This systematic review and meta-analysis provides an overview on possible immunomodulating treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis and estimates their effects on mortality compared with supportive care.
Key Points
Questions
Which systemic immunomodulating therapies are proposed for the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis and what are their effects on mortality compared with supportive care?
Findings
In this meta-analysis of 96 studies comprising 3248 patients, patients were treated with supportive care, glucocorticosteroids, intravenous immunoglobulins, cyclosporine, plasmapheresis, thalidomide, cyclophosphamide, hemoperfusion, tumor necrosis factor inhibitors, and granulocyte colony-stimulating factor. Glucocorticosteroids and cyclosporine were associated with promising survival benefit. This finding was not observed for other treatments.
Meaning
Glucocorticosteroids and cyclosporine are the most promising therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis, although these findings still require further evaluation in prospective studies.
Abstract
Importance
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are rare but severe adverse reactions with high mortality. There is no evidence-based treatment, but various systemic immunomodulating therapies are used.
Objectives
To provide an overview on possible immunomodulating treatments for SJS/TEN and estimate their effects on mortality compared with supportive care.
Data Sources
A literature search was performed in December 2012 for articles published in MEDLINE, MEDLINE Daily, MEDLINE Inprocess, Web of Science, EMBASE, Scopus, and the Cochrane Library (Central) from January 1990 through December 2012, and updated in December 2015, in the English, French, Spanish, and German languages looking for treatment proposals for SJS/TEN. Other sources were screened manually.
Study Selection
Initially, 157 randomized and nonrandomized studies on therapies (systemic immunomodulating therapies or supportive care) for SJS/TEN were selected.
Data Extraction and Synthesis
Relevant data were extracted from articles. Authors were contacted for further information. Finally, 96 studies with sufficient information regarding eligibility and adequate quality scores were considered in the data synthesis. All steps were performed independently by 2 investigators. Meta-analyses on aggregated study data (random-effects model) and individual patient data (IPD) (logistic regression adjusted for confounders) were performed to assess therapeutic efficacy. In the analysis of IPD, 2 regression models, stratified and unstratified by study, were fitted.
Main Outcomes and Measures
Therapy effects on mortality were expressed in terms of odds ratios (ORs) with 95% CIs.
Results
Overall, 96 studies (3248 patients) were included. Applied therapies were supportive care or systemic immunomodulating therapies, including glucocorticosteroids, intravenous immunoglobulins, cyclosporine, plasmapheresis, thalidomide, cyclophosphamide, hemoperfusion, tumor necrosis factor inhibitors, and granulocyte colony-stimulating factors. Glucocorticosteroids were associated with a survival benefit for patients in all 3 analyses but were statistically significant in only one (aggregated data: OR, 0.5; 95%% CI, 0.3-1.01; IPD, unstratified: OR, 0.7; 95% CI, 0.5-0.97; IPD, stratified: OR, 0.8; 95% CI, 0.4-1.3). Despite the low patient size, cyclosporine was associated with a promising significant result in the only feasible analysis of IPD (unstratified model) (OR, 0.1; 95% CI, 0.0-0.4). No beneficial findings were observed for other therapies, including intravenous immunoglobulins.
Conclusions and Relevance
Although all analyses, including the unstratified model, had limitations, glucocorticosteroids and cyclosporine were the most promising systemic immunomodulating therapies for SJS/TEN. Further evaluation in prospective studies is required. However, this work provides a comprehensive overview on proposed systemic immunomodulating treatments for SJS/TEN, which is of great relevance for treating physicians.
Introduction
Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) are rare, severe cutaneous adverse reactions that are associated with high mortality. SJS/TEN can be characterized by the detachment of necrotic epidermis and erosions of mucous membranes with different degrees of severity. The programmed cell death of the epidermis is believed to be induced by cytotoxic T cells and mediated by various cytokines. However, mainly because of their rareness, there is still a lack of an evidence-based standard treatment protocol for SJS/TEN. This review is a step toward such a protocol and reveals hypotheses on the most promising therapies essential for future studies.
Because of the severity of SJS/TEN, hospital admission is required for these patients. One of the first actions in the treatment is to identify the most likely cause and the early withdrawal of the potentially inducing agent. Because of the skin-related symptoms, supportive care has highest priority. Moreover, because of the underlying immune-mediated mechanism, different systemic immunomodulating treatments (SITs) are proposed with the intent of stopping the progression of skin necrosis. However, an evidence-based evaluation is missing. The aims of this project are therefore to (1) provide a comprehensive overview on proposed SITs and (2) estimate their effect on mortality compared with supportive care.
To acknowledge the specific situation in SJS/TEN, randomized and nonrandomized studies were considered. Furthermore, aggregated study data (meta-analysis at the study level) and individual patient data (IPD) (meta-analysis at the patient level) were used to obtain effect estimates for different SITs.
