Key Points
Question
Are the outcomes of patients with melanomas in situ treated with Mohs micrographic surgery (MMS) different from those treated with wide local excision (WLE)?
Findings
In this cohort study of 662 patients with melanoma in situ treated with either MMS or WLE, there was no significant difference in recurrence at 5 years, overall survival, or melanoma-specific survival.
Meaning
These data indicate that MMS was as effective as WLE for treatment of MIS.
This cohort study assesses the outcomes of melanomas in situ treated with Mohs micrographic surgery compared with those treated with wide local excision.
Abstract
Importance
Melanoma in situ (MIS) is increasing in incidence, and expert consensus opinion recommends surgical excision for therapeutic management. Currently, wide local excision (WLE) is the standard of care. However, Mohs micrographic surgery (MMS) is now used to treat a growing subset of individuals with MIS. During MMS, unlike WLE, the entire cutaneous surgical margin is evaluated intraoperatively for tumor cells.
Objective
To assess the outcomes of patients with MIS treated with MMS compared with those treated with WLE.
Design, Setting, and Participants
Retrospective review of a prospective database. The study cohort consisted of 662 patients with MIS treated with MMS or WLE per standard of care in dermatology and surgery (general surgery, otolaryngology, plastics, oculoplastics, surgical oncology) at an academic tertiary care referral center from January 1, 1978, to December 31, 2013, with follow-up through 2015.
Exposure
Mohs micrographic surgery or WLE.
Main Outcomes and Measures
Recurrence, overall survival, and melanoma-specific survival.
Results
There were 277 patients treated with MMS (mean [SD] age, 64.0 [13.1] years; 62.1% male) and 385 treated with WLE (mean [SD] age, 58.5 [15.6] years; P < .001 for age; 54.8% male). Median follow-up was 8.6 (range, 0.2-37) years. Compared with WLE, MMS was used more frequently on the face (222 [80.2%] vs 141 [36.7%]) and scalp and neck (23 [8.3%] vs 26 [6.8%]; P < .001). The median (range) year of diagnosis was 2008 (1986-2013) for the MMS group vs 2003 (1978-2013) for the WLE group (P < .001). Overall recurrence rates were 5 (1.8%) in the MMS group and 22 (5.7%) in the WLE group (P = .07). Mean (SD) time to recurrence after MMS was 3.91 (4.4) years, and after WLE, 4.45 (2.7) years (P = .73). The 5-year recurrence rate was 1.1% in the MMS group and 4.1% in the WLE group (P = .07). For WLE-treated tumors, the surgical margin taken was greater for tumors that recurred compared with tumors that did not recur (P = .003). Five-year overall survival for MMS was 92% and for WLE was 94% (P = .28). Melanoma-specific mortality for the MMS group was 2 vs 13 patients for the WLE group, with mean (SD) survival of 6.5 (4.8) and 6.1 (0.8) years, respectively (P = .77).
Conclusions and Relevance
No significant differences were found in the recurrence rate, overall survival, or melanoma-specific survival of patients with MIS treated with MMS compared with WLE.
Introduction
Melanoma in situ (MIS) often presents treatment challenges, especially when located on photodamaged skin, where lesions can be large, clinical margins can be ill defined, and there is often subclinical extension of atypical melanocytes. Incidence of MIS is increasing in the United States, where 68 480 cases of MIS are anticipated in 2016. However, only 1 randomized clinical trial for MIS treatment has been reported, which only assessed topical therapies.
The current standard of care recommended by expert panels is wide local excision (WLE), in which the tumor is excised with a standard margin of clinically uninvolved skin, and a small proportion of the surgical margin is examined histopathologically. Due to the challenges in clearing MIS, the recommendations for WLE margins were increased in 2014 from 0.5 cm to between 0.5 and 1.0 cm, and the usefulness of alternative surgical techniques enabling complete assessment of the entire cutaneous surgical margin, such as Mohs micrographic surgery (MMS), in which the margin is examined intraoperatively by staining frozen sections, has become increasingly recognized.
