Abstract
Atypical antipsychotics increase the risk of diabetes and cardiovascular disease through their side effects of insulin resistance and weight gain. The populations for which atypical antipsychotics are used carry a baseline risk of metabolic dysregulation prior to medication which has made it difficult to fully understand whether atypical antipsychotics cause insulin resistance and weight gain directly. The purpose of this work was to conduct a systematic review and meta-analysis of atypical antipsychotic trials in healthy volunteers to better understand their effects on insulin sensitivity and weight gain. Furthermore, we aimed to evaluate the occurrence of insulin resistance with or without weight gain and with treatment length by using subgroup and meta-regression techniques. Overall, the meta-analysis provides evidence that atypical antipsychotics decrease insulin sensitivity (standardized mean difference = −0.437, p<0.001) and increase weight (standardized mean difference = 0.591, p<0.001) in healthy volunteers. It was found that decreases in insulin sensitivity were potentially dependent on treatment length but not weight gain. Decreases in insulin sensitivity occurred in multi-dose studies less than 13 days while weight gain occurred in studies 14 days and longer (max 28 days). These findings provide preliminary evidence that atypical antipsychotics cause insulin resistance and weight gain directly, independent of psychiatric disease and may be associated with length of treatment. Further, well-designed studies will be needed to assess the co-occurrence of insulin resistance and weight gain and to understand the mechanisms and sequence by which they occur are required.
Keywords: Antipsychotic, insulin, weight, meta-analysis
1. INTRODUCTION
Atypical antipsychotic (AAP) use has been linked to an increased risk of metabolic syndrome, diabetes and cardiovascular disease (1–4). These conditions are, in part, due to the insulin resistance and weight gain caused by these medications (5, 6). The resulting cardiovascular illness leads to elevated rates of mortality in psychiatric populations treated with AAPs compared to the general population (7, 8). Although these drugs provide clear therapeutic benefits, their metabolic side effects can create unfavorable outcomes.
Within the general population, obesity is a significant risk factor for insulin resistance and the two are strongly associated (9). Exceptions exist where either obese individuals are not insulin resistant and/or lean individuals exhibit insulin resistance (10, 11). Many of the AAPs cause significant weight gain and insulin resistance with extended treatment yet, not all AAPs cause metabolic dysfunction to the same degree (5, 12, 13). Additionally, the first generation, or typical, antipsychotics can also cause weight gain so this effect is not exclusive to AAPs (5). Some evidence has suggested AAPs, when compared to typical antipsychotics, may have lower discontinuation rates due to any cause as well as greater weight gain and diabetes however, more work is needed to clarify these comparisons (4, 14). It has been debated whether AAPs cause insulin resistance secondary to, or independent of, weight gain. Pre-clinical models have demonstrated an acute and direct effect of AAPs on insulin sensitivity independent of weight gain which has spurred further hypotheses regarding their effects in humans (15). Indeed, work has shown that patients with schizophrenia on long-term AAP treatment may exhibit insulin resistance even with a body mass index below 30 (i.e.,., non-obese)(6).
Despite data suggesting that AAPs may cause insulin resistance independent of weight gain, the psychiatric diseases for which AAPs are used has also been linked to significant glucose dysregulation prior to AAP treatment (16, 17). This makes drawing conclusions on the role of AAPs in inducing direct insulin resistance in psychiatric populations more difficult. To address this potentially moderating issue, studies have been performed in healthy volunteers to investigate the effect of AAPs on insulin sensitivity in healthy volunteers while simultaneously assessing changes in weight. Although these studies may have reduced external validity, since they exclude individuals that would generally be treated with AAPs, they may provide evidence into the direct effect of AAPs irrespective of the complex disease states they are intended to treat. Ultimately the results of healthy volunteer studies should be taken in context with the results in psychiatric populations to better our understanding of AAP-induced insulin resistance and weight gain.
The primary aim of this meta-analysis was to review the available evidence that AAPs cause insulin resistance and weight gain independent of psychiatric disease based on results from trials in healthy volunteers. Additionally, we aimed to use subgroup and meta-regression techniques to better understand if concurrent weight changes or treatment length could account for changes in insulin sensitivity. The ability to understand if AAPs cause direct insulin resistance prior to and without weight gain is critical for informing future investigations. Such investigations will ultimately identify the therapeutic targets that can potentially prevent the sequelae of AAP-induced insulin resistance (e.g., cardiovascular disease and diabetes) and prevent premature death.
2. MATERIALS AND METHODS
This systematic review followed the guidelines put forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. This review was registered with PROSPERO (protocol # CRD42017063804).
2.1 Inclusion Criteria
To estimate the effect of AAPs on insulin sensitivity and weight independent of psychiatric disease we restricted our analyses to controlled prospective, interventional studies including healthy human subjects (listed as inclusion/exclusion criteria within each study). The treatment could be any AAP approved from any country. The comparator group could be either placebo, a typical antipsychotic or ziprasidone. These non-placebo comparators were permitted to increase study inclusion and because such comparators would be included by investigators as active treatment controls due to their expected low rate of metabolic dysfunction(13). It should be noted, that typical antipsychotics still carry a risk of weight gain and other metabolic side effects making their use as a proper comparator difficult to assess. Due to these limitations, we have included sensitivity analyses detailed below. Finally, studies must have measured both changes in weight and insulin sensitivity by commonly accepted methods (e.g., weight in kilograms, body mass index, hyperinsulinemic euglycemic clamp, etc). Reviews have been conducted on the strengths and limitations of accepted insulin sensitivity measures in clinical populations(18). Single dose studies assessing changes in insulin sensitivity were allowed.
2.2 Search Strategy and Information Sources
Potential studies for inclusion were searched in MEDLINE, EMBASE and PSYCHINFO from earliest record to the date of search (November 2017). Primary language was not a restriction. Keywords used in the search included varying combinations of: “atypical antipsychotic”, “antipsychotic”, “second generation antipsychotic”, “neuroleptic”, “tranquilizer”, the generic names for every individual atypical antipsychotic, “weight”, “insulin resistance”, “insulin sensitivity”, “glucose”, and “insulin”. An original search strategy utilized “healthy subject”, and “healthy volunteer” however we found that this was an ineffective search strategy (for MEDLINE only) that did not yield any appropriate hits (search results of 136 potential papers with no inclusions). Therefore, a broader search was undertaken excluding those two terms. Limits that were defined in the searches were: Clinical Trials and Humans.
2.3 Data Extraction
Included primary studies were searched and data was extracted for both weight and insulin sensitivity for effect size calculations. Extraction was undertaken by two authors and disputes were clarified by a third. Study level variables including AAP used, length of treatment, blinding status, randomization methods, comparator used (placebo or other), and weight or insulin sensitivity measurement used. The final variable was categorized as dynamic (e.g., tests that administer glucose/insulin to measure changes in glucose and insulin) versus non-dynamic (e.g., fasting values only) since dynamic measurements have higher reliability.