Methods
Systematic Review
A systematic search was performed in December 2012 for articles published from January 1990 through December 2012 in the English, French, German, and Spanish languages on therapies (SIT or supportive care) for SJS/TEN in MEDLINE, MEDLINE Daily, MEDLINE Inprocess, Web of Science, EMBASE, Scopus, and the Cochrane Library (Central) by staff of the library at the Institute for Medical Biometry and Statistics, Medical Center – University of Freiburg, Freiburg, Germany, under the supervision of the head librarian (E.M.), who is experienced in literature search for systematic reviews. Articles published before 1990 were excluded because the internationally accepted consensus definition for diagnosing SJS/TEN was developed in 1990. Duplicate references were excluded. Subsequently, all titles were screened to remove obviously irrelevant publications. In addition, a manual search in other sources was performed (eMethods 1 in the Supplement). For processing of identified references, results of the different searches were imported into Endnote.
After articles were obtained, studies were assessed according to the following eligibility criteria (eMethods 2 in the Supplement): (1) clearly described type of study, (2) diagnostic accuracy of SJS/TEN, (3) sufficient description of treatment, (4) information on mortality, and (5) at least 5 participants per study. A slightly modified instrument proposed by the Cochrane group was applied to all remaining publications that assigned a respective quality score to each study (eMethods 3 in the Supplement). Moreover, data from each study were extracted using a predefined instrument (eMethods 4 in the Supplement). The different steps to identify and assess the literature were independently performed by 2 of us (S.Z., M.V.). Any disagreement was solved by means of consensus. Subsequently, authors were approached to obtain additional information. Finally, all studies that fullfilled eligiblity criteria and had quality scores larger than the lowest tertile of the observed distribution of scores (≥5 points) were considered in the data synthesis. Furthermore, duplicate publications of the same study population were excluded.
To incorporate more recent literature, the search was repeated in December 2015 using the same search strategy except for modifications partially requested because of changes on the search platforms. In addition, identified references were similarly processed to detect new therapeutic proposals (aim 1).
Data Synthesis
All extracted data of the selected studies were imported into SAS statistical software, version 9.2 (SAS Institute Inc). A descriptive analysis of proposed SITs was performed to provide a comprehensive overview. To estimate therapy effects, 2 meta-analytic approaches were considered using aggregated data or IPD. The analyses were performed for each immunomodulating therapy separately with supportive care as the comparison group. Therapy effects were expressed in terms of odds ratios (ORs) with 95% CIs.
For the meta-analysis at the study level, only studies that compared SITs and supportive care and reported therapy effects or provided data for its calculation could contribute to this analysis. A random-effects model was fitted to estimate a pooled treatment effect on the mortality of SJS/TEN using the function metagen of the R-package meta (R version 2.15.3; R Foundation for Statistical Computing). Studies were weighted by the inverse-variance method. Results are presented in forest plots. Heterogeneity was quantified using I2. Funnel plots were used to assess for the presence of publication bias.
For the analysis of IPD, information on therapy, outcome, age, and severity of SJS/TEN was requested from each patient. When identified, duplicate patients were excluded. A logistic regression model was fitted to estimate the treatment effect of each SIT separately compared with supportive care (with at least 10 patients per group) on mortality. Analyses were adjusted for age (<40 or ≥40 years) and severity of disease (SJS, SJS/TEN overlap, or TEN). Moreover, models were fitted with and without consideration of the source of patients (ie, estimates from analyses stratified and unstratified by study were obtained). The stratified model has the advantage of adjusting estimation for potential differences among studies but limits the amount of data that can be used. To make use of all IPD, an unstratified model was fitted as well.