While the use of MMS for MIS has increased in recent years, almost all lesions (90%) are treated with WLE. Mohs micrographic surgery as a technique may be better suited for the removal of ill-defined skin cancers such as MIS on sun-damaged skin, which make up approximately 80% of all MIS; however, there is a concern regarding the perceived lack of reliability in detecting atypical melanocytes in MMS frozen sections, and potential resultant litigation. Furthermore, surgeons may not be aware of the growing body of data from case reports and case series supporting MMS as an acceptable alternative to WLE, especially in areas with anatomic functional and cosmetic significance. Because to our knowledge, no study to date has compared outcomes after the 2 procedures, data comparing MMS with WLE for MIS would be valuable to help inform treatment decisions for general and procedural dermatologists, oncologists, and oncological surgeons. In this study, we assessed clinical outcomes for MIS treated with MMS vs WLE.
Methods
Design and Setting
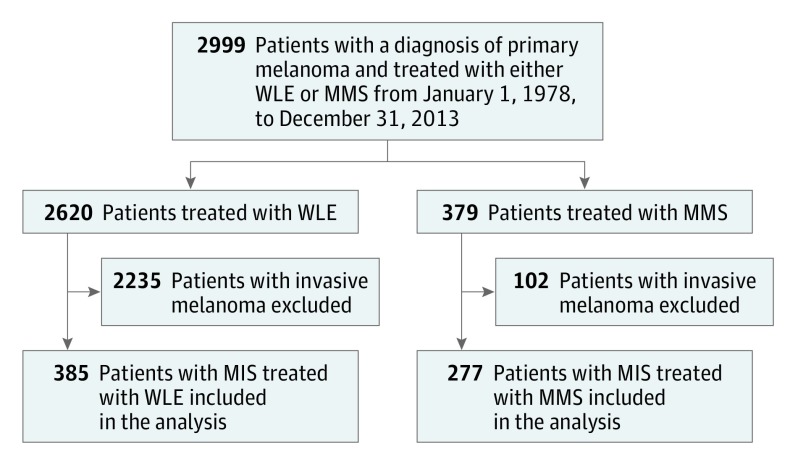
A prospective, single-institution, multidisciplinary database of patients with cancer was retrospectively reviewed. A total of 2999 patients with primary melanoma treated by either WLE or MMS were identified (Figure). Cases were identified through pathology report review, supplemented with discharge summary review of melanoma billing case lists. Data extraction was performed by a certified tumor registrar in accordance with the American College of Surgeons, Commission on Cancer per Facility Oncology Registry Data Standards Manual. Patients who had a biopsy demonstrating MIS, followed by MMS (277 patients) or WLE (385), were included. Patients with invasive disease or multiple melanoma were excluded. Follow-up was performed once every 12 to 15 months from date of last contact, in this order: medical record review, letter to referring physician (including those outside our institution), letter to patient, letter to relative. The study was approved by the Institutional Review Board, University of California, San Francisco. Informed consent was waived due to the retrospective nature of the study.
Figure. Schematic Depicting Inclusion Criteria for Study Participants.
Derived from consecutive patients who received a diagnosis of melanoma in situ (MIS) during the period 1978 through 2013. MMS indicates Mohs micrographic surgery; WLE, wide local excision.
Measures
Outcomes were recurrence, overall survival, and melanoma-specific survival. Recurrence was defined as follows: after a disease-free period, clinical and/or pathologic evidence of melanoma recurrence, which was not stated to be a new primary melanoma, at a body location identical to that of the primary tumor. The date of recurrence was the date of biopsy of the recurrent lesion. Recurrences were identified by medical record review and letters to physicians and patients. Physicians were asked to indicate “new areas of recurrence and/or metastasis since diagnosis: (date and site)”. Patients were asked, “Have you had any further treatment for the condition for which you were treated?” Two dermatologists reviewed the medical records of patients with missing data and added recurrence information when available. Survival was determined by tracking deaths through notification by family, by patients’ physicians, or from state registry of death certificate files, Department of Motor Vehicles registration, or voter registration. Before 2001, the Social Security death index was used to confirm deaths not provided by direct death certificate match, and from 2001 onward, an Internet obituary search was performed to confirm any report of death other than from the state registry.
