Key Points
Question
What are the characteristics and findings of preapproval and mandated postapproval trials of drugs granted US Food and Drug Administration (FDA) accelerated approval between 2009 and 2013?
Findings
The FDA granted accelerated approval to 22 drugs for 24 indications. Clinical trials conducted before (n=30) and after (n=18) approval had similar design characteristics, such as lack of blinding, randomization, and comparator groups. Of the required postapproval confirmatory studies, half were completed a minimum of 3 years after approval, and of these, most showed some benefit but relied on surrogate measures rather than clinical outcomes.
Meaning
Among 24 indications granted accelerated approval by the FDA in 2009-2013, 42% had efficacy confirmed in postapproval trials a minimum of 3 years after approval, although both preapproval and postapproval trials had limitations in the study designs and end points used.
Abstract
Importance
Drugs treating serious or life-threatening conditions can receive US Food and Drug Administration (FDA) accelerated approval based on showing an effect in surrogate measures that are only reasonably likely to predict clinical benefit. Confirmatory trials are then required to determine whether these effects translate to clinical improvements.
Objective
To characterize preapproval and confirmatory clinical trials of drugs granted accelerated approval.
Design and Setting
Publicly available FDA documents were reviewed to identify the preapproval trials leading to accelerated approval between 2009 and 2013. Information on the status and findings of required confirmatory studies was extracted from the FDA’s database of postmarketing requirements and commitments, ClinicalTrials.gov, and matched peer-reviewed publications. Follow-up ended on April 7, 2017.
Exposures
Granting of accelerated approval.
Main Outcomes and Measures
Characteristics of preapproval and confirmatory studies were compared in terms of study design features (randomization, blinding, comparator, primary end point). Subsequent regulatory decisions and estimated time between accelerated approval and fulfillment of regulatory requirements were summarized.
Results
The FDA granted accelerated approval to 22 drugs for 24 indications (19 for indications involving cancer treatment) between 2009 and 2013. A total of 30 preapproval studies supported the 24 indications. The median number of participants enrolled in the preapproval studies was 132 (interquartile range, 89-224). Eight studies (27%) included fewer than 100 participants and 20 (67%) included fewer than 200. At a minimum 3 years of follow-up, 19 of 38 (50%) required confirmatory studies were completed, including 18 published reports. Twenty-five of the 38 (66%) examined clinical efficacy, 7 (18%) evaluated longer follow-up, and 6 (16%) focused on safety The proportion of studies with randomized designs did not differ before and after accelerated approval (12/30 [40%] vs 10/18 [56%]; difference, 16%; 95% CI, −15% to 46%; P = .31). Postapproval requirements were completed and demonstrated efficacy in 10 of 24 indications (42%) on the basis of trials that evaluated surrogate measures. Among the 14 of 24 indications (58%) that had not yet completed all requirements, at least 1 of the confirmatory studies failed to demonstrate clinical benefit in 2 (8%), were terminated in 2 (8%), and were delayed by more than 1 year in 3 (13%). Studies were progressing according to target timelines for the remaining 7 indications (29%). Clinical benefit had not yet been confirmed for 8 indications that had been initially approved 5 or more years prior.
Conclusions and Relevance
Among 22 drugs with 24 indications granted accelerated approval by the FDA in 2009-2013, efficacy was often confirmed in postapproval trials a minimum of 3 years after approval, although confirmatory trials and preapproval trials had similar design elements, including reliance on surrogate measures as outcomes.
This study uses public US Food and Drug Administration (FDA) data to characterize drug trials used to obtain FDA accelerated approval and to describe the existence, timing, and characteristics of postapproval trials mandated by the FDA as part of the accelerated approval decision.
Introduction
The US Food and Drug Administration (FDA) has several pathways aimed at expediting the development and approval of drugs that address serious or life-threatening conditions. The “accelerated approval” pathway permits the FDA to grant marketing authorization on the basis of surrogate measures, such as biomarkers, laboratory values, or other physical measures that may serve as indicators of clinical outcomes such as symptom control or mortality, that are only “reasonably likely” to predict clinical benefit. Once accelerated approval drugs are granted marketing authorization, the FDA requires that the sponsors complete confirmatory trials to describe and verify clinical efficacy. When these requirements are fulfilled, the drug’s label may be updated to account for the new information.
Although special pathways like accelerated approval can be highly effective in facilitating the approval of certain new drugs, these pathways have also been a source of controversy. Drugs approved via expedited pathways may have greater safety risks to patients. In a recent evaluation of 222 novel therapeutic agents approved by the FDA in 2001-2010, postmarket safety events were more frequent among drugs receiving accelerated approval. There is also uncertainty about whether observed effects on surrogate measures will materialize into clinical improvements. In a review of drugs approved by the FDA between 2005 and 2012 on the basis of limited evidence, a minority showed efficacy in clinical trials in the postapproval period. Confirmatory trials evaluating the clinical benefit of drugs in the accelerated approval pathway can also be substantially delayed. In a previous evaluation of accelerated approval of 35 oncology drugs, clinical benefit was demonstrated in confirmatory studies for approximately half of new indications, but drugs granted accelerated approval quickly become standard of care despite the limited evidence on which they were approved.
Implementation of the accelerated approval pathway in recent years has not been characterized. This study was designed to compare the evidence gathered on qualifying drugs before and after approval, including the extent to which confirmatory studies were completed and determined whether the drugs demonstrated clinically meaningful benefits. The time between accelerated approval and fulfillment of postapproval requirements was also assessed.
Methods
Sample Identification
Two investigators (H.N. and K.R.S.) reviewed publicly available FDA documents (CDER Drug and Biologic Accelerated Approvals as of 30 June 2016 and Novel Drug Approvals for 2011-2013) to identify drugs granted accelerated approval between January 1, 2009, and December 31, 2013. The CDER Drug and Biologic Accelerated Approvals list is compiled by the FDA’s Center for Drug Evaluation and Research (CDER). The Novel Drug Approvals report is an annual catalog of approved new molecular entities. The sample included drugs that received accelerated approval as new therapeutic agents and as supplemental approvals (products already approved for other indications). Drugs that received original marketing authorization prior to 2009 were also included if they received a supplemental accelerated approval for a new indication during the study period. Accelerated approvals for new formulations (eg, tablet vs injection) of already approved agents were excluded (n = 1). To ensure that no approvals were missed, the consistency of the sample was confirmed with a previously published report on FDA approvals. Drugs approved up to 2013 were included, allowing at least 3 years for the completion and publication of confirmatory clinical studies (median, 5 years).
