Key Points
Question
Can propranolol be used as a first-line treatment for infantile hemangioma (IH)?
Findings
In this noninferiority randomized clinical trial of 34 infants, the treatment response rate in the propranolol group was 95.65%, and that of the steroid group was 91.94%. Because the difference in response rate between the groups was 3.71%, propranolol was considered noninferior to steroid in therapeutic effects in IH, and there was no difference between the groups in safety outcomes.
Meaning
Propranolol can be used as a first-line treatment for IH.
Abstract
Importance
There are limited data from randomized clinical trials comparing propranolol and steroid medication for treatment of infantile hemangioma (IH).
Objective
To determine the efficacy and safety of propranolol compared with steroid as a first-line treatment for IH.
Design, Setting, and Participants
This randomized clinical noninferiority trial tested the efficacy and safety of propranolol vs steroid treatment for IH at a single academic hospital. All participants were diagnosed with IH between June 2013 and October 2014, had normal heart function, and had not been previously treated for IH.
Interventions
The participants were randomly assigned to either the propranolol group or the steroid group. In the propranolol group, the patients were admitted, observed for adverse effects for 3 days after treatment initiation, and then released and treated as outpatients for 16 weeks (2 mg/kg/d). In the steroid group, the patients were seen as outpatients from the beginning and were also treated for 16 weeks (2 mg/kg/d).
Main Outcomes and Measures
The primary efficacy variable was the response to treatment at 16 weeks, which was evaluated by the hemangioma volume using magnetic resonance imaging before and at 16 weeks after treatment initiation. While comparing the effect of medication between the groups, we monitored the adverse effects of both drugs.
Results
A total of 34 patients (15 boys, 19 girls; mean age, 3.3 months; range, 0.3-8.2 months) were randomized to receive either propranolol or steroid treatment (17 in each treatment group). Guardians for 2 patients in the steroid group withdrew their consent, and 1 patient in the propranolol group did not complete the efficacy test. The intention-to-treat analysis, applying multiple imputations, found the treatment response rate in the propranolol group to be 95.65%, and that of the steroid group was 91.94%. Because the difference in response rate between the groups was 3.71%, propranolol was considered noninferior. We found that there was no difference between the groups in safety outcomes.
Conclusions and Relevance
Our trial demonstrated that propranolol was not inferior to steroid with respect to therapeutic effects in IH.
Trial Registration
clinicaltrials.gov Identifier: NCT01908972
This randomized clinical trial compares propranolol vs steroid therapies for efficacy and safety in the treatment of infantile hemangioma.
Introduction
Infantile hemangioma (IH) is the most common tumor of infants and young children. Although small IH lesions typically do not cause problems, they often require treatment because of the site of occurrence and associated complications such as abnormal eye development or vision problems, airway obstruction, and bowel obstruction. Historically, steroids have been used as the primary treatment for IH. Steroids have been shown to be antiangiogenic in a number of in vitro settings and also have shown good therapeutic effects clinically. However, the use of steroids may lead to various complications including gastroesophageal reflux and growth disorders, although these complications are associated with long-term use and high dose. A type of anticancer drug or immunomodulator, interferon alfa, may be used for severe IH in cases where patients did not respond to steroids. However, interferon alfa also has several possible adverse effects, including fever, muscle pain, systemic myalgia, and in severe cases, liver damage, blood toxic effects, thyroid hormonal abnormality, and neurological and neurodevelopmental toxic effects. Because of concerns about these adverse effects, many guardians of pediatric patients prefer to wait rather than accept treatment.
A study published in 2008 reported that an infant with IH showed signs of improvement after receiving the β-blocker propranolol. After the study, many centers conducted studies and published reports about the use of propranolol for IH. Although propranolol has been used worldwide as an effective treatment for IH, the majority of the studies conducted on this off-label use of the drug have been insufficient to determine the efficacy and safety of propranolol compared with steroid. In 2015, a randomized clinical trial verified the effect of propranolol. However, the study results did not provide evidence that propranolol could be used as a first-line treatment. Therefore, the purpose of this study was to determine the efficacy and safety of propranolol compared with steroid as a first-line treatment for IH by randomized clinical trial.
Methods
The trial protocol is provided as Supplement 1.
Study Population
Among patients aged 0 to 9 months diagnosed with IH at the Seoul National University Hospital, those with normal heart function and who had never been treated for IH were the target population. At least 1 of 3 IH characteristics was required for patient inclusion in the study: 10% to 20% volume increase in 2-4 weeks, IH-related organ dysfunction, and IH-related aesthetic problem.