Surgery
Mohs micrographic surgery, vs WLE, was chosen to try to minimize margins and/or ensure clear margins before complex closure was performed. If a lesion was relatively large, especially on the face, then it was more likely that a flap or graft would be required, and MMS was selected as the excision modality. For MMS, borders were delineated using a UVA light (Wood lamp) and a 5-mm margin was drawn around the tumor, less in areas of high cosmetic or functional significance or if the tumor was very small and well defined. The tumor was then debulked with a 3-mm margin and sent for permanent sectioning to assess for an invasive component. Next, a first Mohs stage was removed with a 2-mm margin and processed for intraoperative frozen section examination; immunohistochemical analysis was not used. Margins were considered positive if clusters of atypical melanocytes, and not just single melanocytes, were identified, using hematoxylin-eosin staining. The presence of melanocytes above the dermoepidermal junction, and crowded melanocytes along the stratum basale and extending down follicles were considered positive margins. Additional stages of similar width were removed if the margins were positive for MIS after the first stage; wider margins may have been taken subsequently, based on the degree of margin positivity. Once margins were deemed clear, the defect was repaired by means of standard techniques. A sample MMS case for MIS was reviewed each quarter by dermatopathologists. Intraoperatively, if there was any question in the reading of a particular slide, an immediate consultation with a dermatopathologist was obtained. For WLE, standard surgical excision was performed with the recommended margins.
Statistical Analysis
Continuous variables were described using means and standard deviations, and proportions were calculated for categorical variables. We compared characteristics of patients receiving MMS or WLE procedures using either the Mann-Whitney test or χ2 test as appropriate. Kaplan-Meier curves were used to examine time to recurrence and time to death. Groups were compared using the log rank test.
Both traditional and propensity-adjusted Cox proportional hazard regression models were used to estimate hazard ratios for both mortality and recurrence comparing the MMS and WLE groups. Because the number of events for the recurrence outcome was low, we used propensity-adjusted Cox models to enable us to control for the full set of possible confounding variables. We developed a model for the probability of MMS and then controlled for that probability in the Cox models. Based on review of the literature, the following potential predictors were considered: age at diagnosis, sex, anatomic site (head and neck or other), race, tumor diameter, and year of diagnosis. Diameter was only available for 181 tumors (27.3%), so this variable was not included in the primary propensity model.
Logistic regression was used to estimate the probability (propensity score) of receiving MMS for each person conditional on the aforementioned predictors. The quintiles of the estimated propensity score were used to create a categorical variable, which was used as a predictor in the subsequent Cox models. Results for the traditional and propensity-adjusted Cox models for mortality were similar, so we present only the results of the propensity-adjusted Cox models for both outcomes.
Tumor diameter is an important factor in determining whether WLE or MMS is used; an additional propensity-adjusted Cox model was run for the subgroup of patients with tumor diameter measurements. A propensity score for MMS, which included tumor diameter in addition to the predictors used previously, was constructed, and a quintile version of the score was used as a predictor in the Cox model for death. The propensity-adjusted Cox model for recurrence was not run because only 2 patients had a recurrence.
In the recurrence models, patients were censored for date of last contact (mean dates were November 8, 2013, for MMS and December 10, 2012, for WLE) or death. In the mortality models, patients were censored for end of follow-up. Adjusted melanoma-specific survival could not be ascertained due to the small number of cases. Analyses were run using SAS, version 9.4.
Results
Patient and Tumor Characteristics
Six hundred sixty-two eligible patients with primary MIS were identified; 385 (58.2%) patients were treated with WLE and 277 (41.8%) were treated with MMS. The median follow-up interval for the entire cohort was 8.6 years (range, 0.2-37 years). There was no statistically significant difference in the sex, race, or clinically assessed tumor diameter (Table 1). In both groups, the individuals were predominantly white men; for tumors with size measurements, mean (SD) tumor diameter was 1.8 (1.5) cm for tumors treated with MMS and 1.6 (1.1) cm for tumors treated with WLE (P = .21).