Identification of Preapproval Studies
The clinical studies underlying accelerated approval were identified and characterized. For all drugs in the sample, the medical review reports and product labels from the Drugs@FDA database were examined to identify preapproval studies that established the drug’s efficacy. Drugs@FDA is a publicly available database of all FDA-approved products and contains the approval history for each product, including links to communications from the FDA to the sponsor, and product label updates. When available, medical review reports were used to gather information about preapproval trial characteristics. Medical review reports provide a comprehensive overview of the efficacy and safety of a drug. When medical reviews were not available (as can be the case for supplemental approvals), the product labels that describe the key clinical studies that supported the accelerated approval for a new indication were used.
Identification of Postapproval Confirmatory Studies
The FDA’s approval letters available in the Drugs@FDA database were reviewed to identify the confirmatory study requirements at the time of accelerated approval. Postmarketing study requirements focusing on safety evaluations alone under FDAAA Section 505(o)(3) regulations were excluded. Information reported in product labels and FDA’s approval letters were used to summarize how the FDA characterized the main limitation of the available data at the time of accelerated approval and whether required postmarketing studies assessed efficacy, safety, or long-term follow-up.
Two sources of information were reviewed to determine the status of postapproval study requirements. First, the FDA’s publicly available database of postmarketing requirements and commitments was searched. This database specifies the clinical studies that satisfy postmarketing requirements and commitments to gather additional information about a product’s safety, efficacy, or optimal use. For agents with confirmatory studies, whether the study was ongoing, delayed, submitted, or fulfilled was noted. Consistent with previous reports, a substantial proportion (n = 18 [47%]) of indications did not have matching postmarketing requirements listed in the FDA database. Second, ClinicalTrials.gov, a publicly available clinical study registry and results database developed and maintained by the US National Library of Medicine, was screened for all confirmatory study requirements. Since 2007, Section 801 of the FDA Amendments Act has required the registration of clinical studies subject to FDA regulation, including studies that satisfy postmarketing requirements. For each registered study, ClinicalTrials.gov specifies the status (eg, still recruiting, ongoing but no longer recruiting, completed), as well as start and end date. Whether the confirmatory study was completed or ongoing per specified timelines in the FDA’s approval letters was assessed. Studies were considered to be delayed if the estimated primary completion date in ClinicalTrials.gov was at least 1 year later than that specified in the FDA approval letters. When there was a discrepancy between the FDA’s public database and ClinicalTrials.gov, information from ClinicalTrials.gov was used to determine the status of postapproval study requirements.
Identification of Published Reports
Using a stepwise approach, published reports of completed confirmatory studies were identified. First, whether a publication link was available on the ClinicalTrials.gov file was assessed for each study. ClinicalTrials.gov periodically searches PubMed to identify corresponding publications; study investigators can also add publication links manually. Second, PubMed was searched using the ClinicalTrials.gov identification number. Third, PubMed and Google were searched using the name of the principal investigator of the study (when available in ClinicalTrials.gov) in combination with the condition and drug name. Identified publications were matched to the corresponding postmarketing study based on the condition, comparator(s), enrollment, and primary and secondary outcome measures.
Data Extraction
Information extracted from each preapproval and confirmatory study included design (randomized vs nonrandomized), comparator(s), participant enrollment, and primary end point. Comparators were classified as active (in trials comparing drugs A vs B), add-on (in trials comparing drugs A + B vs drug B alone), placebo, or none. Drugs tested in single-intervention-group trials were classified as having no comparators. Type of blinding (double blind vs open label) was recorded. Study findings were summarized in terms of the specified primary end point. In confirmatory studies, whether the findings demonstrated verification of clinical benefit was assessed. All data extraction was performed independently by 2 investigators (H.N. and K.R.S.) and disagreements resolved by consensus.
Assessment of Regulatory Outcomes
To determine whether drugs granted accelerated approvals later had their labels updated, changes to product labels and the accompanying regulatory letters were assessed. The FDA’s correspondence with product sponsors on topics related to the approval and changes in status of their products is publicly available in the Drugs@FDA database. Regulatory letters were systematically screened for either confirmation of the fulfillment of the requirements or the lack of regulatory action as of the end of our data collection (April 7, 2017). The time between the granting of accelerated approval and the associated label update was determined.
Statistical Analysis
Descriptive statistics were used to characterize the clinical studies supporting the accelerated approval of drugs included in the sample. Wilcoxon-Mann-Whitney and t tests were used as appropriate to examine differences in study features between preapproval and confirmatory studies, including enrollment, design, comparator(s), and primary end points. A 2-tailed P<.005 was considered statistically significant, taking into account the 10 comparisons made between the 2 groups of studies. All analyses were performed using Stata version 14 (Stata Corp).
Results
Between 2009 and 2013, the FDA granted accelerated approval to 22 drugs for 24 indications, with 2 products granted the designation for 2 indications (Table 1). The 2 drugs with 2 indications were everolimus and brentuximab vedotin. Thirteen approvals were for novel therapeutic agents and 11 were for supplemental indications for previously approved drugs. Cancer treatment accounted for 19 of the indications. The remaining indications included a range of conditions including transfusion- and non–transfusion-dependent iron overload, multidrug-resistant tuberculosis, and Hunter syndrome.
