Abstract
Introduction
Breast cancer survivors face dual challenges: long term sequelae of treatment, and risk of recurrent disease. Obesity and a sedentary lifestyle complicate both challenges. The WISER Survivor trial assessed the effects of exercise and/or weight-loss on lymphedema, biomarkers of breast cancer recurrence, and quality of life. We report on the innovative transdisciplinary design of this trial and report attrition rates.
Methods
This one year trial randomized breast cancer survivors who had a BMI of ≥25 kg/m2, were sedentary and had breast-cancer-related-lymphedema to 1) exercise (weight training and aerobic exercise) 2) weight-loss 3) exercise and weight-loss 4) or control group. Innovative aspects included: adaptation of a community-based weight training program to a largely home-based program; use of a commercial meal replacement system as part of the lifestyle modification weight-loss program; inclusion of measures of cost-effectiveness to enable economic evaluations; and alignment with a parallel mouse model for breast cancer recurrence to enable transdisciplinary research. In this model, mice bearing dormant residual tumor cells, which spontaneously relapse, were placed on a high-fat diet. Overweight animals were randomly assigned to exercise, calorie restriction, both, or control group and followed for cancer recurrence. The animal model will guide mechanistic biomarkers to be tested in the human trial.
Results & discussion
351 participants were randomized; 13 experienced breast cancer recurrence during the trial. Of the 338 participants without recurrence, 83% completed the trial. The WISER Survivor trial will show the effects of exercise and weight-loss on lymphedema outcomes, biomarkers of recurrence and quality of life.
NCT ClinicalTrials.gov registration #: NCT01515124
Keywords: Lymphedema, Biomarkers, Breast cancer, Relapse
1. Introduction
Breast cancer survivors face many challenges, typically divided into two broad categories: persistent adverse effects of treatment, and risk of recurrent disease [1]. The WISER Survivor trial was designed to address both challenges by assessing the effects of exercise and/or weight-loss on lymphedema (a persistent treatment effect), and biomarkers for recurrence. Additionally, it assessed effects on quality of life.
Breast cancer-related lymphedema incidence varies from 20 to 35% largely depending on whether patients are treated with sentinel lymph node biopsy or axillary dissection [2]. Lymphedema is associated with discomfort, depression, and disability, and compromises medical, social, functional, vocational, and psychological status [3–6]. Obesity increases the risk of breast cancer-related lymphedema [7–9]. Moreover, obesity and lymphedema may act synergistically to erode health-related quality of life [10–12].
Randomized trials demonstrated that upper body strength training is safe and increases muscular strength among survivors suffering from lymphedema [13,14]. Our group previously demonstrated that a twice-weekly slowly-progressive weight training program halved the incidence of clinical events that required medical care for lymphedema [15], but had no effect on the cardinal feature of lymphedema: arm swelling. Two small studies suggest that weight-loss can be beneficial with regard to lymphedema outcomes [16,17]. However, the combined effect of weight-loss and weight training on arm swelling has not yet been studied.
Large observational studies have consistently observed reduced breast cancer recurrence among women who are physically active, with maximal benefits seen for an amount of activity roughly equivalent to 30 min of walking per day [18,19]. In contrast, observational data on breast cancer recurrence and weight change are not fully consistent. For example, the Nurses’ Health Study suggested that weight gain was associated with increased risk of recurrence, but the LACE cohort found no association [20,21]. Several large intervention studies in breast cancer survivors are currently ongoing to study the effect of weight-loss on breast cancer outcomes [22].
Previous studies in breast cancer survivors have examined the effects of exercise and weight-loss on mechanistic pathways [23–28], mainly focusing on insulin and insulin-like growth factors, inflammation, and the immune system. Based on research of mechanistic pathways in primary breast cancer, other pathways may also be potentially relevant [29,41–52]: these include those involving insulin resistance, growth factors and adipokines; sex steroid hormones; pathogenic angiogenesis; and inflammation/oxidative stress. So far few, if any, studies have examined effects on all potentially relevant pathways.
The WISER Survivor trial was designed to test the effects of exercise and/or weight-loss on lymphedema, biomarkers for recurrence and quality of life. The hypothesis is that exercise and weight loss will affect these outcomes, but that the combined effect will be larger. WISER Survivor was performed as part of the Transdisciplinary Research on Energetics and Cancer (TREC) initiative [29,30]. The Penn TREC Survivor center focused on three interwoven projects to span the translational research continuum from basic animal research through clinical research to economic evaluation and dissemination (Fig. 1). This setting signifies the unique features of the WISER Survivor trial: the alignment of a human trial with a parallel mouse study and an economic evaluation of the intervention. The current paper describes the innovative design and methods of the WISER Survivor trial, provides a description of baseline characteristics of participants, and reports retention rates.
Fig. 1.
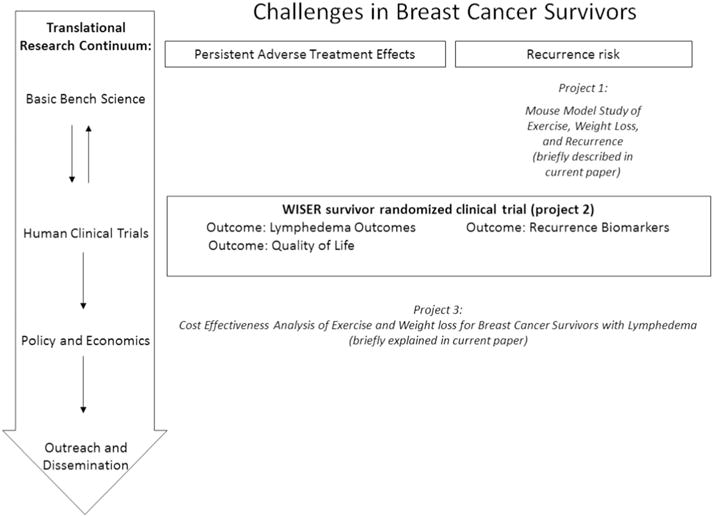
Overview of three interwoven projects conducted at the Penn TREC center of which the WISER Survivor trial was part. Project 1 was an animal model of breast cancer recurrence. Project 2 was the WISER Survivor trial. Project 3 was a cost-effectiveness analysis of the WISER Survivor trial.
2. Methods
2.1. Study population and recruitment
Eligible participants were female sedentary breast cancer survivors aged 80 years or younger with a BMI of ≥25 kg/m2 with breast cancer-related lymphedema who had completed curative treatment at least six months prior to randomization and were currently free of cancer. Exclusion criteria were: grade 4 lymphedema, medical conditions or medications that would prohibit participation in an exercise program, inability to walk for 6 min unaided, extreme obesity (body mass index > 50 kg/m2), plans for additional (e.g. curative or reconstructive) surgery during the study period, self-report of weight training within the past year, already engaging in 3 or more times weekly aerobic activity of moderate intensity (e.g. brisk walking, step aerobics or bicycling), planning to move away from the area over the next year, current use of weight-loss medication (OTC or prescription), self-report of alcohol or substance abuse within the past 12 months, including at-risk drinking (current consumption of > 14 alcoholic drinks per week), and weight-loss > 10% of body weight in the past 3 months. In addition, we specified that participants experiencing a cancer recurrence (except for non-melanoma skin cancers) during the study would be removed from the study at the time of recurrence.
Recruitment strategies were targeted to increase the participation of racial/ethnic minorities as described earlier [31]. Over a 39-month period, participants were recruited through both active strategies such as community education events supplemented and passive approaches, such as mailings to survivors identified through hospital and state cancer registries.