Table 1. Patient and Tumor Characteristics.
| Variable | MMS (n = 277) |
WLE (n = 385) |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 64.0 (13.1) | 58.5 (15.6) | <.001 |
| Sex, No. (%) | |||
| Male | 172 (62.1) | 211 (54.8) | .06 |
| Female | 105 (37.9) | 174 (45.2) | |
| Race, No. (%) | |||
| White | 252 (91.0) | 354 (91.9) | .66 |
| Other/unknown | 25 (9.0) | 31 (8.1) | |
| Year of diagnosis | |||
| Mean (SD) | 2007 (3.9) | 2003 (5.6) | <.001 |
| Median (range) | 2008 (1986-2013) | 2003 (1978-2013) | |
| Tumor diameter,a cm | |||
| Mean (SD) | 1.8 (1.5) | 1.6 (1.1) | .21 |
| Median (IQR) | 1.4 (0.5-9.0) | 1.3 (0.1-8.3) | |
| Anatomic site, No. (%)b | |||
| Face | 222 (80.2) | 141 (36.7) | <.001 |
| Scalp/neck | 23 (8.3) | 26 (6.8) | |
| Trunk | 10 (3.6) | 73 (19.0) | |
| Upper extremity | 13 (4.7) | 85 (22.1) | |
| Lower extremity | 9 (3.2) | 59 (15.4) | |
| WLE surgical margins, mean (SD), cm | NA | 0.6 (0.3) | |
| Recurrences, No. (%) | 5 (1.8) | 22 (5.7) | .07 |
| WLE margin taken, mean (SD), cm | |||
| Recurred | NA | 0.9 (0.2) | .003 |
| Not recurred | NA | 0.6 (0.04) | |
| Time to recurrence, mean (SD), y | 3.91 (4.4) | 4.45 (2.7) | .73 |
| Deaths, No. (%)c | |||
| All cause | 37 (13.4) | 75 (19.5) | |
| Melanoma specific | 2 (0.7) | 13 (3.4) | |
| Time to death, mean (SD), y | |||
| All cause | 18.4 (0.8) | 17.4 (0.4) | .28 |
| Melanoma specific | 6.5 (4.8) | 6.1 (0.8) | .77 |
Abbreviations: IQR, interquartile range; MMS, Mohs micrographic surgery; NA, not applicable; WLE, wide local excision.
Preoperative assessment of 107 MMS-treated patients and 74 WLE-treated patients.
Assessment of 277 MMS-treated patients and 384 WLE-treated patients.
P values not given because a statistical test comparing the raw counts/percentages does not account for differential follow-up time (see time to death analyses).
The 2 treatment groups differed significantly in age at diagnosis, anatomic site of procedure, and year of diagnosis. Patients treated with MMS were typically older than those treated by WLE (mean [SD] age, 64.0 [13.1] and 58.5 [15.6] years, respectively; P < .001). Both procedures were performed on a wide variety of anatomical sites and the use of the procedures varied by site (P < .001): as expected, MMS was used more frequently on the face and scalp/neck while WLE was used more often on the trunk and extremities. The majority of the MISs in our cohort were found on the head and neck (62% of all MIS). Use of WLE vs MMS shifted from primarily WLE before 2005 (270 [79%] were treated with WLE through 2004) to primarily MMS from 2005 onward (204 [64%] were treated with MMS from 2005 onward) (eTable 1 in the Supplement); the median year of diagnosis was 2008 for MMS (range, 1986-2013) and 2003 for WLE (1978-2013) (P < .001).
Tumor Recurrence
Twenty-seven tumors recurred: 22 of the 385 WLE patients had a recurrence (5.7%) whereas only 5 of the 277 MMS patients had a recurrence (1.8%). For tumors treated by MMS, the calculated 5-, 10-, and 15-year recurrence rates were 1.1% (95% CI, 0.4%-3.4%), 1.8% (95% CI, 0.7%-5.1%), and 5.0% (95% CI, 1.4%-17.3%), respectively (Table 2). For tumors treated by WLE, the 5-, 10-, and 15-year recurrence rates were 4.1% (95% CI, 2.5%-6.8%), 6.8% (95% CI, 4.4%-10.2%), and 7.3% (95% CI, 4.8%-11.0%), respectively (P = .07).
