Key Points
Question
Can targeted sequencing of 13 kindreds with bathing suit ichthyosis (BSI) reveal novel mutations and provide evidence of temperature sensitivity of specific TGM1 mutations?
Findings
We report 1 novel TGM1 indel mutation (Ile469_Cys471delinsMetLeu) and 8 TGM1 missense mutations that have not been previously found in BSI: 5 have been previously described in non–temperature-sensitive forms of congenital ichthyosis, and 3 are novel mutations. We also provide evidence for temperature sensitivity of Arg264Trp, Arg286Gln, Arg307Gly, Arg315Leu, Arg315His, and Phe495Leu, highlighting the importance of these residues in the pathogenesis of BSI.
Meaning
Our findings expand the genotypic spectrum of BSI.
Abstract
Importance
Bathing suit ichthyosis (BSI) is a rare congenital disorder of keratinization characterized by restriction of scale to sites of relatively higher temperature such as the trunk, with cooler areas remaining unaffected. Fewer than 40 cases have been reported in the literature. Bathing suit ichthyosis is caused by recessive, temperature-sensitive mutations in the transglutaminase-1 gene (TGM1). Clear genotype-phenotype correlations have been difficult to establish because several of the same TGM1 mutations have been reported in BSI and other forms of congenital ichthyosis. We identify novel and recurrent mutations in 16 participants with BSI.
Objective
To expand the genotypic spectrum of BSI, identifying novel TGM1 mutations in patients with BSI, and to use BSI genotypes to draw inferences about the temperature sensitivity of TGM1 mutations.
Design, Setting, and Participants
A total of 16 participants with BSI from 13 kindreds were identified from 6 academic medical centers. A detailed clinical history was obtained from each participant, including phenotypic presentation at birth and disease course. Each participant underwent targeted sequencing of TGM1.
Main Outcomes and Measures
Phenotypic and genotypic characteristics in these patients from birth onward.
Results
Of the 16 participants, 7 were male, and 9 were female (mean age, 12.6 years; range, 1-39 years). We found 1 novel TGM1 indel mutation (Ile469_Cys471delinsMetLeu) and 8 TGM1 missense mutations that to our knowledge have not been previously reported in BSI: 5 have been previously described in non–temperature-sensitive forms of congenital ichthyosis (Arg143Cys, Gly218Ser, Gly278Arg, Arg286Gln, and Ser358Arg), and 3 (Tyr374Cys, Phe495Leu, and Ser772Arg) are novel mutations. Three probands were homozygous for Arg264Trp, Arg286Gln, or Arg315Leu, indicating that these mutations are temperature sensitive. Seven of 10 probands with a compound heterozygous TGM1 genotype had a mutation at either arginine 307 or 315, providing evidence that mutations at these sites are temperature sensitive and highlighting the importance of these residues in the pathogenesis of BSI.
Conclusions and Relevance
Our findings expand the genotypic spectrum of BSI and the understanding of temperature sensitivity of TGM1 mutations. Increased awareness of temperature-sensitive TGM1 genotypes should aid in genetic counseling and provide insights into the pathophysiology of TGM1 ichthyoses, transglutaminase-1 enzymatic activity, and potential therapeutic approaches.
This cohort study uses targeted sequencing of the TGM1 gene to evaluate mutations for their specificity to bathing suit ichthyosis and their temperature sensitivity.
Introduction
Autosomal recessive congenital ichthyosis (ARCI) is a heterogeneous group of disorders of keratinization linked by the common finding of generalized hyperkeratosis and often accompanied by erythroderma. ARCI is rare, with an incidence of approximately 1 in 200 000 births.
The major phenotypic subtypes of ARCI include lamellar ichthyosis (LI), congenital ichthyosiform erythroderma, and harlequin ichthyosis. While ARCI is genetically heterogeneous, with at least 9 different genes causative for the most common forms, approximately 30% of the heritability of ARCI is explained by mutations in the TGM1 gene, which encodes transglutaminase-1 (TGase-1), an enzyme involved in the formation of the cornified envelope.