2.2. Randomization and design of the interventions
WISER Survivor trial was a 12-month, four-arm, randomized controlled intervention trial. The four equally sized arms were: 1) exercise (weight training and aerobic exercise), 2) weight-loss through lifestyle modification, 3) both exercise and weight-loss and 4) control group. The primary aim was to determine the effect of the interventions on change in interlimb volume difference. The secondary aims were to assess the effects of the intervention on biomarkers of breast cancer recurrence and to assess effects of the interventions on health-related quality of life. An additional project leveraged the WISER Survivor study to assess cost-effectiveness of the three tested interventions. The trial was executed in 15 waves throughout a four year period; within each wave, women were assigned to one of the four groups in equal numbers, using the process of minimization [32]. Minimization ensures balanced groups over a set of possible confounding factors. We defined the following factors, each of which is hypothesized to be associated with the clinical course of lymphedema: baseline BMI (above or below BMI of 37.5 kg/m2), age (above or below 65 years), radiation (yes or no), number of nodes removed (above or below 5), lymphedema severity (grade 1, 2, 3 or 4), and trunk edema only (yes or no). With a trial that was executed in waves, a stratified, blocked, blinded randomization was infeasible. We used the software program MINIM (MINIM, version 1.5) [53] for the minimization process. All measurement staff remained blinded throughout the study.
2.3. Exercise intervention: weight training and aerobic exercise
The exercise intervention combined a twice-weekly weight training intervention with 180 min per week aerobic exercise (mostly walking). The intervention was based on our earlier completed trials, which were mainly community-based [15,33–36]. In the current WISER Survivor trial, we adapted the intervention into a largely home-based setting, see Table 1 for an overview of the exercise intervention. The weight training was designed based on the PAL trial [15]. However, the PAL trial exercise program took place within fitness centers. Qualitative research from the PAL trial indicated that a home based program might be preferred by breast cancer survivors. A pilot study was undertaken that successfully translated the intervention to the home setting (unpublished data). Revisions to the PAL trial intervention to make it home based were also included in the physical therapy adaptation of the PAL intervention, called Strength After Breast Cancer [37]. This version of the intervention has now been documented to be equally effective for lymphedema outcomes [37] and is broadly disseminated (> 400 centers across the U.S.), given the availability of an online training to prepare allied health professionals to deliver the Strength After Breast Cancer program [38]. Further, we included aerobic exercise as component of the exercise intervention to be able to study whether adding this component to the previously established, effective intervention for lymphedema of the PAL trial would have additional benefits on lymphedema outcomes and on biomarkers.
Table 1.
Overview of the dosage of exercise prescribed by intervention week for participants in the exercise or exercise and weight-loss arms of the WISER Survivor trial.
| Week | Supervision | Weight training sessions per week | Weight training sets/exercise | Weight training time per session | Aerobic sessions per week | Aerobic time per session | Total exercise time/week |
|---|---|---|---|---|---|---|---|
| 1–3 | One supervised, one unsupervised weight training session per week | 2 | 2 sets of 10 reps/exercise | 70–90 min | 3 | 30 min | 230–270 |
| 2–4 | 2 | 2 sets of 10 reps/exercise | 70–90 min | 4 | 30 min | 260–300 | |
| 5–6 | 2 | 3 sets of 10 reps/exercise | 70–90 min | 5 | 30 min | 290–330 | |
| 7–52 | Weekly calls for motivation & support for first 6 months, then monthly for months 6–12. Monthly group meetings for ongoing behavioral support group weight training | 2 | 3 sets of 10 reps/exercise | 60–80 min | 6 | 30 min | 300–340 |
In weeks 1–6, participants were trained by certified fitness professionals on the weight training intervention and how to safely increase their aerobic exercise activity to 180 min per week; weight training was group-based. In addition, participants were instructed to do one unsupervised weight training session per week in weeks 1–6. Adjustable dumbbells (PowerBlocks, Inc., Owatanna, MN) were shipped directly to participants’ homes upon randomization to an exercise group. Women were required to wear their compression garment during all weight lifting activities. They were not required to wear it during aerobic exercise.
During the first 6 weeks of the intervention, there were weekly group-based sessions to teach participants the exercise program. The group-based weight training sessions during the first 6 weeks of the intervention provided the participants with: instruction on basic weight training technique, and on the nine weight training exercises, documentation of exercise logs, review of accurate tracking procedures, and provided the contact information of the exercise physiologist for questions and concerns throughout the study.
Each supervised weight training session consisted of five parts:
A warm up session of at least 10 min at low to moderate intensity (brisk walking, bicycling or steps aerobics). Level intensity was defined as moderate using the Borg scale [39]
Stretching exercises for each of the major muscle groups, 15 s per stretch.
Core training exercises of abdominal and lower back muscles, including one stabilization, one flexion, and one extension core exercise ten repetitions per set per exercise.
Weight training exercises. No more than three weight training exercises were introduced per session; the full protocol was introduced over four weeks. Exercises included nine exercises: chest-presses, squats on a chair, one-arm rowing exercise, side-raises, step-ups, kickbacks, split-leg lunges, side lunges, and bicep-curls. Participants received an instruction booklet with color pictures of the exercises and things to remember when doing the exercises. Participants were instructed to perform the exercise in the order as instructed during the supervised sessions, to do two sets of ten repetitions for each exercise during the first month, and to add a third set per exercise starting in week 5 and for the remainder of the study. Participants were instructed to start with light weights and to increase the resistance by the smallest available increment (e.g. 1 lb) for each exercise when they could do three sets of the exercise with proper form for two consecutive sessions. If there were no changes in lymphedema-related or other symptoms, participants could follow this incremental progression. If there was worsening of lymphedema symptoms, the upper body exercises were removed from the exercise program for a week until symptoms resolved. If there were musculoskeletal symptoms or other reasons why a person could not do a certain exercise, the exercise was skipped until symptoms resolved, or replaced with a different exercise that did not give symptoms if symptoms did not resolve.
Cool down consisted of stretching exercises for each of the major muscle groups for at least 30 s per side.
During weeks 7–52, participants were instructed to continue with the weight training using the same routine as during the first 6 weeks, including gradual progression of resistance. The home-based exercises during weeks 7–52 were unsupervised, with additional supervised monthly in-person sessions. Monthly in-person sessions consisted of a meeting with the fitness professional to address questions and concerns, and to provide behavioral counseling to improve intervention adherence and exercise-related behavior. According to the preference of the participants, they did their weight training in their homes or in a community gym facility except for the first six weekly sessions and monthly check-in sessions which were completed at the intervention site closest to the participant’s home [31].
For the exercise intervention, all fitness professionals were trained in motivational interviewing and used the six supervised sessions at the beginning of the intervention to establish the participants’ motivations and expectations for participation. This information was used to enhance adherence to the exercise intervention if necessary. Confidence to complete upcoming exercise sessions was assessed during all in person and phone counseling sessions, and appropriate discussion regarding what would increase confidence and reminders of what they did when they were successful were used, as per standard motivational interviewing techniques [40]. Participants in the exercise groups completed exercise logs. During weeks 1–6, fitness professionals collected and reviewed these logs during the group-based sessions. Weeks 7–52, exercise logs were collected and reviewed during the monthly group-based sessions. If participants missed one of these sessions, the fitness professionals called the participants. In addition during weeks 7–52 there were weekly calls for motivation and support for the first 6 months, then monthly for the remainder of that period. These brief phone calls were positive, encouraging, empathetic and non-confrontational. As with our prior studies, these calls were generally brief, rarely lasting longer than 15 min. Participants randomized to this group were referred to the American Cancer Society website if they had diet-related questions.