Table 2. Recurrence Rates.
| Procedure | Recurrence Rate | P Value | |||||
|---|---|---|---|---|---|---|---|
| 5-Year | 10-Year | 15-Year | |||||
| % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | No. | ||
| Mohs micrographic surgery | 1.1 (0.4-3.4) | 3 | 1.8 (0.7-5.1) | 4 | 5.0 (1.4-17.3) | 5 | .07 |
| Wide local excision | 4.1 (2.5-6.8) | 14 | 6.8 (4.4- 10.2) | 21 | 7.3 (4.8-11.0) | 22 | |
Mean (SD) time for recurrence after MMS procedure was 3.91 (4.4) years and after WLE was 4.45 (2.7) years (P = .73) (Table 1). Recurrence was nonsignificantly less likely in the MMS-treated group in the unadjusted model (hazard ratio [HR], 0.41; 95% CI, 0.15-1.09) (Table 3). In the propensity-adjusted Cox proportional hazards model, recurrence was also nonsignificantly less likely in MMS-treated patients (HR, 0.55; 95% CI, 0.18-1.70).
Table 3. Hazard of Recurrence, Patients With Melanoma In Situ Treated With Mohs Micrographic Surgery (MMS) Compared With Wide Local Excision (WLE).
| Risk of Recurrence, MMS vs WLE | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Unadjusted | 0.41 (0.15-1.09) | .07 |
| Adjusteda | 0.55 (0.18-1.70) | .31 |
Adjusted for age at diagnosis, sex, race, anatomic site, year of diagnosis, and by quintile of propensity score for performance of MMS.
Survival
Thirty-seven patients (13.4%) died in the MMS group and 75 patients (19.5%) died in the WLE group (Table 1). Mean (SD) time to all-cause death was 18.4 (0.8) years for individuals treated with MMS and 17.4 (0.4) years for those treated with WLE (P = .28) (Table 1). There was no statistically significant difference in overall survival for patients treated with MMS compared with WLE in the unadjusted (HR, 1.25; 95% CI, 0.83-1.88) or propensity-adjusted model (HR, 0.45; 95% CI, 0.16-1.29) (Table 4). The results for survival and recurrences with and without the propensity analysis were similar (data not shown).
Table 4. Survival Analysis, Patients With Melanoma In Situ Treated With Mohs Micrographic Surgery vs Wide Local Excision.
| Survival | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Unadjusted overall survival | 1.25 (0.83-1.88) | .28 |
| Adjusted overall survivala | 0.45 (0.16-1.29) | .14 |
| Unadjusted melanoma-specific survivalb | 0.80 (0.17-3.80) | .77 |
Adjusted for age, sex, race, anatomic site, year of diagnosis, tumor diameter, and by quintile of propensity score for performance of Mohs micrographic surgery.
Adjusted melanoma-specific survival was not calculated due to small number of events.
Two patients (0.7%) died of melanoma in the MMS group and 13 (3.4%) in the WLE group (Table 1). Mean (SD) time to death due to melanoma was 6.5 (4.8) years for individuals treated with MMS and 6.1 (0.8) years for WLE (P = .77) (Table 1). Unadjusted melanoma-specific survival (Table 4) was nonsignificantly better for MMS-treated compared with WLE-treated patients (HR, 0.80; 95% CI, 0.17-3.80). Adjusted melanoma-specific survival could not be ascertained due to the small number of events.
Survival and recurrence outcomes were also analyzed using only cases treated between 1996 and 2013, an interval when MMS was more commonly used, and no significant difference in outcomes was found vs 1978 through 2013 (data not shown).