While mutations in TGM1 most commonly cause a spectrum of LI and congenital ichthyosiform erythroderma phenotypes of varying severity, they also underlie bathing suit ichthyosis (BSI), a very rare form of ARCI with fewer than 40 reported cases characterized by lamellar scaling restricted primarily to the trunk, neck and scalp. Affected infants are typically born as collodion babies and develop more localized scaling after shedding of the membrane. Bathing suit ichthyosis is due to the temperature sensitivity of certain TGM1 mutations. Clear genotype-phenotype correlations have been difficult to establish owing to the rarity of BSI and because many of the BSI mutations have also been reported in individuals with more generalized forms of ARCI. The present study of 16 individuals from 13 kindreds expands the spectrum of TGM1 mutations known to occur in patients with BSI and the understanding of mutations related to temperature sensitivity.
Methods
Participants and Samples
The study was approved by the Yale human investigation committee, consistent with the Declaration of Helsinki guidelines, and written informed consent was provided by all 16 participants (7 male and 9 female; mean age, 12.6 years; range, 1-39 years) or their parents. A detailed clinical history was obtained from each participant, including phenotypic presentation at birth and evolution of disease when available. Self-reporting of ethnicity was obtained to evaluate for a founder effect. Saliva samples were obtained from all participants for genetic analysis.
Genetic Analysis
Genetic analysis was performed on DNA isolated from the saliva of participants and both parents, if available. The DNA was extracted using standard procedures. Samples were analyzed in 1 of 2 ways: (1) they were screened for mutations in 11 genes (ABCA12, ALOXE3, ALOX12B, CYP4F22, NIPAL4, PNPLA1, SPINK5, TGM1, KRT1, KRT2E, and KRT10) via multiplex polymerase chain reaction and next-generation sequencing; or (2) the coding exons of TGM1 were amplified using polymerase chain reaction and subsequently examined via Sanger sequencing.
Results
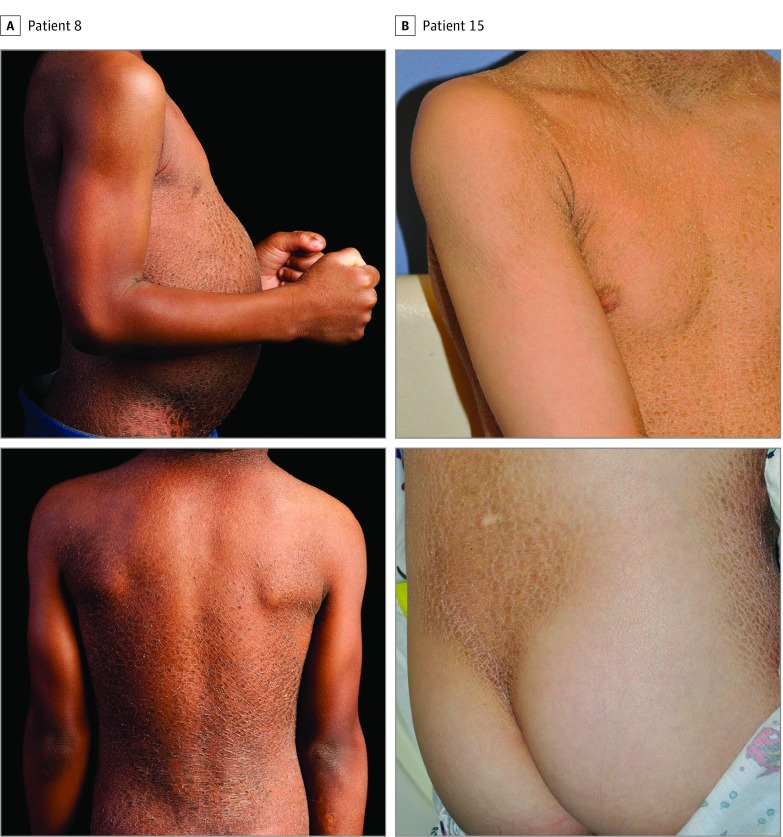
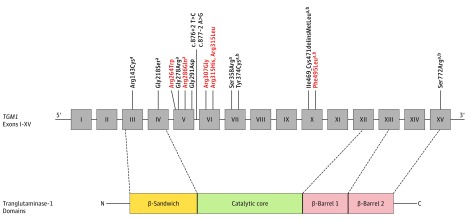
The BSI phenotypes and TGM1 genotypes of each participant are summarized in the Table. Representative photographs are provided in Figure 1 (patients 8 and 15), and the locations of the mutations relative to TGM1 protein domains are shown in Figure 2.