Study Oversight
This study was a single-institution, randomized, clinical, noninferiority trial to test the efficacy and safety of propranolol vs steroid for IH treatment. In this study, the steroid group was set as the control group to evaluate the noninferiority of the experimental group (propranolol). The treatment response after 16 weeks of treatment was used to determine the therapeutic index. Volume of the IH lesions was measured using magnetic resonance imaging (MRI).
We received institutional review board approval from Seoul National University Hospital (H-1212-016-446) and approval for an investigational new drug from the Korea Food & Drug Administration (20130047039). Guardians of all participants provided their written informed consent.
Study Design
Eligible patients were randomly assigned at a 1:1 ratio to the propranolol group or steroid group. The Medical Research Collaborating Center at Seoul National University Hospital generated a randomization list and implemented randomization using an interactive web-based system.
We measured the IH lesion volume using MRI while the patient was under sedation with oral chloral hydrate (50 mg/kg) prior to treatment initiation. The anteroposterior diameter, width, and height were measured from T2 axial and coronal images. We took medical photographs of all lesions and recorded the surface area, color, ulceration, and reepithelialization.
Propranolol Group
In the experimental group, we used a powdered form of propranolol, total daily dose 2 mg/kg/d (prepared from Indenol, 10-mg tablets) divided into 3 daily oral administrations. Children who met the baseline criteria were admitted to the hospital. The treatment dose was gradually reached through the induction treatment schedule. One hour after treatment initiation, we measured vital signs and glucose levels and monitored whether the patient showed any of the following complications: decreased heart rate, low blood pressure, hypoglycemia, or difficulty breathing. After 3 days, the patient was released from the hospital and followed up as an outpatient 1, 4, 8, 12, 16, and 20 weeks after initial treatment.
After 16 weeks, the drug dose was adjusted by the tapering schedule. In cases where the child’s guardian requested treatment or treatment was required because of remaining IH, the study protocol was not followed, and the researcher used discretion to adjust the drug dose, depending on the therapeutic response. In cases where a complication was observed, treatment advice from a pediatrician was sought immediately.
Steroid Group
The control group was observed throughout the study as outpatients. We used a single daily dose of an orally administered prednisolone syrup (PRD Suspension, 1 mg/mL syrup), 2 mg/kg/d. One hour after the treatment initiation, vital signs and glucose levels were measured, and signs of complications were monitored. The patient observation protocol, drug-tapering regimen, and posttreatment management were the same as for the experimental group.
Outcome Measures
The primary efficacy variable was the clinical response at 16 weeks, classified as follows: when the volume did not increase or decreased by less than 25% after treatment began, we defined it as stop of progression; when the volume decreased by 25% or more compared with the original size, we defined it as regression. Both stop of progression and regression were defined as reaction. If the volume at the primary efficacy evaluation point was greater than the size measured when treatment started, we called it an increase. Increase was defined as a nonreaction.
The secondary efficacy variables included volume change, surface area, and color of the IH lesion; the presence of ulceration; presence of reepithelialization; progression stop point; regression point; and treatment compliance. The independent, centralized, blinded evaluations of standardized photographs were performed at every visit. Once a week, the patient’s guardian measured and recorded the surface area of the IH lesions and ulceration size even if they did not come to the hospital.
For safety evaluation, we checked heart rate, blood pressure, glucose level, and the presence of any complication such as gastroesophageal reflux, hypertension, growth disability, and adverse event.
Statistical Analysis
The primary efficacy variable was the proportion of reaction at 16 weeks. The planned sample size allowing for a 10% dropout rate was 34 patients to show the noninferiority of propranolol treatment with 80% power. We assumed the reaction rates of the propranolol group and steroid group to be 85% and 65%, respectively. The noninferiority margin was set at −20%. To show that the reaction rate in the propranolol group was noninferior to that in the steroid group, a noninferiority analysis with a 1-sided test was applied at the 0.025 level; that is, the hypothesis of inferiority would be rejected in the lower boundary of the 95% confidence interval (CI) for pT-pC (experimental group’s treatment reaction rate − control group’s treatment reaction rate) if it was larger than −20%. The primary analysis was based on the intention-to-treat (ITT) population, and missing outcomes were imputed using a multiple imputation (MI) method. The variables included in the imputation model were the surface area of the IH lesion, the progression stop point, regression time point, color, ulceration size, and presence of reepithelialization. Sensitivity analyses were performed to assess the effect of missing values on the primary outcome in 2 ways: imputing all missing values as reaction and as nonreaction. The analysis based on the per protocol (PP) population was performed as a sensitivity analysis to determine whether the PP analysis provided results consistent with those of the ITT analysis. The exact CI for differences between groups was estimated using R software (R for Windows, version 3.1.2/R package–ExactCIdiff) when the proportion of reaction was 100%.