Discussion
To our knowledge, this is the first study to directly compare the outcomes for MIS treated with WLE vs MMS. We found that the 5-, 10-, and 15-year recurrence rates of MIS treated with MMS were not different than for WLE, the currently widely accepted and preferred mode of therapy. Patients who underwent MMS had similar outcomes by several measures compared with those who underwent WLE: rate of recurrence, mean time to all-cause and melanoma-specific death, overall survival, and melanoma-specific survival.
Prior studies assessing outcomes for MIS treated with either MMS or WLE, without direct comparison of the 2 modalities, reported recurrence rates between 0% and 6% for MMS and somewhat higher rates of 6% to 20% for WLE; other studies were limited by low patient numbers and short follow-up times, or focused only on the subset of MIS occurring on sun-damaged skin (also known as lentigo maligna). Our study is notable for its larger cohort size and lengthier follow-up period (662 individuals with a mean follow-up of 8.6 years). A previous study by Hou et al assessed lentigo maligna treated with MMS or WLE but did not directly compare outcomes. Their results were similar to ours reported here, with overall recurrence rates, over the course of their study, of 1.9% for lentigo maligna treated with MMS vs 5.9% for those treated with WLE; their reported calculated rates for 5-, 10-, and 15-year recurrence-free survival were also comparable to ours.
There is some variability to the Mohs technique, for example, in the intraoperative tissue-staining techniques as well as the width of the debulking and staging margins taken. Barriers to wide-spread acceptance of MMS as an effective treatment for MIS include the perceived limited reliability of detecting atypical melanocytes on frozen section and the need for immunohistochemical stains. A notable difference in our surgical approach compared to that of Hou et al is that they elected to use intraoperative immunohistochemistry in 37% of cases treated with MMS. For our cohort, immunohistochemical stains were not used. In our experience, hematoxylin-eosin does not limit the identification of atypical melanocytes and this technique has previously been shown to be reliable. Furthermore, the use of immunostains may result in unnecessary extension of margins due to the presence of melanocytic hyperplasia in sun-damaged skin, rather than biologically active tumor cells. The validity of our technique is supported by the low recurrence rates for MIS treated with MMS in our cohort; similarly low recurrence rates have been reported for cohorts in which immunostains were not used at all or were not used uniformly. Whereas it is possible that detection of atypical melanocytes is simpler with the use of immunostains, it does not seem to change outcomes.
Several studies have shown that the formerly recommended 0.5-cm surgical margins for removal of MIS were inadequate for clearing a substantial proportion of MIS. Because of this, recently the National Comprehensive Cancer Network recommendations for margins for MIS were increased to 0.5 to 1.0 cm in 2014, in particular for sun-damaged skin such as on the head and neck. In our approach to MMS, a 5-mm margin is usually taken and includes the margin taken for the debulking specimen and the first stage. There are cases in which a smaller initial margin is taken, such as for lesions on the ear, lip, eyelid, or nasal ala. In these anatomically sensitive areas, achieving the recommended 1-cm margins can cause significant functional and cosmetic impairment and surgical approaches offering complete cutaneous margin assessment, such as MMS, offer the potential for tissue preservation while clearing margins, which is also desirable before complex wound repair is undertaken.
Similar to other reports, our surgical technique begins with a Wood lamp (UVA light) examination to define the tumor borders; this step is important for highlighting the extent of pigmented lesions. When MMS is performed, the initial debulking specimen is fixed in formalin and processed for permanent sectioning to assess for an invasive component. This step has been shown to result in upstaging of the tumor in more than 8% of the cases.