2.4. Weight-loss intervention
The weight-loss intervention was based on several earlier trials [41,42] and was tailored to the needs of breast cancer survivors [43,44], see Table 2 for an overview of the weight-loss intervention. The intervention started with a 24 week intensive phase that included weekly 1 h group meetings and provision of meals and snacks from a commercial manufacturer (NutriSystem®, Inc., Fort Washington, PA). The in-person meetings included a weigh-in, discussion of adherence to the planned dietary approach, and a behavioral modification lesson. The dietary approach included four NutriSystem® meals per day (breakfast, lunch, dinner, dessert), one protein shake per day, four servings of vegetables, three servings of fruit, two servings of low-fat dairy or lean protein such as fish or meat, and one serving of fat, such as butter/spreads/oils/nuts/salad dressing/seeds. During the 24 week intensive phase, participants paid $105 each month for the NutriSystem® meals (75% discount compared with commercial costs of the meals); scholarships were provided to women who expressed concern about paying. Each in-person session included a behavioral modification lesson on topics such as goal setting, problem solving, preparing and practicing for difficult situations (e.g. holiday parties) etc. During each in-person session a different topic was addressed. Table 2 displays the specific topics that were covered. During the first 20 weeks, daily caloric intake was restricted to 1200–1500 kcals/day, representing a reduction of 40–50% from usual caloric intake. During weeks 20–24, participants were instructed to gradually incorporate “conventional foods” available in their grocery stores with a goal of staying at the same number of calories per day until reaching the study defined goal of 10% weight-loss. After week 24, there was a gradual increase in caloric intake of no > 500 kcal/day, which resulted in caloric intake between 1700 and 2000 kcal/day. From week 24 onwards, the aim was maintenance of a stable body weight throughout the remainder of the intervention. In this period, participants took part in monthly group meetings and had weekly phone contact with the dietitian. Groups of 2–6 participants were led by registered dietitians experienced with the NutriSystem® program. Group meetings took place in the community gym facilities where the exercise interventions also took place. Throughout the intervention, participants used paper or electronic food diaries to self-monitor their dietary intake. Dietitians reviewed these diaries either in-person or over the phone.
Table 2.
Overview of the weight-loss intervention by intervention week for participants in the weight-loss or exercise and weight-loss arms of the WISER Survivor trial Lymphedema Care for Participants.
| Week | Supervision | Frequency of sessions | Meeting length | NutriSystem® meal replacements provided | Caloric intake |
|---|---|---|---|---|---|
| 1–19 | Weekly meetings/lessonsa led by registered dietitian | 1 per week | 60 min | 4 daily meals: breakfast, lunch, dinner, dessert | 1200–1500 kcal/day |
| 20–24 | Weekly meetings/lessonsb led by registered dietitian | 1 per week | 60 min | Gradually decreasing from 3 in week 20 to none in week 24, gradually increasing conventional foods | 1200–1500 kcal/day |
| Remainder of weeks | Monthly meetings/lessonsc led by registered dietitian, weekly phone calls or emails from registered dietitian between meetings | 1 per month | 60 min | None | Gradually increasing to 1700–2000 kcal/day |
Lessons discussed the following topics 1: Introduction to the program, 2: External cues, 3: Overweight as a risk factor, 4: Problem Solving, 5: Fruits and Vegetables, 6: Stress and Emotional Eating, 7: Dealing with Negative Thoughts, 8: Dealing with Lapse and Relapse, 9: Social Support, 10: Healthy Dining Out, 11: Alcohol, 12: Handling Holidays/Vacations, 13: Coping with Cravings, 14: Calcium and Vitamin D, 15: Continuum of Cancer Care, 16: Body Image, 17: Mood, Hunger, and Overeating, 18: Dietary Fat, 19: Fad Diets.
Lessons discussed the following: 20: Conventional Foods/Tipping the Calorie Balance, 21: Ways to Eat Fewer Calories, 22: Grocery Shopping, 23: Healthy Eating, 24: Ingredients and Recipes.
Lessons discussed the following: 25: Volumetrics/Caloric Density, 26: Tune Up, 27: Ways to Stay Motivated, 28: Maintaining Energy Balance, 29: Becoming a Weight Loss Expert, 30: Preventing a Weight Relapse.
2.5. Exercise and weight-loss intervention
Participants randomized to this group received both the exercise intervention and the weight-loss intervention. These participants started with the group-based weight training sessions during the first six weeks. After these six weeks they continued this routine at home or at a gym as described above and additionally began with the weight-loss program.
2.6. Control group
Participants randomized to the control group were referred to the American Cancer Society website if they had diet-related questions. In addition, they were referred to their physician to discuss which types of exercise would be safe for them. The study staff instructed the participants to continue whatever exercise program they had been undertaking prior to enrollment in the study, but not to increase exercise, begin weight training, or engage in a supervised weight-loss program over the period of study participation.
Throughout the trial, the following strategies were in place to reduce the possibility that these factors would introduce bias affecting the findings of the trial. These included strategies to limit heterogeneity in the study population regarding basic knowledge of lymphedema, access to compression garments, and access to lymphedema treatment by a certified lymphatic therapist as needed. All participants, including the control group, attended a lymphedema education session prior to the start of any study related activities to provide information on how best to manage their lymphedema. This one-hour lecture was developed for the Physical Activity and Lymphedema trial [33]. The content included anatomy and function of the lymphatic system, as well as review of the National Lymphedema Network guidelines for lymphedema risk reduction, diagnosis and treatment, self-care, exercise, and air travel [45]. In addition, all study participants were fitted for and provided with two free custom fitted lymphedema compression garments (arm sleeve, glove, or gauntlet) (BSN Medical, Charlotte, NC). Finally, all study participants were given access to certified lymphatic therapists for evaluation and treatment of lymphedema flares. Women who noted a change in symptoms lasting a week or longer were referred to certified lymphatic therapists for evaluation. If the therapist deemed treatment was needed, it was provided, free of charge, for as many sessions as clinically indicated.
2.7. Intervention adherence
Adherence to the intervention was assessed in several ways. Adherence to the dietary intervention was recorded by attendance at the in-person sessions. Adherence to the weight training and to the aerobic exercise was by self-report on the logs provided to participants by the exercise professionals.
2.8. Consent/IRB/DSMB
Approval was obtained from the IRB of the University of Pennsylvania. Written informed consent was obtained from all participants prior to enrollment. A Data Safety Monitoring Board was assembled to monitor unexpected adverse effects of the experimental intervention and, if needed, to implement safeguards to decrease or eliminate future risks. The DSMB included a biostatistician, an obesity intervention expert, a rehabilitation physician with expertise in oncology rehabilitation, and an exercise scientist. The group met quarterly by phone.
2.9. Outcomes
In person measurements took place at the University of Pennsylvania. All staff members who were involved in taking measurements were blinded to the group assignment of the participants. Participants were instructed not to reveal their group assignment to the study staff. An overview of all measurements over time is presented in Fig. 2.
Fig. 2.