2.4 Meta-analysis, Sensitivity analysis, Subgroup analysis and Meta-Regression Analysis
Data was entered into Comprehensive Meta-Analysis (CMA) data software Version 3 for estimation of effect size (standardized mean difference) with 95% confidence intervals through calculations between the treatment and comparator groups. As weight and insulin sensitivity represent continuous variables a standardized mean difference was calculated for each study by the calculating change in value (insulin sensitivity or weight measurement) from provided baseline and endpoint values, endpoint scores (for crossover trials that compare differences between treatment arms) or from provided mean change values, depending on what was reported in each study. For the meta-analysis of insulin sensitivity, the direction of effect size was negative for a treatment that demonstrated reduced insulin sensitivity versus the comparator group. For the meta-analysis of weight, the direction of effect was positive for if the treatment resulted in weight gain versus the comparator group. For studies with multiple treatments and a single comparator, the strategy suggested by Borenstein and colleagues was utilized by computing a combined, overall effect of AAP treatment with the control group (19). This method utilizes the number of participants treated in each group to compute a combined, weighted, effect size while accounting for sharing of the control group in the variance since the groups cannot be assumed to be independent. A 2-sided, random effects meta-analysis was performed with a p<0.05 indicating statistical significance. Heterogeneity was assessed with the Q and I2 parameters to better understand true versus random dispersion of effects across studies. A chi-square test was performed using the heterogeneity parameters to assess statistical significance. An I2 greater than 50% with a p-value<0.05 was considered statistically significant for heterogeneity.
A sensitivity analysis was performed to assess the effect of excluding studies that did not utilize dynamic measurements of insulin sensitivity due to the potentially lower precision. A sensitivity analysis of only studies including a placebo group was performed due to the potential issues with an active comparator and the higher-quality design when utilizing a placebo comparator. Additionally, due to some studies having multiple treatment groups, we conducted a sensitivity analysis of only olanzapine versus placebo extracted values to estimate the effect of an AAP (non-combined effect size) on insulin sensitivity and weight. Olanzapine was chosen as it was the most commonly utilized AAP across studies.
Subgroup analyses were performed to assess study level variables influence on insulin sensitivity or weight. Subgroup analysis was performed by length of treatment within trials and statistically significant weight gain within trials. Length of trials were broken down to three groups: 1) single dose, 2) 13 days and less and 3) 14 days or more. This breakdown was chosen to break the multiple-dose trials at half the length of the longest included trial (28 days). Statistically significant weight gain within trials was defined as a “yes” if the study reported statistically significant weight gain versus comparator (p<0.05) and a “no” if it was reported to be non-statistically significant (p≥0.05). A mixed-effect analysis was used to assess if subgrouping had a significant effect on insulin sensitivity and/or weight. Meta-regression was performed to analyze the influence of weight change on insulin sensitivity with our hypothesis being that weight gain is responsible for a significant amount of AAP’s effect on insulin sensitivity. A Post-Hoc meta-regression analysis using length of trial and weight gain effect size (calculated from the weight meta-analysis) was performed to further understand the effect of this variable on the observed variance for insulin sensitivity. A final meta-regression was performed to assess the effect of weight measurement type (kilograms versus body mass index (BMI)) on each meta-analysis. Study quality and risk of bias was assessed using the Cochrane Risk of Bias Tool and Jadad Scale(20). Publication bias was evaluated via funnel plots, a 2-sided Begg-Maxumdar and Egger’s test and the “trim and fill” method by Duval and Tweedie (21)
3. RESULTS
3.1 Study Selection Results and Description
The preliminary search (from all databases) returned 10142 results (Supplementary Figure 1). Screening of search results by title and abstract excluded a total of 10081 articles. Most exclusions were due to the study being performed in population with mental illness or another disease which was expected based on our expanded search strategy detailed in the methods. Of the 61 remaining articles, 45 were excluded mainly due to the study evaluating non-metabolic outcomes (e.g., pharmacokinetics). Full texts of 16 articles were requested and searched for inclusion and exclusion criteria. Articles were excluded due to not evaluating insulin/glucose/weight outcomes (n=1), not having a comparator group (n=2 studies compared oral versus sublingual formulations of olanzapine) and previously reported results (n=2).
The 11 studies included a total of 304 subjects (22–32). Of the 11 studies, only one study did not include at least olanzapine as the AAP that was evaluated. Additional antipsychotics evaluated versus placebo included sulpride, amisulpride, risperidone, and aripiprazole. The length of the studies ranged from single dose to 28 days. Nine of eleven studies included placebo as the comparator group. The other 2 studies used haloperidol and ziprasidone as a comparator group. Four studies had multiple treatment groups that were compared to a single placebo group (23, 24, 29, 30). Finally, the two single dose studies did not report on weight before and after AAP administration (only baseline values reported) therefore these two studies were excluded from the weight meta-analysis. Further details of the 11 meta-analyzed studies are found in Table 1. The Jadad Scale is in Supplementary Table 1.
Table 1.
Detailed overview of included healthy volunteer trials.