Limitations
Limitations of our study include nonrandomization of patient selection for our treatment groups and limitation of the accuracy of detection of recurrence due to the unknown response rate to the letters sent to physicians and patients. In addition, because the use rate for MMS over the course of the study only increased to match or surpass the rate of use of WLE in 2004 (due to shifting outside physician referral preferences for specific procedures), as reflected in the median year of diagnosis, the mean follow-up interval for MMS-treated patients was shorter. Furthermore, the suggestion of improved melanoma-specific survival for those treated with MMS might be attributed to the fact that most MMS is performed on lesions occurring on the head and neck, where MIS frequently occurs on sun-damaged skin, and perhaps the behavior of these MIS lesions differ from those of lesions occurring elsewhere. The overall number of recurrences and deaths here was low, and a larger study will be needed to demonstrate significant differences in outcomes, or to establish equivalence. Finally, the WLEs were performed with the formerly recommended guidelines for smaller surgical margins for MIS; as the new guidelines for a wider margin are adopted, it may be that patients undergoing WLE may have more favorable outcomes in the future. However, the majority of MISs occur on the head and neck, and this location favors referral for MMS; anatomic considerations may continue to limit the use of the full 1-cm margins.
To date, no randomized clinical trials comparing surgical management of MIS have been performed, even though MMS has been in use for this purpose since 1950. Current consensus expert opinion guidelines recommend consideration of MMS for MIS, particularly on sun-damaged sites, as do the appropriate use guidelines for MMS. Whereas MMS is used by procedural dermatologists and plastic surgeons for skin cancer removal, the use of MMS for MIS has yet to gain widespread acceptance by medical and surgical oncologists, likely because reports on MMS use have been primarily limited to dermatology journals.
Conclusions
Our data indicate that MMS is as effective as WLE for treatment of MIS. This sizable cohort study did not demonstrate differences in recurrence or survival for MIS treated with MMS vs WLE. Future studies, including a randomized study comparing MMS with WLE, are needed, but may be problematic to implement, given patients’ desires to try to preserve anatomic function and cosmesis; a well-designed prospective observational study would be informative. Further study to assess whether there are functional or perceived cosmetic differences or adverse outcomes for patients treated with MMS vs WLE would be illuminating.
eTable. Procedures performed by year
References
- 1.Higgins HW II, Lee KC, Galan A, Leffell DJ. Melanoma in situ: part II. histopathology, treatment, and clinical management. J Am Acad Dermatol. 2015;73(2):193-203. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 3.Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol. 2016;75(4):698-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tzellos T, Kyrgidis A, Mocellin S, Chan AW, Pilati P, Apalla Z. Interventions for melanoma in situ, including lentigo maligna. Cochrane Database Syst Rev. 2014;(12):CD010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bichakjian CK, Halpern AC, Johnson TM, et al. ; American Academy of Dermatology . Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2011;65(5):1032-1047. [DOI] [PubMed] [Google Scholar]
- 6.Coit DG, Thompson JA, Algazi A, et al. Melanoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(4):450-473. [DOI] [PubMed] [Google Scholar]
- 7.Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438-444. [DOI] [PubMed] [Google Scholar]
- 8.Clark GS, Pappas-Politis EC, Cherpelis BS, et al. Surgical management of melanoma in situ on chronically sun-damaged skin. Cancer Control. 2008;15(3):216-224. [DOI] [PubMed] [Google Scholar]
- 9.Coit DG, Thompson JA, Andtbacka R, et al. ; National Comprehensive Cancer Network . Melanoma, version 4.2014. J Natl Compr Canc Netw. 2014;12(5):621-629. [DOI] [PubMed] [Google Scholar]