Table. TGM1 Mutations in Patients With BSI.
| Patient No./Sex/Age, y | Ethnicity | Presentation at Birth | Study Presentationa | Zygosity | Mutation in Coding DNA | Protein Effectb | |||
|---|---|---|---|---|---|---|---|---|---|
| Scalp | Neck | Trunk | Extremities | ||||||
| 1/M/4 | Middle Eastern | Collodion membrane | o | x | x | o | HOM | c.857 G>A | Arg286Glnc |
| 2/F/37 | Middle Eastern | Normal skin at birth, no collodion membrane | x | x | x | x (Flexural) |
HOM | c.790 C>T | Arg264Trp |
| 3/F/32 | Middle Eastern | Normal skin at birth, no collodion membrane | x | x | x | x (Flexural) |
HOM | c.790 C>T | Arg264Trp |
| 4/F/39 | White | Collodion membrane | x | x | x | o | HET | c.790 C>T c.832G>A |
Arg264Trp Gly278Argc |
| 5/M/4 | African American | Collodion membrane, thickened skin | x | o | x | o | HOM | c.944 G>T | Arg315Leu |
| 6/F/9 | African American | Collodion membrane, thickened skin | x | o | x | o | HOM | c.944 G>T | Arg315Leu |
| 7/F/11 | African American | Collodion membrane, thickened, fragile skin | x | x | x | x (Very mild) |
HET | c.944 G>T c.2316 C>A |
Arg315Leu Ser772Argc,d |
| 8/M/8 | African American | Collodion membrane, thickened, fragile skin | x | x | x | x (Mild) |
HET | c.944 G>T c.2316 C>A |
Arg315Leu Ser772Argc,d |
| 9/M/8 | Turkish | Collodion membrane, ectropion as a neonate | x | x | x | x (Flexural) |
HET | c.944 G>A c.832G>A |
Arg315His Gly278Argc |
| 10/M/1 | White | Collodion membrane, thickened skin | x | o | x | o | HET | c.944 G>A c.876 + 2 t > C |
Arg315His splice site |
| 11/F/4 | Irish | Collodion membrane | x | x | x | o | HET | c.919 C>G c.877-2 A>G |
Arg307Gly splice site |
| 12/M/21 | Hispanic | Collodion membrane | x | x | x | o | HET | c.919 C>G c.652 G>A |
Arg307Gly Gly218Serc |
| 13/F/9 | Northern European | Collodion membrane | x | x | x | o | HET | c.919 C>G c.1074 C>G |
Arg307Gly Ser358Argc |
| 14/F/1 | White | Collodion membrane, thickened, fragile skin | x | o | x | o | HET | c.919 C>G c.1121 A>G |
Arg307Gly Tyr374Cysc,d |
| 15/M/13 | White | Collodion membrane | x | x | x | x (Flexural) |
HET | c.427 C>T c.1483 t > C |
Arg143Cysc Phe495Leuc,d |
| 16/F/1 | White | Collodion membrane | x | x | x | o | HET | c.872 G>A c.1407_ 1416del10ins GCTCTGT |
Gly291Asp I469_C471 delinsMLc,d |
Abbreviations: BSI, bathing suit ichthyosis; HET, heterozygous; HOM, homozygous; o, absence of scaling; x, presence of scaling.
No patient had facial involvement.