The secondary efficacy variables were the percent changes in volume of the IH lesions at 16 weeks from baseline, surface area and color of the IH, presence of ulceration and ulceration size, presence of reepithelialization, the point of progression stop, regression, and treatment compliance. The variables measured repeatedly at 0, 1, 4, 8, 12, 16, and 20 weeks were analyzed using a generalized estimating equation model in which the effects of treatment, time, and the interaction between treatment and time were assessed after adjusting values at screening. The time to progression stop or regression was compared between the 2 study groups using the Kaplan-Meier method and log-rank test.
During the trial, the number and percentage of participants who experienced at least 1 adverse event were recorded for each event according to treatment, and were tested using χ2 test or Fisher exact test. Information regarding the extent of adverse events, result, causality, and related measures also was arranged by treatment group.
Results
Participants
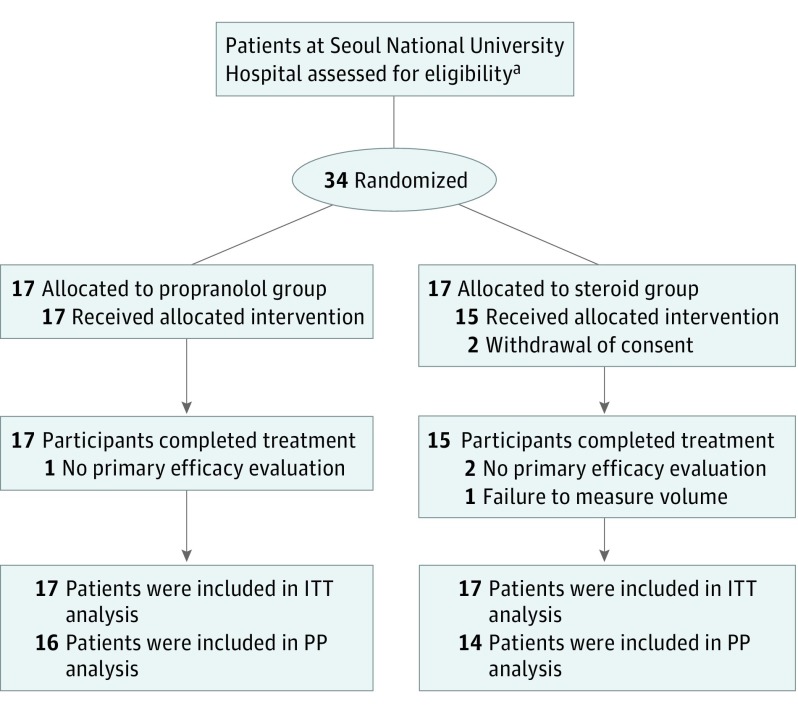
The participants were enrolled from June 2013 to October 2014. The enrollment flowchart is presented in the Figure. Thirty-four patients were enrolled and randomly assigned to either the propranolol group (17 patients) or steroid group (17 patients). The guardians for 2 patients withdrew their consent in the steroid group, and 1 patient in the propranolol did not complete the efficacy test. For 1 patient in the steroid group, the radiologist reported that it was impossible to measure the volume of the IH lesion because of the unclear boundaries between the IH and normal tissue. The efficacy evaluation was conducted in the PP analysis group, which included 30 target participants (16 in the propranolol group, 14 in the steroid group). The safety evaluation was conducted in the safety population, which included 33 patients (17 in the propranolol group, 16 in the steroid group).
Figure. Study Flowchart Illustrating Randomization, Treatment, Follow-up, and Data Analysis of the Study Participants.
PP indicates per protocol; ITT, intention to treat. Note that the efficacy evaluation was conducted in the PP analysis group, which included 30 participants (16 in the propranolol group and 14 in the steroid group). The safety evaluation was conducted in the safety population, which included 33 participants (17 in the propranolol group and 16 in the steroid group).
aNumber of patients not available.
