned 3% of the enrolled patients (3.7% in the azithromycin group vs 2.5% in the placebo group).
Fifth, given the stratification of randomization, selection bias due to violation of the allocation concealment by the investigators is possible; the use of a website by which the strata were automatically computed and the absence of preference of investigators toward the use of azithromycin in these highly complex patients argue against it.
Sixth, the absence of adjustment for multiple comparisons of secondary end points requires these findings to be considered exploratory. Moreover, all results of the secondary and post hoc analyses (including subgroup analyses) were exploratory in nature and should be viewed as hypothesis generating.
Seventh, a nonnegligible number of patients discontinued their treatment before 2 years, and a per-protocol analysis will be considered in the near future to evaluate the effect of treatment among those who were treated as outlined in the protocol.
Conclusions
Among patients undergoing allogeneic HSCT for hematological malignancy, early administration of azithromycin resulted in worse airflow decline–free survival than did placebo; these findings are limited by the early trial termination. The potential for harm related to relapse requires further investigation.
Trial Protocol
eTable 1. Prognostic factors of the hazard of death based on univariate cause-specific Cox models
eTable 1. Prognostic factors of the hazard of death based on univariate cause-specific Cox models
eTable 2. Serious adverse events (SAEs) reported during the trial according to randomized groups
eTable 3. Prognostic factors of the cause-specific hazard of relapse based on univariate cause-specific Cox models
eTable 4. Post Hoc subgroups analyses of airflow decline-free survival, graft-vs-host disease and hematological relapse
eTable 5. Results of the tipping point analyses evaluating the impact of missing outcome data at 2 year
eFigure 1. Estimation of the cumulative incidence of each event of the composite primary endpoint according to the randomization group
eFigure 2. Boxplots of annualized decline in FEV1 according to randomized groups
eFigure 3. Trajectories of pulmonary function parameters (expressed in percentage of predicted values) according to randomized groups
eFigure 4. Survival after relapse according to the randomized groups
eFigure 5. Post-hoc analyses of severe graft-versus-host disease (GVHD) outcomes according to randomized groups
eFigure 6. Graft versus Host Disease (GVHD) outcomes according to randomized groups in acute leukemia patients
eFigure 7. Treatment-by-covariate interaction effects on the cause-specific hazard of hematological
eFigure 8. Subset analyses of treatment effects on the cumulative incidence of hematological relapse according to both the underlying hematological disease (eFigure 8A-C) and the type of transplant (eFigure 8D-F)
eAppendix. Expanded Methods an expanded Results
References
- 1.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370(19):1820-1828. [DOI] [PubMed] [Google Scholar]
- 2.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease, I: the 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389-401.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron A. Late-onset noninfectious pulmonary complications after allogeneic hematopoietic stem cell transplantation. Clin Chest Med. 2017;38(2):249-262. [DOI] [PubMed] [Google Scholar]
- 4.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(7):1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron A, Godet C, Chevret S, et al. Bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: phenotypes and prognosis. Bone Marrow Transplant. 2013;48(6):819-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vieira AG, Funke VA, Nunes EC, Frare R, Pasquini R. Bronchiolitis obliterans in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(6):812-817. [DOI] [PubMed] [Google Scholar]
- 7.Abedin S, Yanik GA, Braun T, et al. Predictive value of bronchiolitis obliterans syndrome stage 0p in chronic graft-versus-host disease of the lung. Biol Blood Marrow Transplant. 2015;21(6):1127-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng GS, Storer B, Chien JW, et al. Lung function trajectory in bronchiolitis obliterans syndrome after allogeneic hematopoietic cell transplant. Ann Am Thorac Soc. 2016;13(11):1932-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergeron A, Chevret S, Chagnon K, et al. Budesonide/formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191(11):1242-1249. [DOI] [PubMed] [Google Scholar]
- 10.Williams KM, Cheng GS, Pusic I, et al. Fluticasone, azithromycin, and montelukast treatment for new-onset bronchiolitis obliterans syndrome after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016;22(4):710-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cramer CL, Patterson A, Alchakaki A, Soubani AO. Immunomodulatory indications of azithromycin in respiratory disease: a concise review for the clinician. Postgrad Med. 2017;129(5):493-499. [DOI] [PubMed] [Google Scholar]
- 12.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225-245. [DOI] [PubMed] [Google Scholar]
- 13.Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J. 2011;37(1):164-172. [DOI] [PubMed] [Google Scholar]
- 14.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 15.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168(2):208-214. [DOI] [PubMed] [Google Scholar]
- 16.Holbro A, Lehmann T, Girsberger S, et al. Lung histology predicts outcome of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(6):973-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlving HH, Andersen CB, Christensen IJ, et al. Biopsy-verified bronchiolitis obliterans and other noninfectious lung pathologies after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(3):531-538. [DOI] [PubMed] [Google Scholar]