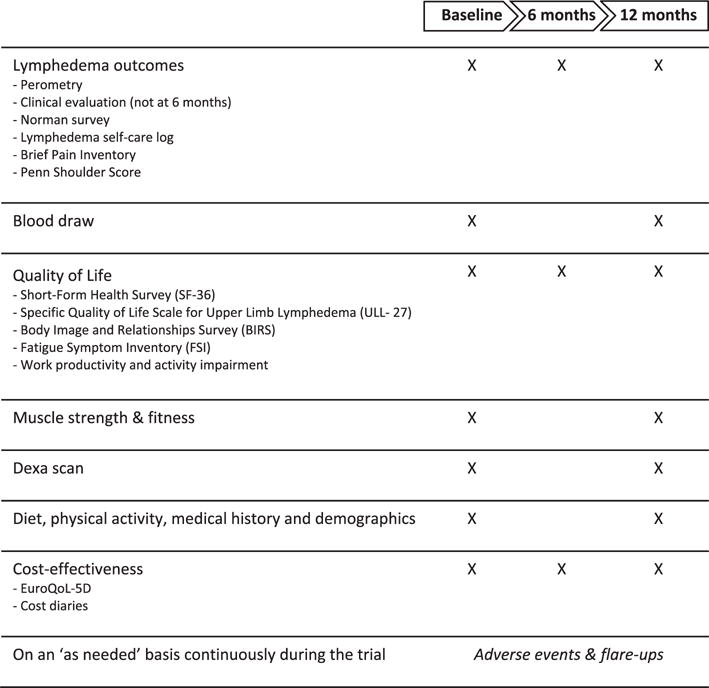
Timeline explaining assessment of various outcomes, surveys and tests in the WISER Survivor trial.
2.9.1. Primary outcome: lymphedema
The primary outcome of the study is change in interlimb volume difference from baseline to 12 months. Additionally, we assessed several other lymphedema related outcomes, to be able to understand potential complexities of the effects of the intervention on lymphedema outcomes. Many measures of lymphedema are affected by factors that generally affect limb swelling, including ambient temperature, recent physical activity, humidity, barometric pressure, hydration status, alcohol intake, caffeine intake, and time of day [20]. These factors were documented for each patient and each measurement.
For the primary outcome, it was hypothesized that reductions in interlimb volume differences would be largest in the weight loss and exercise group, smaller in the weight-loss group, smaller in the exercise group, and smallest in the control group. Interlimb volume difference was assessed using perometry. The Optoelectronic Perometer (Juzo USA, Cuyahoga Falls, OH) was used to assess limb dimension and volume [46–48]. The device uses infrared light beams to scan the limb length and circumference; from these measurements volume and percent differences between arms is calculated. For all participants, the compression garment was removed at least one hour before perometer measurement. The protocol included assessment of the arm length at which we would halt the measurement of arm volume, specific arm and hand positioning (fisted hand), and use of a motorized table on which the perometer was positioned so that the table could be raised and lowered to ensure that the extended arm to be measured was parallel to the floor. Test re-test evaluation of our perometer measurements indicated excellent reliability, with intra-observer correlation of 94% and inter-observer correlation of 98%.
As additional lymphedema related outcomes, we assessed the occurrence of therapist-delivered lymphedema flare treatment, cellulitic infections, a standardized clinical evaluation, and self-reported symptoms and pain.
2.9.2. Flare and cellulitic infections
Participants who reported a change in flare symptoms lasting one week or longer, regardless of group assignment, were evaluated by a certified lymphedema therapist. Flare evaluation was standardized and included changes in swelling by perometry, symptoms, and tissue tone and texture. In addition, the area of pain was documented along with the sensation (throbbing, aching, dull), duration and frequency of flares. Participants who reported a change in systemic symptoms consistent with possible cellulitic infections were referred to a designated physician within 72 h of onset of symptoms for evaluation.
2.9.3. Standardized clinical evaluation
All participants had a clinical evaluation at baseline and at 12 months with a certified lymphedema therapist. This therapist reviewed symptoms and the arm volume results measured by research staff prior to this appointment; assessed tissue tone and texture, symmetry between affected and unaffected sides, anatomic architecture (ability to see normal skinfolds, bones, joints); and conducted a neck and shoulder orthopedic screen. The therapist rated the extent of lymphedema according to the Common Toxicity Criteria version 3.0 [21].
2.9.4. Self-report of lymphedema symptoms
Symptoms were assessed using the Norman lymphedema questionnaire [49]. This survey has previously been shown to have a specificity of 0.90 and sensitivity between 0.86 and 0.92 to detect at least moderate lymphedema. In addition, we used the same lymphedema self-care log as used in earlier trials [32] to monitor possible factors that may have let to lymphedema onset. These factors were scored at baseline, 6 months and 12 months, and when participants were evaluated for possible flare.
2.9.5. Pain
Pain was rated using the Brief Pain Inventory [50], a reliable and valid instrument to assess pain across cultures and languages. In addition, the Penn Shoulder Score was used to assess participants’ self-reported levels of pain, satisfaction and function of the shoulder [51]. This score was found to be reliable test-retest intra-class correlation: 0.94 and internally consistent (Cronbach alpha of 0.93).
2.9.6. Secondary outcomes: biomarkers of recurrence
To assess changes in biomarkers in the WISER Survivor trial, blood was drawn from all participants at baseline and 12 months, after a 12 h fast, in the morning. Participants were asked to drink an eight ounce glass of water prior to the blood draw. Plasma and serum samples were prepared, aliquoted and stored at −80 °C until further analysis. In addition, PaxRNA and PaxDNA tubes were collected, for later evaluation of both RNA and DNA based changes.
The trial was designed to study effects of the intervention on biomarkers of mechanistic pathways hypothesized to link energy balance with recurrence risk. The pathways we focused on were based on research on mechanistic pathways in primary breast cancer [52–64], because of the lack of studies in breast cancer recurrence. The following pathways were identified as potentially relevant: insulin resistance & growth factors, sex steroid hormones, pathogenic angiogenesis, and inflammation/oxidative stress. As shown in Fig. 1, the WISER Survivor trial was interwoven with a pre-clinical animal study that assessed the effects of the same interventions (exercise, weight-loss, exercise and weight-loss, or no intervention) on breast cancer recurrence.
In the animal model, mammary tumors were induced in overweight mice by the doxycycline-dependent activation of HER2, an oncogene relevant to human breast cancer [65]. Withdrawal of doxycycline results in tumor regression and in cohorts of mice bearing dormant residual tumor cells, which eventually spontaneously relapse by mechanisms relevant to human breast cancer [66–69]. Overweight mice bearing residual disease were randomly assigned to exercise (aerobic exercise on a treadmill), calorie restriction, both of these interventions, or neither and followed for cancer recurrence. Biomarkers will be evaluated to explore the hypothesized relationship between energy balance and recurrence. To our knowledge, this is the first study to explore the impact of exercise and calorie restriction on cancer recurrence in an animal model. The animal model will guide additional mechanistic biomarkers to be tested in the WISER Survivor trial.
2.9.7. Secondary outcomes: health-related quality of life and psychosocial measures
The trial was designed to study quality of life outcomes, focusing on a lymphedema-related quality of life measures and body image. Quality of life was assessed at baseline, 6 months, and 12 months. Participants were asked to complete several surveys that covered different aspects of health-related quality of life.
2.9.8. Short-form health survey (SF-36) [70–72]
This survey is a widely used health survey which has been used extensively in breast cancer survivors [73–79]. It includes 36 items covering eight health domains: physical functioning, bodily pain, role limitations due to physical health problems, role limitations due to personal or emotional problems, emotional well-being, social functioning, energy/fatigue, and general health perceptions.
2.9.9. Quality of life scale for upper limb lymphedema (ULL-27) [80]
The instrument contains 27 items, divided into three dimensions (physical, psychological, and social quality of life), and is precise, sensitive and accurate on these dimensions [80].