Boldface indicates temperature-sensitive mutations.
Mutations not previously found in BSI.
Novel mutations.
Figure 1. Clinical Features of Bathing Suit Ichthyosis (BSI).
Representative photographs of the BSI phenotype, notable for platelike scaling of the trunk and back, with sparing of the extremities and buttocks.
Figure 2. Protein Schematic of TGM1 Mutations Found in Bathing Suit Ichthyosis (BSI).
Thirteen mutations are located in the catalytic core, 2 in the β-sandwich domain, and 1 in the β-barrel 2 domain. Nine mutations lead to substitution of charged residues. Temperature-sensitive mutations are shown in red.
aMutations not previously described in BSI.
bNovel mutations.
Homozygous TGM1 Mutations and Temperature Sensitivity: Arg264Trp, Arg286Gln, and Arg315Leu TGM1 Mutations in BSI
Patient 1 was the child of first cousins. He was born with a collodion membrane and later developed large brown scales on the back, chest, and groin, with sparing of the face and extremities. He was homozygous for a TGM1 Arg286Gln missense mutation, which has been previously reported in a compound heterozygous state with Gly278Arg in LI (and also found in patient 9), but to our knowledge has not been previously reported in BSI.
Patients 2 and 3 were siblings and the children of second cousins. Both were born with normal skin at birth and no collodion membrane and went on to develop platelike scale restricted to the neck, scalp, trunk, and flexural areas of the upper extremities. Nonscarring alopecia was present in both patients, and both siblings were homozygous for a TGM1 Arg264Trp missense mutation, which has previously been found in a compound heterozygous state in a patient with BSI. It was also found in a heterozygous state in patient 4, a female with TGM1 mutations Arg264Trp and Gly278Arg. The Gly278Arg mutation has been previously described in both LI and in self-healing ichthyosis, a rare form of ARCI characterized by the presence of a collodion membrane at birth with spontaneous healing of the phenotype within the first few weeks.
Patients 5 and 6 were African American siblings with no known consanguinity in the family. Both were born with a collodion membrane and later developed platelike scale restricted to the scalp and trunk. They were both homozygous for a TGM1 Arg315Leu mutation, which has been reported in a cohort of 8 South African patients with BSI.
The observation of homozygous mutations in these 5 patients with BSI provides evidence that these TGM1 mutations (Arg264Trp, Arg286Gln, and Arg315Leu) occurred with temperature sensitivity. All fall within the catalytic core of the transglutaminase-1 enzyme (Figure 2).
Temperature-Sensitive Substitutions at R315 TGM1: Common Compound Heterozygous Mutations in BSI
Patients 7 and 8 were siblings. Both were born with a collodion membrane and were noted to have thickened, fragile skin at birth. They developed dark platelike scaling, most prominent on the back, chest, and neck. Both were compound heterozygous for TGM1 Arg315Leu, a temperature-sensitive mutation, and Ser772Arg, a novel mutation falling within the β-barrel 2 domain (Figure 2).
Patient 9 was born with a collodion membrane and ectropion. At the time of the study he had brown platelike scales most prominent on the neck, scalp, and trunk, with sparing of the face and the extremities. He also exhibited attention-deficit/hyperactivity disorder and developmental delay. He was compound heterozygous for TGM1 (Arg315His and Gly278Arg). While both mutations fall within the catalytic core, Arg315His affects the same residue as Arg315Leu (found in patients 5-8) and has been commonly reported in patients with BSI. The Gly278Arg mutation was also found in patient 4.
Patient 10 was born with a collodion membrane and later developed scaling restricted to the scalp and trunk. He had TGM1 mutations Arg315His and c.876 + 2 T > C, a mutation within the donor splice site of exon 5 previously described in generalized ARCI.
The observation of a missense mutation at R315 in 6 of the 16 participants highlights the prevalence of substitutions at this site in BSI and contributes to evidence that such mutations are temperature sensitive.