As detailed in Table 1, none of the demographic differences between groups was statistically significant at below the 5% level (P < .05). For both groups, the face was the most common location of the IH (10 cases for the propranolol group and 13 for the steroid group). The MRI scans were conducted for all patients at baseline, and the mean IH volume was 14 125 mm3 for the propranolol group and 9349 mm3 for the steroid group. Although the mean volume for the steroid group was smaller, there was no significant difference (P = .33). An image of the lesion was taken for all participants in each group, and the mean area for the propranolol group was 1318 mm2, while the mean area for the steroid group was 1093 mm2. As with the volume, there was no statistically significant difference between the groups (P > .99).
Table 1. Demographic and Baseline Clinical Characteristics of the Study Patientsa.
| Characteristics | Propranolol Group (n = 17) |
Steroid Group (n = 17) |
P Value |
|---|---|---|---|
| Patients | |||
| Age, mo | 3.6 (0.3-8.2) | 3.0 (0.8-8.0) | .37b |
| Boys, No. (%) | 7 (41) | 8 (47) | .73c |
| Weight, kg | 6.45 (1.73) | 6.02 (1.68) | .48b |
| Height, cm | 61.75 (6.05) | 60.51 (5.96) | .55b |
| Blood pressure, mm Hg | |||
| Systolic | 88 (80-112) | 91 (76-108) | .40d |
| Diastolic | 49.82 (10.76) | 50.59(10.69) | .84b |
| Heart rate, beats/min | 128 (109-167) | 140 (118-160) | .11d |
| Respiration rate, breaths/min | 36 (30-40) | 36 (32-44) | .86d |
| Body temperature, °C | 36.89 (0.41) | 36.93 (0.38) | .80b |
| Hemangiomas | |||
| Location, No. (%) of patients | |||
| Scalp | 1 (6) | 2 (12)e | >.99f |
| Face | 10 (59)g | 13 (76) | .46f |
| Chest | 2 (12)g | 0 | .48f |
| Abdomen | 1 (6) | 0 | >.99f |
| Back | 1 (6) | 0 | >.99f |
| Upper extremity | 3 (18) | 2 (12) | >.99f |
| Lower extremity | 0 | 1 (6)e | >.99f |
| Size | |||
| Volume, mm3 by MRI | 14 125.35 (18 246.77) | 9349.54 (16 015.69) | .33d |
| Surface area, mm2 | 1318.06 (1833.07) | 1093.51 (1316.68) | >.99d |
| Height, mm | 4.26 (2.22) | 3.71 (2.49) | .50b |
| Color, No. (%) of patients | |||
| Red | 11 (65) | 14 (82) | .48f |
| Purple | 2 (12) | 1 (6) | NR |
| Blue | 1 (6) | 0 | NR |
| Gray | 0 | 1 (6) | NR |
| Apricot | 0 | 0 | NR |
| Other | 3 (18) | 1 (6) | NR |
| Purple/blue | 1 (6) | 0 | NR |
| Red/blue | 0 | 1 (6) | NR |
| Red to purple | 1 (6) | 0 | NR |
| Reddish purple | 1 (6) | 0 | NR |
| Ulcer, No. (%) of patients | |||
| Yes | 1 (6) | 1 (6) | >.99f |
| Reepithelization, No. (%) of patients | |||
| Yes | 2 (12) | 0 | .48f |
Abbreviations: MRI, magnetic resonance imaging, NR, not reported.
Unless otherwise indicated, data are reported as mean (range) or mean (SD).
Independent t test.
χ2 Test.
Wilcoxon rank-sum test.
Two lesions in 1 patient in the propranolol group (both the scalp and lower extremity).
Fisher exact test.
Two lesions in 1 patient in the steroid group (both the face and chest).
Efficacy Outcomes
In the ITT analysis after applying MI, propranolol was not inferior to steroid with respect to the proportion of reaction (95.65% vs 91.94%, a difference of 3.71%; 95% CI, −15.43% to 22.84%). In addition, sensitivity analyses showed results consistent with ITT analysis (Table 2).