| Reference | Antipsychotic(s) and Comparator (dose in mg/day)* |
Study Design | Duration of Treatment | Number of Subjects | Risk of biasa |
Procedures Used | Funding |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baptista 1997(22) | Sulpride (200) | Randomized, openlabel, placebo-controlled trial | 28 days | Total: 34 | 2 | Oral Glucose Tolerance Test | academic |
| Placebo | Sulpride: 17 | Weight (Kilograms) | |||||
| Placebo: 17 | |||||||
|
| |||||||
| Sowell 2002(23) | Olanzapine(10)* | Randomized, parallel, single-blinded placebo controlled trial. | 15–17 days | Total: 48 | 3 | Hyperglycemic Clamp | Pharmaceutical |
| Risperidone(4)* | Olanzapine: 17 | Body Mass Index | |||||
| Placebo | Inpatient with 9 days of optional outpatient allowed. | Risperidone: 13 | |||||
| Placebo: 18 | |||||||
|
| |||||||
| Sowell 2003(24) | Olanzapine(10)* | Randomized, parallel, single-blinded placebo controlled trial. | 21 days | Total: 55 | 3 | Hyperinsulinemic Euglycemic Clamp | Pharmaceutical |
| Risperidone(4)* | Olanzapine: 22 | ||||||
| Placebo | Inpatient with 9 days of optional outpatient allowed. | Risperidone: 14 | Weight (Kilograms) | ||||
| Placebo: 19 | |||||||
|
| |||||||
| Sacher 2008(25) | Olanzapine(10) | Randomized, parallel, open-label trial | 10 days | Total: 29 | 3 | Pharmaceutical and foundation | |
| Ziprasidone(80) | Olanzapine: 14 | Hyperinsulinemic Euglycemic Clamp | |||||
| Ziprasidone: 15 | Body Mass Index | ||||||
|
| |||||||
| Fountaine 2010(26) | Olanzapine(10)* | Randomized, double-blind, placebo-controlled crossover trial | 15 days of each intervention with 12-day washout in between performed inpatient. | Total: 21 (crossover) | 4 | Hyperinsulinemic Euglycemic Clamp | Pharmaceutical |
| Placebo | Olanzapine: 21 | Weight (Kilograms) | |||||
| Placebo: 21 | |||||||
|
| |||||||
| Vidarsdottir 2010(27) | Olanzapine(10) | Does not specify randomization or blinding characteristics | 8 days | Total: 14 | 1 | Hyperinsulinemic Euglycemic Clamp | Government |
| Haloperidol(3) | Olanzapine: 7 | Weight (Kilograms) | |||||
| Haloperidol: 7 | |||||||
|
| |||||||
| Albaugh 2011(28) | Olanzapine(10) | Randomized, double-blind, placebo-controlled crossover trial | 3-day outpatient interventions with at least 2-week washout period | Total: 15 (crossover) | 5 | Oral Glucose Tolerance Test | Government and Foundation |
| Placebo | Olanzapine: 15 | Weight (Kilograms) | |||||
| Placebo: 15 | |||||||
|
| |||||||
| Kopf 2012(29) | Olanzapine (10) | Randomized, double-blind, single-dose, placebo-controlled, cross-over trial | Single-dose study with 7-day washout period | Total: 10 (crossover) | 3 | Hyperinsulinemic Euglycemic Clamp | Pharmaceutical |
| Amisulpride (200) | Olanzapine: 10 | ||||||
| Placebo | Amisulpride: 10 | ||||||
| Placebo: 10 | |||||||
|
| |||||||
| Teff 2013(30) | Olanzapine(10)* | Randomized, parallel, double-blind, placebo-controlled trial | 9-day inpatient intervention with pre-drug stabilization period | Total: 30 | 4 | Government | |
| Aripiprazole(10)* | Olanzapine: 10 | Hyperinsulinemic Euglycemic Clamp | |||||
| Placebo | Aripiprazole: 10 | Weight (Kilograms) | |||||
| Placebo: 10 | |||||||
|
| |||||||
| Hahn 2013(31) | Olanzapine (10) | Randomized, double-blind, placebo-controlled crossover trial | controlled Single-dose study with 6-day minimum washout period | Total: 15 (crossover) | 3 | Insulin Modified – Frequently Sampled Intravenous Glucose Tolerance Test | Foundation |
| Placebo | Olanzapine: 15 | ||||||
| Placebo: 15 | |||||||
|
| |||||||
| Daurignac 2015(32) | Olanzapine (10)b | Randomized, double-blind, placebo-controlled trial | 14 day with 1-week baseline period | Total: 19 | 3 | Fasting Blood Glucose and Insulin | NA |
| Placebo | Olanzapine: 13 | Weight (Kilograms) | |||||
| Placebo: 6 | |||||||
Gives study -specific description for all 11 included meta-analyzed studies.
indicates titration and taper used.
indicates risk of bias determined with Jadad scale. See supplementary information for details.
dose reduced to 5mg if subjects unable to tolerate
3.2 Effect of Atypical Antipsychotic Treatment on Insulin Sensitivity and Weight in Healthy Individuals
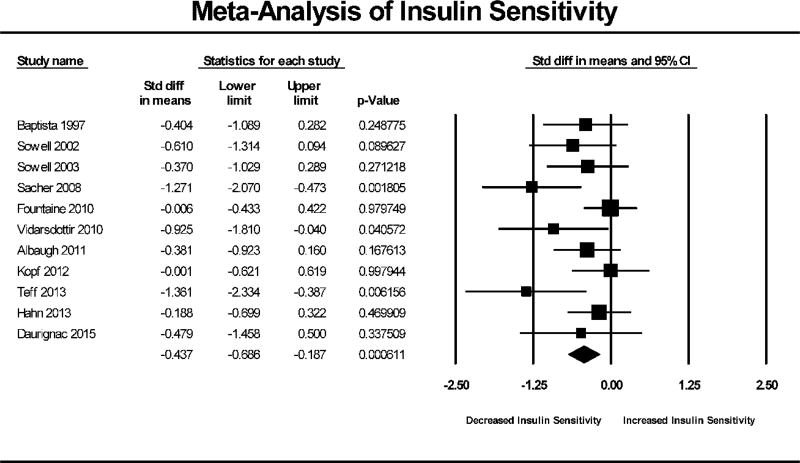
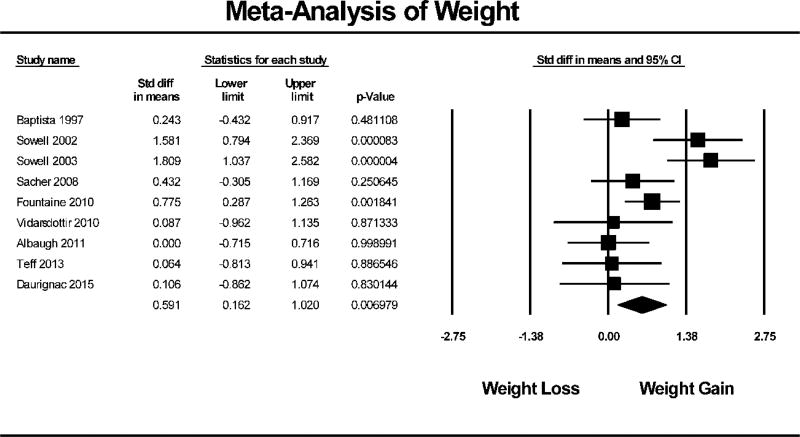
AAP treatment in healthy individuals resulted in an overall significant reduction in insulin sensitivity (N=11, SMD = −0.437, 95% CI: −0.686 to −0.187, p<0.001). Figure 1 shows the Forest Plot of the meta-analysis for insulin sensitivity. Similarly, AAP treatment in healthy individuals also resulted in significant weight gain (N=9, SMD = 0.591, 95% CI: 0.162 to 1.020, p=0.007). The Forest Plot of weight gain is depicted in Figure 2.
Figure 1. Forest Plot of Insulin Sensitivity Meta-Analysis.
Forest plot based on pooled standardized mean differences in insulin sensitivity for each study. Square sizes are proportional to each study’s statistical weight in the meta-analysis. Diamond represents overall estimated effect of AAP on insulin sensitivity.
Figure 2. Forest Plot of Weight Meta-Analysis.
Forest plot based on pooled standardized mean differences in weight for each study. Square sizes are proportional to each study’s statistical weight in the meta-analysis. Diamond represents overall estimated effect of AAP on weight.