Temperature-Sensitive TGM1 Arg307Gly: A Common BSI Mutation
Patient 11 was born with a collodion membrane that peeled at a few weeks of age, and she developed thick dark scale on the scalp, neck, axillae, and groin by age 1 year. She was compound heterozygous for TGM1 Arg307Gly, which has been commonly described in BSI, and c.877-2 A>G, a mutation within the acceptor splice site of exon 6, which has previously been found in BSI in conjunction with Arg307Gly (as in patient 11) as well as with Arg264Trp and Arg315His in the present cohort.
Patient 12 was born with a collodion membrane. At the time of the study he had brown platelike scales most prominent on the neck, scalp, axillae, and trunk. He was compound heterozygous for TGM1 Gly218Ser and Arg307Gly. The Gly218Ser mutation has been previously reported in an individual with LI with a collodion membrane at birth and later development of thick scales and ectropion.
Patient 13 was born with a collodion membrane. At the time of the study she had thick dark scale restricted to the neck, scalp, and trunk. She had TGM1 mutations Arg307Gly and Ser358Arg. The Ser358Arg mutation has been previously reported in 2 siblings with LI who were born with collodion membranes and later developed generalized scaling with facial and palmoplantar involvement.
Patient 14 was born with a collodion membrane and later developed scaling restricted to the trunk and scalp. She had TGM1 mutations Arg307Gly and Tyr374Cys. The Tyr374Cys mutation was within the catalytic domain and to our knowledge has not been described previously.
The observation of the Arg307Gly mutation in 4 out of the 16 study participants contributes to evidence that Arg307Gly is relatively common in BSI and is a temperature-sensitive mutation.
TGM1 Phe495Leu: A Temperature-Sensitive Mutation
Patient 15 was born with a collodion membrane. At the time of the study he had thick scale restricted to the neck, scalp, and trunk, as well as flexural involvement of the extremities. He has TGM1 mutations Arg143Cys and Phe495Leu. Homozygosity for the Arg143Cys mutation has been previously described in 2 patients with LI. We therefore presume that Phe495Leu, which is novel, is the temperature-sensitive mutation in patient 15.
TGM1 Ile469_Cys471delinsMetLeu: A Novel Mutation in the Catalytic Core
Patient 16 was born with a collodion membrane. At the time of the study she had scale restricted to the neck, scalp, and trunk. She had TGM1 mutations Gly291Asp and Ile469_Cys471delinsMetLeu. The Gly291Asp mutation was previously described in a compound heterozygous state in a patient with BSI as well as in a patient with generalized ARCI. The TGM1 Ile469_Cys471delinsMetLeu is a novel in-frame indel mutation that affects the catalytic core.
Discussion
Bathing suit ichthyosis is a rare ARCI phenotype characterized by presentation at birth with a collodion membrane followed by clinical improvement of ichthyosis on the face and extremities during the first few weeks of life. The resulting phenotype of scaling restricted to the trunk, neck, and scalp is a distinguishing feature of BSI and can be differentiated from somatic mosaicism by the lack of a distribution pattern along the Blaschko lines.
Prior to the present report, 21 missense mutations had been reported in patients with BSI. Of these, 9 had been reported exclusively in patients with BSI, while 12 had been observed in both BSI and generalized ARCI. Both truncating mutations (nonsense, splice site, and frameshift) and missense mutations in TGM1 have been found in individuals with BSI. However, while homozygosity or compound heterozygosity for truncating mutations has been observed in generalized forms of ARCI, to our knowledge, such a genotype has never been observed in BSI. This is consistent with the hypothesis that near or total loss of TGase-1 function causes generalized forms of ARCI, while genotypes that include a missense mutation resulting in a partially active, temperature-sensitive TGase-1 result in the more limited BSI phenotype.