Results
Systematic Review
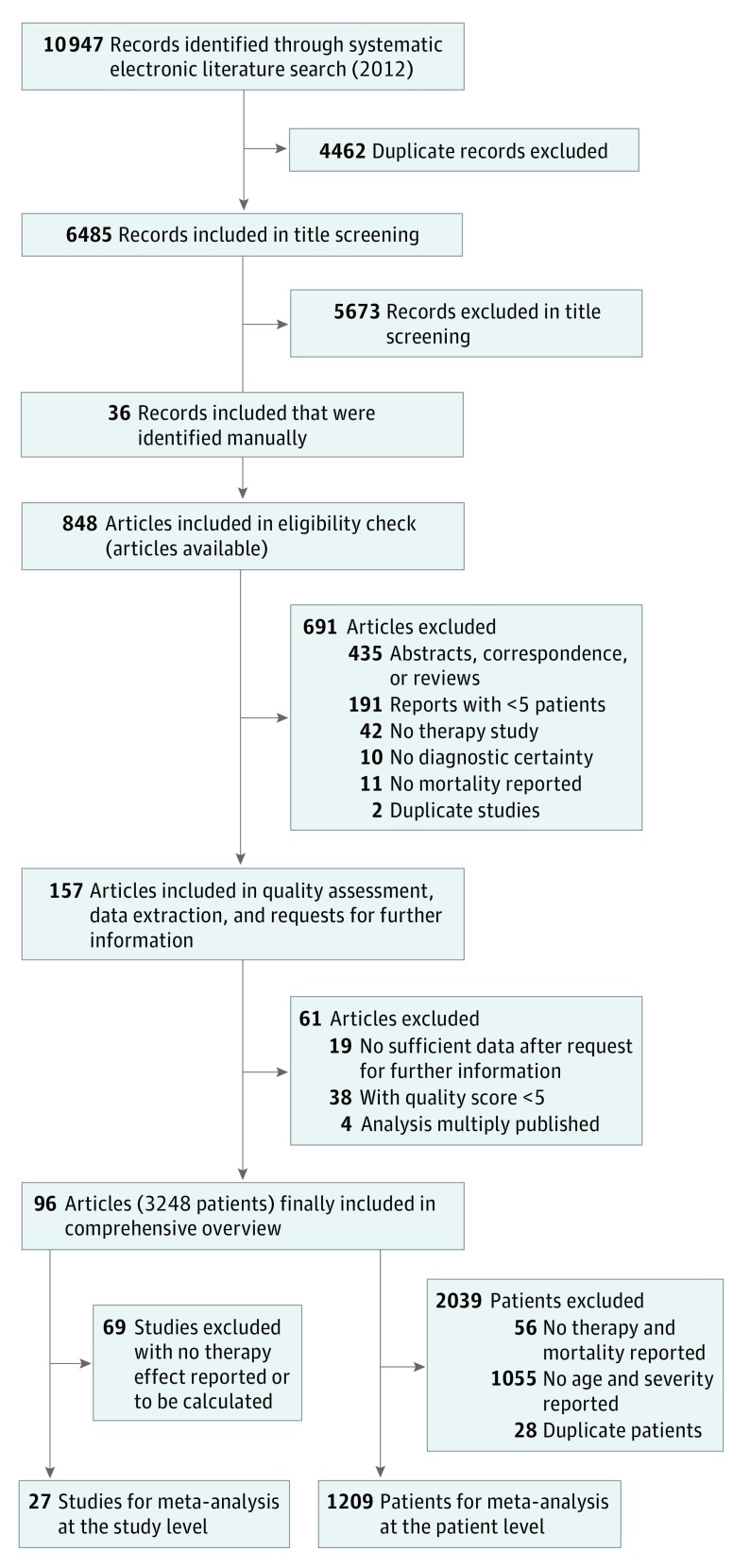
The systematic search in different electronic databases in 2012 yielded 6485 references (duplicates excluded) (Figure 1). After exclusion of unrelated references via title screening and inclusion of additional records identified by manual search, the full text of 848 articles was obtained and subjected to a detailed eligibility check. As a result, 691 articles were excluded at this stage because eligibility criteria were clearly violated. The remaining 157 publications were then included in the quality assessment and data extraction. Completed data extraction sheets were sent to authors of the respective publication to obtain missing information. After sending reminders, answers from 35 study groups (22.3%) were received; however, only 27 groups (17.2%) provided further information. Subsequently, 19 publications were excluded because, even with additionally obtained information, eligibility criteria were not sufficiently fulfilled.
Figure 1. Flowchart of Search Strategy and Study Selection.
eTable 1 in the Supplement provides detailed results of the quality assessment for the remaining 138 publications, whereas eFigure 1 in the Supplement presents the distribution of the respective quality scores. Overall, the observed quality is low (median, 5; range, 1-10.83). Because the aim of the quality assessment was to identify low-quality studies to exclude them from further analysis, all publications with a score below a median score of 5 points (38 [27.5%]) were excluded. Furthermore, 4 publications that reported the results of the same study population were combined with the respective publication. Finally, 96 publications that covered altogether 3248 patients were selected for the data synthesis (eReferences in the Supplement).
Comprehensive Overview
Among the 96 included studies, all except 1 are of nonrandomized nature. The only exception was a randomized clinical trial (RCT) that found a detrimental effect of thalidomide on mortality in patients with TEN. The conduct of RCTs is difficult for several reasons in SJS/TEN (eg, rareness). A former systematic review that attempted to evaluate the effect of SITs compared with supportive care also identified only the mentioned RCT with thalidomide. Most other identified studies are observational studies, especially cohort studies (retrospective, 68 [70.8%]; prospective, 9 [9.5%]; and unclear, 17 [17.7%]). There is just one exception of an interventional study with one treatment arm (1 [1.0%]).
Besides 40 publications that reported findings obtained from case series (1 therapy group), 56 (58.3%) of the 96 studies described 2 or more different therapy groups, which led to a total of 182 therapy groups within the 96 studies. The various treatments are described in Figure 2. Most often, patients with SJS/TEN were treated without SITs (62 [34.1%]), with glucocorticosteroids (45 [24.7%]), or with intravenous immunoglobulins (IVIGs) (37 [20.3%]). Few patients were treated with another SIT, including cyclosporine, plasmapheresis, cyclophosphamide, or thalidomide, or with a combination therapy with more than 1 SIT. Detailed data on all 96 included studies are presented in eTable 2 in the Supplement.
Figure 2. Overview on Systemic Immunomodulating Therapies for Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis Assessed in 182 Therapy Groups From 96 Studies.
Fifty-six studies (58.3%) assessed more than 1 therapy option. For diverse combined therapies, observed combination therapies were glucocorticosteroids and cyclophosphamide (n = 2), glucocorticosteroids and cyclosporine (n = 1), glucocorticosteroids and plasmapheresis (n = 1), glucocorticosteroids and thalidomide (n = 1), and intravenous immunoglobulin (IVIG) and plasmapheresis (n = 1). Therapy not defined indicates therapy groups whose therapy is not clearly defined in the article.