Table 1. Drugs and FDA Accelerated Approval Indications, 2009-2013.
| Agent | Year Approved | Indication at Time of Accelerated Approval | Novel or Supplemental Indications |
|---|---|---|---|
| Bevacizumab | 2009 | Treatment of glioblastoma as a single agent for patients with progressive disease following prior therapy | Supplemental |
| Ofatumumab | 2009 | Treatment of patients with chronic lymphocytic leukemia refractory to fludarabine and alemtuzumab | Novel |
| Pralatrexate | 2009 | Treatment of patients with relapsed or refractory PTCL | Novel |
| Dasatinib | 2010 | Treatment of newly diagnosed adults with Ph+ CML in chronic phase | Supplemental |
| Everolimus | 2010 | Treatment of subependymal giant cell astrocytoma associated with tuberous sclerosis who require therapeutic intervention but are not candidates for curative surgical resection | Supplemental |
| Lapatinib | 2010 | In combination with letrozole for treatment in postmenopausal women of hormone receptor–positive metastatic breast cancer that overexpresses the HER2 receptor for whom hormone therapy is indicated | Supplemental |
| Nilotinib | 2010 | Treatment of adult patients newly diagnosed as having Ph+ CML in chronic phase | Supplemental |
| Brentuximab vedotin | 2011 | Treatment of patients with Hodgkin lymphoma after failure of ASCT or after failure of ≥2 prior multiagent chemotherapy regimens in patients who are not ASCT candidates | Novel |
| Brentuximab vedotin | 2011 | Treatment of patients with systemic anaplastic large cell lymphoma after failure of ≥1 prior multiagent chemotherapy regimen | Supplemental |
| Crizotinib | 2011 | Treatment of patients with locally advanced or metastatic non–small cell lung cancer that is anaplastic lymphoma kinase positive as detected by an FDA-approved test | Novel |
| Deferiprone | 2011 | Treatment of patients with transfusional iron overload due to thalassemia syndromes when current chelation therapy is inadequate | Novel |
| Hydroxyprogesterone caproate | 2011 | To reduce risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth | Novel |
| Romidepsin | 2011 | Treatment of PTCL in patients who have received at least one prior therapy | Supplemental |
| Bedaquiline | 2012 | Indicated as part of combination therapy in adults (aged ≥18 y) with pulmonary multidrug-resistant tuberculosis | Novel |
| Carfilzomib | 2012 | Treatment of patients with multiple myeloma who have received ≥2 prior therapies including bortezomib and an immunomodulatory agent and have demonstrated disease progression on or within 60 d of completion of the last therapy | Novel |
| Everolimus | 2012 | Treatment of adults with renal angiomyolipoma and tuberous sclerosis complex not requiring immediate surgery | Supplemental |
| Omacetaxine mepesuccinate | 2012 | Treatment of adult patients with chronic- or accelerated-phase CML with resistance and/or intolerance to ≥2 TKIs | Novel |
| Ponatinib | 2012 | Treatment of adult patients with chronic-, accelerated-, or blast-phase CML resistant or intolerant to prior TKI therapy or Ph+ ALL resistant or intolerant to prior TKI therapy | Novel |
| Vincristine sulfate liposome | 2012 | Treatment of adult patients with Ph− ALL in second or greater relapse or whose disease has progressed following ≥2 antileukemia therapies | Novel |
| Pomalidomide | 2013 | Patients with multiple myeloma who have received ≥2 prior therapies including lenalidomide and bortezomib and have demonstrated disease progression on or within 60 d of completion of last therapy | Novel |
| Ibrutinib | 2013 | Treatment of patients with mantle cell lymphoma who have received ≥1 prior therapy | Novel |
| Deferasirox | 2013 | Treatment of chronic iron overload in patients aged ≥10 y with non–transfusion-dependent thalassemia syndromes and with liver iron concentration ≥5 mg/g of dry weight and serum ferritin ≥300 µg/L | Supplemental |
| Idursulfase | 2013 | Patients aged 16 mo to 5 y with Hunter syndrome (mucopolysaccharidosis II) | Supplemental |
| Pertuzumab | 2013 | In combination with trastuzumab and docetaxel as neoadjuvant treatment of patients with HER2-positive, locally advanced, inflammatory, or early-stage breast cancer (either >2 cm in diameter or node positive) as part of a complete treatment regimen for early breast cancer | Supplemental |
Abbreviations: ALL, acute lymphoblastic leukemia; ASCT, autologous stem cell transplantation; CML, chronic myeloid leukemia; FDA, US Food and Drug Administration; HER2, human epidermal growth factor; Ph+, Philadelphia chromosome–positive; Ph−, Philadelphia chromosome–negative; PTCL, peripheral T-cell lymphoma; TKI, tyrosine kinase inhibitor.
Features of Preapproval Studies
Thirty preapproval studies supported the 24 indications of interest. Twelve studies (40%) were randomized and 6 (20%) were double blind (Table 2 and Table 3). Six preapproval studies (20%) used placebo controls, 3 (10%) used an active comparator, another 2 (7%) evaluated the active agent as an add-on to a standard treatment regimen, and 19 (63%) had no comparators. The median number of participants enrolled in the preapproval studies was 132 (interquartile range, 89-224). Eight studies (27%) included fewer than 100 participants and 20 (67%) included fewer than 200.
Table 2. Comparison of Preapproval and Postapproval Study Characteristics for Drugs Receiving Accelerated Approval.
| Study Characteristics | No. (%)a | Difference in Proportions, % (95% CI)b | P Value for Difference | |
|---|---|---|---|---|
| Preapproval Studies (n = 30) |
Postapproval Studies (n = 18) |
|||
| Enrollment, median (interquartile range), No. | 132 (89-224) | 345 (111-619) | .17 | |
| Randomized | 12 (40) | 10 (56) | 16 (−15 to 46) | .31 |
| Double blind | 6 (20) | 1 (6) | −14 (−36 to 7) | .17 |
| Comparator | ||||
| Placebo | 6 (20) | 1 (6) | −14 (−36 to 7) | .17 |
| Active | 3 (10) | 7 (39) | 29 (6 to 53) | .02 |
| Add-on | 2 (7) | 2 (11) | 4 (−12 to 21) | .60 |
| None | 19 (63) | 8 (44) | −19 (−49 to 11) | .21 |
| Primary end pointc | ||||
| Disease response | 21 (70) | 9 (50) | −20 (−49 to 9) | .17 |
| Progression-free survival | 1 (3) | 7 (39) | 36 (15 to 56) | .001 |
| Overall survival | 0 | 1 (6)d | 6 (−3 to 14) | .20 |
| Other surrogate | 8 (27) | 2 (11) | −16 (−40 to 9) | .21 |
Data are No. (%) unless otherwise indicated.
Not adjusted for multiple comparisons.
Primary end points do not add up to total number of postapproval studies: 1 study included 2 primary end points.
