Abstract.
Coxiella-like bacteria have been recently proposed as human pathogens. Using molecular techniques, we detected Coxiella-like bacteria in the blood and serum samples of a patient with a scalp eschar, neck lymphadenopathy, severe urticaria, edema, fever, and arthralgia indicating that this organism can provide systemic complications.
INTRODUCTION
A scalp eschar associated with neck lymphadenopathy is considered as an emerging syndrome with multiples causes.1,2 Coxiella-like bacteria are clustered together with Coxiella burnetii and are sharing some degree of identity with C. burnetii but much less than that is needed to be considered the same species.3 Coxiella-like bacteria genotypes vary from tick species to species and are frequently found in many ticks species,4 alone or coinfecting the tick with Rickettsia. Gottlieb et al.5 recently presented the complete genome of Coxiella-like symbiont from Rhipicephalus turanicus ticks named Candidatus Coxiella mudrowiae and showed that its genome is larger than the genomes of most obligate mutualists. Moreover, the comparative analyses between Candidatus C. mudrowiae and Coxiella from Amblyomma americanum ticks revealed that Candidatus C. mudrowiae has a genome reduction and gene decay because of the loss of selection on gene functions.5 To date, Coxiella-like bacteria have been associated with infection in birds;6,7 recently, based on immunofluorescence assay (IFA) and a positive polymerase chain reaction (PCR) of the skin biopsy, we found that Coxiella-like bacteria could be human pathogens that are frequently associated with a scalp eschar and neck lymphadenopathy.8 Here, we report a patient with possible bacteremia due to Coxiella-like bacteria.
CASE REPORT
In May 2016, a previously healthy 23-year-old man from Vendée (western France), was admitted to an emergency department with a 2-day fever history, urticaria, muscle aches, extremities and head edema, pharyngeal pain, and knee and elbow arthritis (Figure 1A). He conducted outdoor activities before one week (fishing in a lake and beneath the trees). He had not traveled abroad in the previous months and was not aware of having been bitten by a tick. At the time of physical examination, he had no hepatoslenomegaly. He had tachycardia and temporary hypotension. Blood collected at the time of admission showed lymphocytopenia (0.97 × 109 cells/L), elevated neutrophils (8 × 109 cells/L), C reactive protein (246 mg/L), and fibrinogen (6 g/L). Platelets (220 × 109/L), hemoglobin (15 g/dL). and serum concentrations of aspartate transaminase and alanine transaminase were normal. A full evaluation for sepsis, including blood cultures, was performed. Treatment by ceftriaxone (1 g/24 hours) was initiated. His fever abated and all the symptoms resolved. C-reactive protein decreased to 69 mg/L. Antibiotic treatment was stopped, and the patient was released from hospital after 3 days. Ten days later, the patient sought care for painful enlarged cervical and occipital nodes with an erythematous and an elevated punctiform scalp lesion. Treatment by using doxycycline was initiated (100 mg/12 hour) for 10 days. The pain became more intense in the area of the potential tick bite after 2 days of antibiotic treatment before disappearing after ten days. Erythema of the scalp has evolved into a crusty scar on the tenth day. After a month, the patient retained a weakness.
Figure 1.