2.9.10. Body image and relationships survey (BIRS) [81,82]
This survey has 32 items measuring attitudes about appearance, health, physical strength, sexuality, relationships, and social functioning. It has satisfactory test-retest reliability ranging from Spearman ρ = 0.41 to 0.80, and internal consistency [81].
2.9.11. Fatigue symptom inventory (FSI) [83,84]
This inventory assesses 14-items related to the severity, frequency, and daily pattern of fatigue as well as its perceived interference with quality of life, and is a valid and reliable measure of fatigue in cancer patients [83]
2.9.12. Work productivity
We used the validated work productivity and activity impairment questionnaire to assess these issues [85].
For the accompanying cost-effectiveness study (project 3 in Fig. 1), patients filled out the EuroQol 5D (EQ-5D[86,87]) at baseline, at 6 months and 12 months. The EuroQoL-5D is a validated instrument that provides a descriptive profile and index value that can be used in the clinical and economic evaluation of health care; it consists of questions in five domains (mobility, self-care, usual activities, pain/discomfort, anxiety/depression). In addition, the participants logged their health-care costs in cost diaries; details about this study will be reported elsewhere.
2.10. Additional physiologic measurements and surveys
2.10.1. Muscle strength
Strength of the arms and legs was tested with standardized bench press and leg press equipment at baseline and after 12 months, using the same protocol as used in earlier trials [9]. The maximum amount of weight that was lifted once (1 Repetition Maximum = 1 RM) was assessed for the bench press and the leg press. Changes in strength were assessed to study whether physical function improved in the intervention groups compared to the control group.
2.10.2. Maximal aerobic fitness
Maximal aerobic fitness was assessed at baseline and after 12 months; heart rate during this test was measured by 12-lead ECG monitoring. The test started with a 5 min rest after which blood pressure, resting heart rate and ECG were measured. Then the participant started with two warmup stages, each lasting 3 min. The first was at 1.7 mph and a 0% grade, and the second was at 1.7 mph and a 5% grade followed by a gradual increase in intensity as specified by the Modified Bruce protocol [74,75] every 3 min until exhaustion. During the last minute of every 3 minute interval, blood pressure, heart rate and ECG were measured.
2.10.3. Height, weight and body composition
Body weight was measured on a calibrated scale at baseline, 6 months and 12 months. Height was assessed at baseline only. Fat mass, fat free mass, bone density were measured by dual energy x-ray absorptiometry (DXA); from those measurements visceral adipose tissue areas and subcutaneous adipose tissue areas were estimated using dedicated software (Hologic, Inc., Bedford, MA) at baseline and after 12 months.
2.10.4. Diet
The Dietary History Questionnaire (DHQ-II) [88] was used to assess usual dietary intake; validation studies indicate that this questionnaire is better in estimating absolute intakes than the Block and Willett food frequency questionnaire [89]. The DHQ-II was administered at baseline and 12 months.
2.10.5. Physical activity
The Modifiable Physical Activity Questionnaire [90] was used to assess physical activity levels. This questionnaire asks about physical activity over the past year in three domains: leisure-time, occupational, and sedentary. Validity of the leisure activity section of the questionnaire was demonstrated through comparisons with the accelerometry (rho = 0.62). It was assessed at baseline and 12 months.
2.10.6. Demographics and other personal factors
Self-report surveys on demographics and other personal factors were developed and used in earlier trials by our group [33,34]. A general survey with questions on marital status, race, income, educational level and occupational status was administered to participants at baseline. Interview-administered surveys on medical history, cancer stage and treatment, medication use and menstrual tracking were taken at baseline and were repeated at twelve months. Additionally, at twelve months, all participants completed an injury history survey to log any major physical or mental health events they experienced over the past year.
2.10.7. Statistical considerations
The study was designed to have 80% power to detect differences in interlimb volume difference −2.62% −5.24% and −7.86% in the exercise, weight-loss, and combined exercise and weight-loss groups, respectively, compared to the control group. Using a Type I error of 0.05 and a Holm-Bonferroni adjustment for multiple comparisons, 79 participants per group were needed. We anticipated a 10% attrition rate based on our earlier trials, and planned to enroll n = 88 participants per group adding up to a total number of 352 participants in the trial. The trial was designed as a four group intervention, and not as a standard 2 × 2 factorial design, because 2 × 2 designs assume a lack of interaction between the two interventions and combine samples to assess the effect of each factor. We wished to allow for the possibility of interaction and therefore designed the study with sufficient power to consider each arm individually.
All primary analyses will be conducted using the intention-to-treat principle, including each participant in the group to which she was randomized regardless of adherence to the assigned strategy. We will use mixed models to test whether changes in interlimb volume difference differ between groups.
3. Results and discussion
During a period of 39 months, we randomized 351 patients into the WISER Survivor trial. The average age of women enrolled in the trial was 59.4 years, and their BMI was 34.0 kg/m2; baseline characteristics, presented in Table 3 were similar between groups. We were successful in recruiting a diverse population: 35% of participants were Black women. The interlimb volume difference was similar for all groups at baseline.
Table 3.
Baseline characteristics of participants in the four arms of the WISER Survivor trial
| Control group n = 90 | Exercise n = 87 | Weight-loss n = 87 | Exercise and weight-loss n = 87 | |
|---|---|---|---|---|
| Personal characteristics | ||||
| Age in years (mean + SD) | 59.0 (8.5) | 59.1 (8.1) | 59.4 (9.2) | 60.0 (9.0) |
| BMI in kg/m2 (mean + SD) | 34.0 (5.7) | 34.0 (6.2) | 33.8 (5.6) | 34.2 (6.3) |
| Race White n (%) | 66 (73%) | 50 (57%) | 52 (60%) | 50 (57%) |
| Black | 22 (24%) | 36 (41%) | 32 (37%) | 32 (37%) |
| Other | 2 (2%) | 1 (1%) | 3 (3%) | 5 (6%) |
| Educational status high school or less n (%) | 19 (21%) | 15 (17%) | 12 (14%) | 18 (21%) |
| Some college | 28 (31%) | 29 (33%) | 36 (41%) | 29 (33%) |
| College Grad or more | 43 (48%) | 43 (49%) | 39 (45%) | 40 (46%) |
| Lifestyle | ||||
| Energy intake at baseline in kcal/day (mean + SD) | 1787 (957) | 1689 (809) | 1727 (1135) | 1665 (946) |
| Physical activity level at baseline in MET-hr./week (median [IQR]) | 5.0 [2.3, 10.3] | 4.0 [1.4, 10.0] | 5.4 [1.5, 14.5] | 2.9 [0.5, 11.3] |
| Clinical characteristics | ||||
| Time since diagnosis in years, mean + SD | 8.1 (5.1) | 7.7 (5.4) | 7.5 (5.6) | 7.3 (5.1) |
| Cancer stage at diagnosisa 0 n (%) | 10 (11%) | 6 (7%) | 5 (6%) | 3 (3%) |
| I | 18 (20%) | 23 (26%) | 17 (20%) | 14 (16%) |
| II | 22 (24%) | 23 (26%) | 28 (32%) | 28 (32%) |
| III | 16 (18%) | 10 (11%) | 18 (21%) | 19 (22%) |
| Unknown | 24 (27%) | 25 (29%) | 19 (22%) | 23 (26%) |
| Number of lymph nodes removed (mean + SD) | 12.3 (9.3) | 12.7 (9.4) | 12.5 (9.9) | 12.0 (8.3) |
| Radiation therapy n (% yes) | 73 (81%) | 73 (84%) | 69 (79%) | 73 (84%) |
| Chemotherapy n (% yes) | 74 (82%) | 65 (75%) | 71 (82%) | 79 (91%) |
| Currently on tamoxifen or aromatase inhibitors n (% yes) | 41 (46%) | 35 (40%) | 32 (37%) | 30 (34%) |
| Breast cancer-related lymphedema characteristics | ||||
| Interlimb volume difference in percentage (mean + SD) | +9.6 (14.4) | +8.8 (16.6) | +8.8 (13.5) | +7.6 (13.7) |
| Self-report of lymphedema (Norman survey [49]) | ||||
| Overall score for extremity | 3.38 (2.72) | 3.47 (2.65) | 3.23 (2.71) | 3.22 (2.71) |
| Overall score for breast/torso | 0.94 (0.88) | 1.10 (1.01) | 0.84 (0.90) | 0.97 (0.95) |
| # of symptoms reported (range 0–14)b | 5.50 (2.81) | 5.74 (2.71) | 5.18 (2.76) | 5.03 (2.81) |
| Symptom severity (range 0–4)2 | 1.92 (0.71) | 1.96 (0.81) | 1.81 (0.74) | 1.84 (0.79) |
Based on self-report.