In 2006, Oji et al investigated TGase-1 enzymatic activity in BSI tissue, assessing uptake of biotinylated cadaverine into cornified envelopes, and found that areas of healthy skin in patients with BSI show nearly normal TGase-1 activity, while affected areas display clearly reduced and abnormal activity. Furthermore, digital thermal imaging showed close association between skin temperature and the degree of scaling in patients with BSI, with warmer body sites exhibiting greater scaling. Functional TGase-1 testing of normal-appearing skin of a patient with BSI and homozygous for the missense mutation Tyr276Asn showed clear temperature sensitivity, with reduction in enzyme activity at 37°C compared with 25°C. This may explain the increased degree of scaling at sites of relatively higher temperature, such as the trunk.
In addition to the TGM1 mutation Tyr276Asn, several other mutations have been previously presumed to be temperature sensitive based on their presence in a homozygous state in individuals with BSI, including TGM1 mutations Ile304Phe, Arg307Gly, Arg315Leu, Arg315His, Val383Met, and Arg687His.
In the present study, we report phenotypic and genotypic data from 16 patients with BSI, the largest known cohort published to date. Aside from a pair of siblings who had normal skin at birth with no collodion membrane (patients 2 and 3), the phenotypes were consistent with prior descriptions of BSI. Of note, while collodion membrane is found in the majority of ARCI due to TGM1 mutation, this finding is not universal. We identified a total of 16 unique mutations in our cohort, including 13 missense mutations, 2 splice-site mutations, and 1 indel mutation. Eight of the missense mutations have not to our knowledge been previously reported for BSI; of these, 5 have been previously described in generalized ARCI (Arg143Cys, Gly218Ser, Gly278Arg, Arg286Gln, and Ser358Arg), while 3 (Tyr374Cys, Phe495Leu, and Ser772Arg) are novel mutations. The indel mutation TGM1 I469_C471delinsML is also novel.
Transglutaminase-1 consists of 3 domains: an N-terminal β-sandwich domain, a catalytic core domain, and 2 C-terminal β-barrel domains. Most BSI mutations have been located in exons 5 and 6 of TGM1, encoding the N-terminal portion of the catalytic core domain. Of the 13 unique missense mutations reported in the present study, only 2 were within the β-sandwich domain (Arg143Cys and Gly218Ser) and 1 was within the β-barrel 2 domain (Ser772Arg). In stark contrast, 10 were within the catalytic core (Arg264Trp, Gly278Arg, Arg286Gln, Gly291Asp, Arg307Gly, Arg315His, Arg315Leu, Ser358Arg, Tyr374Cys, and Phe495Leu), including all 3 of the mutations in our homozygous participants (Figure 2). All of our participants had at least 1 mutation within the catalytic core, and catalytic core mutations represent 88% of the mutations in our unrelated probands (23 of 26 alleles). Given that the catalytic core is only 38% of the total protein, our findings underscore a striking clustering of BSI mutations in this domain.
Based on our observation of patients with BSI homozygous for TGM1 mutations Arg264Trp, Arg286Gln, and Arg315Leu, we conclude that these mutations are temperature sensitive. Furthermore, the recurrence of mutations affecting R307 and R315 in our cohort—which among unrelated probands are present in one-third of homozygotes and seven-tenths of compound heterozygotes, comprising 35% of the mutations (9 of 26 total alleles)—bolsters prior evidence that these mutations are common in BSI (also reported by Bourrat et al) and that they are temperature sensitive. Finally, we hypothesize that the novel mutation Phe495Leu is also temperature sensitive, given that the TGM1 genotype of patient 15 included this mutation along with Arg143Cys. The Arg143Cys mutation is presumably not temperature sensitive, given that patients homozygous for Arg143Cys have been described as exhibiting generalized LI.
Though our findings provide evidence for temperature sensitivity of TGM1 mutations, clear genotype-phenotype correlations have been difficult to establish because several TGM1 mutations have been reported in both BSI and generalized ARCI. For example, homozygosity for Arg315Leu has been found in a pair of twins who were described as having LI and whose phenotype at age 2 months included thick platelike scaling on the trunk and extremities but sparing the face. Another patient described as having characteristic phenotypic findings of LI was found to be compound heterozygous for TGM1 mutations, including Arg286Gln, which we describe here as temperature sensitive.