Co–primary end point with progression-free survival.
Table 3. Characteristics of 30 Preapproval Studies of Drugs Receiving Accelerated Approval.
| Agent | Participant Population | Design | Comparators | Enrollment, No. | Primary End Point |
|---|---|---|---|---|---|
| Bevacizumab | Glioblastoma after prior therapy | Randomized noncomparative study | None | 85 | Objective response rate |
| Glioblastoma after prior therapy | Single-group trial | None | 56 | Objective response rate | |
| Pralatrexate | PTCL after prior therapy | Single-group trial | None | 115 | Overall response rate |
| Ofatumumab | Chronic lymphocytic leukemia after prior therapy | Single-group trial | None | 154 | Objective response rate |
| Lapatinib | Postmenopausal women with HER2-positive metastatic breast cancer with no prior therapy for whom hormone therapy is indicated | Placebo-controlled, double-blind randomized trial | Group 1: lapatinib + letrozole Group 2: letrozole + placebo |
1286 (219 HER2 positive) | Progression-free survival |
| Nilotinib | Newly diagnosed Ph+ chronic-phase CML | Active-comparator, open-label randomized trial | Group 1: nilotinib, 300 mg twice daily Group 2: nilotinib, 400 mg twice daily Group 3: imatinib, 400 mg once daily |
846 | Major molecular response |
| Dasatinib | Newly diagnosed chronic-phase CML | Active-comparator, open-label randomized trial | Group 1: dasatinib Group 2: imatinib |
519 | Complete cytogenetic response |
| Everolimusa | SEGA associated with tuberous sclerosis | Single-group trial | None | 28 | Change in SEGA volume |
| Hydroxyprogesterone caproate | Women with previous singleton spontaneous preterm birth | Placebo-controlled, double-blind randomized trial | Group 1: hydroxyprogesterone Group 2: placebo |
463 | Proportion of deliveries at <37 wk of gestation |
| Romidepsin | PTCL after ≥1 prior therapy | Single-group trial | None | 131 | Complete response rate |
| PTCL after ≥1 prior therapy | Single-group trial | None | 47 | Complete response rate | |
| Brentuximab vedotinb | Hodgkin lymphoma after ASCT of after failure of ≥2 prior multiagent chemotherapy regimens in patients who are not ASCT candidates | Single-group trial | None | 102 | Objective response rate |
| Brentuximab vedotinc | Systemic anaplastic large cell lymphoma after prior therapy | Single-group trial | None | 58 | Objective response rate |
| Crizotinib | Locally advanced or metastatic ALK-positive NSCLC after prior therapy | Single-group trial | None | 136 | Objective response rate |
| Locally advanced or metastatic ALK-positive NSCLC after prior therapy | Single-group trial | None | 119 | Objective response rate | |
| Deferiprone | Transfusion-dependent iron overload after prior therapy | Single-group trial (pooled analysis of 12 studies) | None | 236 | ≥20% Decline in ferritin |
| Everolimusd | Renal angiomyolipoma as a feature of tuberous sclerosis complex or sporadic lymphangioleimyomatosis | Placebo-controlled, double-blind randomized trial | Group 1: everolimus Group 2: placebo |
118 | Angiomyolipoma response rate |
| Carfilzomib | Multiple myeloma after ≥2 prior therapies | Single-group trial | None | 266 | Overall response rate |
| Vincristine sulfate liposome | Ph− ALL after ≥2 prior therapies | Single-group trial | None | 65 | Complete remission and complete remission with incomplete blood count recovery |
| Omacetaxine mepessucinate | Chronic- and accelerated-phase CML after ≥2 TKIs | Single-group trial (pooled analysis of 2 studies) | None | 111 | Major cytogenetic and hematologic response |
| Ponatinib | Chronic-, accelerated-, and blast-phase CML and Ph+ ALL after prior TKI | Single-group trial | None | 449 | Major cytogenetic and hematologic response |
| Bedaquiline | Patients newly diagnosed as having MDR-TB | Placebo-controlled, double-blind randomized trial | Group 1: bedaquiline + other drugs used to treat MDR-TB Group 2: placebo + other drugs used to treat MDR-TB Other drugs: ethionamide, kanamycin, pyrazinamide, ofloxacin, and cycloserine/terizidone or available alternative |
160 | Proportion with sputum culture conversion |
| Patients newly diagnosed as having MDR-TB | Placebo-controlled, double-blind randomized trial | Group 1: bedaquiline + other drugs used to treat MDR-TB Group 2: placebo + other drugs used to treat MDR-TB |
47 | Proportion with sputum culture conversion | |
| Deferasirox | Non–transfusion-dependent thalassemia syndromes and iron overload | Placebo-controlled, double-blind randomized trial | Group 1: deferasirox, 5 mg/kg per d Group 2: deferasirox, 10 mg/kg per d Group 3: placebo |
166 | Mean change in liver iron concentration from baseline (mg/g dry weight) |
| Non–transfusion-dependent thalassemia syndromes and iron overload | Single-group extension of randomized trial | None | 133 | Proportion achieving liver iron concentration <5 mg/g dry weight | |
| Pomalidomide | Refractory multiple myeloma after receiving lenalidomide and bortezomib | Active-comparator, open-label randomized trial | Group 1: pomalidomide Group 2: pomalidomide + low-dose dexamethasone |
221 | Overall response rate |
| Idursulfase | Patients aged 16 mo to 7.5 y with Hunter syndrome | Single-group trial | None | 28 | Adverse reactions (safety trial) |
| Pertuzumab | Patients with operable, locally advanced, or inflammatory HER2-positive breast cancer | Add-on comparator, open-label randomized trial | Group 1: pertuzumab + trastuzumab + docetaxel Group 2: trastuzumab + docetaxel Group 3: pertuzumab + trastuzumab Group 4: pertuzumab + docetaxel |
417 | Pathological complete response rate |
| Patients with operable, locally advanced, or inflammatory HER2-positive breast cancer | Add-on comparator, open-label randomized trial | Group 1: Pertuzumab + trastuzumab + fluorouracil, epirubicin, and cyclophosphamide followed by pertuzumab + trastuzumab + docetaxel Group 2: pertuzumab + trastuzumab + docetaxel following fluorouracil, epirubicin, and cyclophosphamide Group 3: pertuzumab + docetaxel, carboplatin, and trastuzumab |
225 | Cardiac safety (pathological complete response rate was secondary end point) | |
| Ibrutinib | Mantle cell lymphoma after ≥1 therapy | Single-group trial | None | 111 | Overall response rate |
Abbreviations: ALK, anaplastic lymphoma kinase; ALL, acute lymphoblastic leukemia; ASCT, autologous stem cell transplant; CML, chronic myeloid leukemia; HER2, human epidermal growth factor; MDR-TB, multidrug-resistant tuberculosis; NSCLC, non–small cell lung cancer; Ph+, Philadelphia chromosome positive; Ph−, Philadelphia chromosome negative; PTCL, peripheral T-cell lymphoma; SEGA, subependymal giant cell astrocytoma; TKI, tyrosine kinase inhibitor.