Detailed information on treatment modalities, such as applied dosages of SITs, were only occasionally provided and in various ways. For glucocorticosteroids, application is highly diverse regarding used substance and dosages reaching from very low to very high levels. Observations are summarized in eTable 3 in the Supplement. Doses of IVIGs ranged from 1 mg/kg to 2 g/kg for all studies that reported data as a mean or from 0.05 mg/kg to 2.9 g/kg for all studies that reported data as a range. The update of the literature search in 2015 revealed few single studies assessing new SITs: hemoperfusion (similar approach as plasmapheresis), tumor necrosis factor inhibitors (infliximab, etanercept), and granulocyte colony-stimulating factor.
Meta-analyses
Among the 56 publications that describe more than 1 therapy group and are thus potentially suitable for meta-analysis at the study level, less than half provided enough information to be used for the estimation of therapy effects. For a meta-analysis comparing the mortality of a single SIT vs supportive care at the study level, information from more than 1 study is only available for comparison of glucocorticosteroids vs supportive care and IVIGs vs supportive care. With respect to IPD, information on 1209 patients (37.2%) from 55 studies is available, including 396 patients (32.8%) who received supportive care.
Glucocorticosteroids
For the meta-analysis at the study level, 11 studies provided information on 12 independent comparisons of glucocorticosteroids vs supportive care (Figure 3A). Although the combined point estimate reflects a beneficial treatment effect, it was not statistically significant (OR, 0.54; 95% CI, 0.29-1.01). Because no death was observed in one (supportive care) or both therapy groups for 4 comparisons, they did not contribute to the estimation of the combined treatment effect (weight, 0%). Heterogeneity was estimated to be low (I2 = 5.9%). The funnel plot is inconclusive (Figure 3B). Therefore, publication bias cannot be excluded.
Figure 3. Comparison of Glucocorticosteroids and Supportive Care.
DE indicates analysis of the German patient collective; FR, analysis of the French patient collective; NA, not applicable; and OR, odds ratio.
Among the 1209 patients with individual data, 367 (30.4%) from 26 studies (eTable 4 in the Supplement) were treated with glucocorticosteroids. The direction of the estimates is the same as in the meta-analysis at the study level toward beneficial effects of glucocorticosteroids (Figure 3A). Although the result of the unstratified model is significant (OR, 0.7; 95% CI, 0.5-0.97), the result of the stratified model is not (OR, 0.8; 95% CI, 0.4-1.3).
Intravenous Immunoglobulins
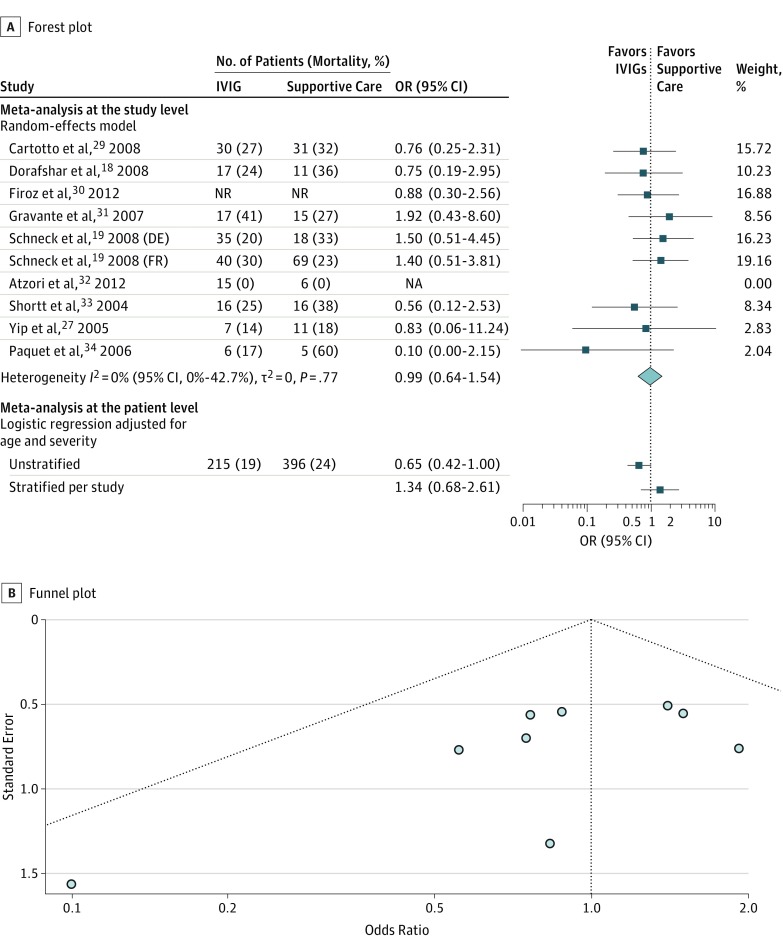
Nine studies provided information on 10 independent comparisons of IVIG vs supportive care (Figure 4A). No difference in mortality was detected in the meta-analysis at the study level (OR, 0.99; 95% CI, 0.64-1.54) and no heterogeneity (I2 = 0%). Again, the funnel plot is inconclusive (Figure 4B). Publication bias cannot be ruled out.