The presence of these mutations in both BSI and generalized ARCI may represent evolution of the phenotype; patients with BSI can present with more generalized scaling earlier in life and then manifest bathing-suit distribution later in childhood. Thus, phenotypic characterization within the first few months of life may lead to misclassification. This dynamic nature of BSI highlights the importance of continued follow-up of patients with presumed temperature-sensitive mutations, including phenotypic reevaluation at multiple ages. Additional environmental or genetic factors that may determine the level of enzyme activity and response to temperature in patients with TGM1 mutations remain unclear.
Patients with BSI typically respond well to agents that improve barrier function and promote desquamation, including keratolytics and topical or systemic retinoids. Topical tazarotene led to substantial improvement in 2 of the present study participants.
Limitations
Since BSI is such a rare disorder, we were unable to recruit a large enough cohort to identify additional genetic modifiers that may contribute to temperature sensitivity in BSI due to TGM1 mutations.
Conclusions
Our findings expand the genotypic spectrum of BSI and provide evidence supporting the temperature sensitivity of specific TGM1 mutations (Arg264Trp, Arg286Gln, Arg307Gly, Arg315Leu, Arg315His, and Phe495Leu), which are clustered in the catalytic core. Although patients respond well to topical and systemic therapies, further research into the pathogenesis of BSI could lead to the development of novel therapeutic approaches targeting enzymatic stability and consideration of environmental modifications that might modify disease severity.
References
- 1.Richard G, Bale SJ. Autosomal recessive congenital ichthyosis In: Pagon RA, Adam MP, Ardinger HH, et al. , eds. GeneReviews(R). Seattle, WA: University of Washington, Seattle; 1993. [Google Scholar]
- 2.Takeichi T, Akiyama M. Inherited ichthyosis: non-syndromic forms. J Dermatol. 2016;43(3):242-251. [DOI] [PubMed] [Google Scholar]
- 3.Fischer J. Autosomal recessive congenital ichthyosis. J Invest Dermatol. 2009;129(6):1319-1321. [DOI] [PubMed] [Google Scholar]
- 4.Elias PM, Schmuth M, Uchida Y, et al. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exp Dermatol. 2002;11(3):248-256. [DOI] [PubMed] [Google Scholar]
- 5.Oji V, Hautier JM, Ahvazi B, et al. Bathing suit ichthyosis is caused by transglutaminase-1 deficiency: evidence for a temperature-sensitive phenotype. Hum Mol Genet. 2006;15(21):3083-3097. [DOI] [PubMed] [Google Scholar]
- 6.Cserhalmi-Friedman PB, Milstone LM, Christiano AM. Diagnosis of autosomal recessive lamellar ichthyosis with mutations in the TGM1 gene. Br J Dermatol. 2001;144(4):726-730. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YL, Yue ZH, Yuan P, et al. Novel compound heterozygous mutations of TGM1 gene identified in a Chinese collodion baby [in Chinese]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2012;29(1):1-4. [DOI] [PubMed] [Google Scholar]
- 8.Raghunath M, Hennies HC, Ahvazi B, et al. Self-healing collodion baby: a dynamic phenotype explained by a particular transglutaminase-1 mutation. J Invest Dermatol. 2003;120(2):224-228. [DOI] [PubMed] [Google Scholar]
- 9.Arita K, Jacyk WK, Wessagowit V, et al. The South African “bathing suit ichthyosis” is a form of lamellar ichthyosis caused by a homozygous missense mutation, p.R315L, in transglutaminase 1. J Invest Dermatol. 2007;127(2):490-493. [DOI] [PubMed] [Google Scholar]
- 10.Jacyk WK. Bathing-suit ichthyosis: a peculiar phenotype of lamellar ichthyosis in South African blacks. Eur J Dermatol. 2005;15(6):433-436. [PubMed] [Google Scholar]