Tuberous sclerosis indication.
Hodgkin lymphoma indication.
Systemic anaplastic large cell lymphoma indication.
Renal angiomyolipoma and tuberous sclerosis complex indication.
The most common surrogate measure used in the preapproval studies was a measure of disease response (n = 21 [70%]), reflecting that most were oncology drugs (Table 2 and Table 3). Other surrogate measures included time-to-event outcomes (eg, time to sputum culture conversion, progression-free survival), change in baseline biomarker levels (eg, liver iron concentration), and acceptable safety.
Nonrandomized, noncomparative single-group studies formed the exclusive basis of accelerated approval for 14 indications (47%), and preapproval studies with fewer than 200 participants supported the accelerated approval of 12 indications (40%).
Status of Required Postapproval Confirmatory Studies
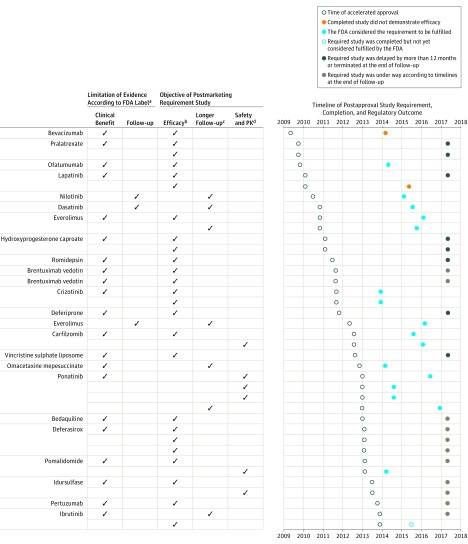
At the time of accelerated approval, the FDA labels emphasized the limitations of the available data (Figure). The majority of labels, 21 of 24 (88%), highlighted the lack of evidence demonstrating an improvement in disease-related symptoms or survival (eTable 1 in the Supplement). To address these limitations, the FDA required the completion of 38 postapproval confirmatory trials for the 24 indications. Twenty-five of the 38 (66%) examined clinical efficacy, 7 (18%) evaluated longer follow-up, and 6 (16%) focused on safety and pharmacokinetics (Figure).
Figure. Key Data Limitations at the Time of FDA Accelerated Approval, Objectives of Postmarketing Requirements, and Timelines for Completion (N=22 Drugs).
FDA indicates US Food and Drug Administration; PK, pharmacokinetics.
aSee eTable 1 in the Supplement for a detailed description of data limitations statements included in the FDA labels at the time of accelerated approval.
bTrials could include expanded patient populations from those in the accelerated approval indication. See eTable 2 in the Supplement for a detailed description of postapproval confirmatory studies.
cTrials were long-term extensions of preapproval studies forming the basis of accelerated approval.
dTrials included both pharmacokinetics and safety evaluations.
Most requirements were for randomized clinical trials (n = 25 [66%]). The remaining 13 requirements (34%) were for single-group studies including long-term extensions of preapproval studies (eTable 2 in the Supplement). Prespecified primary end points were reported in 13 (34%) of the required confirmatory studies from publicly available documents. Among this sample, the most common prespecified end point was progression-free survival (n = 8), followed by overall survival (n = 2) (eTable 2).
Nineteen of 38 confirmatory study requirements (50%) had been fulfilled as of April 7, 2017. Of the remaining 19, 11 were under way according to planned timelines, 6 were reported to be delayed by more than 12 months, and 2 had been terminated (Figure). In most cases, recruitment challenges were cited as the primary reason for reported delays (eTable 2).
Features of Completed and Published Postapproval Confirmatory Studies
Published reports were available for 18 of 19 completed confirmatory studies. Ten (56%) of the completed and published postapproval confirmatory studies were randomized and 1 (6%) was double blind (Table 4). One study included a placebo comparator, 2 evaluated the accelerated approval agent as an add-on to a standard treatment regimen, 7 (39%) had active comparators, and 8 (44%) had no comparators (single-group studies). The majority (14 of 18 [78%]) of completed confirmatory studies included more than 100 participants; the median number of participants was 345 (interquartile range, 111-619).
Table 4. Characteristics and Findings of 18 Completed and Published Postapproval Confirmatory Studies of Drugs Receiving Accelerated Approvala.