Table 2. Efficacy Outcomes.
| Characteristic | Propranolol Group | Steroid Group | pT-pC (95% CI) | P Value |
|---|---|---|---|---|
| Primary Efficacy Evaluation | ||||
| ITT analysis group applying MI, % | ||||
| Reaction | 95.65 | 91.94 | 3.71 (−15.43 to 22.84) | NR |
| Nonreaction | 4.35 | 8.06 | ||
| Missing values replaced with “nonreaction,” No. (%) of patients | ||||
| Reaction | 16 (94) | 14 (82) | 11.76 (−9.53 to 33.06) | NR |
| Nonreaction | 1 (6) | 3 (18) | ||
| Missing values replaced with “reaction,” No. (%) of patients | ||||
| Reaction | 17 (100) | 17 (100) | 0 (−18.65 to 18.65)a | NR |
| Nonreaction | 0 | 0 | ||
| PP population, No. (%) of patients | ||||
| Reaction | 16 (100) | 14 (100) | 0 (−20.59 to 23.16)a | NR |
| Nonreaction | 0 | 0 | ||
| Secondary Efficacy Evaluationb | ||||
| Change in hemangioma volume, mean (SD), % | −55.87 (18.92) | −46.52 (26.24) | NR | .27c |
| Progression stop and regression point | ||||
| Cases, No. | 17 | 15 | NR | .34d |
| Median time, d | 12 | 11 | ||
| Regression point | ||||
| Cases, No. | 13 | 9 | NR | .53d |
| Median time, d | 62 | 120 | ||
| Compliance | ||||
| Duration of medication, mean (SD), d | 120.29 (24.99) | 128.53 (25.86) | NR | .44e |
| Outpatient visits, mean (SD), No. | 7.00 (0.00) | 6.29 (1.99) | NR | .16e |
Abbreviations: ITT, intention to treat; MI, multiple imputation; NR, not reported; PP, per protocol; pT-pC, experimental group’s treatment reaction rate − control group’s treatment reaction rate.
Exact confidence interval.
A complete report of secondary efficacy evaluation data is provided in Supplement 2.
Independent t test.
Log-rank test.
Wilcoxon rank-sum test.
The volume reduction in the propranolol group (55.87%) was greater than in the steroid group (46.52%), but the difference was not statistically different (P = .27). The surface area of the IH after treatment initiation significantly decreased over time in both groups (model-estimated average over time, 1013.6, 960.3, 917.6, 832.7, 781.9 and 736.8 mm2 at 1, 4, 8, 12, 16, and 20 weeks after initial treatment; P = .02), although there was no significant difference between groups (13.2 mm2; 95% CI, −269.1 to 242.6 mm2; P = .92). As detailed in eFigure 1 in Supplement 2, there were no significant differences between the treatment groups and over time in other secondary efficacy evaluations such as IH color, presence of reepithelialization, presence of ulceration, size of ulceration, and medication administration.
The numbers of patients showing progression stop or regression were 17 and 15 in the propranolol group and the steroid group, respectively. The median time to progression stop or regression was 12 days in the propranolol group (95% CI, 11-15 days) and 11 days in the steroid group (95% CI, 10-14 days) after treatment initiation. The occurrence of progression stop or regression over time was not significantly different between the groups (P = .34). When considering regression to be a decrease of at least 25%, 13 and 9 patients experienced regression in the propranolol and steroid groups, respectively, with median respective times of 62 and 120 days after treatment initiation. The occurrence of regression over time was not significantly different between the groups (P = .53).
Safety Outcomes
Safety analysis was conducted for the remaining 33 patients (safety population). The heart rate (131.88 vs 147.63 bpm; P = .003), body temperature (36.66°C vs 36.96°C; P = .04), and blood glucose level (103 vs 121 mg/dL; P = .002) after initial medication administration in the propranolol group were significantly lower than those in the steroid group. Two patients in steroid group showed growth disability. As detailed in Table 3, there was no significant difference between the groups in safety outcomes.
Table 3. Adverse Events in the Safety Population.
| Variable | Study Participants, No. (%) | P Value | |
|---|---|---|---|
| Propranolol Group (n = 17) |
Steroid Group (n = 16) |
||
| Adverse events | |||
| Serious adverse event | 0 | 0 | |
| Any adverse event | 16 (94) | 15 (94) | >.99a |
| Known risks associated with drugsb | |||
| Bradycardia | 0 | 0 | |
| Hypotension | 5 (29) | 1 (7) | .18a |
| Hypoglycemia | 0 | 0 | |
| Trouble breathing | 0 | 0 | |
| Gastroesophageal reflux | 0 | 0 | |
| Hypertension | 7 (41) | 7 (47) | .75c |
| Growth disability | 0 | 2 (13) | .21a |
One or more adverse events were observed in 31 patients (16 in the propranolol group and 15 from the steroid group; P > .90 for the difference between the groups). In the propranolol group, there were 70 adverse events across 16 patients, and in the steroid group, there were 60 adverse events across 15 patients. No serious adverse events occurred. The adverse events that occurred were minor and not related to the treatment.