- 11.Bourrat E, Blanchet-Bardon C, Derbois C, Cure S, Fischer J. Specific TGM1 mutation profiles in bathing suit and self-improving collodion ichthyoses: phenotypic and genotypic data from 9 patients with dynamic phenotypes of autosomal recessive congenital ichthyosis. Arch Dermatol. 2012;148(10):1191-1195. [DOI] [PubMed] [Google Scholar]
- 12.Farasat S, Wei MH, Herman M, et al. Novel transglutaminase-1 mutations and genotype-phenotype investigations of 104 patients with autosomal recessive congenital ichthyosis in the USA. J Med Genet. 2009;46(2):103-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laiho E, Ignatius J, Mikkola H, et al. Transglutaminase 1 mutations in autosomal recessive congenital ichthyosis: private and recurrent mutations in an isolated population. Am J Hum Genet. 1997;61(3):529-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huber M, Yee VC, Burri N, et al. Consequences of seven novel mutations on the expression and structure of keratinocyte transglutaminase. J Biol Chem. 1997;272(34):21018-21026. [DOI] [PubMed] [Google Scholar]
- 15.Rossmann-Ringdahl I, Anton-Lamprecht I, Swanbeck G. A mother and two children with nonbullous congenital ichthyosiform erythroderma. Arch Dermatol. 1986;122(5):559-564. [PubMed] [Google Scholar]
- 16.Hackett BC, Fitzgerald D, Watson RM, Hol FA, Irvine AD. Genotype-phenotype correlations with TGM1: clustering of mutations in the bathing suit ichthyosis and self-healing collodion baby variants of lamellar ichthyosis. Br J Dermatol. 2010;162(2):448-451. [DOI] [PubMed] [Google Scholar]
- 17.Sakai K, Akiyama M, Yanagi T, et al. ABCA12 is a major causative gene for non-bullous congenital ichthyosiform erythroderma. J Invest Dermatol. 2009;129(9):2306-2309. [DOI] [PubMed] [Google Scholar]
- 18.Benmously-Mlika R, Zaouak A, Mrad R, et al. Bathing suit ichthyosis caused by a TGM1 mutation in a Tunisian child. Int J Dermatol. 2014;53(12):1478-1480. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Sakaguchi Y, Itoh M, et al. Bathing suit ichthyosis with summer exacerbation: a temperature-sensitive case. Br J Dermatol. 2012;166(3):672-674. [DOI] [PubMed] [Google Scholar]
- 20.Esposito G, Tadini G, Paparo F, et al. Transglutaminase 1 deficiency and corneocyte collapse: an indication for targeted molecular screening in autosomal recessive congenital ichthyosis. Br J Dermatol. 2007;157(4):808-810. [DOI] [PubMed] [Google Scholar]
- 21.Herman ML, Farasat S, Steinbach PJ, et al. Transglutaminase-1 gene mutations in autosomal recessive congenital ichthyosis: summary of mutations (including 23 novel) and modeling of TGase-1. Hum Mutat. 2009;30(4):537-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petit E, Huber M, Rochat A, et al. Three novel point mutations in the keratinocyte transglutaminase (TGK) gene in lamellar ichthyosis: significance for mutant transcript level, TGK immunodetection and activity. Eur J Hum Genet. 1997;5(4):218-228. [PubMed] [Google Scholar]
- 23.Gånemo A, Pigg M, Virtanen M, et al. Autosomal recessive congenital ichthyosis in Sweden and Estonia: clinical, genetic and ultrastructural findings in eighty-three patients. Acta Derm Venereol. 2003;83(1):24-30. [DOI] [PubMed] [Google Scholar]
- 24.Terrinoni A, Serra V, Codispoti A, et al. Novel transglutaminase 1 mutations in patients affected by lamellar ichthyosis. Cell Death Dis. 2012;3(10):e416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tok J, Garzon MC, Cserhalmi-Friedman P, Lam HM, Spitz JL, Christiano AM. Identification of mutations in the transglutaminase 1 gene in lamellar ichthyosis. Exp Dermatol. 1999;8(2):128-133. [DOI] [PubMed] [Google Scholar]