| Agent | Participant Population | Design | Comparators | Enrollment, No. | Primary End Point | Magnitude of Benefitb |
|---|---|---|---|---|---|---|
| Bevacizumab | Newly diagnosed glioblastomae1 | Double-blind, placebo-controlled randomized trial | Group 1: bevacizumab + radiotherapy-temozolomide Group 2: placebo + radiotherapy-temozolomide |
921 | Progression-free survival and overall survival (co–primary end points) | Median progression-free survival: group 1, 10.6 mo; group 2, 6.2 mo; HR, 0.64 (95% CI, 0.55-0.74); P < .001 Median overall survival: group 1, 16.8 mo; group 2, 16.7 mo; HR, 0.88 (95% CI, 0.76-1.02); P = .10 |
| Ofatumumab | Untreated patients with chronic lymphocytic leukemiae2 | Add-on comparator, open-label randomized trial | Group 1: ofatumumab + chlorambucil Group 2: chrorambucil |
447 | Progression-free survival | Group 1: 22.4 mo; group 2: 13.1 mo; HR, 0.57 (95% CI, 0.45-0.72); P < .001 |
| Lapatinib | Human epidermal growth factor–positive metastatic breast cancere3 | Active-comparator, open-label randomized trial | Group 1: lapatinib + taxane Group 2: trastuzumab + taxane |
652 | Progression-free survival | Group 1: 9.0 mo; group 2: 11.3 mo; HR, 1.33 (95% CI, 1.06-1.67); P = .01 More deaths occurred with lapatinib vs trastuzumab: 102 vs 82; HR, 1.28 (95% CI, 0.95-1.72); P = .11 |
| Nilotinib | Newly diagnosed chronic-phase CMLe4 | Active-comparator, open-label randomized trial Long-term extension of preapproval study |
Group 1: nilotinib, 300 mg twice daily Group 2: nilotinib, 400 mg twice daily Group 2: imatinib, 400 mg once daily |
846 | Major molecular response | Group 1: 217 (77.0%); group 2: 217 (77.2%); group 3: 171 (60.4%) |
| Dasatinib | Newly diagnosed chronic-phase CMLe5 | Active-comparator, open-label randomized trial Long-term extension of preapproval study |
Group 1: dasatinib Group 2: imatinib |
519 | Complete cytogenetic response | Group 1: 28.0%; group 2: 26.0% (frequency data not reported) |
| Everolimusc | Tuberous sclerosis complex–related SEGAe6 | Single-group extension of trial | None | 111 | SEGA response rate | 64 (57.7%) |
| Tuberous sclerosis complex–related SEGAe7 | Single-group trial Long-term extension of preapproval study |
None | 28 | Change in SEGA volume | 0.50 (range, −0.74 to 9.84) cm3 | |
| Crizotinib | Locally advanced or metastatic ALK-positive lung cancer following 1 prior platinum-based regimene8 | Active-comparator, open-label randomized trial | Group 1: crizotinib Group 2: chemotherapy (pemetrexed or docetaxel) |
347 | Progression-free survival | Group 1: 7.7 mo; group 2: 3.0 mo; HR, 0.49 (95% CI, 0.37-0.64); P < .001 |
| Advanced ALK-positive nonsquamous non–small cell lung cancer without previous systemic treatment for advanced diseasee9 | Active-comparator, open-label randomized trial | Group 1: crizotinib Group 2: chemotherapy (pemetrexed + cisplatin or carboplatin) |
343 | Progression-free survival | Group 1: 10.9 mo; group 2: 7.0 mo; HR, 0.45 (95% CI, 0.35-0.60); P < .001 | |
| Everolimusd | Renal angiomyolipoma and diagnosis of tuberous sclerosis complex or lymphangioleimyomatosise10 | Single-group extension of trial Long-term extension of preapproval study |
None | 112 | Angiomyolipoma response rate | 60 (54.0%) |
| Carfilzomib | Relapsed multiple myeloma following 1-3 prior treatmentse11 | Add-on comparator, open-label randomized trial | Group 1: carfilzomib + lenalidomide + dexamethasone Group 2: lenalidomide + dexamethasone |
792 | Progression-free survival | Group 1: 26.3 mo; group 2: 17.6 mo; HR, 0.69 (95% CI, 0.57-0.83); P = .0001 |
| Relapsed or refractory multiple myeloma following 1-3 prior treatmentse12 | Active-comparator, open-label randomized trial | Group 1: carfilzomib + dexamethasone Group 2: bortezomib + dexamethasone |
929 | Progression-free survival | Group 1: 18.7 mo; group 2: 9.4 mo; HR, 0.53 (95% CI, 0.44-0.64); P < .0001 | |
| Omacetaxine mepessucinate | CML after ≥2 approved tyrosine kinase inhibitor therapiese13 | Post hoc pooled analysis of 2 single-group trials Long-term extension of preapproval study |
None | 111 | Major cytogenetic response and major hematologic response | Chronic-phase CML: 14 (18.0%); accelerated-phase CML: 5 (14.0%) |
| Ponatinib | Patients newly diagnosed as having chronic-phase CML, previously untreatede14 | Active-comparator, open-label randomized trial | Group 1: ponatinib Group 2: imatinib |
307 (23 remaining in study at 12 mo had had a molecular assessment) | Major molecular response | Group 1: 8 (80.0%); group 2: 5 (38.0%) Trial was terminated early following concerns about vascular adverse events observed in patients given ponatinib in other trials |
| Healthy adultse15 | Single-group crossover study | None | 20 | Pharmacokinetics | Statistically significant interaction with rifampin | |
| Healthy adultse16 | Single-group crossover study | None | 20 | Pharmacokinetics | Modest reduction in ponatinib concentration | |
| CML and Ph+ ALL after prior therapye17 | Single-group trial Long-term extension of preapproval study |
None | 449 | Major cytogenetic response and major hematologic response | Chronic-phase CML: 158 (59.0%); accelerated-phase CML: 51 (61.0%); blast-phase CML: 19 (31.0%); Ph+ ALL: 13 (41.0%) | |
| Ibrutinib | Relapsed or refractory mantle cell lymphomae18 | Single-group trial Long-term extension of preapproval study |
None | 111 | Overall response rate | 67.0% (Frequency data not reported) |
Abbreviations: ALK, anaplastic lymphoma kinase; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; HR, hazard ratio; Ph+, Philadelphia chromosome positive; Ph−, Philadelphia chromosome negative; SEGA, subependymal giant cell astrocytoma.
eCitations correspond to a list of supporting eReferences in the Supplement.
As reported in primary analysis in publication.
Tuberous sclerosis indication.
Renal angiomyolipoma and tuberous sclerosis complex indication.
Surrogate measures were the primary end points in 17 (94%) of the 18 studies. Disease response was the most common surrogate (n = 9 [50%]), followed by progression-free survival (n = 6 [33%]) and pharmacokinetic measures (n = 2 [11%]). The only confirmatory study that did not test a surrogate had co–primary end points of overall survival and progression-free survival.