Figure 4. Comparison of Intravenous Immunoglobulins (IVIGs) and Supportive Care.
DE indicates analysis of the German patient collective; FR, analysis of the French patient collective; NA, not applicable; NR, not reported; OR, odds ratio.
From IPD, data from 215 of 1209 patients (17.8%) treated with IVIG from 23 studies are available (Figure 4A and eTable 4 in the Supplement). Compared with supportive care, results from unstratified and stratified models are not significant and not uniform regarding the direction of the effect.
Other SITs
No further meta-analysis at the study level could be conducted. On the basis of IPD, regression models were fitted for thalidomide (n = 10), plasmapheresis (n = 16), and cyclosporine (n = 40) compared with supportive care (eFigure 2 in the Supplement). For thalidomide, similar detrimental effects were detected (unstratified model: OR, 12.5; 95% CI, 2.4-66; stratified model: OR, 36.9; 95% CI, 2.5-540) because all but 1 patient originated from the RCT that reported the detrimental effect in the first place. For plasmapheresis, the combined effect estimates are not significant but in line with beneficial effects (unstratified model: OR, 0.3; 95% CI, 0.1-1.3; stratified model: OR, 0.4; 95% CI, 0.0-4.4). Finally, the comparison of cyclosporine revealed an interesting result. Among the 40 patients treated with cyclosporine, no death was observed. To obtain an effect estimate, an unstratified, exact logistic regression model was fitted that revealed a significant and beneficial effect of cyclosporine compared with supportive care on mortality (OR, 0.1; 95% CI, 0.0-0.4). No meta-analysis was possible for cyclophosphamide because of insufficient data.
Discussion
Proposed Therapies
A total of 96 studies that reported data from 182 therapy groups were included. Patients with SJS/TEN were most often treated without the administration of SIT (supportive care) or with glucocorticosteroids or IVIG. Less often administered SITs include cyclosporine, plasmapheresis, cyclophosphamide, or thalidomide. This observation agrees with current textbooks.
Supportive Care
Supportive care is most important in the treatment of patients with SJS/TEN. It consists of maintaining hemodynamic equilibrium and preventing life-threatening complications. Although studies included in the review often lack a detailed description of supportive care, differences were observed, especially in dealing with detached skin and topical treatments. In the presence of no standardized care, these differences may cause differences in outcome mortality.
Glucocorticosteroids
Although the results of the different approaches suggest a beneficial effect, this finding is not conclusive because of the absence of statistical significance in 2 of 3 analyses. Our findings reflect the ongoing debate on the effectiveness of glucocorticosteroids in the literature. However, it is also suggested that a beneficial effect of glucocorticosteroids might exist when specific treatment modalities are applied, such as early administration, pulse therapy, or within selected subgroups. Because the data in the current project do not allow addressing these suggestions, this is an additional point for future studies.
Intravenous Immunoglobulins
Our results do not support the use of IVIGs in the treatment of SJS/TEN. Effect estimates of IVIGs vs supportive care on mortality obtained from the different analysis approaches are not significant and heterogeneous concerning a beneficial or deleterious effect. Because the amount of observed evidence is of major importance, IVIGs cannot be recommended for the treatment of SJS/TEN. The authors of other reviews that focused on IVIGs came to a similar conclusion.
Cyclosporine
Cyclosporine provides an interesting therapeutic option because our results suggest a beneficial effect on mortality. However, our findings reflect essentially the results of the one well-performed interventional study, conducted in Créteil, France, that is limited in its generalizability because only a few, mostly younger patients with SJS/TEN were treated with this agent. Remarkably, the French group still uses cyclosporine in the treatment of SJS/TEN with good results. Moreover, 2 studies assessing cyclosporine and not considered in this review found a positive effect of cyclosporine. However, these retrospective studies have their weaknesses, and the results should be interpreted with caution.
Other SITs
There is not much evidence of the usefulness of other SITs, including thalidomide, phasmapheresis, and cyclophosphamide or any combination of SITs. Of note, because the RCT on thalidomide found a detrimental effect of the therapy, no additional study assessing thalidomide was conducted to our knowledge.
Limitations
Because of the specific situation in SJS/TEN (difficulty to conduct RCTs, mainly small observational studies), adaptations of standard methods were required to successfully conduct this project supported by the German Cochrane group and an experienced librarian (E.M.) and statistician. To achieve this goal, we based the project on a predefined study protocol and a systematic literature search. Assessment of the literature was performed independently by 2 of us (S.Z., M.V.). Although this project has several intriguing aspects, there are also some limitations.
Completeness and Currentness of Data
The literature search was performed in December 2012 in different electronic databases and other important sources. For reasons of practicability and expense, the search was restricted to the English, French, German, and Spanish languages in all databases except Cochrane Library Central and Web of Science. To quantify studies missed because of language restrictions, we estimate to have missed only a small number of studies (eMethods 1 in the Supplement). Thus, language restrictions should not be a major issue for this review. Because of the complexity of data aquisition and analysis, no full update of the literature search was possible after 2012, limiting the currentness of the data and results. However, the search was repeated in 2015 to identify new therapy proposals after 2012.