3.3 Sub Group Analyses
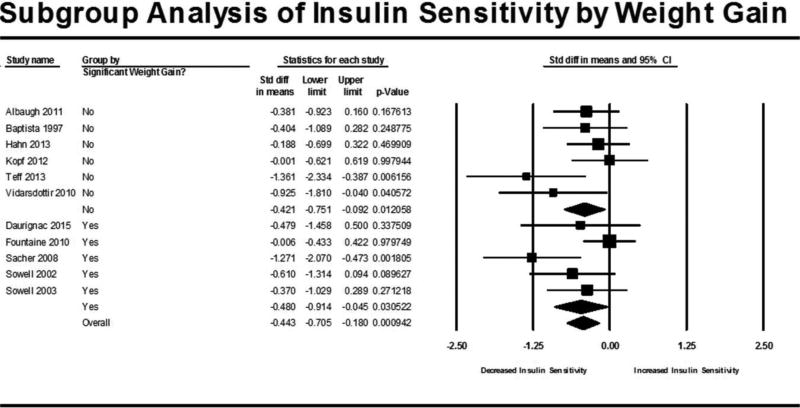
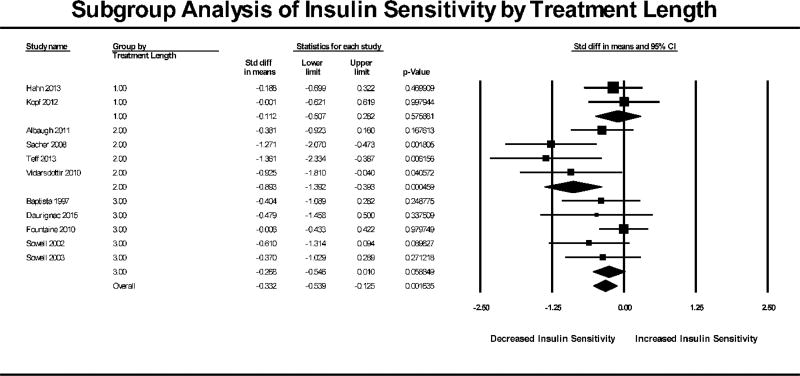
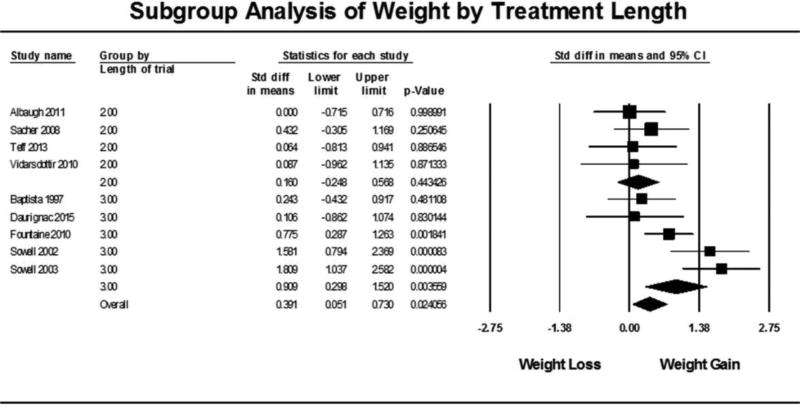
Subgroup analyses were carried out for two conditions: 1) studies showing significant weight gain and 2) length of treatment in each trial defined as single-dose, 13 days or less and 14 days or more. The subgroup analysis based on significant weight gain (Figure 3) showed that there was a reduction in insulin sensitivity for both studies that did not show significant weight gain (N= 6, SMD = −0.421, 95% CI: −0.751 to −0.092, p=0.0126) as well as studies that did have significant weight gain (N = 5, SMD = −0.480, 95% CI: −0.914 to −0.045, p=0.0305). Overall, the effect of subgrouping by weight gain was not statistically significant (p=0.949). The subgroup analysis based on treatment length (Figure 4) showed that insulin sensitivity was not reduced in single dose studies (N=2, SMD = −0.112, 95% CI: −0.507 to 0.282, p=0.576) or in studies 14 days or longer (N=5, SMD = −0.268, 95% CI: −0.546 to 0.010, p=0.0588). Studies that administered the AAP for multiple doses of 13 days or less (trials ranging from 3 to 10 days) had a significant reduction in insulin sensitivity (N=4, SMD = −0.893, 95% CI: −1.392 to −0.393, p<0.001). A chi-square test determined that subgrouping by treatment length had a statistically significant effect on insulin sensitivity (p=0.044). When subgrouping by length of trial for the weight meta-analysis (Figure 5), trials less than 13 days had no significant weight change (N=4, SMD = 0.160, 95% CI: −0.248 to 0.568, p=0.443) while trials 14 days and longer had a significant gain in weight (N=5, SMD = 0.909, 95% CI: 0.298 to 1.520, p=0.004) which was a statistically significant difference between the groups (p=0.046).
Figure 3. Subgroup Analysis of Insulin Sensitivity by Weight Gain.
Forest plot based on pooled standardized mean differences in insulin sensitivity for each study grouped by significant weight gain (determined from meta-analysis by weight). Square sizes are proportional to each study’s statistical weight in the meta-analysis. Diamond represents overall estimated effect of AAP on insulin sensitivity for studies that did not have significant weight gain (labeled as “No”), studies that did have significant weight gain (labeled as “Yes) and all studies combined (labeled as “Overall”).
Figure 4. Subgroup Analysis of Insulin Sensitivity by Treatment Length.
Forest plot based on pooled standardized mean differences in insulin sensitivity for each study grouped by significant treatment length. Group 1.0 were single-dose studies, Group 2.0 were studies 13 days and less and Group 3.0 were studies 14 days and longer (max 28 days). Square sizes are proportional to each study’s statistical weight in the meta-analysis. Diamond represents overall estimated effect of AAP on insulin sensitivity for each subgroup and overall.
Figure 5. Subgroup Analysis of Weight by Treatment Length.
Forest plot based on pooled standardized mean differences in weight for each study grouped by significant treatment length. Group 2.0 were studies 13 days and less and Group 3.0 were studies 14 days and longer (max 28 days). Single-dose studies were not included because studies did not record weight before and after single dose. Square sizes are proportional to each study’s statistical weight in the meta-analysis. Diamond represents overall estimated effect of AAP on weight for each subgroup and overall.