Possible values were zero (did not have symptom) to four (very severe) for each symptom; there were 14 possible symptoms (rings too tight, watch too tight, bracelets too tight, clothing too tight, puffiness, couldn’t see knuckles, couldn’t see veins, skin felt leathery, arm felt tired, pain, pitting, swelling after exercise, difficulty writing, or ‘other’).
Thirteen participants were diagnosed with a cancer recurrence during the trial, and 58 participants dropped out of the study (Table 4). We collected 12 month post-intervention data of 280 participants. Out of the 338 participants without a breast cancer recurrence, 83% completed the trial and participated in the post-intervention measurement. Baseline characteristics of the n = 280 participants with post-intervention data were very similar to the characteristics of the total group of 351 participants that started the trial, with an average age of 59.7 (SD 8.6 years), 63% White women, 34% Black women, and 3% other race, and an interlimb volume difference at baseline of 8.9% (SD 15.1).
Table 4.
Description of the number of participants randomized to WISER Survivor and retention over time.
| Total | Control | Exercise | Weight-loss | Exercise and weight-loss | |
|---|---|---|---|---|---|
| At baseline | 351 | 90 | 87 | 87 | 87 |
| Drop-outs during the trial | 58 | 19 | 13 | 18 | 8 |
| Recurrences during the trial | 13 | 3 | 3 | 1 | 6 |
| Number for analysis at 12 months | 280 | 68 | 71 | 68 | 73 |
| Percentage of participants without recurrence who completed the trial | 83% | 78% | 85% | 79% | 90% |
The primary goal of WISER Survivor is to test the combined effect of exercise and weight-loss on changes in lymphedema. An important strength of the trial is our assessment of a range of lymphedema related outcomes, enabling examination of the effect of the intervention on all aspects of lymphedema. We hypothesize that the combined effects of exercise and weight-loss on lymphedema outcomes will be larger than the separate effects for the following reasons. Weight-loss through caloric restriction may reduce arm swelling by using the fuel in the excess peripheral fatty tissue networks prevalent in affected arms. In addition, compression garments may work better as the subcutaneous fat depots shrink. Moreover, as obesity is considered a state of inflammation, weight-loss may reduce the inflammatory state of the lymphatic system which may translate into improved lymphedema outcomes.
Successful recruitment of 351 breast cancer survivors required active as well as passive recruitment approaches. The exercise intervention used was adapted from a prior study that required women to attend in person sessions at a community gym for a full year [33]. In that prior study, exercise adherence was 79% [15]. Feedback from participants in the prior study and other research by our team [37] led our research team to pilot test a home based adaptation of the intervention. High adherence to this new home adaptation in the pilot (unpublished results) led to the decision to make the WISER Survivor trial exercise intervention largely home based.
The interlimb volume difference at baseline was 8–10%. This was slightly lower than the 15–17% as observed in our earlier trial [15]. In our previous trials, we used slightly different eligible criteria, requiring an interlimb difference of at least 10% or a clinically confirmed diagnosis of lymphedema. For WISER Survivor, we additionally recruited women who were not previously aware that they had lymphedema but whom we identified using a valid, reliable, self-reported survey [49]. If they responded positively to any of the questions on that survey, they were screened for lymphedema by a certified therapist, and if confirmed they were eligible for the trial.
The WISER Survivor trial has several unique features. The first is the use of a commercially available product (NutriSystem®) during the first 24 weeks of the lifestyle modification for weight-loss. Earlier trials primarily used diet and/or physical activity and/or behavioral modification to achieve weight-loss [79].
A second unique feature of the WISER Survivor trial is its conduct within the broader scope of transdisciplinary research as depicted in Fig. 1. This enables the comparison of biomarkers for recurrence in both mice and humans. The pre-clinical study aids in prioritizing which biomarkers will be tested in the WISER Survivor trial by their likelihood of playing a mechanistic role; ideally, it will observe effects of the energy balance interventions on recurrence and changes in biomarkers that will point to specific mechanistic pathways through which this effect works. Using data from WISER Survivor, we will then assess whether similar interventions in women have similar effects on the same biomarkers.
The WISER Survivor trial collected a wealth of data to study potential effects of the interventions on lymphedema outcomes, biomarkers of recurrence, quality of life, and other physiological and functional outcomes. In addition, interrelationships among outcomes can be studied and characterized.
Acknowledgments
State Cancer Registry Representatives: Robin Otto (PA Cancer Registry contact), James Rubertone (PA Cancer Registry Contact), Dr. Lisa Paddock (NJ Cancer Registry), Jie Li (NJ Cancer Registry). Penn Cancer Network staff: Cathy Belt, Cindy Stern. Hospital Partners: Caitlin Beaudoin (Doylestown Hospital), Dr. Beth Dupree (Holy Redeemer Hospital), Dr. Tapan Kikani (Holy Redeemer Hospital), Heidi Volpe (Holy Redeemer Hospital), Cindy Brockway (Chester County Hospital), Sara Hollstein (Paoli Hospital), Margaret Beauchesne (Phoenixville Hospital), Dr. Lawrence Solin (Einstein Medical Center), Jeff Mealey (Einstein Medical Center), Susan Van Loon (Virtua Health System), Dr. Crystal Denlinger (Fox Chase Cancer Center), Louise Baca (Kennedy Cancer Center), Karen Swenson (Kennedy Cancer Center), Jennifer Torres, (St. Mary Medical Center), Dr. Stacy Krisher (St. Mary Medical Center). Fitness Facility Partners: Roger Schwab (Main Line Health and Fitness), Rhonda Hamilton (Upper Main Line YMCA), Lionville YMCA, Joe Fuhrman and Jill Makkay (Hamilton YMCA). NutriSystem®: Mandi Knowles, RDN, LDN and Courtney M McCormick, MPH, RDN, LDN.
Funding
This work was funded by National Cancer Institute grant U54CA155850 (Kathryn Schmitz).