- 18.Schlemmer F, Chevret S, Lorillon G, et al. Late-onset noninfectious interstitial lung disease after allogeneic hematopoietic stem cell transplantation. Respir Med. 2014;108(10):1525-1533. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 20.Weatherall M, Marsh S, Shirtcliffe P, Williams M, Travers J, Beasley R. Quality of life measured by the St George’s Respiratory Questionnaire and spirometry. Eur Respir J. 2009;33(5):1025-1030. [DOI] [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables (with discussion). J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 22.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141-1154. [Google Scholar]
- 23.Genolini C, Falissard B. KmL: a package to cluster longitudinal data. Comput Methods Programs Biomed. 2011;104(3):e112-e121. [DOI] [PubMed] [Google Scholar]
- 24.Armand P, Gibson CJ, Cutler C, et al. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120(4):905-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhédin N, Huynh A, Maury S, et al. ; GRAALL group . Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486-2496. [DOI] [PubMed] [Google Scholar]
- 26.Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41(2):361-372. [PubMed] [Google Scholar]
- 27.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corris PA, Ryan VA, Small T, et al. A randomised controlled trial of azithromycin therapy in bronchiolitis obliterans syndrome (BOS) post lung transplantation. Thorax. 2015;70(5):442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruttens D, Verleden SE, Vandermeulen E, et al. Prophylactic azithromycin therapy after lung transplantation: post hoc analysis of a randomized controlled trial. Am J Transplant. 2016;16(1):254-261. [DOI] [PubMed] [Google Scholar]
- 30.Iwamoto S, Azuma E, Kumamoto T, et al. Efficacy of azithromycin in preventing lethal graft-versus-host disease. Clin Exp Immunol. 2013;171(3):338-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radhakrishnan SV, Palaniyandi S, Mueller G, et al. Preventive azithromycin treatment reduces noninfectious lung injury and acute graft-versus-host disease in a murine model of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015;21(1):30-38. [DOI] [PubMed] [Google Scholar]
- 32.Williams KM. How I treat bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Blood. 2017;129(4):448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diab M, ZazaDitYafawi J, Soubani AO. Major pulmonary complications after hematopoietic stem cell transplant. Exp Clin Transplant. 2016;14(3):259-270. [DOI] [PubMed] [Google Scholar]
- 34.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555-562. [PubMed] [Google Scholar]
- 35.Lin SJ, Kuo ML, Hsiao HS, Lee PT. Azithromycin modulates immune response of human monocyte-derived dendritic cells and CD4(+) T cells. Int Immunopharmacol. 2016;40:318-326. [DOI] [PubMed] [Google Scholar]
- 36.Lin SJ, Yan DC, Lee WI, Kuo ML, Hsiao HS, Lee PY. Effect of azithromycin on natural killer cell function. Int Immunopharmacol. 2012;13(1):8-14. [DOI] [PubMed] [Google Scholar]
- 37.Vos R, Vanaudenaerde BM, Verleden SE, et al. Anti-inflammatory and immunomodulatory properties of azithromycin involved in treatment and prevention of chronic lung allograft rejection. Transplantation. 2012;94(2):101-109. [DOI] [PubMed] [Google Scholar]
- 38.Ishimatsu Y, Kadota J, Iwashita T, et al. Macrolide antibiotics induce apoptosis of human peripheral lymphocytes in vitro. Int J Antimicrob Agents. 2004;24(3):247-253. [DOI] [PubMed] [Google Scholar]
- 39.Miller JS, Warren EH, van den Brink MR, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol Blood Marrow Transplant. 2010;16(5):565-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368-1379. [DOI] [PubMed] [Google Scholar]
- 41.Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol. 2017;35(15):1650-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sivan A, Corrales L, Hubert N, et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanik G, Cooke KR. The lung as a target organ of graft-versus-host disease. Semin Hematol. 2006;43(1):42-52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Prognostic factors of the hazard of death based on univariate cause-specific Cox models
eTable 1. Prognostic factors of the hazard of death based on univariate cause-specific Cox models
eTable 2. Serious adverse events (SAEs) reported during the trial according to randomized groups
eTable 3. Prognostic factors of the cause-specific hazard of relapse based on univariate cause-specific Cox models
eTable 4. Post Hoc subgroups analyses of airflow decline-free survival, graft-vs-host disease and hematological relapse
eTable 5. Results of the tipping point analyses evaluating the impact of missing outcome data at 2 year
eFigure 1. Estimation of the cumulative incidence of each event of the composite primary endpoint according to the randomization group
eFigure 2. Boxplots of annualized decline in FEV1 according to randomized groups
eFigure 3. Trajectories of pulmonary function parameters (expressed in percentage of predicted values) according to randomized groups
eFigure 4. Survival after relapse according to the randomized groups
eFigure 5. Post-hoc analyses of severe graft-versus-host disease (GVHD) outcomes according to randomized groups
eFigure 6. Graft versus Host Disease (GVHD) outcomes according to randomized groups in acute leukemia patients
eFigure 7. Treatment-by-covariate interaction effects on the cause-specific hazard of hematological
eFigure 8. Subset analyses of treatment effects on the cumulative incidence of hematological relapse according to both the underlying hematological disease (eFigure 8A-C) and the type of transplant (eFigure 8D-F)
eAppendix. Expanded Methods an expanded Results