3.4 Sensitivity Analysis
We performed a sensitivity analysis by removing trials with a non-placebo comparator. Studies that included placebo still showed significant reductions in insulin sensitivity (N=9, SMD = −0.298, 95% CI: −0.511 to −0.085, p=0.006) and increases in weight (N=7, SMD = 0.666, 95% CI: −0.141 to 1.191, p=0.013). Of note, the two studies that did use haloperidol and ziprasidone as comparators had significant reductions in insulin sensitivity (N=2, SMD = −0.925, 95% CI: −1.810 to −0.040, p<0.001) but not increases in weight (N=2, SMD = 0.318, 95% CI: −0.285 to 0.920, p=0.302). Another sensitivity analysis was performed by removing the one study that did not utilize dynamic assessments of insulin sensitivity as they can have lower reliability(32). The removal of this study did not substantially change the estimated reduction on insulin sensitivity (N=10, SMD = −0.442, 95% CI: −0.710 to −0.175, p=0.001). Finally, we performed an analysis of only olanzapine versus placebo data due to our strategy to combine multiple treatments in some studies. This meta-analysis showed a reduction in insulin sensitivity (N=8, SMD = −0.330, 95% CI: −0.577 to −0.084, p=0.008) and increase in weight (N=6, SMD = 0.759, 95% CI: 0.126 to 1.392, p=0.018).
3.5 Meta-regression
Meta-regressions were performed utilizing the variables of weight gain effect size (calculated from the meta-analysis of weight gain for each study) and treatment length. Weight gain did not have a significant effect on the variance of insulin sensitivity (p=0.9377) while treatment length, as reflected in the subgroup analysis above, significantly affected insulin sensitivity (p=0.0229). Running the meta-regression model with both variables entered did not improve the fit or explain additional variance (p=0.0647). A meta-regression was also conducted to assess if the type of measurement of weight (change in kilograms or BMI) influenced the weight or insulin sensitivity meta-analyses. Meta-regression including this variable did not identify a statistically significant effect on the weight meta-analysis (p=0.3304) or the insulin sensitivity meta-analysis (p=0.0680).
3.6 Publication Bias
The funnel plot (Supplementary Figure 2) and Egger’s test for all studies included in the insulin sensitivity meta-analysis indicated a possibility for publication bias (p=0.003). A trim and fill analysis trimmed 4 studies and reduced the estimated random effects standardized mean difference from the original −0.437 (95% CI: −0.686 to −0.187) to −0.224 (95% CI: −0.510 to − 0.0623) (Supplementary Figure 3). Publication bias was not evident for the weight meta-analysis (p=0.620) although some asymmetry can be detected in the funnel plot (Supplementary Figure 4). A trim and fill analysis of the weight meta-analysis trimmed 1 study and adjusted the random effects standardized mean difference estimated from the original 0.591 (95% CI: 0.162 to 1.020) to 0.669 (95% CI:0.261 to 1.08) (Supplementary Figure 5). When publication bias was assessed after subgrouping by treatment length in the insulin sensitivity analyses, publication bias was not significant (all p>0.06 except single dose studies did not have a high enough N for publication bias analyses). The Cochrane Risk of Bias Tool is found in Supplementary Table 2.
3.7 Heterogeneity
Statistically significant heterogeneity was not evident amongst all 11 studies included in the insulin sensitivity analysis (p=0.114, I2=35.6%). Meta-regression showed that using length of treatment as a moderator explained an additional 29% of the variance in the insulin sensitivity analysis leaving an I2 of 6.0%. Using weight as a moderator in the meta-regression for insulin sensitivity did not explain any additional variance from the original 35.6%.
In contrast, statistically significant heterogeneity was evident in the 9 studies included in the weight meta-analysis (p=0.003, I2=65.5). When using length of trial as a moderator for the weight meta-analysis, only an additional 11% of variance was accounted for (I2=54.7%). No other study-level variables could account for the observed heterogeneity.
4. DISCUSSION
4.1 Overall Findings
Our meta-analysis of 11 healthy volunteer studies with a total of 304 subjects suggests that treatment with an AAP significantly reduces insulin sensitivity in healthy volunteers which may suggest a direct effect of the drug. An additional meta-analysis in 9 of the 11 studies using multiple dosing also suggests that AAP treatment leads to significant weight gainin healthy volunteers. The overall standardized mean difference was −0.437 for insulin sensitivity and 0.591 for weight. It has been suggested that a mean difference of around 0.5 SD equates to a medium effect size. Due to the differences in reporting insulin sensitivity (methods of measurement) and weight gain (BMI versus kg change) it is difficult to pool raw values and yield an overall mean change in either meta-analysis. The findings here are like those conducted in psychiatric populations treated with AAPs. AAPs have been shown to cause weight gain in first-episode patients previously naive to the AAPs (33). These studies identified rapid weight gain in previously AAP-naive patients (mean increase of 1.8 BMI in 4–8 weeks) that was not only sustained but increased with extended treatment (mean increase of 3.87 BMI with 24–48 weeks of treatment) (33). Similar studies on the rapid changes in insulin resistance in AAP-naive patients are not available. However, several case reports exist of rapid-onset hyperglycemia, diabetic ketoacidosis and new onset diabetes with AAP treatment in previously AAP-naive patients (34–37). Taken together, the findings from this meta-analysis and the literature suggest that psychiatric populations that are treated with AAPs are at an increased baseline risk and treatment with AAPs cause early and sustained insulin resistance and weight gain. It should be noted that, to our knowledge, there are no studies that attempt to estimate the moderating effect of psychiatric diagnosis on AAP-induced insulin resistance and weight gain which should be pursued in future work.
Clinically meaningful reductions in insulin sensitivity are difficult to define as there are no uniform cutoffs for insulin resistance. However, given the statistical significance found within the meta-analysis coupled with the data that demonstrates antipsychotics increase the risk of diabetes 2 to 3-fold, it can be hypothesized that this acute change in insulin sensitivity is clinically meaningful. For weight, an effect size of 0.5 SD is generally considered a moderate effect and longer studies in psychiatric populations suggest that weight gain continues to accumulate. Two studies (23, 25) utilized change in weight, measured by body mass index, as the outcome which could influence the outcomes of the weight meta-analysis. Our study pooled these outcomes to estimate the effect of any AAP on weight however, performing a meta-regression including type of weight measurement (i.e., weight in kg versus BMI) did not have a statistically significant effect (p=0.3304). This meta-analysis only had studies one month or shorter likely due to procedural and ethical limitations of using antipsychotics in healthy volunteers. It may be possible that a longer treatment period (>4 weeks) could lead to higher effect sizes in healthy volunteers and some AAPs may contribute a larger effect compared to others (5, 38).
Within this meta-analysis, we aimed to understand if changes in insulin sensitivity 1) occur in healthy volunteers and 2) could be accounted for by changes in weight. These studies may provide preliminary evidence that AAPs cause insulin resistance and weight gain irrespective of psychiatric disease which supports previous work in both clinical and pre-clinical models. However, both our subgroup and meta-regression techniques did not conclusively show that weight gain accounts for changes in insulin sensitivity. This should be interpreted with caution due to the limited number of included studies and other possibilities. For example, this does not rule out a correlation between reductions in insulin sensitivity and weight gain. Indeed, a few reports included such findings (22, 23, 30, 32). Future studies should consider reporting such correlations and consider multiple (e.g., daily) measurements to better understand how the two-metabolic variables co-occur and potentially influence each other.