Accuracy of Diagnosis
Patients with SJS/TEN partly have symptoms that can also be seen in other diseases, such as erythema multiforme majus. However, since 1993, clear diagnostic criteria are available. To avoid any bias through mixture of patients with different diseases, studies published before 1990 were excluded during the literature search. In addition, a respective criterion was included in the eligibility check.
Selective Reporting
Because of the rareness and severity of the disease, care and treatment of a patient with SJS/TEN are still something special in the professional life of most physicians. If a new finding is observed (eg, a new potentially causative drug), there is a tendency to publish a case report, maybe in combination with few earlier observed cases. If the reason for publication is associated with mortality, such studies may introduce a bias to the results of the current meta-analysis at the patient level. The meta-analysis at the study level should not be affected because such studies usually report only one therapy option. Therefore, we had decided to exclude any study that reported on only 5 or fewer patients.
However, the decision to exclude small studies may affect the amount of proposed therapies. Although we do not believe that a new therapy is truly proposed in any such publication, we may have missed it. Therefore, we also checked therapies of studies not included in this review with small patient sizes. We found studies that reported tumor necrosis factor inhibitors (infliximab, etanercept) as treatment for SJS/TEN. Larger studies were published after 2012. Moreover, one study reported that pentoxifylline had been administered to patients with SJS/TEN.
Poor Quality of Studies, Poor Reporting, and Study Design
The main source of evidence in this context is built by observational studies that are prone to bias for several reasons. Thus, assessment of the risk of bias (quality assessment) is urgently required to identify studies of poor quality. For this reason, we applied an instrument that also allows assigning a quality score to each study (eMethods 3 in the Supplement). Although the Cochrane group no longer recommends the use of quality scores, we decided to exclude studies of lowest quality based on the score to avoid biased results in the meta-analysis. Because we had no previous experience in the quality assessment of observational studies, we assessed the scoring results in a sample of studies and checked whether the score reflects our opinion of study quality (eMethods 3 in the Supplement). The scores are sensible in this sample.
Overall, the quality of studies was rather low (eFigure 1 in the Supplement). Of note, publications are often poorly reported (eg, study groups did not provide sufficient data on treatment modalities). It is imperative that authors follow available reporting guidelines for observational studies, such as Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
Estimation of Therapy Effects
For the estimation of therapy effects, we used different approaches: meta-analysis at the study level and meta-analysis at the patient level (stratified or unstratified by study). Each of them has its advantages and disadvantages. Although meta-analysis on aggregated data represents the state-of-the-art analysis when combining results from RCTs, meta-analysis on IPD is an approach that gained importance when evidence is assembled from nonrandomized studies. By application of the 3 different approaches, we were able to obtain as much information from the data as possible to draw conclusions carefully.
Proposed combinations of SITs were not considered in the analysis because information is limited and insufficient to provide a sensible estimate of the effect. In addition, the effect of potential interactions among the treatments cannot be considered.
Conclusions
This is the first major review, to our knowledge, of SJS/TEN that includes observational studies to provide a comprehensive overview on SITs for these patients. Among different proposals, glucocorticosteroids and cyclosporine are the most promising SITs in the treatment of SJS/TEN. Still, further evaluation is required because the current data included in the meta-analysis are limited in amount and validity. Multinational efforts may be especially helpful in this situation of rare diseases to develop prospective studies of high quality.
eMethods 1. Literature search: strategies and results
eMethods 2. Eligibility check: form and definition of criteria
eMethods 3. Quality assessment: instrument, form, and score definition
eMethods 4. Data extraction
eTable 1. Overview on the result of the quality assessment of published studies (N = 138)
eTable 2. Overview on included studies (N = 96)
eTable 3. Glucocorticosteroids: substances and dosages (N = 48)
eTable 4. Overview on studies contributing individual patient data for analysis
eFigure 1. Frequency distribution of total quality scores obtained for 138 eligible studies
eFigure 2. Forest plot: comparison of other systemic immunomodulating therapies vs supportive care
eAcknowledgments. Authors who provided additional information
eReferences
References
- 1.Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13(7):625-645. [DOI] [PubMed] [Google Scholar]