Most completed postapproval studies showed that the drug had some benefit on the surrogate measure (15/18 [83%]), including 2 trials that evaluated pharmacokinetics (Table 4). The remaining 3 studies (18%) either failed to demonstrate efficacy or were terminated early. In a randomized clinical trial, the addition of bevacizumab to radiotherapy improved progression-free survival but did not extend overall survival in patients with glioblastoma multiforme. Lapatinib combined with taxane showed shorter progression-free survival compared with trastuzumab as first-line therapy for human epidermal growth factor–positive metastatic breast cancer. One of the required confirmatory studies of ponatinib among previously untreated patients with chronic myeloid leukemia was terminated early owing to higher rates of arterial occlusive events observed in patients receiving ponatinib in other trials.
Table 2 shows the comparison of preapproval and published postapproval trial characteristics. The proportion with randomized designs was not statistically significantly different before and after accelerated approval (12/30 vs 10/18; difference, 16%; 95% CI, −15% to 46%; P = .31). The confirmatory studies were more likely to use the surrogate measure of progression-free survival as the primary trial end point (1/30 vs 7/18; difference, 36%; 95% CI, 15%-56%; P = .001).
Regulatory Outcomes for Accelerated Approval Drugs
Of 24 indications treated by the drugs granted accelerated approval between 2009 and 2013, 10 (42%) fulfilled their postmarketing requirements and had their labels updated (Figure). All of the label updates were based on postmarketing studies evaluating surrogate measures. Label changes were supported by response rate in 6 indications (25%) and progression-free survival in 4 (17%).
Among the remaining 14 indications with no label updates, at least 1 of the confirmatory studies failed to demonstrate clinical benefit in 2 (8%) and at least 1 of the studies were terminated in 2 (8%). Studies for the remaining 10 indications remained ongoing, with 7 progressing according to target timelines and 3 reported to be delayed by more than 1 year.
The Figure shows the duration of time elapsed between accelerated approval and follow-up actions by our study end date. Time from accelerated approval to fulfillment of requirements ranged from 1.3 years to 5.3 years among the 10 indications for which the requirements were fulfilled. Time elapsed since accelerated approval was 5 years or more for 8 indications.
Discussion
The clinical trial evidence for therapeutic agents granted accelerated approval by the FDA between 2009 and 2013 shows that 14 of 24 indications for these drugs entered the market on the basis of single-intervention-group studies that enrolled a median of 132 patients, which some investigators would consider a small number. Half of required confirmatory studies were completed a minimum of 3 years after the approved drug was on the market. The quality and quantity of postmarketing studies required by the FDA to confirm clinical benefit varied widely across indications. There were few statistically detectable differences in the key design features of trials conducted before and after approval. Nonrandomized studies were common in the accelerated approval pathway both before (60%) and after (44%) market entry. Even though the majority of completed studies showed positive results in the postmarketing period, all completed confirmatory studies demonstrating drug benefit evaluated surrogate measures of disease activity rather than clinical outcomes.
Drugs granted accelerated approval receive market authorization on the basis of fewer studies, smaller patient populations, shorter follow-up, and less-established surrogate measures than drugs approved via the traditional pathway. In these cases, postapproval confirmation of clinical benefit is essential. For the 10 accelerated approvals between 2009 and 2013 that have since had their requirements fulfilled and labels updated, all of which were for cancer indications, the studies used to confirm clinical benefit tested surrogate measures. The FDA’s senior scientists consider overall survival to be the most dependable end point in clinical trials of cancer drugs. Yet overall survival was among the prespecified primary end points in only 5% of required confirmatory studies. Disease response was the most common end point in postapproval trials, and although disease response may be an appropriate surrogate measure in hematological malignancies, its adequacy depends on several factors, such as the magnitude and duration of effect. In the remaining cases, postmarketing requirements were fulfilled based on improvements in progression-free survival, which may not be a statistically validated surrogate for survival in all settings. These findings are consistent with previous research showing that cancer drugs approved on the basis of surrogate measures may not show survival benefit in the postmarketing period.
Another finding from the current study is the slow progression of some postapproval studies. A recent Government Accountability Office report criticized the FDA’s oversight of drugs approved on the basis of surrogate measures. Although the fulfillment of postmarketing commitments and requirements improved overall from 2009 to 2011, the number of studies with delays doubled during the same period. For 14 (58%) of 24 indications granted accelerated approval from 2009 to 2013, results from required confirmatory studies were not available after a median of 5 years of follow-up, and 8 (42%) of 19 incomplete confirmatory studies were either terminated or delayed by more than 1 year.
Confirmatory studies failed to demonstrate clinical benefit in 2 indications granted accelerated approval between 2009 and 2013. According to the Code of Federal Regulations, the FDA may withdraw a therapeutic agent if confirmatory studies fail to verify its clinical benefit. However, according to publicly available documents, the FDA has neither rescinded its approval nor imposed additional requirements for these 2 indications. Historically, the FDA has rarely withdrawn an indication during the 25 years since the accelerated approval pathway was established. For bevacizumab, which received accelerated approval in 2008 on the basis of progression-free survival for patients with metastatic breast cancer, the FDA later rescinded its approval for this indication after multiple postmarketing trials revealed no improvement in survival and increased toxicity.
Limitations
This study has several limitations. First, the analysis was limited to the preapproval and confirmatory studies presented to the FDA. There may be other studies that evaluated the clinical benefit of therapeutic agents granted accelerated approvals, but if those studies were rigorous and reflected strongly on the utility of the product, it is likely that the manufacturer would have presented them to the FDA and used them to contribute to any label updates. When safety-related postmarketing requirements under FDAAA Section 505(o)(3) generated efficacy data, this information was captured if it was used to inform label changes. These findings are supported by another large investigation of drugs approved on the basis of surrogate measures or single trials, which showed that postapproval studies rarely evaluate efficacy using clinical outcomes. Second, the adequacy of the confirmatory studies in addressing questions about the drugs that the FDA considered to be unresolved was not examined because such insights are not available from the FDA documents.
Third, the study examined a recent cohort of drugs that had received approvals, and the minimum 3 years of follow-up may not be adequate for completing some postapproval studies. However, the findings were consistent with a previous review of accelerated approvals in oncology, which showed a similar proportion of incomplete confirmatory studies. Fourth, this assessment focused on the trials’ sample size, comparators, end points, and findings. Data on other important characteristics, including risk of bias and trial duration, were not consistently reported in FDA documents and published reports. Fifth, the comparisons between preapproval and postapproval study characteristics are underpowered to detect statistically significant differences.