4.2 Treatment Length May Influence Insulin Sensitivity and Weight
Using sub-group and meta-regression analyses, this meta-analysis identified that statistically significant changes in insulin sensitivity and weight may depend on treatment length in healthy volunteers. Data demonstrated that a single dose of an atypical antipsychotic does not cause a reduction in insulin sensitivity however, this subgroup was limited in the number of studies (N=2) so this data is inconclusive. Additionally, weight was not reported in single dose studies so we cannot report on a potential effect. Included multi-dose studies ranged from 3 to 28 days and our subgroup analysis observed a significant reduction in insulin sensitivity in studies ranging from 3–13 days but not in studies ranging from 14–28 days. In contrast, weight did not significantly increase in studies ranging from 3–13 days but did increase in studies greater than 14 days. These results may support the hypothesis that insulin resistance occurs prior to significant weight gain. Future studies are required to assess if significant shifts in adiposity, that may not be detected through weight measurement, occur prior, concurrent or after insulin resistance. Additionally, not all studies recorded dietary and caloric intake or measured energy expenditure which could have important implications on the pathogenesis of antipsychotic-induced insulin resistance and weight gain. We hypothesize that antipsychotic-induced insulin resistance occurs acutely (< 2 weeks) followed by perhaps compensatory mechanisms that lead to compensation or masking of insulin resistance and significant weight gain from 2 to 4 weeks. Work will be needed to confirm this hypothesized sequence of events as the findings from this subgroup analysis should be considered preliminary in nature.
4.3 Bias and Heterogeneity
Publication bias occurs when studies that yield negative results are less likely to be published. Based on funnel plot, Egger’s test and Trim and Fill analysis we identified a potential for publication bias in the insulin sensitivity meta-analysis but not the weight meta-analysis. When the studies were sub-grouped by treatment length, this bias was reduced suggesting that there is some correlation between publication bias and treatment length. Our search strategies were broad and inclusive so we believe that we have identified all relevant work to be included. It is not clear what impact non-full text articles may have on outcomes, nevertheless, we cannot rule out an overestimation of effect size in this meta-analysis due to publication bias (39). Therefore, the meta-analysis findings presented here require follow-up upon publication of further studies.
Along with publication bias, another important factor in systematic reviews and meta-analyses is heterogeneity which is a measure the variation from the true effect size. Within our meta-analysis we identified less heterogeneity for insulin sensitivity while higher heterogeneity was observed for the weight meta-analysis. Heterogeneity is generally due to between study methodological differences such as patient populations, outcomes, treatment type and treatment length (see Table 1 and Supplementary Table 1). The included studies did indeed have varying treatment lengths, measurement strategies of insulin sensitivity, follow-up strategies and comparator treatments. Based on our meta-regression analyses we observed that the variable of treatment length accounted for some (29%) of the observed heterogeneity in the insulin sensitivity analysis, reducing I2 from 35.6% to 6%. It is difficult to know if this reduction is due to treatment length, subgrouping in general or other, unknown factors. Additional study-level variables did not improve the observed heterogeneity (data not shown) for the insulin sensitivity meta-analysis and no variable improved the heterogeneity observed in the weight gain meta-analysis.
4.4 Limitations
A few limitations of the meta-analysis should be noted when interpreting the findings. First, although the search strategy was broad and comprehensive, publication bias was still detected in the insulin sensitivity meta-analysis and the total number of studies included was limited. Nevertheless, the findings here do support the findings from psychiatric populations and pre-clinical models (6, 40). The meta-analysis was limited to interventional studies in healthy volunteers to assess the effect of AAPs independent of psychiatric disease. This may reduce the validity of the findings in populations that are treated with AAPs (e.g., psychiatric illness) however, it strengthens the hypothesis that these side effects are medication driven irrespective of specific disease states. It has also been hypothesized that the psychiatric populations for which AAPs are used may be “metabolically vulnerable” which could lead pronounced effects (e.g., further metabolic dysregulation). Although we identified an association between treatment length, weight gain and insulin resistance, this should be interpreted cautiously as these findings were with study-level variables. Future work is needed to simultaneously assess both outcomes, their occurrence during treatment and correlation, if any.
The total number of studies, total number of participants and treatment length was limited in the included studies. This limits the power in subgroup analyses, could cause an erroneous non-significant finding and limits generalizability to practice. Excluding single-dose studies from our subgroup analysis of insulin sensitivity based on treatment length did not alter the findings. The statistical difference between studies <14 days and greater than 14 days was increased (p=0.032) when removing single-dose studies versus when they were included in subgrouping (p=0.044) at described in the results. The limitations in terms of total studies, participants and treatment length could be due to the ethical implications of treating healthy volunteers with antipsychotics and due to the intense nature of the insulin sensitivity measurements (e.g., dynamic testing requires two intravenous lines with glucose/insulin pushes or active infusions) used in 8 of the included studies. Therefore, the suggested findings from this meta-analysis can only be viewed in terms of acute treatment of AAPs and not long-term effects.
The heterogeneity across the studies was higher for the weight analysis and some clear methodological differences can be observed. We could reduce this heterogeneity when accounting for certain study-level variables such as treatment length however this increased heterogeneity remained for the weight meta-analysis. Our extraction strategy for cross-over studies utilized data reported from both periods of the cross-over (i.e., differences in endpoint between treatment groups). This could lead to bias due to carryover effects or possibly period effects (41). Each study utilized endpoint scores from each period to compare treatments. Hahn et. al reported that no sequence effects were detected. Fountaine et. al. study included strategies to test and control for possible carryover effects on their primary outcome of food intake. We utilized an approach to compute a combined, weighted effects size for studies utilizing multiple treatments versus a single comparator. This method allowed us to assess the general effect of AAPs, however, it does not assess individual effects of AAPs or compare between AAPs so this is study only assumes a general effect by AAPs. Most of the studies utilized olanzapine which is anecdotally considered the “standard” AAP for causing metabolic side effects. Our sensitivity analysis of only olanzapine versus placebo data suggested a reduction in insulin sensitivity and an increase in weight, like the overall meta-analysis. Nevertheless, some studies have suggested that insulin resistance may be present with AAPs thought to be lower risk (30). Given this finding, research utilizing highly sensitive strategies for measuring insulin sensitivity and weight should be pursued for other AAPs and studies should be designed to compare the effects between AAPs.
5. Conclusions
Overall, this meta-analysis provides evidence that antipsychotic-induced insulin resistance and weight gain may occur acutely and directly. The findings regarding treatment length effects may have been underpowered therefore they should be considered preliminary and follow-up work is needed. The evidence outlined in this meta-analysis is clinically meaningful because even short trials (e.g., 4 weeks and less) with an antipsychotic could potentially induce metabolic dysregulations that require monitoring and intervention. Future work is needed to better understand the correlations between these side effects, their dependence on treatment length and in understanding their mechanisms.