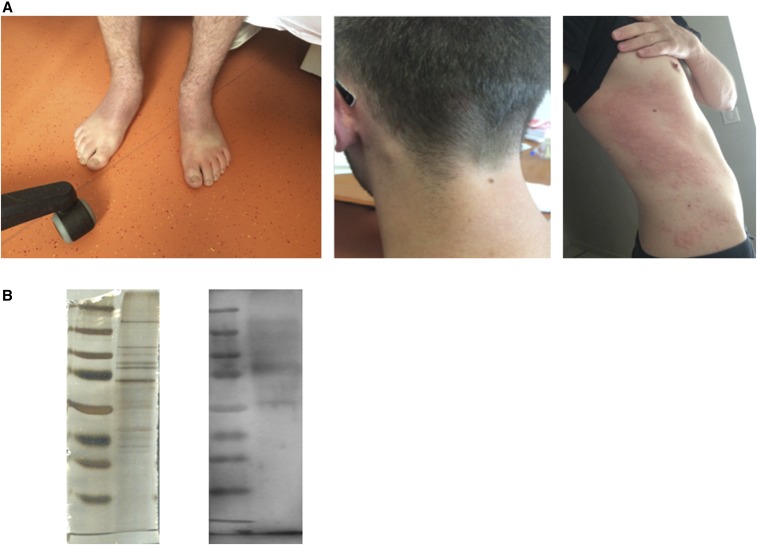
(A) Patient’scervical adénopathy and urticaria; (B) western blot using antigens from Rhipicephalus sanguineus ticks infected with Coxiella-like bacteria. This figure appears in color at www.ajtmh.org.
The blood and serum sample collected at the time of admission were sent to our center (Marseille, France) and tested for Coxiella burnetti, Bartonella sp., Rickettsia sp., Borrelia sp., Ehrlichia sp., Tropheryma whipplei, Francisella tularensis, Mycobacteria sp., S. aureus, Streptococcus pyogenes, Leishmania sp., orthopoxvirus, and parapoxvirus by quantitative PCR (qPCR).8,9 The samples were also screened for the presence of bacteria by PCR amplification and sequencing, targeting the 16S rRNA gene using the methods previously described.9 For both samples, all molecular assays were negative except the qPCR assay targeting Coxiella-like bacteria that was positive in both samples. Both samples were cultured in human embryonic lung fibroblasts in L929 cell line cells and XTC cells using the centrifugation-shell vial technique (Sterilin- Felthan-England, 3.7 mL) as previously described.10,11 Cultures were surveyed for 8 weeks, and bacterial growth was assessed every 7 days on cover slips directly inside the shell vial using Gimenez and immunofluorescence staining. However, all cultures remained negative.
The serum sample was further tested with a previously described IFA for Coxiella-like bacteria.8 IFA was positive with titers for IgG, IgM, and IgA to be 800, 0, and 0, respectively. Finally, the patient serum was diluted to 1:200 in Tris-buffered saline –3% nonfat milk and analyzed by western blotting using antigens from five spawns of Rhipicephalus sanguineus ticks infected with Coxiella-like bacteria and was found positive (Figure 1B). Molecular assays indicated that these spawns were negative for Rickettsia, Borrelia, Bartonella, Ehrlichia spp.; F. tularensis; and C. burnetii.
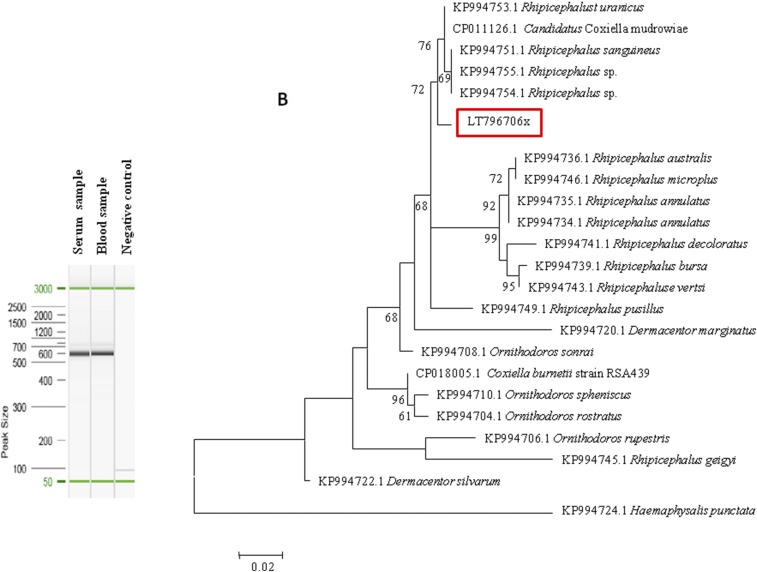
Both DNA samples were then tested for the presence of Coxiella-like bacteria using a nested PCR assay of the 23S ribosomal RNA (23S rRNA) gene using Coxiella-specific primers as previously described.12 For both samples, the PCR products were positive when electrophoresed on 1.5% agarose gel (Figure 2A). We then amplified and sequenced the 23S rRNA gene and compared the sequences with the 23S rRNA gene sequences of Coxiella-like strains detected in ticks and those available in GenBank. Phylogenetic analysis revealed that they were closely related to Coxiella-like strains of Rhipicephalus spp. (Figure 2B and Supplemental Figure 1).