Discussion
After propranolol was introduced for IH treatment in 2008, it has been used worldwide because it is considered more effective and safer than steroid treatment. However, there has been limited evidence for propranolol’s efficacy and safety because of the lack of randomized clinical trials. Very recently, 2 randomized clinical trials for propranolol and steroid were reported. However, they were not designed as confirmative studies: the sample size was not predetermined for enough statistical power, and inflation of type I error in the studies was not considered. In addition, the studies had a high dropout rate: only 11 of 30 patients in the one trial and 11 of 19 in the other completed treatment. Moreover, the steroid group had a higher dropout rate in both trials (6 patients of 10 patients in one study, 6 patients of 8 patients in the other). Another well-planned, randomized clinical trial was reported very recently. However, the authors could not prove that propranolol can be used as a first-line treatment for IH because propranolol was compared with placebo. Moreover, only 19 of 55 patients completed treatment in the placebo group.
We enrolled 34 patients to show noninferiority of propranolol and made the minimum dropout rate. We had set the reaction rate based on response rates in published studies that used the same primary efficacy variables. However, the reaction rate in the steroid group was higher than expected. We thought that the reaction rate, which was evaluated by IH volume, could not exactly match the results of previous studies that evaluated the surface area of IH. The sensitivity for the reaction seemed to be higher when we used volumetrics to measure response.
To our knowledge, this is the first well-designed randomized clinical trial evaluating the efficacy and safety of propranolol compared with steroid in the treatment of IH. The first strength is that this study was performed in a single center; therefore, all participants received their treatments in the same environment. The second strength is that we used MRI to measure IH lesion volume. Most clinical trials use photography and measure surface area of the IH because MRI has cost and convenience issues even though it is most useful for IH evaluation. Moreover, the radiologist evaluated the volume independently and blindly. Third, this study had a very low dropout rate compared with other studies. It is very difficult to get permission from parents because the participants are very young infants. Moreover, young parents have knowledge about IH and treatment. As a result, they already prefer propranolol treatment over the use of steroid. We overcame these circumstances by cordial, thoughtful explanation of the experiment before permission was obtained and during follow-up. In addition, there were no serious complications or adverse events observed during the trial. Other studies have reported that adverse effects greatly influenced the dropout rate.
This trial showed that the therapeutic effects of propranolol were not inferior to that of steroid. In addition, we found that there was no significant difference between the 2 groups in terms of safety outcomes. The 2 previously published randomized clinical trials conducted on the use of propranolol and steroid showed conflicting results regarding the efficacy and speed of treatment response. In one study, propranolol was shown to have better efficacy: the adverse effects profile and speed of treatment response were both superior. However, in another study, both medications showed similar efficacy, and propranolol had significantly fewer severe adverse events, although the treatment response to steroid was faster. We believe that our results provide stronger evidence of the noninferiority of propranolol for treatment of IH.
Limitations
Although the sample size in the present study was predetermined to show noninferiority, it is difficult to provide strong evidence of safety outcomes. According to a recent meta-analysis, the most commonly reported adverse effects of steroids were altered growth and moon facies, and the incidence of overall adverse effects was approximately 17.6%. The most common adverse effects of propranolol were hypotension, bradycardia, and hypoglycemia, and it was reported that about 13.7% of patients experienced adverse effects with propranolol therapy. The incidence of complications varied among studies and depended on the definition of complication. As safety cannot be assessed based on the complication rate alone, physicians must make appropriate clinical decisions.
There are other factors that should be considered when using propranolol. Physicians should consider complications, hospital admission, vital sign monitoring, and cost before administering propranolol, even though many centers are now eliminating inpatient initiation and reducing monitoring, making the costs comparable to those for steroid use. The concerns about neurocognitive issues caused by propranolol have led to atenolol being considered as an alternative for the treatment of IH. While there was little published research about the use of atenolol for IH at the time the present trial was ongoing, several studies have since been reported. The risk of pulmonary adverse effects and hypoglycemia are decreased because of the characteristics of atenolol as a selective β1-blocker. Moreover, atenolol is less likely to produce central nervous system–related adverse effects. However, studies in patients with IH are lacking. We believe that further studies on the use of atenolol as a treatment for IH are needed.