Supplementary Material
HIGHLIGHTS.
This meta-analysis presents evidence that atypical antipsychotics cause insulin resistance and weight gain independent of psychiatric disease.
Decreases in insulin sensitivity occurred both with and without significant weight gain when analyzed by subgrouping.
Insulin resistance and weight gain may be associated with treatment length however this requires further research.
Further work is needed to understand the interplay of insulin resistance and weight gain with atypical antipsychotic use and the mechanisms by which they occur.
Acknowledgments
This work was supported by NIH/NIDDK R01DK081750 (ZY), R01DK107666 (ZY), American College of Clinical Pharmacy Futures Research Grant (KB), Michigan Diabetes Research Center NIH Grant 2P30-DK020572 (KB) and a Wayne State University Faculty Research Award Program Grant (KB, ZY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have conflicts of interest to disclose relating to the content of the article.
Ethical Statement
All authors made contributions to the idea and concept of the study. All authors assisted in manuscript preparation, editing and approved the final form of the manuscript. KB and BS completed the systematic review and data collection. PB served as the tertiary reviewer during systematic review. BS and AM provided expertise in the collection of data and assessment relating to clinical endocrinology methods utilized in studies. PB, RK and ZY assisted with meta-analysis methodology and interpretation.
References
- 1.Silva A, Ribeiro M, Sousa-Rodrigues CF, Barbosa FT. Association between antipsychotics and cardiovascular adverse events: A systematic review. Revista da Associacao Medica Brasileira (1992) 2017;63(3):261–7. doi: 10.1590/1806-9282.63.03.261. Epub 2017/05/11. [DOI] [PubMed] [Google Scholar]
- 2.Vancampfort D, Correll CU, Galling B, Probst M, De Hert M, Ward PB, Rosenbaum S, Gaughran F, Lally J, Stubbs B. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World psychiatry : official journal of the World Psychiatric Association (WPA) 2016;15(2):166–74. doi: 10.1002/wps.20309. Epub 2016/06/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S, Correll CU. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World psychiatry : official journal of the World Psychiatric Association (WPA) 2015;14(3):339–47. doi: 10.1002/wps.20252. Epub 2015/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith M, Hopkins D, Peveler RC, Holt RI, Woodward M, Ismail K. First-v. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. The British journal of psychiatry : the journal of mental science. 2008;192(6):406–11. doi: 10.1192/bjp.bp.107.037184. Epub 2008/06/03. [DOI] [PubMed] [Google Scholar]
- 5.Bak M, Fransen A, Janssen J, van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PloS one. 2014;9(4):e94112. doi: 10.1371/journal.pone.0094112. Epub 2014/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, Weber MT, Anderson EJ, Allison DB, Daley TB, Schoenfeld D, Goff DC. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Archives of general psychiatry. 2005;62(1):19–28. doi: 10.1001/archpsyc.62.1.19. Epub 2005/01/05. [DOI] [PubMed] [Google Scholar]
- 7.Murray-Thomas T, Jones ME, Patel D, Brunner E, Shatapathy CC, Motsko S, Van Staa TP. Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database. Cardiovascular psychiatry and neurology. 2013;2013:247486. doi: 10.1155/2013/247486. Epub 2014/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, Thapa-Chhetri N, Fornaro M, Gallicchio D, Collantoni E, Pigato G, Favaro A, Monaco F, Kohler C, Vancampfort D, Ward PB, Gaughran F, Carvalho AF, Stubbs B. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World psychiatry : official journal of the World Psychiatric Association (WPA) 2017;16(2):163–80. doi: 10.1002/wps.20420. Epub 2017/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez KE, Tucker LA, Bailey BW, LeCheminant JD. Expanded Normal Weight Obesity and Insulin Resistance in US Adults of the National Health and Nutrition Examination Survey. J Diabetes Res. 2017;2017:9502643. doi: 10.1155/2017/9502643. Epub 2017/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diabetes & vascular disease research. 2005;2(3):105–12. doi: 10.3132/dvdr.2005.017. Epub 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 11.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Archives of internal medicine. 2008;168(15):1609–16. doi: 10.1001/archinte.168.15.1609. Epub 2008/08/13. [DOI] [PubMed] [Google Scholar]
- 12.Gianfrancesco F, Grogg A, Mahmoud R, Wang RH, Meletiche D. Differential effects of antipsychotic agents on the risk of development of type 2 diabetes mellitus in patients with mood disorders. Clinical therapeutics. 2003;25(4):1150–71. doi: 10.1016/s0149-2918(03)80073-5. Epub 2003/06/18. [DOI] [PubMed] [Google Scholar]
- 13.Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, Kissling W, Davis JM, Leucht S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophrenia research. 2010;123(2–3):225–33. doi: 10.1016/j.schres.2010.07.012. Epub 2010/08/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2013;16(6):1205–18. doi: 10.1017/s1461145712001277. Epub 2012/12/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ader M, Kim SP, Catalano KJ, Ionut V, Hucking K, Richey JM, Kabir M, Bergman RN. Metabolic dysregulation with atypical antipsychotics occurs in the absence of underlying disease: a placebo-controlled study of olanzapine and risperidone in dogs. Diabetes. 2005;54(3):862–71. doi: 10.2337/diabetes.54.3.862. Epub 2005/03/01. [DOI] [PubMed] [Google Scholar]
- 16.Greenhalgh AM, Gonzalez-Blanco L, Garcia-Rizo C, Fernandez-Egea E, Miller B, Arroyo MB, Kirkpatrick B. Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naive patients with nonaffective psychosis. Schizophrenia research. 2017;179:57–63. doi: 10.1016/j.schres.2016.09.026. Epub 2016/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pillinger T, Beck K, Gobjila C, Donocik JG, Jauhar S, Howes OD. Impaired Glucose Homeostasis in First-Episode Schizophrenia: A Systematic Review and Meta-analysis. JAMA psychiatry. 2017;74(3):261–9. doi: 10.1001/jamapsychiatry.2016.3803. Epub 2017/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. American journal of physiology Endocrinology and metabolism. 2008;294(1):E15–26. doi: 10.1152/ajpendo.00645.2007. Epub 2007/10/25. [DOI] [PubMed] [Google Scholar]