Conclusions
Among 22 drugs with 24 indications granted accelerated approval by the FDA in 2009-2013, 42% had efficacy confirmed in postapproval trials a minimum of 3 years after approval, although confirmatory trials and preapproval trials had similar design elements, including reliance on surrogate measures as outcomes.
eTable 1. Key Data Limitations at the Time of Accelerated Approval
eTable 2. Confirmatory Study Details, Status and Associated Regulatory Outcomes
eReferences
References
- 1.US Food and Drug Administration Guidance for Industry: Expedited Programs for Serious Conditions—Drugs and Biologics May 2014. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf. Accessed May 7, 2017.
- 2.New drug, antibiotic, and biological drug product regulations; accelerated approval—FDA: final rule. Fed Regist. 1992;57(239):58942-58960. [PubMed] [Google Scholar]
- 3.US Food and Drug Administration Postmarketing requirements and commitments. https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Post-marketingPhaseIVCommitments/default.htm. Accessed March 31, 2017.
- 4.Kesselheim AS, Wang B, Franklin JM, Darrow JJ. Trends in utilization of FDA expedited drug development and approval programs, 1987-2014: cohort study. BMJ. 2015;351:h4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downing NS, Aminawung JA, Shah ND, Braunstein JB, Krumholz HM, Ross JS. Regulatory review of novel therapeutics—comparison of 3 regulatory agencies. N Engl J Med. 2012;366(24):2284-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downing NS, Zhang AD, Ross JS. Regulatory review of new therapeutic agents—FDA vs EMA, 2011-2015. N Engl J Med. 2017;376(14):1386-1387. [DOI] [PubMed] [Google Scholar]
- 7.Downing NS, Shah ND, Aminawung JA, et al. . Postmarket safety events among novel therapeutics approved by the US Food and Drug Administration between 2001 and 2010. JAMA. 2017;317(18):1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming TR. Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood). 2005;24(1):67-78. [DOI] [PubMed] [Google Scholar]
- 9.Kim C, Prasad V. Cancer drugs approved on the basis of a surrogate end point and subsequent overall survival: an analysis of 5 years of us food and drug administration approvals. JAMA Intern Med. 2015;175(12):1992-1994. [DOI] [PubMed] [Google Scholar]
- 10.Pease AM, Krumholz HM, Downing NS, Aminawung JA, Shah ND, Ross JS. Postapproval studies of drugs initially approved by the FDA on the basis of limited evidence: systematic review. BMJ. 2017;357:j1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JR, Ning Y-M, Farrell A, Justice R, Keegan P, Pazdur R. Accelerated approval of oncology products: the Food and Drug Administration experience. J Natl Cancer Inst. 2011;103(8):636-644. [DOI] [PubMed] [Google Scholar]
- 12.Naci H, Wouters OJ, Gupta R, Ioannidis JPA. Timing and characteristics of cumulative evidence available on novel therapeutic agents receiving Food and Drug Administration accelerated approval. Milbank Q. 2017;95(2):261-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Accelerated approval. https://www.fda.gov/forpatients/approvals/fast/ucm405447.htm. Accessed June 30, 2017.
- 14.US Food and Drug Administration Accelerated and restricted approvals under Subpart H (drugs) and Subpart E (biologics). https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/ucm121597.htm. Accessed August 19, 2015.
- 15.Darrow JJ, Kesselheim AS. Drug development and FDA approval, 1938-2013. N Engl J Med. 2014;370(26):e39. [DOI] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed August 21, 2015.
- 17.US Food and Drug Administration Guidance for Industry: Postmarketing Studies and Clinical Trials—Implementation of Section 505(o)(3) of the Federal Food, Drug, and Cosmetic Act April 2011. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM172001.pdf. Accessed June 30, 2017.
- 18.US Food and Drug Administration CFR—Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=314&showFR=1&subpartNode=21:5.0.1.1.4.8. Accessed August 24, 2015.
- 19.US Government Accountability Office FDA Expedites Many Applications, But Data for Postapproval Oversight Need Improvement. January 14, 2016. https://www.gao.gov/products/GAO-16-192. Accessed July 7, 2017.
- 20.US Government Accountability Office New Drug Approval: FDA Needs to Enhance Its Oversight of Drugs Approved on the Basis of Surrogate Endpoints September 2009. http://www.gao.gov/new.items/d09866.pdf. Accessed August 24, 2015.
- 21.US National Library of Medicine ClinicalTrials.gov background. https://clinicaltrials.gov/ct2/about-site/background. Accessed August 20, 2015.
- 22.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344:d7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chinot OL, Wick W, Mason W, et al. . Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709-722. [DOI] [PubMed] [Google Scholar]
- 25.Gelmon KA, Boyle FM, Kaufman B, et al. . Lapatinib or trastuzumab plus taxane therapy for human epidermal growth factor receptor 2-positive advanced breast cancer: final results of NCIC CTG MA.31. J Clin Oncol. 2015;33(14):1574-1583. [DOI] [PubMed] [Google Scholar]
- 26.Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton JH, Chuah C, Guerci-Bresler A, et al. ; EPIC Investigators . Ponatinib vs imatinib for newly diagnosed chronic myeloid leukaemia: an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(5):612-621. [DOI] [PubMed] [Google Scholar]
- 28.McKee AE, Farrell AT, Pazdur R, Woodcock J. The role of the US Food and Drug Administration review process: clinical trial endpoints in oncology. Oncologist. 2010;15(suppl 1):13-18. [DOI] [PubMed] [Google Scholar]
- 29.US Food and Drug Administration Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics May 2007. https://www.fda.gov/downloads/drugsGuidanceComplianceRegulatoyInformation/Guidance/UCM071590.pdf. Accessed April 6, 2017.
- 30.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365(2):e3. [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration Guidance for Industry: Clinical Studies Section of Labeling for Human Prescription Drug and Biological Products—Content and Format January 2006. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM075059.pdf. Accessed June 30, 2017.
- 32.Fain K, Daubresse M, Alexander GC. The Food and Drug Administration Amendments Act and postmarketing commitments. JAMA. 2013;310(2):202-204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Key Data Limitations at the Time of Accelerated Approval
eTable 2. Confirmatory Study Details, Status and Associated Regulatory Outcomes
eReferences