The present study was not a double-blind trial. Since only the patients in the propranolol group were hospitalized for the study, each was aware of the group in which they were placed. We chose to give up a double-blind trial to be faithful to each drug protocol. And it was difficult to evaluate safety outcomes based solely on this study. Another limitation was that we did not evaluate which dose would be most effective in this study, and instead used 2 mg/kg/d for both drugs because this dose was used in the current literature. Some studies have reported that 3 mg/kg/d is the best dose of steroid for treating IH, but no randomized clinical trials have been conducted to confirm the dose-response relationship for steroids. However, a randomized clinical trial found propranolol to be more effective at 3 mg/kg/d than at 1 mg/kg/d. Although increasing the dose typically yields a better response rate, it also could increase the likelihood of complications.
Conclusions
Our trial demonstrated that propranolol was not inferior to steroid with respect to therapeutic effects in IH.
Trial Protocol
eTable 1 through eTable 32. Various data reports
eFigure 1. Secondary efficacy variables
eReferences.
References
- 1.Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25(2):168-173. [DOI] [PubMed] [Google Scholar]
- 2.Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170(4):907-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions: proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22(5):383-406. [DOI] [PubMed] [Google Scholar]
- 4.Haggstrom AN, Drolet BA, Baselga E, et al. ; Hemangioma Investigator Group . Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. J Pediatr. 2007;150(3):291-294. [DOI] [PubMed] [Google Scholar]
- 5.Zarem HA, Edgerton MT. Induced resolution of cavernous hemangiomas following prednisolone therapy. Plast Reconstr Surg. 1967;39(1):76-83. [DOI] [PubMed] [Google Scholar]
- 6.Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985;230(4732):1375-1378. [DOI] [PubMed] [Google Scholar]
- 7.George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Arch Dermatol. 2004;140(8):963-969. [DOI] [PubMed] [Google Scholar]
- 8.Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326(22):1456-1463. [DOI] [PubMed] [Google Scholar]
- 9.Wasserman JD, Mahant S, Carcao M, Perlman K, Pope E. Vincristine for successful treatment of steroid-dependent infantile hemangiomas. Pediatrics. 2015;135(6):e1501-e1505. [DOI] [PubMed] [Google Scholar]
- 10.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649-2651. [DOI] [PubMed] [Google Scholar]
- 11.Léauté-Labrèze C, Taïeb A. Efficacy of beta-blockers in infantile capillary haemangiomas: the physiopathological significance and therapeutic consequences [in French]. Ann Dermatol Venereol. 2008;135(12):860-862. [DOI] [PubMed] [Google Scholar]
- 12.Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics. 2009;124(3):e423-e431. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann AP, Wiegand S, Werner JA, Eivazi B. Propranolol therapy for infantile haemangiomas: review of the literature. Int J Pediatr Otorhinolaryngol. 2010;74(4):338-342. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz RA, Sidor MI, Musumeci ML, Lin RL, Micali G. Infantile haemangiomas: a challenge in paediatric dermatology. J Eur Acad Dermatol Venereol. 2010;24(6):631-638. [DOI] [PubMed] [Google Scholar]
- 15.Buckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. Laryngoscope. 2010;120(4):676-681. [DOI] [PubMed] [Google Scholar]
- 16.Price CJ, Lattouf C, Baum B, et al. Propranolol vs corticosteroids for infantile hemangiomas: a multicenter retrospective analysis. Arch Dermatol. 2011;147(12):1371-1376. [DOI] [PubMed] [Google Scholar]
- 17.Bertrand J, McCuaig C, Dubois J, Hatami A, Ondrejchak S, Powell J. Propranolol versus prednisone in the treatment of infantile hemangiomas: a retrospective comparative study. Pediatr Dermatol. 2011;28(6):649-654. [DOI] [PubMed] [Google Scholar]
- 18.Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128(2):e259-e266. [DOI] [PubMed] [Google Scholar]
- 19.Holmes WJ, Mishra A, Gorst C, Liew SH. Propranolol as first-line treatment for rapidly proliferating infantile haemangiomas. J Plast Reconstr Aesthet Surg. 2011;64(4):445-451. [DOI] [PubMed] [Google Scholar]