- 19.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Multiple Comparisons within a Study. Introduction to Meta-Analysis: John Wiley & Sons, Ltd. 2009:239–42. [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled clinical trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. Epub 1996/02/01. [DOI] [PubMed] [Google Scholar]
- 21.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. doi: 10.1111/j.0006-341x.2000.00455.x. Epub 2000/07/06. [DOI] [PubMed] [Google Scholar]
- 22.Baptista T, Molina MG, Martinez JL, de Quijada M, Calanche de Cuesta I, Acosta A, Paez X, Martinez JM, Hernandez L. Effects of the antipsychotic drug sulpiride on reproductive hormones in healthy premenopausal women: relationship with body weight regulation. Pharmacopsychiatry. 1997;30(6):256–62. doi: 10.1055/s-2007-979503. Epub 1998/01/27. [DOI] [PubMed] [Google Scholar]
- 23.Sowell MO, Mukhopadhyay N, Cavazzoni P, Shankar S, Steinberg HO, Breier A, Beasley CM, Jr, Dananberg J. Hyperglycemic clamp assessment of insulin secretory responses in normal subjects treated with olanzapine, risperidone, or placebo. The Journal of clinical endocrinology and metabolism. 2002;87(6):2918–23. doi: 10.1210/jcem.87.6.8599. Epub 2002/06/07. [DOI] [PubMed] [Google Scholar]
- 24.Sowell M, Mukhopadhyay N, Cavazzoni P, Carlson C, Mudaliar S, Chinnapongse S, Ray A, Davis T, Breier A, Henry RR. Evaluation of insulin sensitivity in healthy volunteers treated with olanzapine, risperidone, or placebo: a prospective, randomized study using the two-step hyperinsulinemic, euglycemic clamp. The Journal of Clinical Endocrinology & Metabolism. 2003;88(12):5875–80. doi: 10.1210/jc.2002-021884. [DOI] [PubMed] [Google Scholar]
- 25.Sacher J, Mossaheb N, Spindelegger C, Klein N, Geiss-Granadia T, Sauermann R, Lackner E, Joukhadar C, Muller M, Kasper S. Effects of olanzapine and ziprasidone on glucose tolerance in healthy volunteers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(7):1633–41. doi: 10.1038/sj.npp.1301541. Epub 2007/08/23. [DOI] [PubMed] [Google Scholar]
- 26.Fountaine RJ, Taylor AE, Mancuso JP, Greenway FL, Byerley LO, Smith SR, Most MM, Fryburg DA. Increased food intake and energy expenditure following administration of olanzapine to healthy men. Obesity (Silver Spring, Md) 2010;18(8):1646–51. doi: 10.1038/oby.2010.6. Epub 2010/02/06. [DOI] [PubMed] [Google Scholar]
- 27.Vidarsdottir S, de Leeuw van Weenen JE, Frolich M, Roelfsema F, Romijn JA, Pijl H. Effects of olanzapine and haloperidol on the metabolic status of healthy men. The Journal of clinical endocrinology and metabolism. 2010;95(1):118–25. doi: 10.1210/jc.2008-1815. Epub 2009/11/13. [DOI] [PubMed] [Google Scholar]
- 28.Albaugh VL, Singareddy R, Mauger D, Lynch CJ. A double blind, placebo-controlled, randomized crossover study of the acute metabolic effects of olanzapine in healthy volunteers. PloS one. 2011;6(8):e22662. doi: 10.1371/journal.pone.0022662. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopf D, Gilles M, Paslakis G, Medlin F, Lederbogen F, Lehnert H, Deuschle M. Insulin secretion and sensitivity after single-dose amisulpride, olanzapine or placebo in young male subjects: double blind, cross-over glucose clamp study. Pharmacopsychiatry. 2012;45(6):223–8. doi: 10.1055/s-0031-1301365. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 30.Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–40. doi: 10.2337/db13-0430. Epub 2013/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn MK, Wolever TM, Arenovich T, Teo C, Giacca A, Powell V, Clarke L, Fletcher P, Cohn T, McIntyre RS, Gomes S, Chintoh A, Remington GJ. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. Journal of clinical psychopharmacology. 2013;33(6):740–6. doi: 10.1097/JCP.0b013e31829e8333. Epub 2013/10/09. [DOI] [PubMed] [Google Scholar]
- 32.Daurignac E, Leonard KE, Dubovsky SL. Increased lean body mass as an early indicator of olanzapine-induced weight gain in healthy men. International clinical psychopharmacology. 2015;30(1):23–8. doi: 10.1097/yic.0000000000000052. Epub 2014/10/29. [DOI] [PubMed] [Google Scholar]
- 33.Tarricone I, Ferrari Gozzi B, Serretti A, Grieco D, Berardi D. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychological medicine. 2010;40(2):187–200. doi: 10.1017/s0033291709990407. Epub 2009/08/07. [DOI] [PubMed] [Google Scholar]
- 34.Porras-Segovia A, Krivoy A, Horowitz M, Thomas G, Bolstridge M, Ion D, Shergill SS. Rapid-onset clozapine-induced loss of glycaemic control: case report. BJPsych open. 2017;3(3):138–40. doi: 10.1192/bjpo.bp.117.004481. Epub 2017/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vuk A, Kuzman MR, Baretic M, Osvatic MM. Diabetic ketoacidosis associated with antipsychotic drugs: case reports and a review of literature. Psychiatria Danubina. 2017;29(2):121–35. doi: 10.24869/psyd.2017.121. Epub 2017/06/22. [DOI] [PubMed] [Google Scholar]
- 36.Lipscombe LL, Austin PC, Alessi-Severini S, Blackburn DF, Blais L, Bresee L, Filion KB, Kawasumi Y, Kurdyak P, Platt RW, Tamim H, Paterson JM. Atypical antipsychotics and hyperglycemic emergencies: multicentre, retrospective cohort study of administrative data. Schizophrenia research. 2014;154(1–3):54–60. doi: 10.1016/j.schres.2014.01.043. Epub 2014/03/04. [DOI] [PubMed] [Google Scholar]
- 37.Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(9):1997–2004. doi: 10.1038/npp.2010.78. Epub 2010/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tek C, Kucukgoncu S, Guloksuz S, Woods SW, Srihari VH, Annamalai A. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early intervention in psychiatry. 2016;10(3):193–202. doi: 10.1111/eip.12251. Epub 2015/05/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmucker CM, Blumle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, von Elm E, Briel M, Meerpohl JJ. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PloS one. 2017;12(4):e0176210. doi: 10.1371/journal.pone.0176210. Epub 2017/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyda HN, Tse L, Procyshyn RM, Honer WG, Barr AM. Preclinical models of antipsychotic drug-induced metabolic side effects. Trends Pharmacol Sci. 2010;31(10):484–97. doi: 10.1016/j.tips.2010.07.002. Epub 2010/08/03. [DOI] [PubMed] [Google Scholar]
- 41.Li T, Yu T, Hawkins BS, Dickersin K. Design, Analysis, and Reporting of Crossover Trials for Inclusion in a Meta-Analysis. PloS one. 2015;10(8):e0133023. doi: 10.1371/journal.pone.0133023. Epub 2015/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.