- 2.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129(1):92-96. [PubMed] [Google Scholar]
- 3.Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343-1350. [DOI] [PubMed] [Google Scholar]
- 4.Heng YK, Lee HY, Roujeau JC. Epidermal necrolysis: 60 years of errors and advances. Br J Dermatol. 2015;173(5):1250-1254. [DOI] [PubMed] [Google Scholar]
- 5.Sekula P, Dunant A, Mockenhaupt M, et al. ; RegiSCAR study group . Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197-1204. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136(3):323-327. [DOI] [PubMed] [Google Scholar]
- 7.Roujeau JC, Bastuji-Garin S. Systematic review of treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis using the SCORTEN score as a tool for evaluating mortality. Ther Adv Drug Saf. 2011;2(3):87-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AM. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technol Assess. 2000;4(34):1-154. [PubMed] [Google Scholar]
- 9.Sutton A. Methods for Meta-analysis in Medical Research. Chichester, NY: John Wiley & Sons; 2000. [Google Scholar]
- 10.Hosmer D, Lemeshow S. Applied Logistic Regression. Hoboken, NJ: John Wiley & Sons; 2000. [Google Scholar]
- 11.Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet. 1998;352(9140):1586-1589. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar S, Mockenhaupt M, Roujeau J, Townshend A. Interventions for toxic epidermal necrolysis. Cochrane Database Syst Rev. 2002;(4):CD001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valeyrie-Allanore L, Wolkenstein P, Brochard L, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163(4):847-853. [DOI] [PubMed] [Google Scholar]
- 14.Abela C, Hartmann CE, De Leo A, et al. Toxic epidermal necrolysis (TEN): the Chelsea and Westminster Hospital wound management algorithm. J Plast Reconstr Aesthet Surg. 2014;67(8):1026-1032. [DOI] [PubMed] [Google Scholar]
- 15.Paquet P, Jennes S, Rousseau AF, Libon F, Delvenne P, Piérard GE. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis: a proof-of-concept study. Burns. 2014;40(8):1707-1712. [DOI] [PubMed] [Google Scholar]
- 16.Paradisi A, Abeni D, Bergamo F, Ricci F, Didona D, Didona B. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71(2):278-283. [DOI] [PubMed] [Google Scholar]
- 17.Wang YM, Tao YH, Feng T, Li H. Beneficial therapeutic effects of hemoperfusion in the treatment of severe Stevens-Johnson syndrome/toxic epidermal necrolysis: preliminary results. Eur Rev Med Pharmacol Sci. 2014;18(23):3696-3701. [PubMed] [Google Scholar]
- 18.Dorafshar AH, Dickie SR, Cohn AB, et al. Antishear therapy for toxic epidermal necrolysis: an alternative treatment approach. Plast Reconstr Surg. 2008;122(1):154-160. [DOI] [PubMed] [Google Scholar]
- 19.Schneck J, Fagot JP, Sekula P, Sassolas B, Roujeau JC, Mockenhaupt M. Effects of treatments on the mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis: a retrospective study on patients included in the prospective EuroSCAR Study. J Am Acad Dermatol. 2008;58(1):33-40. [DOI] [PubMed] [Google Scholar]
- 20.Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H, Tripathi C. Drug-induced Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS-TEN overlap: a multicentric retrospective study. J Postgrad Med. 2011;57(2):115-119. [DOI] [PubMed] [Google Scholar]
- 21.Brand R, Rohr JB. Toxic epidermal necrolysis in Western Australia. Australas J Dermatol. 2000;41(1):31-33. [DOI] [PubMed] [Google Scholar]
- 22.Ioannides D, Vakali G, Chrysomallis F, et al. Toxic epidermal necrolysis: a study of 22 cases. J Eur Acad Dermatol Venereol. 1994;3:266-275. [Google Scholar]
- 23.Kamaliah MD, Zainal D, Mokhtar N, Nazmi N. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in northeastern Malaysia. Int J Dermatol. 1998;37(7):520-523. [DOI] [PubMed] [Google Scholar]
- 24.Koh MJ-A, Tay Y-K. Stevens-Johnson syndrome and toxic epidermal necrolysis in Asian children. J Am Acad Dermatol. 2010;62(1):54-60. [DOI] [PubMed] [Google Scholar]
- 25.Léauté-Labrèze C, Lamireau T, Chawki D, Maleville J, Taïeb A. Diagnosis, classification, and management of erythema multiforme and Stevens-Johnson syndrome. Arch Dis Child. 2000;83(4):347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethuraman G, Sharma VK, Pahwa P, Khetan P. Causative drugs and clinical outcome in Stevens Johnson Syndrome (SJS), Toxic Epidermal Necrolysis (TEN), and SJS-TEN overlap in children. Indian J Dermatol. 2012;57(3):199-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yip LW, Thong BY, Tan AW, Khin LW, Chng HH, Heng WJ. High-dose intravenous immunoglobulin in the treatment of toxic epidermal necrolysis: a study of ocular benefits. Eye (Lond). 2005;19(8):846-853. [DOI] [PubMed] [Google Scholar]
- 28.Azfar NA, Zia MA, Malik LM, Khan AR, Jahangir M. Role of systemic steroids in the outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis. JPAD. 2010;20:158-162. [Google Scholar]
- 29.Cartotto R, Mayich M, Nickerson D, Gomez M. SCORTEN accurately predicts mortality among toxic epidermal necrolysis patients treated in a burn center. J Burn Care Res. 2008;29(1):141-146. [DOI] [PubMed] [Google Scholar]
- 30.Firoz BF, Henning JS, Zarzabal LA, Pollock BH. Toxic epidermal necrolysis: five years of treatment experience from a burn unit. J Am Acad Dermatol. 2012;67(4):630-635. [DOI] [PubMed] [Google Scholar]