Figure 2.
(A) 23S ribosomal RNA gene nested PCR assay electrophoresis in agarose gel; (B) maximum likelihood (ML) phylogenetic tree based on 23S ribosomal RNA gene sequences including Coxiella-like strains of ticks. The sequences were aligned using CLUSTALW. Phylogenetic inferences were performed with the maximum likelihood and neighbor-joining (NJ, see Supplemental Figure 1) methods under the Kimura 2-parameter model and complete deletion. Both trees were run using MEGA software.16 The robustness of the nodes in both ML and NJ trees was estimated through bootstrap analyses of 500 and 1,000 replicates, respectively. GenBank accession numbers are indicated in the beginning, followed by the tick host. This figure appears in color at www.ajtmh.org.
DISCUSSION
We describe a patient with a scalp eschar associated with neck lymphadenopathy and bacteremia due to Coxiella-like bacteria. Our molecular assays were sensitive and versatile and have previously been evaluated for the detection of Coxiella-like bacteria.3,12 Only Coxiella-like bacteria DNA was present in both samples, indicating that these bacteria were the only infectious agents. The culture was negative for both samples, although a limitation of our study was that the samples were only cultured in mammalian cell-lines and not in arthropod cell-lines. Another limitation was that we did not receive a sample of the eschar to test for Coxiella-like bacteria. Indeed, the diagnosis of vector-borne diseases is considered difficult because of physicians’ unfamiliarity with rare diseases because they may commonly masquerade as other clinical syndromes. Finally, our IFA and western blotting serology were supplementary assays for the diagnosis. However, the fact that the serum tested demonstrated only IgG does not eliminate the possibility of previous exposure because these bacteria are common in ticks.4,5,8 Doxycycline is the treatment of choice for many tick-transmitted infections, and physicians commonly prescribe doxycycline or amoxicillin prophylaxis for patients after a tick bite.13 In our study, most patients were empirically treated with doxycycline.
Rickettsia slovaca and Rickettsia raoultii have been considered the most common agents of scalp eschar and neck lymphadenopathy, and sporadic cases of Candidatus Rickettsia rioja, Rickettsia massiliae, Rickettsia sibirica mongolitimonae, Bartonella henselae, C. burnetii, and F. tularensis have also been reported.2,14 Moreover, we recently found that Coxiella-like bacteria were the second most common agent of this clinical entity after R. slovaca.8 However, in contrast to the previously described cases, both Coxiella-like bacteria patients presented a discrete erythema that was modified to an eschar after several days of antibiotic treatment. Moreover, the patient mentioned an intense and increased pain even after the beginning of the antibiotic treatment. Indeed, he evoked neuropathic pain such as electric shocks leading to a cessation of work and declined slowly over a period of 10 days. Finally, to the best of our knowledge, this is the first time that a scalp eschar and neck lymphadenopathy have been associated with the appearance of an initial bacteremic phase with severe urticaria, edema, fever, and arthralgia. Previously, fever, asthenia, and edema have also been associated with a scalp eschar and neck lymphadenopathy due to R. slovaca and R. raoultii.15 This Coxiella-like bacterial infection could easily go unrecognized because of the absence of systematic research of these bacteria and the nonspecific clinical manifestations. We empirically treated our patient with doxycycline because it is the treatment of choice for many tick-transmitted infections.13
In conclusion, we confirm that Coxiella-like bacteria are a new agent of human infections. These bacteria can possibly lead to an atypical scalp eschar and neck lymphadenopathy syndrome with delayed evolution to crust eschar in the area of the tick-bite, intense and prolonged local pain, and slow recovery despite the use of antibiotics. Considering this example and the fact that all the previously known scalp eschar and neck lymphadenopathy pathogens can provide systemic complications, physicians should be aware of any abnormal symptom.