References
- 1.Institute of Medicine and National Research CouncilHewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. The National Academies Press; Washington, DC: 2006. p. 534. [Google Scholar]
- 2.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27(3):390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women’s Health Study. J Clin Oncol. 2008;26(35):5689–5696. doi: 10.1200/JCO.2008.16.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter BJ. Women’s experiences of lymphedema. Oncol Nurs Forum. 1997;24(5):875–882. [PubMed] [Google Scholar]
- 5.Passik SD, McDonald MV. Psychosocial aspects of upper extremity lymphedema in women treated for breast carcinoma. Cancer. 1998;83(12 Suppl American):2817–2820. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2817::aid-cncr32>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Sneeuw KC, Aaronson NK, Yarnold JR, Broderick M, Regan J, Ross G, et al. Cosmetic and functional outcomes of breast conserving treatment for early stage breast cancer. 2. Relationship with psychosocial functioning. Radiother Oncol. 1992;25(3):160–166. doi: 10.1016/0167-8140(92)90262-s. [DOI] [PubMed] [Google Scholar]
- 7.Helyer LK, Varnic M, Le LW, Leong W, McCready D. Obesity is a risk factor for developing postoperative lymphedema in breast cancer patients. Breast J. 2010;16(1):48–54. doi: 10.1111/j.1524-4741.2009.00855.x. [DOI] [PubMed] [Google Scholar]
- 8.Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19(6):853–857. doi: 10.1007/s00520-011-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Werner RS, McCormick B, Petrek J, Cox L, Cirrincione C, Gray JR, et al. Arm edema in conservatively managed breast cancer: obesity is a major predictive factor. Radiology. 1991;180(1):177–184. doi: 10.1148/radiology.180.1.2052688. [DOI] [PubMed] [Google Scholar]
- 10.Cheville AL, Almoza M, Courmier JN, Basford JRA. Prospective cohort study defining utilities using time trade-offs and the Euroqol-5D to assess the impact of cancer-related lymphedema. Cancer. 2010;116(15):3722–3731. doi: 10.1002/cncr.25068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13(11):904–911. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 12.Ridner SH, Dietrich MS, Kidd N. Breast cancer treatment-related lymphedema self-care: education, practices, symptoms, and quality of life. Support Care Cancer. 2011;19(5):631–637. doi: 10.1007/s00520-010-0870-5. [DOI] [PubMed] [Google Scholar]
- 13.Cheema BS, Kilbreath SL, Fahey PP, Delaney GP, Atlantis E. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148(2):249–268. doi: 10.1007/s10549-014-3162-9. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz KH. Balancing lymphedema risk: exercise versus deconditioning for breast cancer survivors. Exerc Sport Sci Rev. 2010;38(1):17–24. doi: 10.1097/JES.0b013e3181c5cd5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361(7):664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 16.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110(8):1868–1874. doi: 10.1002/cncr.22994. [DOI] [PubMed] [Google Scholar]
- 17.Shaw C, Mortimer P, Judd PA. Randomized controlled trial comparing a low-fat diet with a weight-reduction diet in breast cancer-related lymphedema. Cancer. 2007;109(10):1949–1956. doi: 10.1002/cncr.22638. [DOI] [PubMed] [Google Scholar]
- 18.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 19.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 21.Caan BJ, Emond JA, Natarajan L, Castillo A, Gunderson EP, Habel L, et al. Post-diagnosis weight gain and breast cancer recurrence in women with early stage breast cancer. Breast Cancer Res Treat. 2006;99(1):47–57. doi: 10.1007/s10549-006-9179-y. [DOI] [PubMed] [Google Scholar]
- 22.Chlebowski RT, R MM. Weight Loss, Randomized intervention trials in female cancer survivors. J Clin Oncol. 2016;34(35):4238–4248. doi: 10.1200/JCO.2016.69.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomark Prev. 2003;12(8):721–727. [PubMed] [Google Scholar]
- 24.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol (1985) 2005;98(4):1534–1540. doi: 10.1152/japplphysiol.00566.2004. [DOI] [PubMed] [Google Scholar]
- 25.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Martin BS, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun. 2005;19(5):381–388. doi: 10.1016/j.bbi.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Irwin ML, Varma K, Alvarez-Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomark Prev. 2009;18(1):306–313. doi: 10.1158/1055-9965.EPI-08-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. J Clin Oncol. 2008;26(6):907–912. doi: 10.1200/JCO.2007.12.7357. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomark Prev. 2005;14(7):1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Gehlert S, Patterson RE, Colditz GA, Chavarro JE, Hu FB, et al. TREC to WHERE? Transdisciplinary research on energetics and cancer. Clin Cancer Res. 2016;22(7):1565–1571. doi: 10.1158/1078-0432.CCR-14-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson RE, Colditz GA, Hu FB, Schmitz KH, Ahima RS, Brownson RC, et al. The 2011–2016 transdisciplinary research on energetics and cancer (TREC) initiative: rationale and design. Cancer Causes Control. 2013;24(4):695–704. doi: 10.1007/s10552-013-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sturgeon KM, Hackley R, Fornash A, Dean LT, Laudermilk M, Brown JC, et al. Strategic Recruitment of an Ethnically Diverse Cohort of Overweight Breast Cancer Survivors with Lymphedema. Cancer Res. 2017 doi: 10.1002/cncr.30935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 33.Schmitz KH, Troxel AB, Cheville A, Grant LL, Bryan CJ, Gross CR, et al. Physical activity and lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemporary Clinical Trials. 2009;30(3):233–245. doi: 10.1016/j.cct.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitz KH, Williams NI, Kontos D, Kurzer MS, Schnall M, Domchek S, et al. Women in steady exercise research (WISER) sister: study design and methods. Contemporary Clinical Trials. 41(2015):17–30. doi: 10.1016/j.cct.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Arikawa AY, O’Dougherty M, Kaufman BC, Smith AJ, Thomas W, Warren M, et al. Women in steady exercise research (WISER): study design and methods. Contemporary Clinical Trials. 2010;31(5):457–465. doi: 10.1016/j.cct.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beidas RS, Paciotti B, Barg F, Branas AR, Brown JC, Glanz K, et al. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. JNCI Monogr. 2014;2014(50):338–345. doi: 10.1093/jncimonographs/lgu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beidas RS, Paciotti B, Barg F, Branas AR, Brown JC, Glanz K, et al. A hybrid effectiveness-implementation trial of an evidence-based exercise intervention for breast cancer survivors. J Natl Cancer Inst Monogr. 2014;2014(50):338–345. doi: 10.1093/jncimonographs/lgu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Online training strength after breast cancer. updated last accessed 6/26/2017. Available from http://klosetraining.com/course/online/strength-abc/
- 39.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 40.Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 2009;23(4):325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 41.Look Ahead Research Group. Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14(5):737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132(2):631–639. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Befort CA, Klemp JR, Sullivan DK, Shireman T, Diaz FJ, Schmitz K, et al. Weight loss maintenance strategies among rural breast cancer survivors: the rural women connecting for better health trial. Obesity (Silver Spring) 2016;24(10):2070–2077. doi: 10.1002/oby.21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Lymphedema Network (NLN) Medical Advisory Committee. Position statement of the national lymphedema network: lymphedema risk reduction practices. 2012 Available from http://www.lymphnet.org/pdfDocs/nlnriskreduction.pdf.