- 31.Gravante G, Delogu D, Marianetti M, Trombetta M, Esposito G, Montone A. Toxic epidermal necrolysis and Steven Johnson syndrome: 11-years experience and outcome. Eur Rev Med Pharmacol Sci. 2007;11(2):119-127. [PubMed] [Google Scholar]
- 32.Atzori L, Pinna AL, Mantovani L, et al. Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department–Cagliari University (Italy). J Eur Acad Dermatol Venereol. 2012;26(11):1424-1430. [DOI] [PubMed] [Google Scholar]
- 33.Shortt R, Gomez M, Mittman N, Cartotto R. Intravenous immunoglobulin does not improve outcome in toxic epidermal necrolysis. J Burn Care Rehabil. 2004;25(3):246-255. [DOI] [PubMed] [Google Scholar]
- 34.Paquet P, Kaveri S, Jacob E, Pirson J, Quatresooz P, Piérard GE. Skin immunoglobulin deposition following intravenous immunoglobulin therapy in toxic epidermal necrolysis. Exp Dermatol. 2006;15(5):381-386. [DOI] [PubMed] [Google Scholar]
- 35.Allanore L, Roujeau J. Clinic and pathogenesis of severe bullous skin reactions: Stevens-Johnson syndrome, toxic epidermal necrolysis In: Drug Hypersensitivity. Basel, Switzerland: Karger; 2007:267-277. [Google Scholar]
- 36.Mockenhaupt M. Stevens-Johnson Syndrome and toxic epidermal necrolysis In: Life-threatening Dermatoses and Emergencies in Dermatology. Berlin, Germany: Springer; 2009:87-95. [Google Scholar]
- 37.Struck MF, Hilbert P, Mockenhaupt M, Reichelt B, Steen M. Severe cutaneous adverse reactions: emergency approach to non-burn epidermolytic syndromes. Intensive Care Med. 2010;36(1):22-32. [DOI] [PubMed] [Google Scholar]
- 38.Chantaphakul H, Sanon T, Klaewsongkram J. Clinical characteristics and treatment outcome of Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Ther Med. 2015;10(2):519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirahara K, Kano Y, Sato Y, et al. Methylprednisolone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis: clinical evaluation and analysis of biomarkers. J Am Acad Dermatol. 2013;69(3):496-498. [DOI] [PubMed] [Google Scholar]
- 40.Kardaun SH, Jonkman MF. Dexamethasone pulse therapy for Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2007;87(2):144-148. [DOI] [PubMed] [Google Scholar]
- 41.Roongpisuthipong W, Prompongsa S, Klangjareonchai T. Retrospective analysis of corticosteroid treatment in Stevens-Johnson syndrome and/or toxic epidermal necrolysis over a period of 10 years in Vajira Hospital, Navamindradhiraj University, Bangkok. Dermatol Res Pract. 2014;2014(6):237821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su P, Aw CW. Severe cutaneous adverse reactions in a local hospital setting: a 5-year retrospective study. Int J Dermatol. 2014;53(11):1339-1345. [DOI] [PubMed] [Google Scholar]
- 43.Barron SJ, Del Vecchio MT, Aronoff SC. Intravenous immunoglobulin in the treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis: a meta-analysis with meta-regression of observational studies. Int J Dermatol. 2015;54(1):108-115. [DOI] [PubMed] [Google Scholar]
- 44.Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol. 2012;167(2):424-432. [DOI] [PubMed] [Google Scholar]
- 45.Valeyrie-Allanore L, Ingen-Housz-Oro S, Chosidow O, Wolkenstein P. French referral center management of Stevens-Johnson syndrome/toxic epidermal necrolysis. Dermatologica Sinica. 2013;31(4):191-195. [Google Scholar]
- 46.Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71(5):941-947. [DOI] [PubMed] [Google Scholar]
- 47.Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013;79(5):686-692. [DOI] [PubMed] [Google Scholar]
- 48.Fischer M, Fiedler E, Marsch WC, Wohlrab J. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146(4):707-709. [DOI] [PubMed] [Google Scholar]
- 49.Gubinelli E, Canzona F, Tonanzi T, Raskovic D, Didona B. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36(3):150-153. [DOI] [PubMed] [Google Scholar]
- 50.Hunger RE, Hunziker T, Buettiker U, Braathen LR, Yawalkar N. Rapid resolution of toxic epidermal necrolysis with anti-TNF-alpha treatment. J Allergy Clin Immunol. 2005;116(4):923-924. [DOI] [PubMed] [Google Scholar]
- 51.Redondo P, Ruiz de Erenchun F, Iglesias ME, Monedero P, Quintanilla E. Toxic epidermal necrolysis: treatment with pentoxifylline. Br J Dermatol. 1994;130(5):688-689. [DOI] [PubMed] [Google Scholar]
- 52.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. [DOI] [PubMed] [Google Scholar]
- 53.Riley RD, Sauerbrei W, Altman DG. Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond. Br J Cancer. 2009;100(8):1219-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Literature search: strategies and results
eMethods 2. Eligibility check: form and definition of criteria
eMethods 3. Quality assessment: instrument, form, and score definition
eMethods 4. Data extraction
eTable 1. Overview on the result of the quality assessment of published studies (N = 138)
eTable 2. Overview on included studies (N = 96)
eTable 3. Glucocorticosteroids: substances and dosages (N = 48)
eTable 4. Overview on studies contributing individual patient data for analysis
eFigure 1. Frequency distribution of total quality scores obtained for 138 eligible studies
eFigure 2. Forest plot: comparison of other systemic immunomodulating therapies vs supportive care
eAcknowledgments. Authors who provided additional information
eReferences