- 20.Park YW, Yeom KB, Choi JW, Kim DY, Shin H, Kim KH. Effect of propranolol on the treatment of infantile hemangiomas: a single tertiary center 3-year experience. J Dermatolog Treat. 2014;25(5):391-395. [DOI] [PubMed] [Google Scholar]
- 21.Izadpanah A, Izadpanah A, Kanevsky J, Belzile E, Schwarz K. Propranolol versus corticosteroids in the treatment of infantile hemangioma: a systematic review and meta-analysis. Plast Reconstr Surg. 2013;131(3):601-613. [DOI] [PubMed] [Google Scholar]
- 22.Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Double-blind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants <4 months of age. Br J Dermatol. 2013;169(1):181-183. [DOI] [PubMed] [Google Scholar]
- 23.Malik MA, Menon P, Rao KL, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: a randomized controlled study. J Pediatr Surg. 2013;48(12):2453-2459. [DOI] [PubMed] [Google Scholar]
- 24.Bauman NM, McCarter RJ, Guzzetta PC, et al. Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2014;140(4):323-330. [DOI] [PubMed] [Google Scholar]
- 25.Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372(8):735-746. [DOI] [PubMed] [Google Scholar]
- 26.Bagazgoitia L, Torrelo A, Gutiérrez JC, et al. Propranolol for infantile hemangiomas. Pediatr Dermatol. 2011;28(2):108-114. [DOI] [PubMed] [Google Scholar]
- 27.Artman M, Grayson M, Boerth RC. Propranolol in children: safety-toxicity. Pediatrics. 1982;70(1):30-31. [PubMed] [Google Scholar]
- 28.Love JN, Sikka N. Are 1-2 tablets dangerous? beta-blocker exposure in toddlers. J Emerg Med. 2004;26(3):309-314. [DOI] [PubMed] [Google Scholar]
- 29.Love JN, Howell JM, Klein-Schwartz W, Litovitz TL. Lack of toxicity from pediatric beta-blocker exposures. Hum Exp Toxicol. 2006;25(6):341-346. [DOI] [PubMed] [Google Scholar]
- 30.Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163(2):269-274. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki GH, Pang CY, Wittliff JL. Pathogenesis and treatment of infant skin strawberry hemangiomas: clinical and in vitro studies of hormonal effects. Plast Reconstr Surg. 1984;73(3):359-370. [DOI] [PubMed] [Google Scholar]
- 32.Enjolras O, Riche MC, Merland JJ, Escande JP. Management of alarming hemangiomas in infancy: a review of 25 cases. Pediatrics. 1990;85(4):491-498. [PubMed] [Google Scholar]
- 33.Bennett ML, Fleischer AB Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol. 2001;137(9):1208-1213. [DOI] [PubMed] [Google Scholar]
- 34.Ratitch B, Lipkovich I, O’Kelly M Combining analysis results from multiply imputed categorical data: PharmaSUG 2013. –paper SP03. http://pharmasug.org/proceedings/2013/SP/PharmaSUG-2013-SP03.pdf). Accessed March 9, 2017.
- 35.Ábarzúa-Araya A, Navarrete-Dechent CP, Heusser F, Retamal J, Zegpi-Trueba MS. Atenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled study. J Am Acad Dermatol. 2014;70(6):1045-1049. [DOI] [PubMed] [Google Scholar]
- 36.Zaher H, Rasheed H, El-Komy MM, et al. Propranolol versus captopril in the treatment of infantile hemangioma (IH): a randomized controlled trial. J Am Acad Dermatol. 2016;74(3):499-505. [DOI] [PubMed] [Google Scholar]
- 37.Aly MM, Hamza AF, Abdel Kader HM, Saafan HA, Ghazy MS, Ragab IA. Therapeutic superiority of combined propranolol with short steroids course over propranolol monotherapy in infantile hemangioma. Eur J Pediatr. 2015;174(11):1503-1509. [DOI] [PubMed] [Google Scholar]
- 38.Raphaël MF, de Graaf M, Breugem CC, Pasmans SG, Breur JM. Atenolol: a promising alternative to propranolol for the treatment of hemangiomas. J Am Acad Dermatol. 2011;65(2):420-421. [DOI] [PubMed] [Google Scholar]
- 39.de Graaf M, Raphael MF, Breugem CC, et al. Treatment of infantile haemangiomas with atenolol: comparison with a historical propranolol group. J Plast Reconstr Aesthet Surg. 2013;66(12):1732-1740. [DOI] [PubMed] [Google Scholar]
- 40.Greene AK, Couto RA. Oral prednisolone for infantile hemangioma: efficacy and safety using a standardized treatment protocol. Plast Reconstr Surg. 2011;128(3):743-752. [DOI] [PubMed] [Google Scholar]
- 41.Chim H, Gosain AK. Discussion: oral prednisolone for infantile hemangioma: efficacy and safety using a standardized treatment protocol. Plast Reconstr Surg. 2011;128(3):753-754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1 through eTable 32. Various data reports
eFigure 1. Secondary efficacy variables
eReferences.