- 46.Labs KH, Tschoepl M, Gamba G, Aschwanden M, Jaeger KA. The reliability of leg circumference assessment: a comparison of spring tape measurements and optoelectronic volumetry. Vasc Med. 2000;5(2):69–74. doi: 10.1177/1358836X0000500202. [DOI] [PubMed] [Google Scholar]
- 47.Stanton AW, Northfield JW, Holroyd B, Mortimer PS, Levick JR. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997;30(2):77–97. [PubMed] [Google Scholar]
- 48.Tierney S, Aslam M, Rennie K, Grace P. Infrared optoelectronic volumetry, the ideal way to measure limb volume. Eur J Vasc Endovasc Surg. 1996;12(4):412–417. doi: 10.1016/s1078-5884(96)80005-0. [DOI] [PubMed] [Google Scholar]
- 49.Norman SA, Miller LT, Erikson HB, Norman MF, McCorkle R. Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther. 2001;81(6):1192–1205. [PubMed] [Google Scholar]
- 50.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Annals of the Academy of Medicine Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 51.Leggin BG, Michener LA, Shaffer MA, Brenneman SK, Iannotti JP, Williams GR., Jr The Penn shoulder score: reliability and validity. J Orthop Sports Phys Ther. 2006;36(3):138–151. doi: 10.2519/jospt.2006.36.3.138. [DOI] [PubMed] [Google Scholar]
- 52.Ozet A, Arpaci F, Yilmaz MI, Ayta H, Ozturk B, Komurcu S, et al. Effects of tamoxifen on the serum leptin level in patients with breast cancer. Jpn J Clin Oncol. 2001;31(9):424–427. doi: 10.1093/jjco/hye097. [DOI] [PubMed] [Google Scholar]
- 53.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150(2):161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 54.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89(4):1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 55.Thomson CA, Giuliano AR, Shaw JW, Rock CL, Ritenbaugh CK, Hakim IA, et al. Diet and biomarkers of oxidative damage in women previously treated for breast cancer. Nutr Cancer. 2005;51(2):146–154. doi: 10.1207/s15327914nc5102_4. [DOI] [PubMed] [Google Scholar]
- 56.Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Tehard B, et al. Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes. 2006;30(11):1623–1631. doi: 10.1038/sj.ijo.0803324. [DOI] [PubMed] [Google Scholar]
- 57.Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu CL, et al. Adipocytokines and breast cancer risk. Chin Med J. 2007;120(18):1592–1596. [PubMed] [Google Scholar]
- 58.Hursting SD, Lashinger LM, Colbert LH, Rogers CJ, Wheatley KW, Nunez NP, et al. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr Cancer Drug Targets. 2007;7(5):484–491. doi: 10.2174/156800907781386623. [DOI] [PubMed] [Google Scholar]
- 59.Pasanisi P, Venturelli E, Morelli D, Fontana L, Secreto G, Berrino F. Serum insulin-like growth factor-I and platelet-derived growth factor as biomarkers of breast cancer prognosis. Cancer Epidemiol Biomark Prev. 2008;17(7):1719–1722. doi: 10.1158/1055-9965.EPI-07-0654. [DOI] [PubMed] [Google Scholar]
- 60.Rock CL, Flatt SW, Laughlin GA, Gold EB, Thomson CA, Natarajan L, et al. Reproductive steroid hormones and recurrence-free survival in women with a history of breast cancer. Cancer Epidemiol Biomark Prev. 2008;17(3):614–620. doi: 10.1158/1055-9965.EPI-07-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai Q, Gao YT, Shu XO, Yang G, Milne G, Cai Q, et al. Oxidative stress, obesity, and breast cancer risk: results from the Shanghai Women’s Health Study. J Clin Oncol. 2009;27(15):2482–2488. doi: 10.1200/JCO.2008.19.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naumov GN, Folkman J, Straume O. Tumor dormancy due to failure of angiogenesis: role of the microenvironment. Clin Exp Metastasis. 2009;26(1):51–60. doi: 10.1007/s10585-008-9176-0. [DOI] [PubMed] [Google Scholar]
- 63.Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27(21):3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114(1):155–167. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2(6):451–461. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 66.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8(3):197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Alvarez James V, Pan TC, Ruth J, Feng Y, Zhou A, Pant D, et al. Par-4 downregulation promotes breast cancer recurrence by preventing multinucleation following targeted therapy. Cancer Cell. 2013;24(1):30–44. doi: 10.1016/j.ccr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng Y, Pan TC, Pant DK, Chakrabarti KR, Alvarez JV, Ruth JR, et al. SPSB1 promotes breast cancer recurrence by potentiating c-MET signaling. Cancer Discov. 2014;4(7):790–803. doi: 10.1158/2159-8290.CD-13-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abravanel DL, Belka GK, Pan TC, Pant DK, Collins MA, Sterner CJ, et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J Clin Investig. 2015;125(6):2484–2496. doi: 10.1172/JCI74883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 71.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 73.Bouskill K, Kramer M. The impact of cancer and quality of life among long-term survivors of breast cancer in Austria. Support Care Cancer. 2016;24(11):4705–4712. doi: 10.1007/s00520-016-3319-7. [DOI] [PubMed] [Google Scholar]
- 74.Connor AE, Baumgartner RN, Pinkston CM, Boone SD, Baumgartner KB. Obesity, ethnicity, and quality of life among breast cancer survivors and women without breast cancer: the long-term quality of life follow-up study. Cancer Causes Control. 2016;27(1):115–124. doi: 10.1007/s10552-015-0688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunitake H, Russell MM, Zheng P, Yothers G, Land SR, Petersen L, et al. Quality of life and symptoms in long-term survivors of colorectal cancer: results from NSABP protocol LTS-01. J Cancer Surviv. 2017;11(1):111–118. doi: 10.1007/s11764-016-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kvale EA, Huang CHS, Meneses KM, Demark-Wahnefried W, Bae S, Azuero CB, et al. Patient-centered support in the survivorship care transition: outcomes from the Patient-Owned Survivorship Care Plan Intervention. Cancer. 2016;122(20):3232–3242. doi: 10.1002/cncr.30136. [DOI] [PubMed] [Google Scholar]
- 77.Larsson YH, Speck R, Schmitz KH, Johansson K, Gyllensten AL. The body image and relationship scale: a Swedish translation, cultural adaptation, and reliability and validity testing. Eur J Phys. 2014;16(2):67–75. [Google Scholar]
- 78.Nesvold IL, Foss SD, Holm I, Naume B, Dahl AA. Arm/shoulder problems in breast cancer survivors are associated with reduced health and poorer physical quality of life. Acta Oncol. 2010;49(3):347–353. doi: 10.3109/02841860903302905. [DOI] [PubMed] [Google Scholar]
- 79.Rogers LQ, Courneya KS, Carter SJ, Anton PM, Verhulst S, Vicari SK, et al. Effects of a multicomponent physical activity behavior change intervention on breast cancer survivor health status outcomes in a randomized controlled trial. Breast Cancer Res Treat. 2016;159(2):283–291. doi: 10.1007/s10549-016-3945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Launois R, Megnigbeto AC, Pocquet K, Alliot F, editors. Progress in Lymphology XVIII International Congress of Lymphology Sept 2001. 2001. A specific quality of life scale in upper limb Lymphoedema: the ULL-27 questionnaire. [Google Scholar]
- 81.Hormes JM, Lytle LA, Gross CR, Ahmed RL, Troxel AB, Schmitz KH. The body image and relationships scale: development and validation of a measure of body image in female breast cancer survivors. J Clin Oncol. 2008;26(8):1269–1274. doi: 10.1200/JCO.2007.14.2661. [DOI] [PubMed] [Google Scholar]
- 82.Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, et al. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421–430. doi: 10.1007/s10549-009-0550-7. [DOI] [PubMed] [Google Scholar]
- 83.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9(7):847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 84.Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 85.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4(5):353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 86.Pickard AS, Wilke CT, Lin HW, Lloyd A. Health utilities using the EQ-5D in studies of cancer. PharmacoEconomics. 2007;25(5):365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 87.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 88.National Institutes of Health Epidemiology and Genomics Research Program: National Cancer Institute. Diet History Questionnaire, Version 2.0. 2010. [Google Scholar]
- 89.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 90.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]