- 10.Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72(6):1060-1065. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SM, Baker DR, Coldiron BM, et al. ; Ad Hoc Task Force; Ratings Panel . AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67(4):531-550. [DOI] [PubMed] [Google Scholar]
- 12.Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990-2000. J Invest Dermatol. 2005;125(4):685-691. [DOI] [PubMed] [Google Scholar]
- 13.Prieto VG, Argenyi ZB, Barnhill RL, et al. Are en face frozen sections accurate for diagnosing margin status in melanocytic lesions? Am J Clin Pathol. 2003;120(2):203-208. [DOI] [PubMed] [Google Scholar]
- 14.Bene NI, Healy C, Coldiron BM. Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases. Dermatol Surg. 2008;34(5):660-664. [DOI] [PubMed] [Google Scholar]
- 15.Iorizzo LJ III, Chocron I, Lumbang W, Stasko T. Importance of vertical pathology of debulking specimens during Mohs micrographic surgery for lentigo maligna and melanoma in situ. Dermatol Surg. 2013;39(3, pt 1):365-371. [DOI] [PubMed] [Google Scholar]
- 16.Mangold AR, Skinner R, Dueck AC, Sekulic A, Pockaj BA. Risk factors predicting positive margins at primary wide local excision of cutaneous melanoma. Dermatol Surg. 2016;42(5):646-652. [DOI] [PubMed] [Google Scholar]
- 17.Bliss JM, Ford D, Swerdlow AJ, et al. ; International Melanoma Analysis Group (IMAGE) . Risk of cutaneous melanoma associated with pigmentation characteristics and freckling: systematic overview of 10 case-control studies. Int J Cancer. 1995;62(4):367-376. [DOI] [PubMed] [Google Scholar]
- 18.Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969-1999. JAMA. 2002;288(14):1719-1720. [DOI] [PubMed] [Google Scholar]
- 19.Dawn ME, Dawn AG, Miller SJ. Mohs surgery for the treatment of melanoma in situ: a review. Dermatol Surg. 2007;33(4):395-402. [DOI] [PubMed] [Google Scholar]
- 20.Hou JL, Reed KB, Knudson RM, et al. Five-year outcomes of wide excision and Mohs micrographic surgery for primary lentigo maligna in an academic practice cohort. Dermatol Surg. 2015;41(2):211-218. [DOI] [PubMed] [Google Scholar]
- 21.Bhardwaj SS, Tope WD, Lee PK. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma using Mel-5 immunostaining: University of Minnesota experience. Dermatol Surg. 2006;32(5):690-696. [DOI] [PubMed] [Google Scholar]
- 22.Bienert TN, Trotter MJ, Arlette JP. Treatment of cutaneous melanoma of the face by Mohs micrographic surgery. J Cutan Med Surg. 2003;7(1):25-30. [DOI] [PubMed] [Google Scholar]
- 23.Temple CL, Arlette JP. Mohs micrographic surgery in the treatment of lentigo maligna and melanoma. J Surg Oncol. 2006;94(4):287-292. [DOI] [PubMed] [Google Scholar]
- 24.Cohen LM, McCall MW, Zax RH. Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma: a follow-up study. Dermatol Surg. 1998;24(6):673-677. [DOI] [PubMed] [Google Scholar]
- 25.Bricca GM, Brodland DG, Ren D, Zitelli JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52(1):92-100. [DOI] [PubMed] [Google Scholar]
- 26.Clayton BD, Leshin B, Hitchcock MG, Marks M, White WL. Utility of rush paraffin-embedded tangential sections in the management of cutaneous neoplasms. Dermatol Surg. 2000;26(7):671-678. [DOI] [PubMed] [Google Scholar]
- 27.Felton S, Taylor RS, Srivastava D. Excision margins for melanoma in situ on the head and neck. Dermatol Surg. 2016;42(3):327-334. [DOI] [PubMed] [Google Scholar]
- 28.Zitelli JA, Moy RL, Abell E. The reliability of frozen sections in the evaluation of surgical margins for melanoma. J Am Acad Dermatol. 1991;24(1):102-106. [DOI] [PubMed] [Google Scholar]
- 29.Huilgol SC, Selva D, Chen C, et al. Surgical margins for lentigo maligna and lentigo maligna melanoma: the technique of mapped serial excision. Arch Dermatol. 2004;140(9):1087-1092. [DOI] [PubMed] [Google Scholar]
- 30.Robinson JK. Margin control for lentigo maligna. J Am Acad Dermatol. 1994;31(1):79-85. [DOI] [PubMed] [Google Scholar]
- 31.Mohs FE. Chemosurgical treatment of melanoma; a microscopically controlled method of excision. Arch Derm Syphilol. 1950;62(2):269-279. [DOI] [PubMed] [Google Scholar]
- 32.Cabbabe EB. Quality care and the plastic surgeon’s role in the treatment of skin lesions. Plast Reconstr Surg. 2015;136(5):719e-720e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Procedures performed by year