Supplementary Material
Note: Supplemental figure appears at www.ajtmh.org.
REFERENCES
- 1.Dubourg G, Socolovschi C, Del Giudice P, Fournier PE, Raoult D, 2014. Scalp eschar and neck lymphadenopathy after tick bite: an emerging syndrome with multiple causes. Eur J Clin Microbiol Infect Dis 33: 1449–1456. [DOI] [PubMed] [Google Scholar]
- 2.Angelakis E, Pulcini C, Waton J, Imbert P, Socolovschi C, Edouard S, Dellamonica P, Raoult D, 2010. Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin Infect Dis 50: 549–551. [DOI] [PubMed] [Google Scholar]
- 3.Mediannikov O, Ivanov L, Nishikawa M, Saito R, Sidelnikov YN, Zdanovskaya NI, Tarasevich IV, Suzuki H, 2003. Molecular evidence of Coxiella-like microorganism harbored by Haemaphysalis concinnae ticks in the Russian far east. Ann N Y Acad Sci 990: 226–228. [DOI] [PubMed] [Google Scholar]
- 4.Zhong J, 2012. Coxiella-like endosymbionts. Adv Exp Med Biol 984: 365–379. [DOI] [PubMed] [Google Scholar]
- 5.Gottlieb Y, Lalzar I, Klasson L, 2015. Distinctive genome reduction rates revealed by genomic analyses of two Coxiella-like endosymbionts in ticks. Genome Biol Evol 7: 1779–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vapniarsky N, Barr BC, Murphy B, 2012. Systemic Coxiella-like infection with myocarditis and hepatitis in an eclectus parrot (Eclectus roratus). Vet Pathol 49: 717–722. [DOI] [PubMed] [Google Scholar]
- 7.Shivaprasad HL, Cadenas MB, Diab SS, Nordhausen R, Bradway D, Crespo R, Breitschwerdt EB, 2008. Coxiella-like infection in psittacines and a toucan. Avian Dis 52: 426–432. [DOI] [PubMed] [Google Scholar]
- 8.Angelakis E, Mediannikov O, Jos SL, Berenger JM, Parola P, Raoult D, 2016. Candidatus Coxiella massiliensis infection. Emerg Infect Dis 22: 285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safont M, Angelakis E, Richet H, Lepidi H, Fournier PE, Drancourt M, Raoult D, 2014. Bacterial lymphadenitis at a major referral hospital in France from 2008 to 2012. J Clin Microbiol 52: 1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouriet F, Fenollar F, Patrice JY, Drancourt M, Raoult D, 2005. Use of shell-vial cell culture assay for isolation of bacteria from clinical specimens: 13 years of experience. J Clin Microbiol 43: 4993–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelakis E, Richet H, Rolain JM, La SB, Raoult D, 2012. Comparison of real-time quantitative PCR and culture for the diagnosis of emerging Rickettsioses. PLoS Negl Trop Dis 6: e1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duron O, et al. 2015. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog 11: e1004892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray T, Feder HM, Jr, 2001. Management of tick bites and early Lyme disease: a survey of Connecticut physicians. Pediatrics 108: 1367–1370. [DOI] [PubMed] [Google Scholar]
- 14.Angelakis E, Richet H, Raoult D, 2016. Rickettsia sibirica mongolitimonae infection, France, 2010–2014. Emerg Infect Dis 22: 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parola P, Rovery C, Rolain JM, Brouqui P, Davoust B, Raoult D, 2009. Rickettsia slovaca and R. raoultii in tick-borne rickettsioses. Emerg Infect Dis 15: 1105–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S, 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.